3D Bioprinting of Polycaprolactone-Based Scaffolds for Pulp-Dentin Regeneration: Investigation of Physicochemical and Biological Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of BG Powder
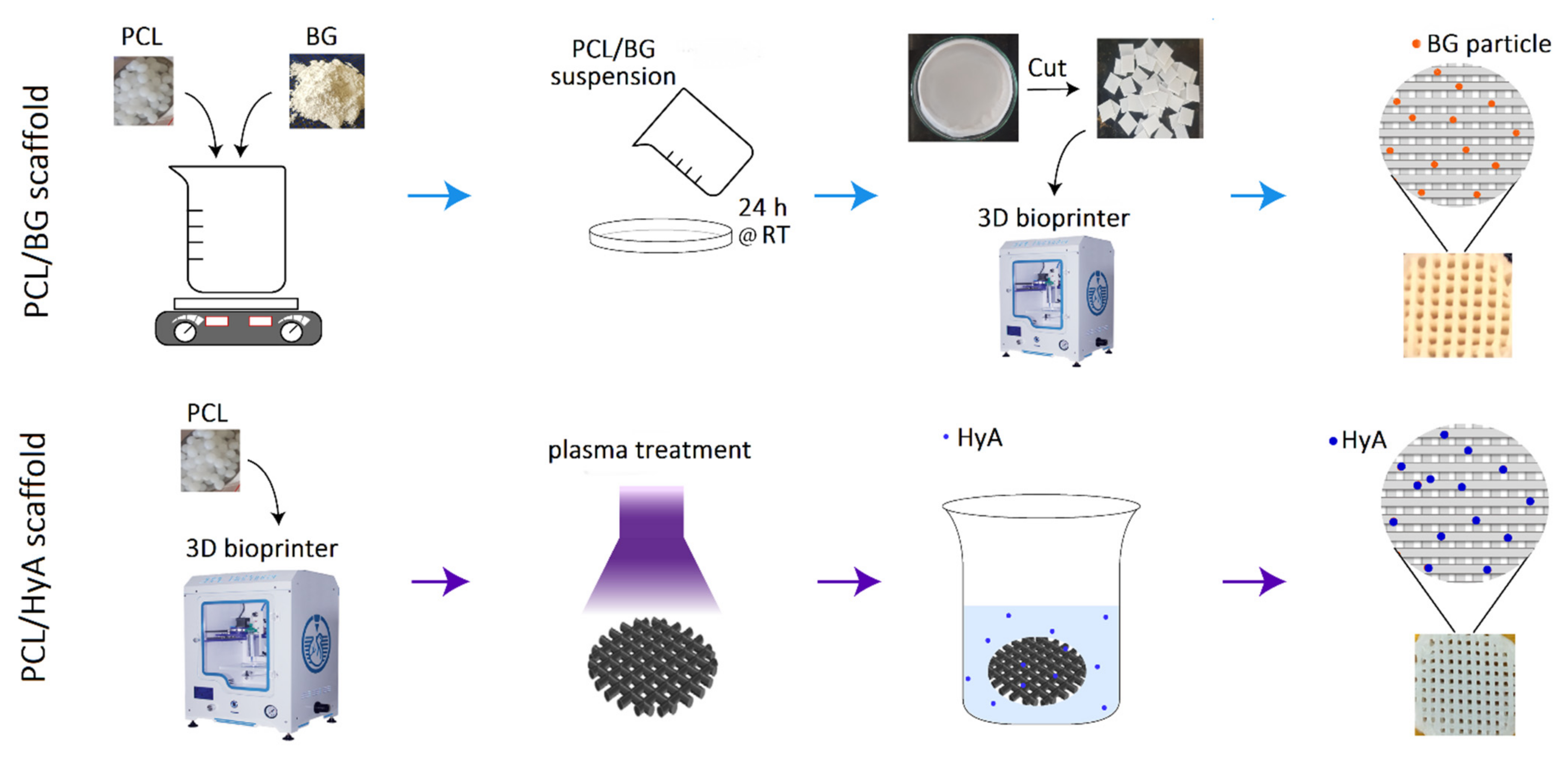
2.2. Fabrication of 3D-Printed PCL, PCL/HyA, and PCL/BG Scaffolds
2.3. Characterization of 45S5 Bioglass Powder
2.4. Characterization of Scaffolds
2.5. In Vitro Cell Viability Assay
2.6. Cell Adhesion Assay
2.7. Gene Expression Analysis
2.8. Statistical Analysis
3. Results and Discussion
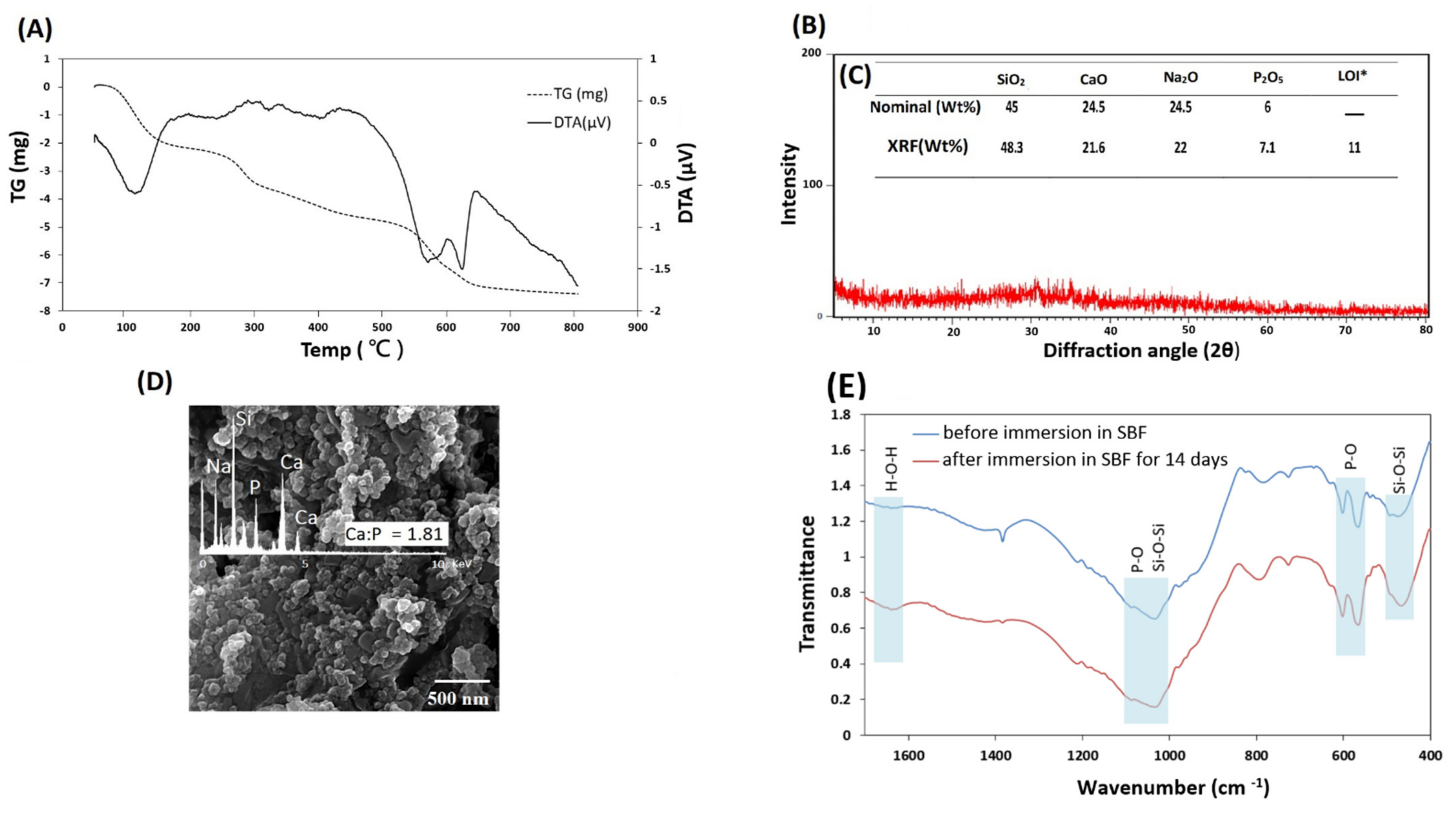
3.1. Characterization of 45S5 Bioglass Powder
3.2. Physicocheimical Characterization of Scaffolds
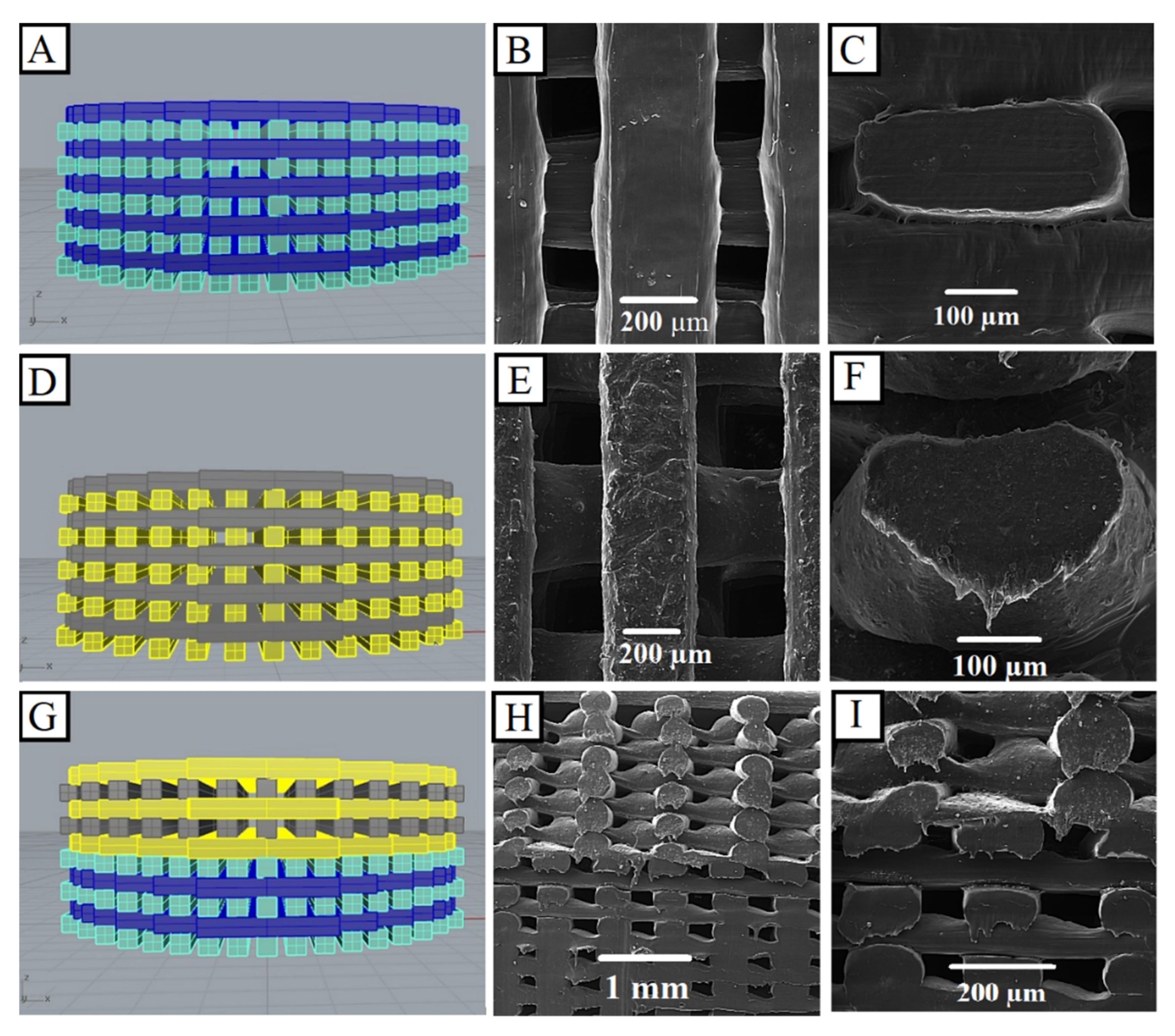
3.2.1. Morphology Observations
3.2.2. Atomic Force Microscopy (AFM)
3.2.3. Static Water Contact Angle
3.2.4. Mechanical Properties of 3D-Printed Scaffolds
3.3. Cytotoxicity Assay
3.4. Cell Adhesion and Morphology Assay
3.5. Gene Expression Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, C.; Kim, S.; Sun, T.; Cho, Y.-B.; Song, M. Pulp-dentin regeneration: Current approaches and challenges. J. Tissue Eng. 2019, 10, 2041731418819263. [Google Scholar] [CrossRef] [Green Version]
- EzEldeen, M.; Loos, J.; Mousavi Nejad, Z.; Cristaldi, M.; Murgia, D.; Braem, A.; Jacobs, R. 3D-printing-assisted fabrication of chitosan scaffolds from different sources and cross-linkers for dental tissue engineering. Eur. Cell Mater. 2021, 41, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Alshehadat, S.A.; Thu, H.A.; Hamid, S.S.A.; Nurul, A.A.; Rani, S.A.; Ahmad, A. Scaffolds for dental pulp tissue regeneration: A review. Int. Dent. Med J. Adv. Res. 2016, 2, 1–12. [Google Scholar]
- Shahrezaie, M.; Moshiri, A.; Shekarchi, B.; Oryan, A.; Maffulli, N.; Parvizi, J. Effectiveness of tissue engineered three-dimensional bioactive graft on bone healing and regeneration: An in vivo study with significant clinical value. J. Tissue Eng. Regen. Med. 2018, 12, 936–960. [Google Scholar] [CrossRef]
- Moshiri, A.; Oryan, A.; Shahrezaee, M. An overview on bone tissue engineering and regenerative medicine: Current challenges, future directions and strategies. J. Sports Med. Doping Stud. 2015, 5, e144. [Google Scholar]
- Sohrabian, M.; Vaseghi, M.; Khaleghi, H.; Dehrooyeh, S.; Kohan, M.S.A. Structural Investigation of Delicate-Geometry Fused Deposition Modeling Additive Manufacturing Scaffolds: Experiment and Analytics. J. Mater. Eng. Perform. 2021, 30, 6529–6541. [Google Scholar] [CrossRef]
