Closed-Cell Rigid Polyimide Foams for High-Temperature Applications: The Effect of Structure on Combined Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nadimide End-Capped Polyimide Oligomers (NPOs)
2.3. Preparation of Closed-Cell Rigid PI Foams
2.4. Measurements
3. Results and Discussion
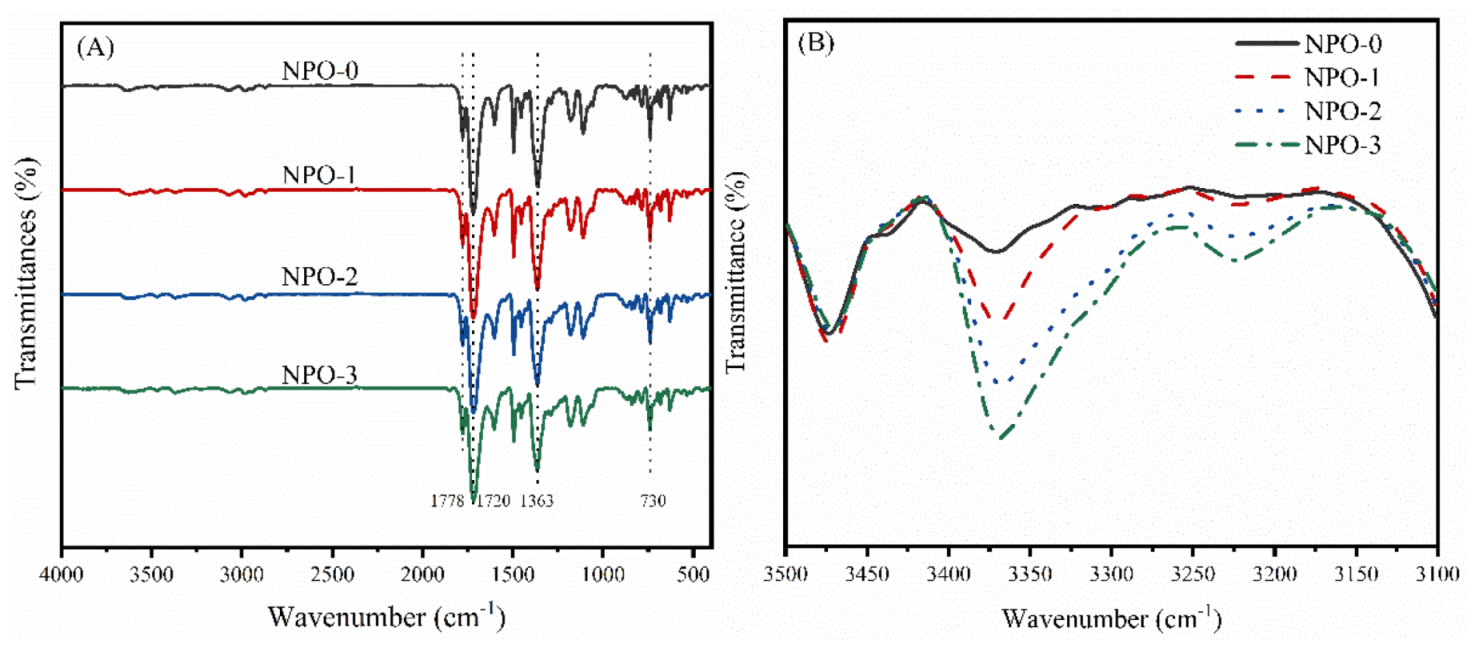
3.1. Characterization
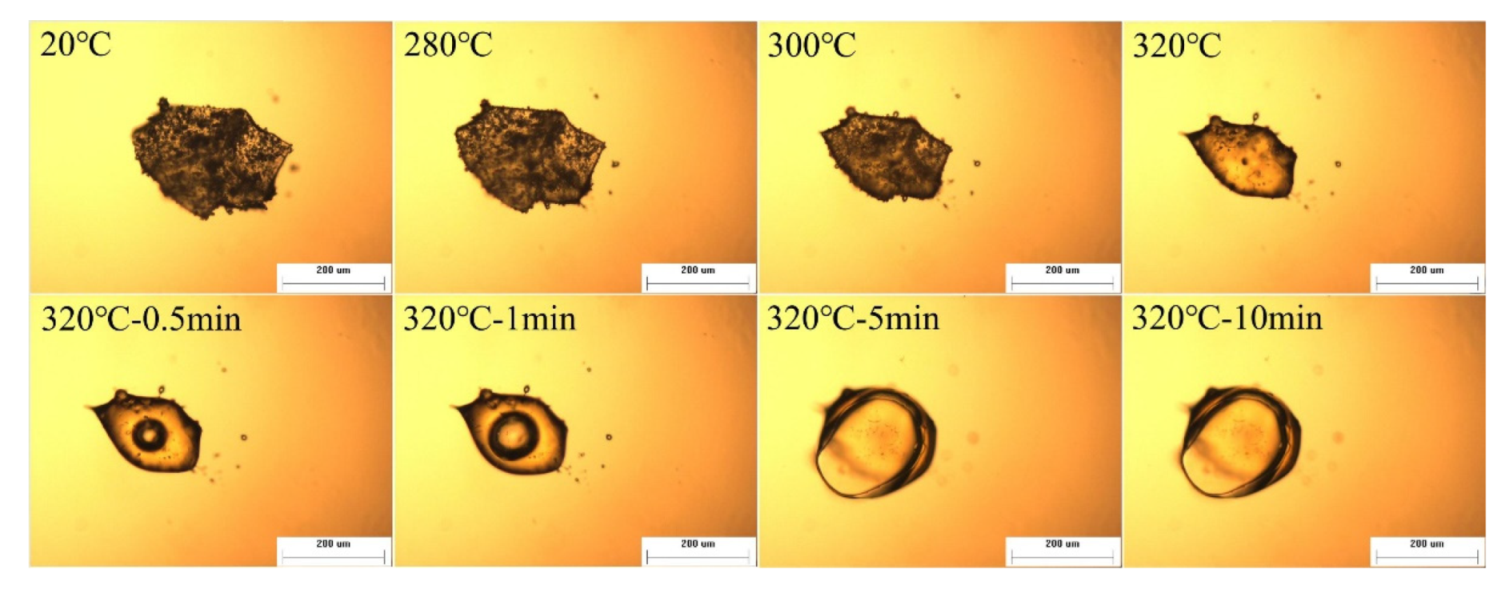
