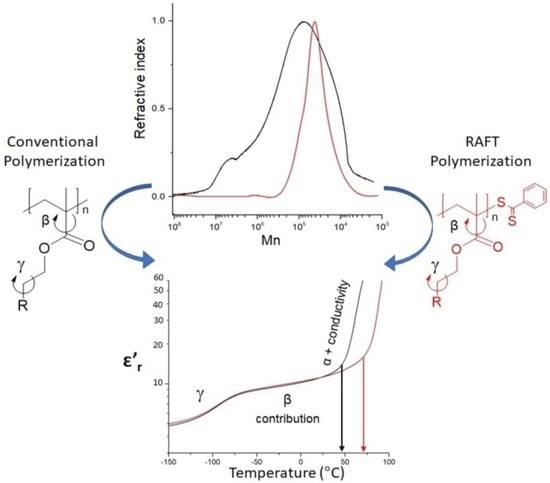
Molecular Weight Enables Fine-Tuning the Thermal and Dielectric Properties of Polymethacrylates Bearing Sulfonyl and Nitrile Groups as Dipolar Entities
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
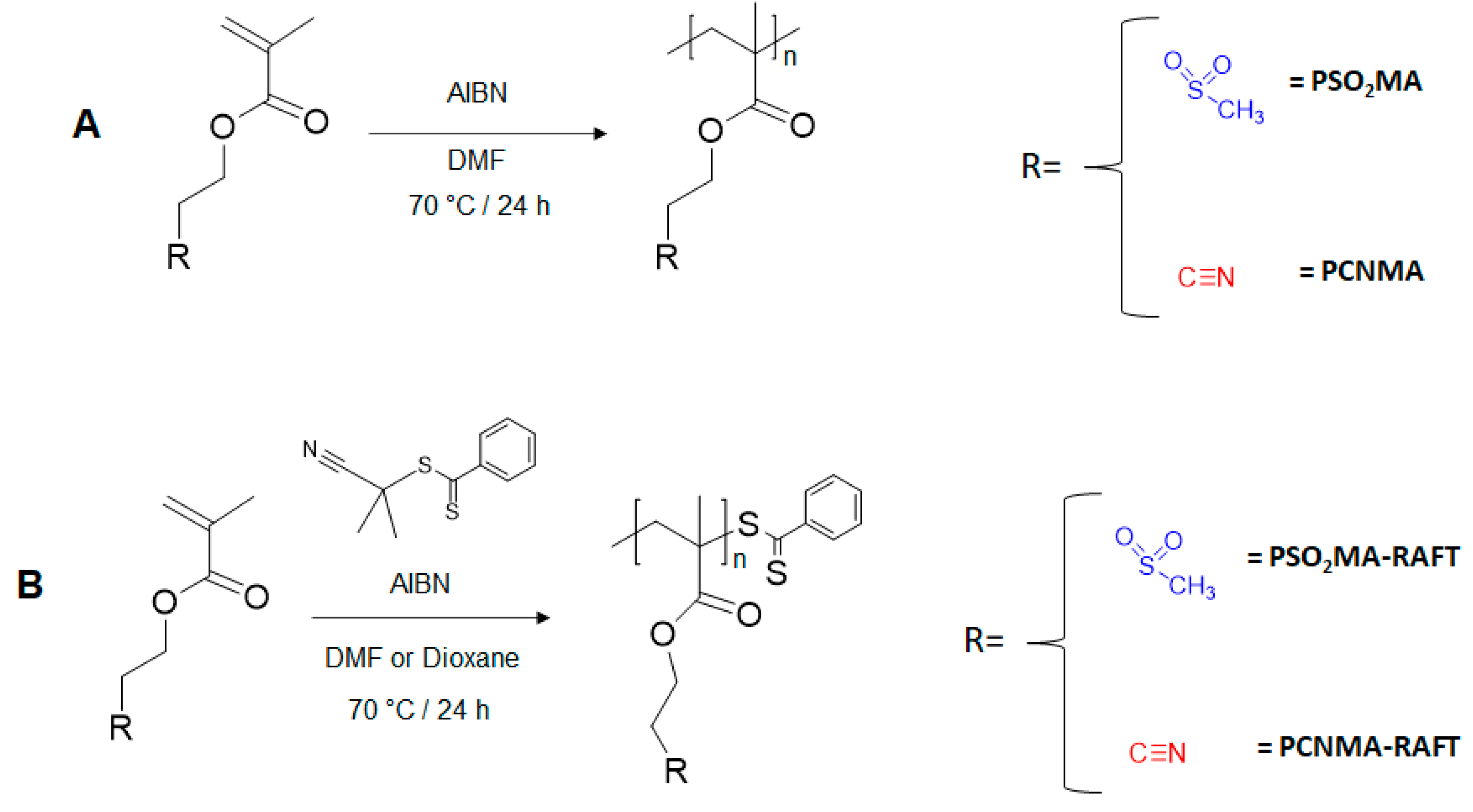
2.2. Monomer Synthesis
2.3. Polymer Synthesis
2.3.1. Conventional Radical Polymerization of Poly(2-cyanoethyl methacrylate) (PCNMA) and Poly(2-(methylsulfonyl)ethyl methacrylate) (PSO2MA)
2.3.2. Reversible Addition-Fragmentation Chain-Transfer Polymerization of Poly(2-cyanoethyl methacrylate) (PCNMA-RAFT) and Poly(2-(methylsulfonyl)ethyl methacrylate) (PSO2MA-RAFT)
2.4. Characterization Techniques
3. Results and Discussion
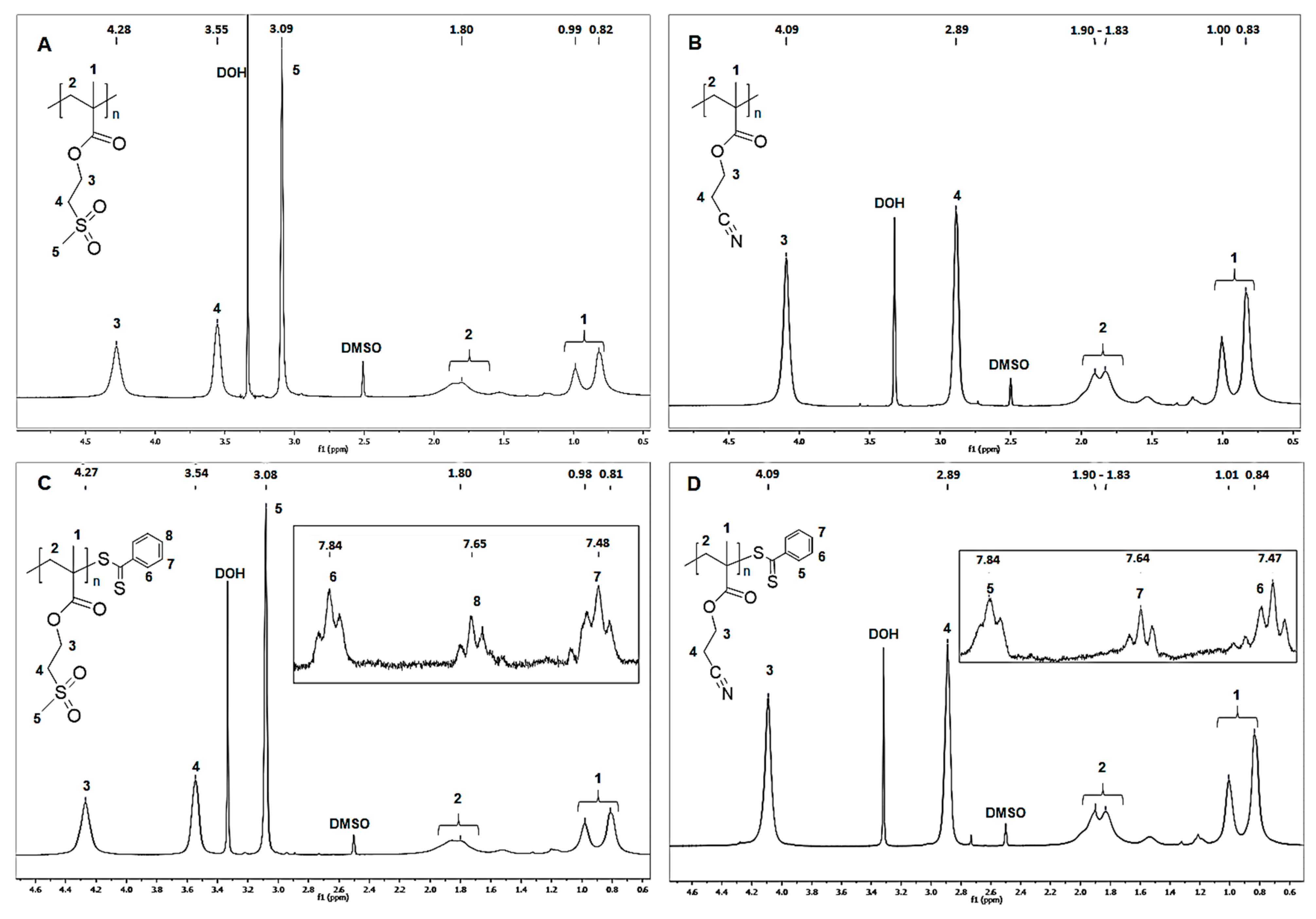
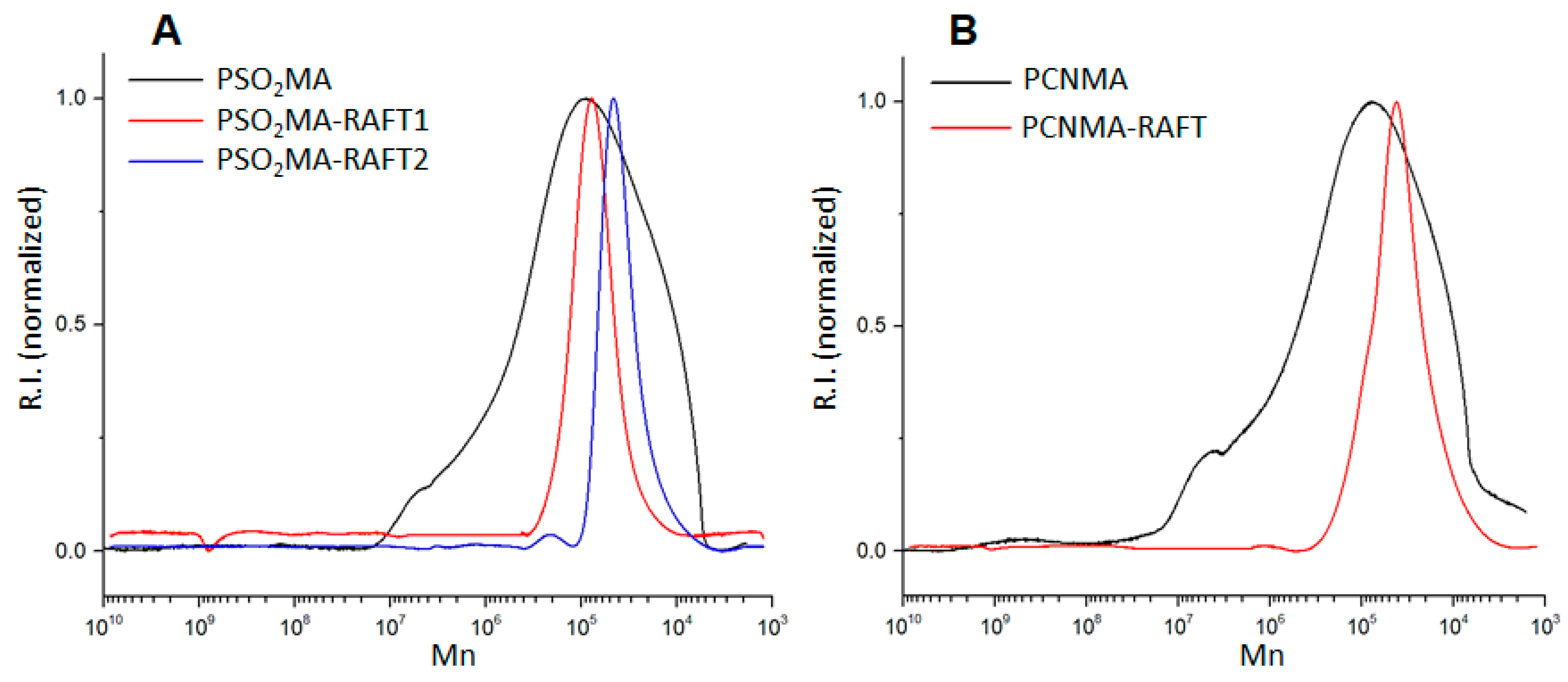
3.1. Structural Characterization
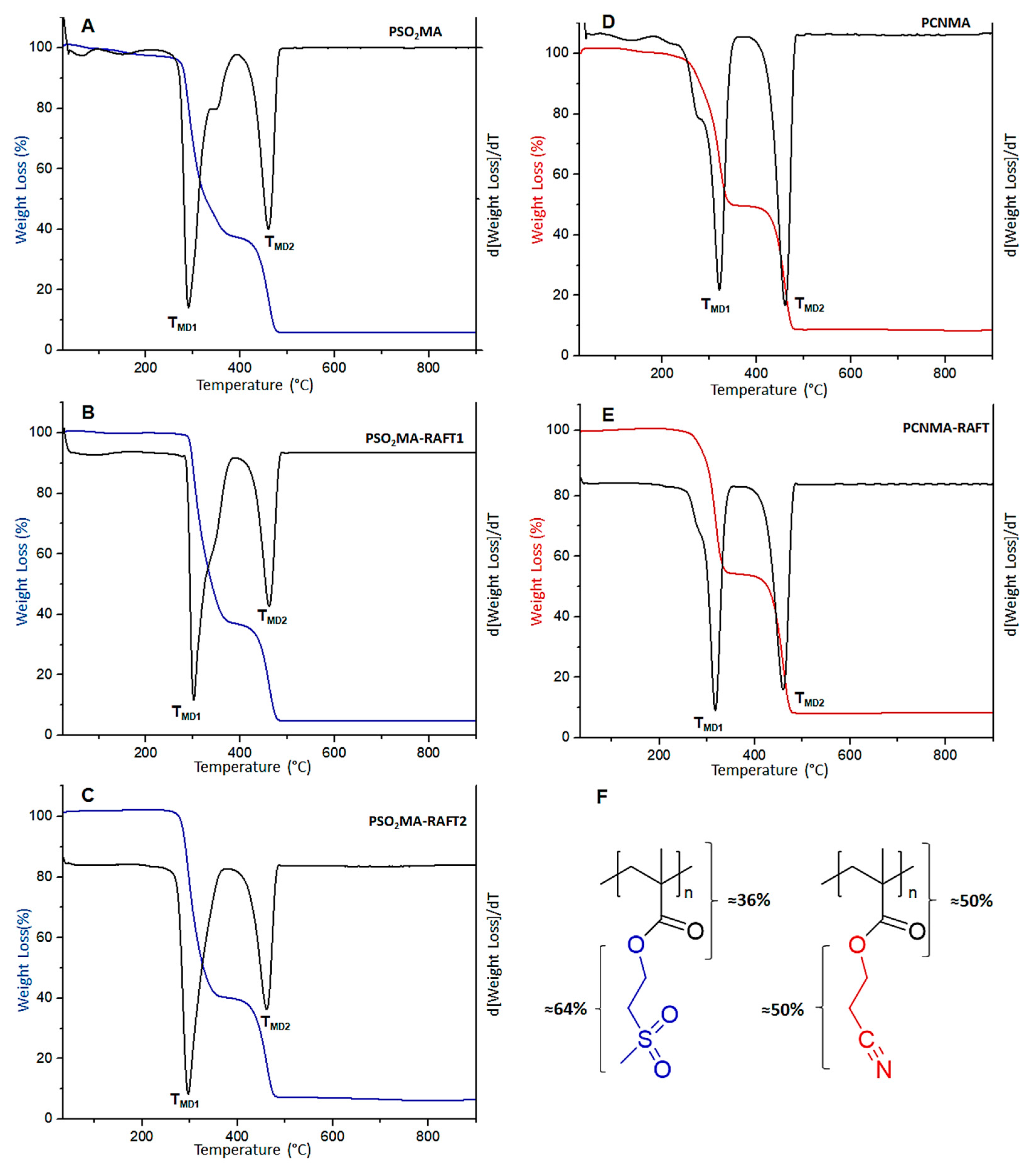
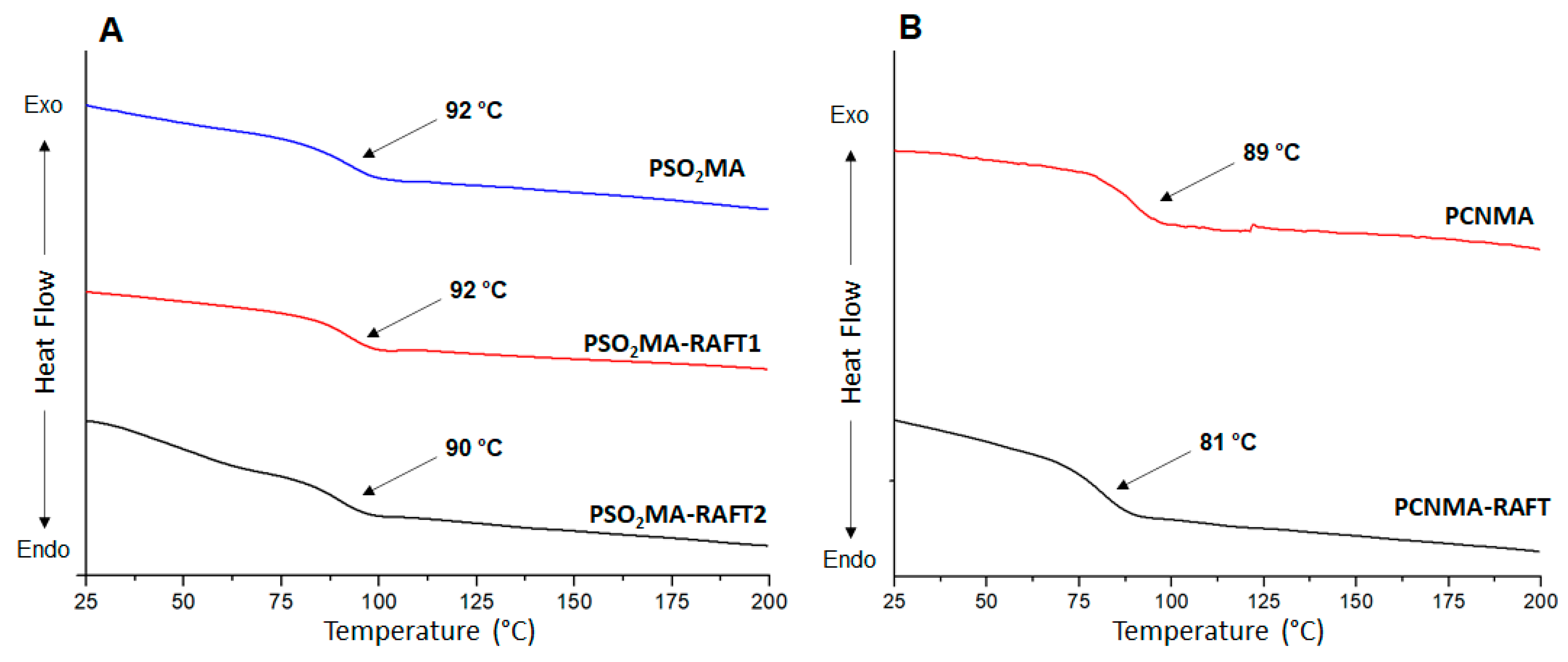
3.2. Thermal Characterization
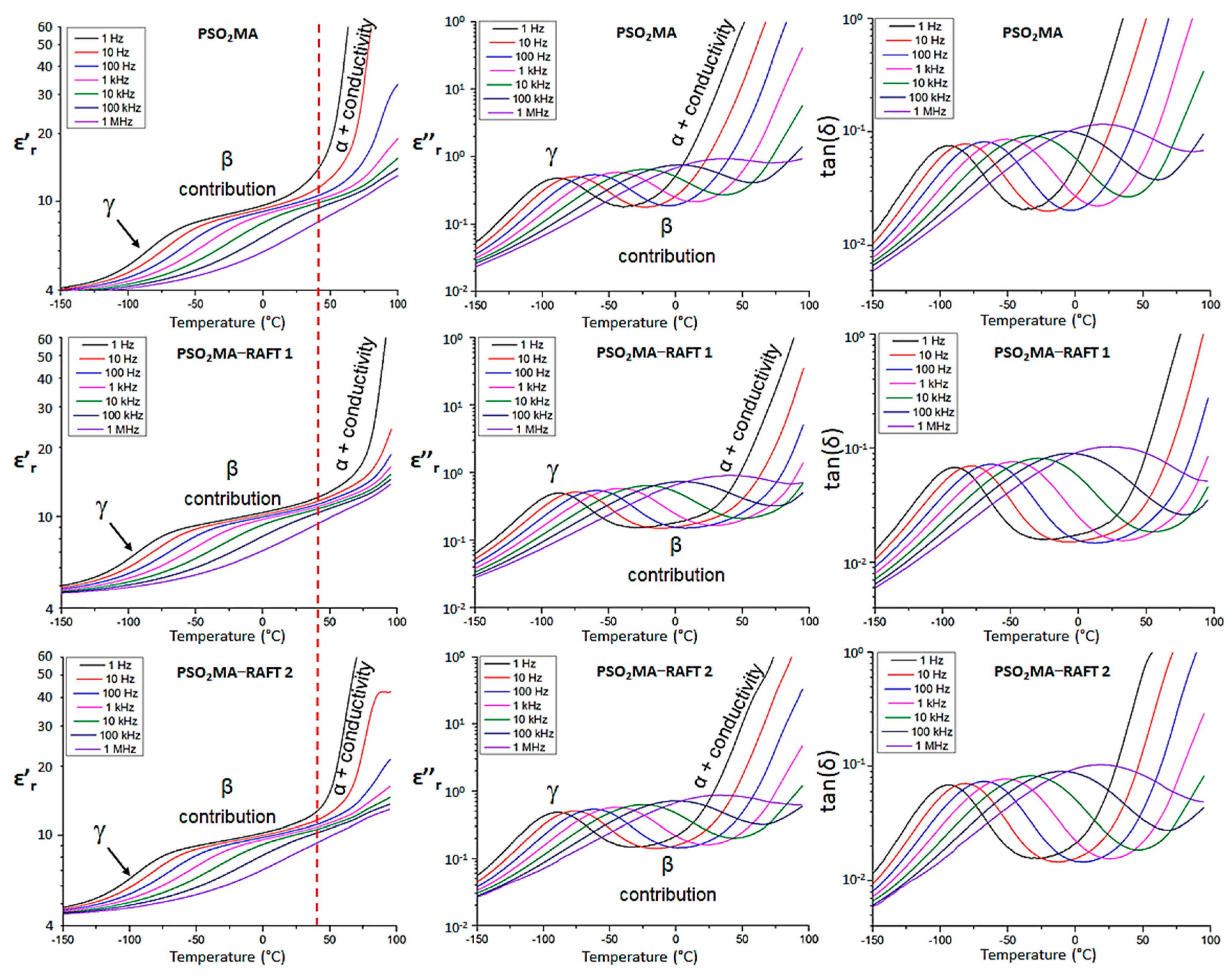
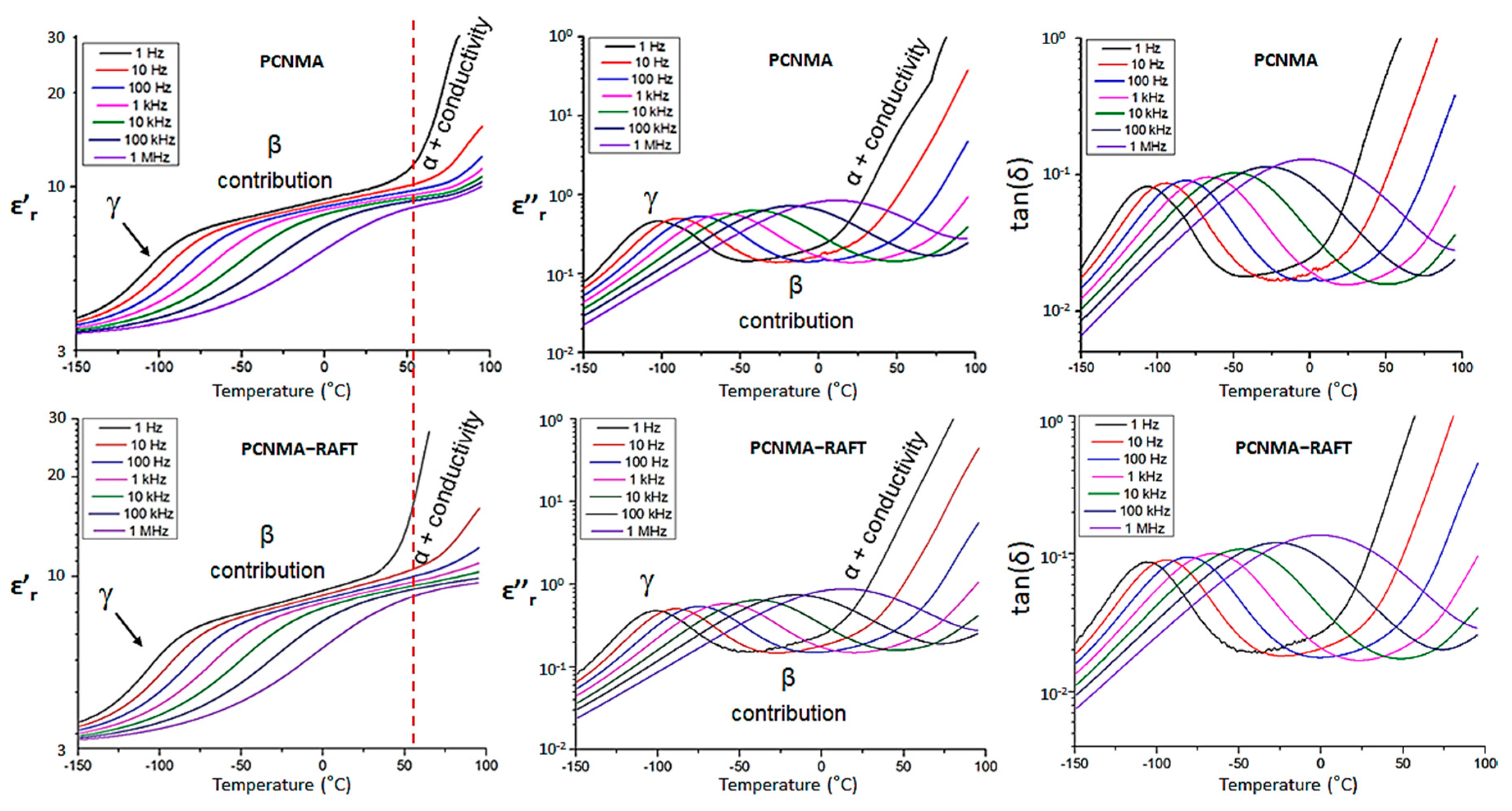
3.3. Dielectric Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, J.; Zhang, Z.; Tseng, J.-K.; Treufeld, I.; Liu, X.; Litt, M.H.; Zhu, L. Achieving High Dielectric Constant and Low Loss Property in a Dipolar Glass Polymer Containing Strongly Dipolar and Small-Sized Sulfone Groups. ACS Appl. Mater. Interfaces 2015, 7, 5248–5257. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, M.; Zhao, Y.; Zhang, X.; Lu, J.; Zhang, Z. Manipulating H-bonds in glassy dipolar polymers as a new strategy for high energy storage capacitors with high pulse discharge efficiency. J. Mater. Chem. A 2019, 7, 19407–19414. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Z.; Pejić, S.; Li, R.; Fukuto, M.; Zhu, L.; Sauvé, G. High dielectric constant semiconducting poly (3-alkylthiophene) s from side chain modification with polar sulfinyl and sulfonyl groups. Macromolecules 2018, 51, 9368–9381. [Google Scholar] [CrossRef]