- Ghorbani, F.; Sahranavard, M.; Mousavi Nejad, Z.; Li, D.; Zamanian, A.; Yu, B. Surface functionalization of three dimensional-printed polycaprolactone-bioactive glass scaffolds by grafting GelMA under UV irradiation. Front. Mater. 2020, 7, 348. [Google Scholar] [CrossRef]
- Mousavi, S.-M.; Nejad, Z.M.; Hashemi, S.A.; Salari, M.; Gholami, A.; Ramakrishna, S.; Chiang, W.-H.; Lai, C.W. Bioactive Agent-Loaded Electrospun Nanofiber Membranes for Accelerating Healing Process: A Review. Membranes 2021, 11, 702. [Google Scholar] [CrossRef]
- Wang, F.; Xie, C.; Ren, N.; Bai, S.; Zhao, Y. Human Freeze-dried Dentin Matrix as a Biologically Active Scaffold for Tooth Tissue Engineering. J. Endod. 2019, 45, 1321–1331. [Google Scholar] [CrossRef]
- Kanimozhi, K.; Basha, S.K.; Kaviyarasu, K.; SuganthaKumari, V. Salt leaching synthesis, characterization and in vitro cytocompatibility of chitosan/poly (vinyl alcohol)/methylcellulose–ZnO nanocomposites scaffolds using L929 fibroblast cells. J. Nanosci. Nanotechnol. 2019, 19, 4447–4457. [Google Scholar] [CrossRef] [PubMed]
- Sola, A.; Bertacchini, J.; D’Avella, D.; Anselmi, L.; Maraldi, T.; Marmiroli, S.; Messori, M. Development of solvent-casting particulate leaching (SCPL) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Mater. Sci. Eng. C 2019, 96, 153–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, F.; Yuan, Z.; Li, M.; Yu, F.; Fang, X.; Jiang, B.; Wen, Y.; Zhang, P. Expanded 3D nanofibre sponge scaffolds by gas-foaming technique enhance peripheral nerve regeneration. Artif. Cells Nanomed. Biotechnol. 2019, 47, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Pourhaghgouy, M.; Zamanian, A.; Shahrezaee, M.; Masouleh, M.P. Physicochemical properties and bioactivity of freeze-cast chitosan nanocomposite scaffolds reinforced with bioactive glass. Mater. Sci. Eng. C 2016, 58, 180–186. [Google Scholar] [CrossRef]
- Namdarian, P.; Zamanian, A.; Asefnejad, A.; Saeidifar, M. Evaluation of Freeze-Dry Chitosan-Gelatin Scaffolds with Olibanum Microspheres Containing Dexamethasone for Bone Tissue Engineering. Polym. Korea 2018, 42, 982–993. [Google Scholar] [CrossRef]
- Rashtchian, M.; Hivechi, A.; Bahrami, S.H.; Milan, P.B.; Simorgh, S. Fabricating alginate/poly (caprolactone) nanofibers with enhanced bio-mechanical properties via cellulose nanocrystal incorporation. Carbohydr. Polym. 2020, 233, 115873. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, Z.; Kordestani, S.; Kuth, S.; Schubert, D.W.; Detsch, R.; Roether, J.A.; Blunk, T.; Boccaccini, A.R. Preparation and Characterization of Electrospun Blend Fibrous Polyethylene Oxide: Polycaprolactone Scaffolds to Promote Cartilage Regeneration. Adv. Eng. Mater. 2020, 22, 2000131. [Google Scholar] [CrossRef]