- Bonardd, S.; Alegria, Á.; Ramirez, O.; Saldías, C.; Leiva, Á.; Kortaberria, G. New poly (itaconate) s with bulky pendant groups as candidates for “all-polymer” dielectrics. React. Funct. Polym. 2019, 140, 1–13. [Google Scholar] [CrossRef]
- Bonardd, S.; Alegria, Á.; Saldías, C.; Leiva, Á.; Kortaberria, G. Synthesis of new poly (itaconate) s containing nitrile groups as high dipolar moment entities for the development of dipolar glass polymers with increased dielectric constant. Thermal and dielectric characterization. Eur. Polym. J. 2019, 114, 19–31. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, J.; Premasiri, K.; Kwok, M.-H.; Li, Q.; Li, R.; Zhang, S.; Litt, M.H.; Gao, X.P.A.; Zhu, L. High-κ polymers of intrinsic microporosity: A new class of high temperature and low loss dielectrics for printed electronics. Mater. Horizons 2020, 7, 592–597. [Google Scholar] [CrossRef]
- Bonardd, S.; Alegria, A.; Saldias, C.; Leiva, A.; Kortaberria, G. Polyitaconates: A new family of “all-polymer” dielectrics. ACS Appl. Mater. Interfaces 2018, 10, 38476–38492. [Google Scholar] [CrossRef]
- Wei, J.; Meng, X.; Chen, X.; Bai, Y.; Song, J.; Yan, N.; Zhu, L.; Shen, A. Facile synthesis of fluorinated poly (arylene ether nitrile) and its dielectric properties. J. Appl. Polym. Sci. 2018, 135, 46837. [Google Scholar] [CrossRef]
- Tong, H.; Fu, J.; Ahmad, A.; Fan, T.; Hou, Y.; Xu, J. Sulfonyl-Containing Polyimide Dielectrics with Advanced Heat Resistance and Dielectric Properties for High-Temperature Capacitor Applications. Macromol. Mater. Eng. 2019, 304, 1800709. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, D.H.; Litt, M.H.; Tan, L.; Zhu, L. High-temperature and high-energy-density dipolar glass polymers based on sulfonylated poly (2, 6-dimethyl-1, 4-phenylene oxide). Angew. Chem. 2018, 130, 1544–1547. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Zhang, Z.; Litt, M.H.; Zhu, L. High Dielectric Constant Sulfonyl-Containing Dipolar Glass Polymers with Enhanced Orientational Polarization. Macromolecules 2018. [Google Scholar] [CrossRef]
- Zhu, L. Exploring Strategies for High Dielectric Constant and Low Loss Polymer Dielectrics. J. Phys. Chem. Lett. 2014, 5, 3677–3687. [Google Scholar] [CrossRef] [PubMed]
- Baer, E.; Zhu, L. 50th Anniversary Perspective: Dielectric Phenomena in Polymers and Multilayered Dielectric Films. Macromolecules 2017, 50, 2239–2256. [Google Scholar] [CrossRef]
- Nasreen, S.; Treich, G.M.; Baczkowski, M.L.; Mannodi-Kanakkithodi, A.K.; Cao, Y.; Ramprasad, R.; Sotzing, G. Polymer Dielectrics for Capacitor Application. Kirk Othmer Encycl. Chem. Technol. 2000, 1–29. [Google Scholar] [CrossRef]
- Qiao, Y.; Yin, X.; Zhu, T.; Li, H.; Tang, C. Dielectric polymers with novel chemistry, compositions and architectures. Prog. Polym. Sci. 2018, 80, 153–162. [Google Scholar] [CrossRef]
- Fan, B.; Zhou, M.; Zhang, C.; He, D.; Bai, J. Polymer-based materials for achieving high energy density film capacitors. Prog. Polym. Sci. 2019, 97, 101143. [Google Scholar] [CrossRef]
- Bonardd, S.; Moreno-Serna, V.; Kortaberria, G.; Díaz Díaz, D.; Leiva, A.; Saldías, C. Dipolar glass polymers containing polarizable groups as dielectric materials for energy storage applications. A Minireview. Polymers 2019, 11, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Zhu, L. Intrinsic polymer dielectrics for high energy density and low loss electric energy storage. Prog. Polym. Sci. 2020, 101254. [Google Scholar] [CrossRef]
- Cowie, J.M.G.; Arrighi, V. Polymers: Chemistry and Physics of Modern Materials; CRC Press: Boca Raton, FL, USA, 2007; ISBN 1420009877. [Google Scholar]
- Bonardd, S.; Alegria, Á.; Saldías, C.; Leiva, Á.; Kortaberria, G. Increasing the temperature range of dipolar glass polymers through copolymerization: A first approach to dipolar glass copolymers. Polymer 2020, 203, 122765. [Google Scholar] [CrossRef]
- Wang, D.H.; Kurish, B.A.; Treufeld, I.; Zhu, L.; Tan, L. Synthesis and characterization of high nitrile content polyimides as dielectric films for electrical energy storage. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 422–436. [Google Scholar] [CrossRef]
- Treufeld, I.; Wang, D.H.; Kurish, B.A.; Tan, L.-S.; Zhu, L. Enhancing electrical energy storage using polar polyimides with nitrile groups directly attached to the main chain. J. Mater. Chem. A 2014, 2, 20683–20696. [Google Scholar] [CrossRef]
- Dünki, S.J.; Cuervo-Reyes, E.; Opris, D.M. A facile synthetic strategy to polysiloxanes containing sulfonyl side groups with high dielectric permittivity. Polym. Chem. 2017, 8, 715–724. [Google Scholar] [CrossRef] [Green Version]
- Bonardd, S.; Robles, E.; Barandiaran, I.; Saldías, C.; Leiva, Á.; Kortaberria, G. Biocomposites with increased dielectric constant based on chitosan and nitrile-modified cellulose nanocrystals. Carbohydr. Polym. 2018, 199, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Bendler, J.T.; Boyles, D.A.; Edmondson, C.A.; Filipova, T.; Fontanella, J.J.; Westgate, M.A.; Wintersgill, M.C. Dielectric properties of bisphenol A polycarbonate and its tethered nitrile analogue. Macromolecules 2013, 46, 4024–4033. [Google Scholar] [CrossRef]
- Corrigan, N.; Jung, K.; Moad, G.; Hawker, C.J.; Matyjaszewski, K.; Boyer, C. Reversible-deactivation radical polymerization (Controlled/living radical polymerization): From discovery to materials design and applications. Prog. Polym. Sci. 2020, 111, 101311. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization—A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Keddie, D.J. A guide to the synthesis of block copolymers using reversible-addition fragmentation chain transfer (RAFT) polymerization. Chem. Soc. Rev. 2014, 43, 496–505. [Google Scholar] [CrossRef]