- Sahranavard, M.; Zamanian, A.; Ghorbani, F.; Shahrezaee, M.H. A critical review on three dimensional-printed chitosan hydrogels for development of tissue engineering. Bioprinting 2019, 17, e00063. [Google Scholar] [CrossRef]
- McGivern, S.; Boutouil, H.; Al-Kharusi, G.; Little, S.; Dunne, N.J.; Levingstone, T.J. Translational application of 3D bioprinting for cartilage tissue engineering. Bioengineering 2021, 8, 144. [Google Scholar] [CrossRef]
- El Magri, A.; Vanaei, S.; Shirinbayan, M.; Vaudreuil, S.; Tcharkhtchi, A. An Investigation to Study the Effect of Process Parameters on the Strength and Fatigue Behavior of 3D-Printed PLA-Graphene. Polymers 2021, 13, 3218. [Google Scholar] [CrossRef]
- Vanaei, H.R.; Shirinbayan, M.; Deligant, M.; Khelladi, S.; Tcharkhtchi, A. In-Process Monitoring of Temperature Evolution during Fused Filament Fabrication: A Journey from Numerical to Experimental Approaches. Thermo 2021, 1, 332–360. [Google Scholar] [CrossRef]
- Vanaei, H.R.; Shirinbayan, M.; Vanaei, S.; Fitoussi, J.; Khelladi, S.; Tcharkhtchi, A. Multi-scale damage analysis and fatigue behavior of PLA manufactured by fused deposition modeling (FDM). Rapid Prototyp. J. 2021, 27, 371–378. [Google Scholar] [CrossRef]
- Vanaei, S.; Parizi, M.S.; Vanaei, S.; Salemizadehparizi, F.; Vanaei, H.R. An Overview on Materials and Techniques in 3D Bioprinting Toward Biomedical Application. Eng. Regen. 2021, 2, 1–18. [Google Scholar] [CrossRef]
- Hilkens, P.; Bronckaers, A.; Ratajczak, J.; Gervois, P.; Wolfs, E.; Lambrichts, I. The angiogenic potential of DPSCs and SCAPs in an in vivo model of dental pulp regeneration. Stem Cells Int. 2017, 2017, 2582080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Azmi, D.F.; Rosa, V.; Fawzy, A.S.; Fuh, J.Y.; Wong, Y.S.; Lu, W.F. Fabrication of dentin-like scaffolds through combined 3D printing and bio-mineralisation. Cogent Eng. 2016, 3, 1222777. [Google Scholar] [CrossRef]