- Fujii, S.; McCarthy, T.J. Sulfone-containing methacrylate homopolymers: Wetting and thermal properties. Langmuir 2016, 32, 765–771. [Google Scholar] [CrossRef]
- Sánchez-Chaves, M.; Arranz, F. Synthesis of amidoxime-containing modified dextran. Polymer 1996, 37, 4403–4407. [Google Scholar] [CrossRef]
- Jo, W.H.; Cruz, C.A.; Paul, D.R. FTIR investigation of interactions in blends of PMMA with a styrene/acrylic acid copolymer and their analogs. J. Polym. Sci. Part B Polym. Phys. 1989, 27, 1057–1076. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, J.P.; Sekhon, S.S. FTIR study of ion dissociation in PMMA based gel electrolytes containing ammonium triflate: Role of dielectric constant of solvent. Eur. Polym. J. 2005, 41, 2718–2725. [Google Scholar] [CrossRef]
- Koiry, B.P.; Ponnupandian, S.; Choudhury, S.; Singha, N.K. Syntheses and morphologies of fluorinated diblock copolymer prepared via RAFT polymerization. J. Fluor. Chem. 2016, 189, 51–58. [Google Scholar] [CrossRef]
- Mccord, E.F.; Anton, W.L.; Wilczek, L.; Ittel, S.D.; Nelson, L.T.J.; Raffell, K.D.; Hansen, J.E.; Berge, C. 1H and 13C NMR of PMMA macromonomers and oligomers-end groups and tacticity. In Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA; Hüthig & Wepf Verlag: Basel, Switzerland, 1994; Volume 86, pp. 47–64. [Google Scholar]
- Quinting, G.R.; Cai, R. High-Resolution NMR Analysis of the Tacticity of Poly (n-butyl methacrylate). Macromolecules 1994, 27, 6301–6306. [Google Scholar] [CrossRef]
- Ferguson, R.C.; Ovenall, D.W. High resolution NMR analysis of the stereochemistry of poly (methyl methacrylate). Macromolecules 1987, 20, 1245–1248. [Google Scholar] [CrossRef]
- Park, J.H.; Hwang, D.K.; Lee, J.; Im, S.; Kim, E. Studies on poly(methyl methacrylate) dielectric layer for field effect transistor: Influence of polymer tacticity. Thin Solid Films 2007, 515, 4041–4044. [Google Scholar] [CrossRef]
- Li, Q.; Yao, F.-Z.; Liu, Y.; Zhang, G.; Wang, H.; Wang, Q. High-Temperature Dielectric Materials for Electrical Energy Storage. Annu. Rev. Mater. Res. 2018, 48, 219–243. [Google Scholar] [CrossRef]
- Schnabel, W. Polymer Degradation: Principles and Practical Applications; Carl Hanser Verlag: Munich, Germany, 1981. [Google Scholar]
- Areizaga, J.; Milagros Cortázar, M.; Elorza, J.M.; Iruin, J.J. Polímeros; Editorial Síntesis: Madrid, Spain, 2002; ISBN 9788497560269. [Google Scholar]
- Snegirev, A.Y.; Talalov, V.A.; Stepanov, V.V.; Korobeinichev, O.P.; Gerasimov, I.E.; Shmakov, A.G. Autocatalysis in thermal decomposition of polymers. Polym. Degrad. Stab. 2017, 137, 151–161. [Google Scholar] [CrossRef]
- Ye, L.; Liu, M.; Huang, Y.; Zhang, Z.; Yang, J. Effects of Molecular Weight on Thermal Degradation of Poly (α-methyl styrene) in Nitrogen. J. Macromol. Sci. Part B 2015, 54, 1479–1494. [Google Scholar] [CrossRef]
- Inaba, A.; Kashiwagi, T.; Brown, J.E. Effects of initial molecular weight on thermal degradation of poly (methyl methacrylate): Part 1—model 1. Polym. Degrad. Stab. 1988, 21, 1–20. [Google Scholar] [CrossRef]
- Rogers, S.; Mandelkern, L. Glass transitions of the poly-(n-alkyl methacrylates). J. Phys. Chem. 1957, 61, 985–991. [Google Scholar] [CrossRef]
- Young, R.J.; Lovell, P.A. Introduction to Polymers; CRC Press: Boca Raton, FL, USA, 2011; ISBN 1439894159. [Google Scholar]
- Fox, T.G.; Flory, P.J. The glass temperature and related properties of polystyrene. Influence of molecular weight. J. Polym. Sci. 1954, 14, 315–319. [Google Scholar] [CrossRef]