- Athirasala, A.; Tahayeri, A.; Thrivikraman, G.; França, C.M.; Monteiro, N.; Tran, V.; Ferracane, J.; Bertassoni, L.E. A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication 2018, 10, 024101. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, E.; Rindone, A.; Dorafshar, A.; Grayson, W.L. Comparison of 3D-printed poly-ɛ-caprolactone scaffolds functionalized with tricalcium phosphate, hydroxyapatite, bio-oss, or decellularized bone matrix. Tissue Eng. Part A 2017, 23, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, F.; Zamanian, A.; Sahranavard, M. Mussel-inspired polydopamine-mediated surface modification of freeze-cast poly (ε-caprolactone) scaffolds for bone tissue engineering applications. Biomed. Eng. /Biomed. Tech. 2020, 65, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Nejad, Z.M.; Torabinejad, B.; Davachi, S.M.; Zamanian, A.; Garakani, S.S.; Najafi, F.; Nezafati, N. Synthesis, physicochemical, rheological and in-vitro characterization of double-crosslinked hyaluronic acid hydrogels containing dexamethasone and PLGA/dexamethasone nanoparticles as hybrid systems for specific medical applications. Int. J. Biol. Macromol. 2019, 126, 193–208. [Google Scholar] [CrossRef]
- Ahmadian, E.; Eftekhari, A.; Dizaj, S.M.; Sharifi, S.; Mokhtarpour, M.; Nasibova, A.N.; Khalilov, R.; Samiei, M. The effect of hyaluronic acid hydrogels on dental pulp stem cells behavior. Int. J. Biol. Macromol. 2019, 140, 245–254. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Kraxner, J.; Michalek, M.; Romero, A.R.; Elsayed, H.; Bernardo, E.; Boccaccini, A.R.; Galusek, D. Porous bioactive glass microspheres prepared by flame synthesis process. Mater. Lett. 2019, 256, 126625. [Google Scholar] [CrossRef]
- Bertuola, M.; Aráoz, B.; Gilabert, U.; Gonzalez-Wusener, A.; Pérez-Recalde, M.; Arregui, C.O.; Hermida, É.B. Gelatin–alginate–hyaluronic acid inks for 3D printing: Effects of bioglass addition on printability, rheology and scaffold tensile modulus. J. Mater. Sci. 2021, 56, 15327–15343. [Google Scholar] [CrossRef]
- Singh, B.N.; Veeresh, V.; Mallick, S.P.; Jain, Y.; Sinha, S.; Rastogi, A.; Srivastava, P. Design and evaluation of chitosan/chondroitin sulfate/nano-bioglass based composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Badr-Mohammadi, M.-R.; Hesaraki, S.; Zamanian, A. Mechanical properties and in vitro cellular behavior of zinc-containing nano-bioactive glass doped biphasic calcium phosphate bone substitutes. J. Mater. Sci. Mater. Med. 2014, 25, 185–197. [Google Scholar] [CrossRef]
- Feng, S.; Liu, J.; Ramalingam, M. 3D printing of stem cell responsive ionically-crosslinked polyethylene glycol diacrylate/alginate composite hydrogels loaded with basic fibroblast growth factor for dental pulp tissue engineering: A preclinical evaluation in animal model. J. Biomater. Tissue Eng. 2019, 9, 1635–1643. [Google Scholar] [CrossRef]
- Monteiro, N.; Smith, E.E.; Angstadt, S.; Zhang, W.; Khademhosseini, A.; Yelick, P.C. Dental cell sheet biomimetic tooth bud model. Biomaterials 2016, 106, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Kokubo, T.; Hata, K.; Nakamura, T.; Yamamuro, T. Apatite formation on ceramics, metals and polymers induced by a CaO SiO2 based glass in a simulated body fluid. In Bioceramics; Elsevier: Amsterdam, The Netherlands, 1991; pp. 113–120. [Google Scholar]
- Kılıç, S.; Okullu, S.Ö.; Kurt, Ö.; Sevinç, H.; Dündar, C.; Altınordu, F.; Türkoğlu, M. Efficacy of two plant extracts against acne vulgaris: Initial results of microbiological tests and cell culture studies. J. Cosmet. Dermatol. 2019, 18, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Salehi, G.; Behnamghader, A.; Pazouki, M.; Houshmand, B.; Mozafari, M. Synergistic reinforcement of glass-ionomer dental cements with silanized glass fibres. Mater. Technol. 2020, 35, 433–445. [Google Scholar] [CrossRef]
- Chatzistavrou, X.; Zorba, T.; Kontonasaki, E.; Chrissafis, K.; Koidis, P.; Paraskevopoulos, K. Following bioactive glass behavior beyond melting temperature by thermal and optical methods. Phys. Status Solidi 2004, 201, 944–951. [Google Scholar] [CrossRef]
- ElBatal, H.; Azooz, M.; Khalil, E.; Monem, A.S.; Hamdy, Y. Characterization of some bioglass–ceramics. Mater. Chem. Phys. 2003, 80, 599–609. [Google Scholar] [CrossRef]
- El-Ghannam, A.; Hamazawy, E.; Yehia, A. Effect of thermal treatment on bioactive glass microstructure, corrosion behavior, ζ potential, and protein adsorption. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2001, 55, 387–395. [Google Scholar]
- Lefebvre, L.; Chevalier, J.; Gremillard, L.; Zenati, R.; Thollet, G.; Bernache-Assolant, D.; Govin, A. Structural transformations of bioactive glass 45S5 with thermal treatments. Acta Mater. 2007, 55, 3305–3313. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Dehiya, B.S.; Sindhu, A.; Kumar, V. Synthesis and Characterization of Nano Bioglass for the Application of Bone Tissue Engineering. J. Nanosci. Technol. 2018, 4, 471–474. [Google Scholar] [CrossRef]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive glasses: Where are we and where are we going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnettler, R.; Alt, V.; Dingeldein, E.; Pfefferle, H.-J.; Kilian, O.; Meyer, C.; Heiss, C.; Wenisch, S. Bone ingrowth in bFGF-coated hydroxyapatite ceramic implants. Biomaterials 2003, 24, 4603–4608. [Google Scholar] [CrossRef]