- Cowie, J.M.G.; Ferguson, R. Possible origins of the β-relaxation in poly (methyl methacrylate) and related structures from molecular mechanics calculations. Polymer 1987, 28, 503–508. [Google Scholar] [CrossRef]
- McCrum, N.; Read, B.; Williams, G. Anelastic and Dielectric Effects in Polymeric Solids; Wiley Subscription Services, Inc., Wiley Company: New York, NY, USA, 1967. [Google Scholar]
- Runt, J.P.; Fitzgerald, J.J. Dielectric Spectroscopy of Polymeric Materials; American Chemical Society: Washington, DC, USA, 1997; ISBN 0841233357. [Google Scholar]
- Blythe, T.; Bloor, D. Electrical Properties of Polymers; Cambridge University Press: Cambridge, UK, 2005; Volume 11, ISBN 9780521552196. [Google Scholar]
- Ku, C.C.; Liepins, R. Electrical Properties of Polymers—Chemical Principles; Carl Hanser Verlag: Munich, Germany, 1988; ISBN 0-446-14280-0. [Google Scholar]
- Claude, J.; Lu, Y.; Wang, Q. Effect of molecular weight on the dielectric breakdown strength of ferroelectric poly (vinylidene fluoride-chlorotrifluoroethylene) s. Appl. Phys. Lett. 2007, 91, 212984. [Google Scholar] [CrossRef]

| PSO2MA | PSO2MA−RAFT1 | PSO2MA−RAFT2 | PCNMA | PCNMA−RAFT | |
|---|---|---|---|---|---|
| Syndiotactic | 62 | 57 | 59 | 62 | 64 |
| atactic | 35 | 37 | 35 | 34 | 33 |
| isotactic | 3 | 6 | 6 | 4 | 3 |
| Sample | Mn (g/mol) | Đ (Mw/Mn) |
|---|---|---|
| PSO2MA | 1.23 × 105 | 2.18 |
| PSO2MA−RAFT1 | 7.10 × 104 | 1.18 |
| PSO2MA−RAFT2 | 3.94 × 104 | 1.12 |
| PCNMA | 1.13 × 105 | 2.36 |
| PCNMA−RAFT | 3.25 × 104 | 1.23 |
| Sample | Ti (°C) | TMD1 (°C) | TMD2 (°C) | Residue (%) |
|---|---|---|---|---|
| PSO2MA | 280 | 291 | 460 | 5.8 |
| PSO2MA−RAFT1 | 295 | 301 | 461 | 4.9 |
| PSO2MA−RAFT2 | 283 | 297 | 461 | 6.4 |
| PCNMA | 265 | 320 | 460 | 8.5 |
| PCNMA−RAFT | 282 | 317 | 461 | 8.4 |