- Chatzistavrou, X.; Velamakanni, S.; DiRenzo, K.; Lefkelidou, A.; Fenno, J.C.; Kasuga, T.; Boccaccini, A.R.; Papagerakis, P. Designing dental composites with bioactive and bactericidal properties. Mater. Sci. Eng. C 2015, 52, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, C.; Kushitani, H.; Kokubo, T.; Kotani, S.; Yamamuro, T. Apatite formation on the surface of Ceravital-type glass-ceramic in the body. J. Biomed. Mater. Res. 1991, 25, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.d.M.; Clark, A.; Hench, L. Calcium phosphate formation on sol-gel-derived bioactive glasses in vitro. J. Biomed. Mater. Res. 1994, 28, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Notingher, I.; Jones, J.; Verrier, S.; Bisson, I.; Embanga, P.; Edwards, P.; Polak, J.; Hench, L. Application of FTIR and Raman spectroscopy to characterisation of bioactive materials and living cells. J. Spectrosc. 2003, 17, 275–288. [Google Scholar] [CrossRef] [Green Version]
- Theodorou, G.; Goudouri, O.; Kontonasaki, E.; Chatzistavrou, X.; Papadopoulou, L.; Kantiranis, N.; Paraskevopoulos, K. Comparative bioactivity study of 45S5 and 58S bioglasses in organic and inorganic environment. Bioceram. Dev. Appl. 2011, 1, 1–4. [Google Scholar] [CrossRef]
- Brauer, D.S.; Karpukhina, N.; O’Donnell, M.D.; Law, R.V.; Hill, R.G. Fluoride-containing bioactive glasses: Effect of glass design and structure on degradation, pH and apatite formation in simulated body fluid. Acta Biomater. 2010, 6, 3275–3282. [Google Scholar] [CrossRef] [Green Version]
- Felisberto, M.D.; Laranjeira, M. Preparation and characterization of hydroxyapatite-coated iron oxide particles by spray-drying technique. An. Da Acad. Bras. De Ciências 2009, 81, 179–186. [Google Scholar]
- Fernandes, J.; Deus, A.M.; Reis, L.; Vaz, M.F.; Leite, M. Study of the influence of 3D printing parameters on the mechanical properties of PLA. In Proceedings of the 3rd International Conference on Progress in Additive Manufacturing (Pro-AM 2018), Singapore, 14–17 May 2018; pp. 547–552. [Google Scholar]
- Guarino, V.; Gloria, A.; Raucci, M.G.; De Santis, R.; Ambrosio*, L. Bio-inspired composite and cell instructive platforms for bone regeneration. Int. Mater. Rev. 2012, 57, 256–275. [Google Scholar] [CrossRef]
- Khiabani, A.B.; Ghanbari, A.; Yarmand, B.; Zamanian, A.; Mozafari, M. Improving corrosion behavior and in vitro bioactivity of plasma electrolytic oxidized AZ91 magnesium alloy using calcium fluoride containing electrolyte. Mater. Lett. 2018, 212, 98–102. [Google Scholar] [CrossRef]
- Syakur, A.; Sutanto, H. Determination of Hydrophobic Contact Angle of Epoxy Resin Compound Silicon Rubber and Silica. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Semarang, Indonesia, 23–25 November 2016; p. 012025. [Google Scholar]
- Bruyas, A.; Lou, F.; Stahl, A.M.; Gardner, M.; Maloney, W.; Goodman, S.; Yang, Y.P. Systematic characterization of 3D-printed PCL/β-TCP scaffolds for biomedical devices and bone tissue engineering: Influence of composition and porosity. J. Mater. Res. 2018, 33, 1948–1959. [Google Scholar] [CrossRef] [PubMed]
- Keivani, F.; Shokrollahi, P.; Zandi, M.; Irani, S.; Shokrolahi, F.; Khorasani, S. Engineered electrospun poly (caprolactone)/polycaprolactone-g-hydroxyapatite nano-fibrous scaffold promotes human fibroblasts adhesion and proliferation. Mater. Sci. Eng. C 2016, 68, 78–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bossard, C.; Granel, H.; Wittrant, Y.; Jallot, É.; Lao, J.; Vial, C.; Tiainen, H. Polycaprolactone/bioactive glass hybrid scaffolds for bone regeneration. Biomed. Glasses 2018, 4, 108–122. [Google Scholar] [CrossRef]
- Yu, H.S.; Park, J.; Lee, H.-S.; Park, S.A.; Lee, D.-W.; Park, K. Feasibility of polycaprolactone scaffolds fabricated by three-Dimensional printing for tissue engineering of tunica albuginea. World J. Men’s Health 2018, 36, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Seyedsalehi, A.; Daneshmandi, L.; Barajaa, M.; Riordan, J.; Laurencin, C.T. Fabrication and characterization of mechanically competent 3D printed polycaprolactone-reduced graphene oxide scaffolds. Sci. Rep. 2020, 10, 22210. [Google Scholar] [CrossRef]
- Roohani-Esfahani, S.; Nouri-Khorasani, S.; Lu, Z.; Appleyard, R.; Zreiqat, H. Effects of bioactive glass nanoparticles on the mechanical and biological behavior of composite coated scaffolds. Acta Biomater. 2011, 7, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Tamjid, E. Three-dimensional polycaprolactone-bioactive glass composite scaffolds: Effect of particle size and volume fraction on mechanical properties and in vitro cellular behavior. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 1005–1015. [Google Scholar] [CrossRef]