| Sample | ε’r (1 Hz) | ε’r (1 kHz) | tan(δ) (1 kHz) |
|---|---|---|---|
| PSO2MA | 11.9 | 10.0 | 0.020 |
| PSO2MA−RAFT1 | 11.3 | 10.3 | 0.016 |
| PSO2MA−RAFT2 | 11.3 | 10.5 | 0.016 |
| PCNMA | 9.8 | 9.0 | 0.016 |
| PCNMA−RAFT | 9.9 | 8.9 | 0.017 |
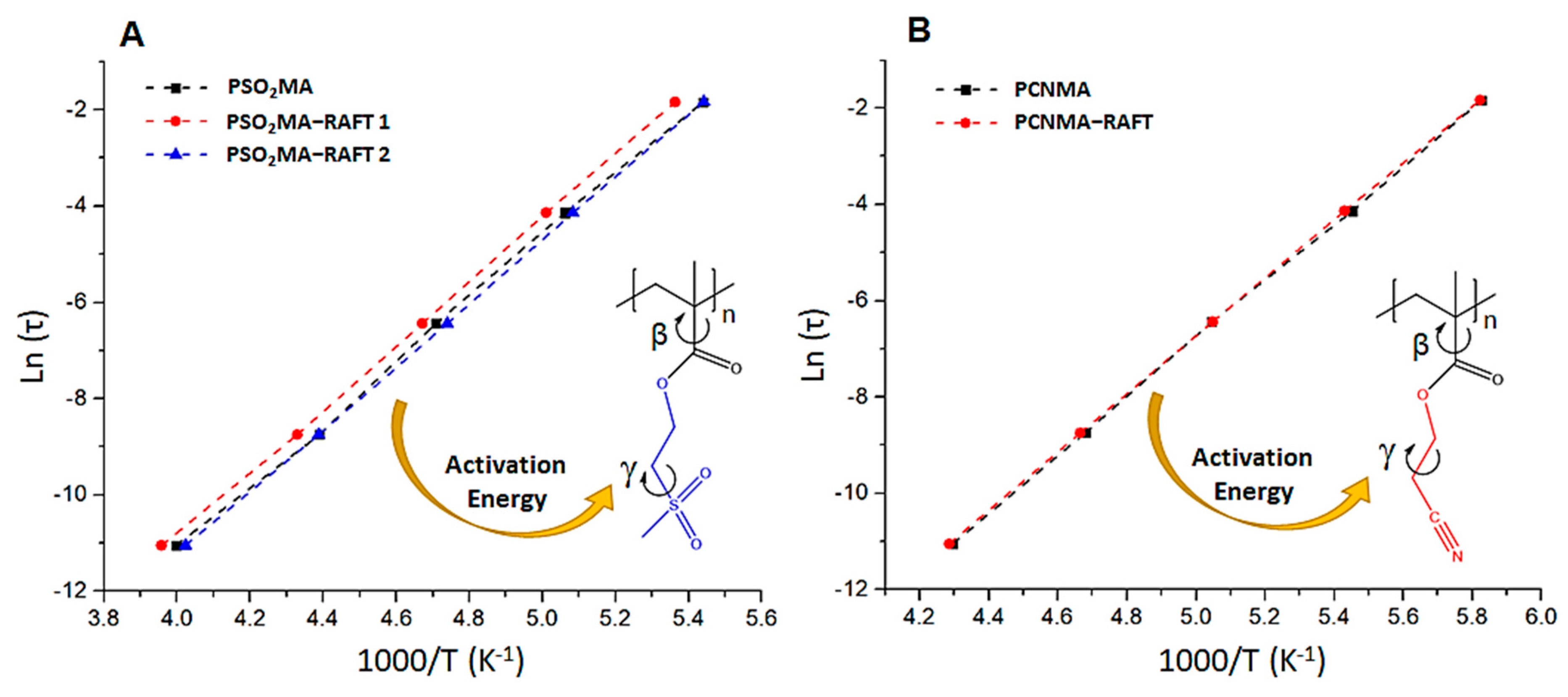
| Sample | γ Transition Temperature (°C) a | Onset Temperature of Paraelectric State (°C) b | Dipolar Glass Polymer Temperature Range (°C) c |
|---|---|---|---|
| PSO2MA | −89 | 48 | 137 |
| PSO2MA-RAFT1 | −87 | 76 | 163 |
| PSO2MA-RAFT2 | −89 | 49 | 138 |
| PCNMA | −101 | 47 | 148 |
| PCNMA-RAFT | −101 | 42 | 138 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonardd, S.; Saldías, C.; Leiva, Á.; Díaz Díaz, D.; Kortaberria, G. Molecular Weight Enables Fine-Tuning the Thermal and Dielectric Properties of Polymethacrylates Bearing Sulfonyl and Nitrile Groups as Dipolar Entities. Polymers 2021, 13, 317. https://doi.org/10.3390/polym13030317
Bonardd S, Saldías C, Leiva Á, Díaz Díaz D, Kortaberria G. Molecular Weight Enables Fine-Tuning the Thermal and Dielectric Properties of Polymethacrylates Bearing Sulfonyl and Nitrile Groups as Dipolar Entities. Polymers. 2021; 13(3):317. https://doi.org/10.3390/polym13030317
Chicago/Turabian StyleBonardd, Sebastian, Cesar Saldías, Ángel Leiva, David Díaz Díaz, and Galder Kortaberria. 2021. "Molecular Weight Enables Fine-Tuning the Thermal and Dielectric Properties of Polymethacrylates Bearing Sulfonyl and Nitrile Groups as Dipolar Entities" Polymers 13, no. 3: 317. https://doi.org/10.3390/polym13030317
APA StyleBonardd, S., Saldías, C., Leiva, Á., Díaz Díaz, D., & Kortaberria, G. (2021). Molecular Weight Enables Fine-Tuning the Thermal and Dielectric Properties of Polymethacrylates Bearing Sulfonyl and Nitrile Groups as Dipolar Entities. Polymers, 13(3), 317. https://doi.org/10.3390/polym13030317