- Anderson, A.S. MTT Proliferation Assay. Proc. West Va. Acad. Sci. 2020, 92, 12498–12508. [Google Scholar]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Jensen, J.; Kraft, D.C.E.; Lysdahl, H.; Foldager, C.B.; Chen, M.; Kristiansen, A.A.; Rölfing, J.H.D.; Bünger, C.E. Functionalization of polycaprolactone scaffolds with hyaluronic acid and β-TCP facilitates migration and osteogenic differentiation of human dental pulp stem cells in vitro. Tissue Eng. Part A 2015, 21, 729–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandelousi, P.S.; Rabiee, S.M.; Jahanshahi, M.; Nasiri, F. The effect of bioactive glass nanoparticles on polycaprolactone/chitosan scaffold: Melting enthalpy and cell viability. J. Bioact. Compat. Polym. 2019, 34, 97–111. [Google Scholar] [CrossRef]
- Kudryavtseva, V.; Stankevich, K.; Kozelskaya, A.; Kibler, E.; Zhukov, Y.; Malashicheva, A.; Golovkin, A.; Mishanin, A.; Filimonov, V.; Bolbasov, E.; et al. Magnetron plasma mediated immobilization of hyaluronic acid for the development of functional double-sided biodegradable vascular graft. Appl. Surf. Sci. 2020, 529, 147196. [Google Scholar] [CrossRef]


| Gene | Primer Sequence | |
|---|---|---|
| Forward | Reverse | |
| OCN | 5′-GCAAAGGTGCAGCCTTTGTG-3′ | 5′-GGCTCCCAGCCATTGATACAG-3′ |
| DSPP | 5′-CCATTCCAGTTCCTCAAAGC-3′ | 5′-TGGCATTTAACTCCTGTA C-3′ |
| DMP1 | 5′-TTCTTTGTGAACTACGGAGG-3′ | 5′-TTGATACCTGGTTACTGGGA-3′ |
| β-actin | 5′-CTTCCTTCCTGGGCATG-3′ | 5′-GTCTTTGCGGATGTCCAC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi Nejad, Z.; Zamanian, A.; Saeidifar, M.; Vanaei, H.R.; Salar Amoli, M. 3D Bioprinting of Polycaprolactone-Based Scaffolds for Pulp-Dentin Regeneration: Investigation of Physicochemical and Biological Behavior. Polymers 2021, 13, 4442. https://doi.org/10.3390/polym13244442
Mousavi Nejad Z, Zamanian A, Saeidifar M, Vanaei HR, Salar Amoli M. 3D Bioprinting of Polycaprolactone-Based Scaffolds for Pulp-Dentin Regeneration: Investigation of Physicochemical and Biological Behavior. Polymers. 2021; 13(24):4442. https://doi.org/10.3390/polym13244442
Chicago/Turabian StyleMousavi Nejad, Zohre, Ali Zamanian, Maryam Saeidifar, Hamid Reza Vanaei, and Mehdi Salar Amoli. 2021. "3D Bioprinting of Polycaprolactone-Based Scaffolds for Pulp-Dentin Regeneration: Investigation of Physicochemical and Biological Behavior" Polymers 13, no. 24: 4442. https://doi.org/10.3390/polym13244442
APA StyleMousavi Nejad, Z., Zamanian, A., Saeidifar, M., Vanaei, H. R., & Salar Amoli, M. (2021). 3D Bioprinting of Polycaprolactone-Based Scaffolds for Pulp-Dentin Regeneration: Investigation of Physicochemical and Biological Behavior. Polymers, 13(24), 4442. https://doi.org/10.3390/polym13244442