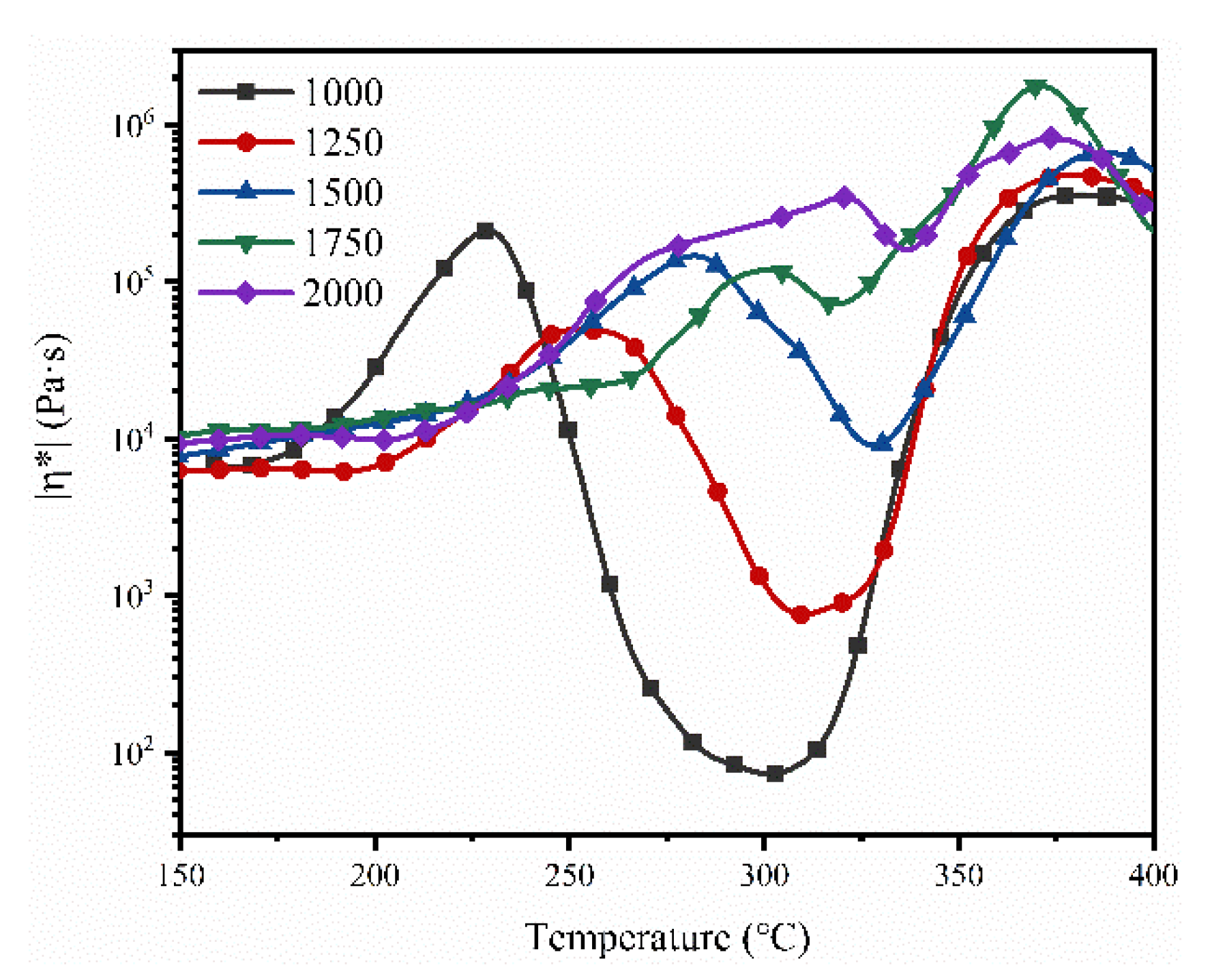
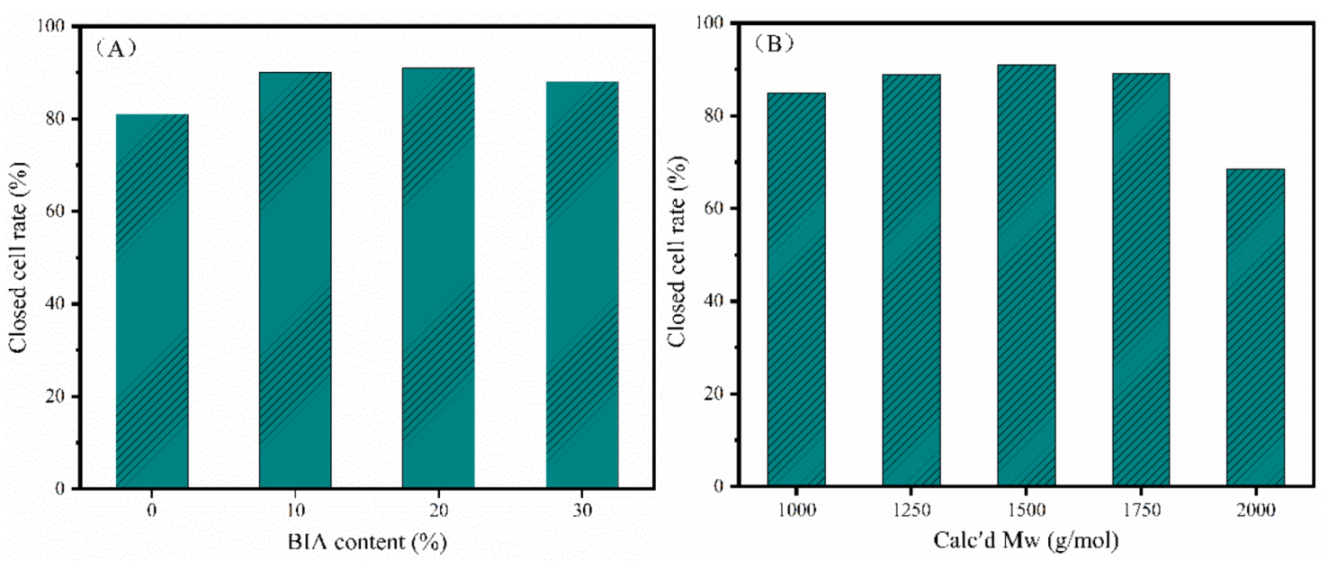
3.2. Thermal Foaming Processability
3.3. Thermal Properties of the Rigid PI Foams
3.4. Mechanical Properties of the Rigid PI Foams
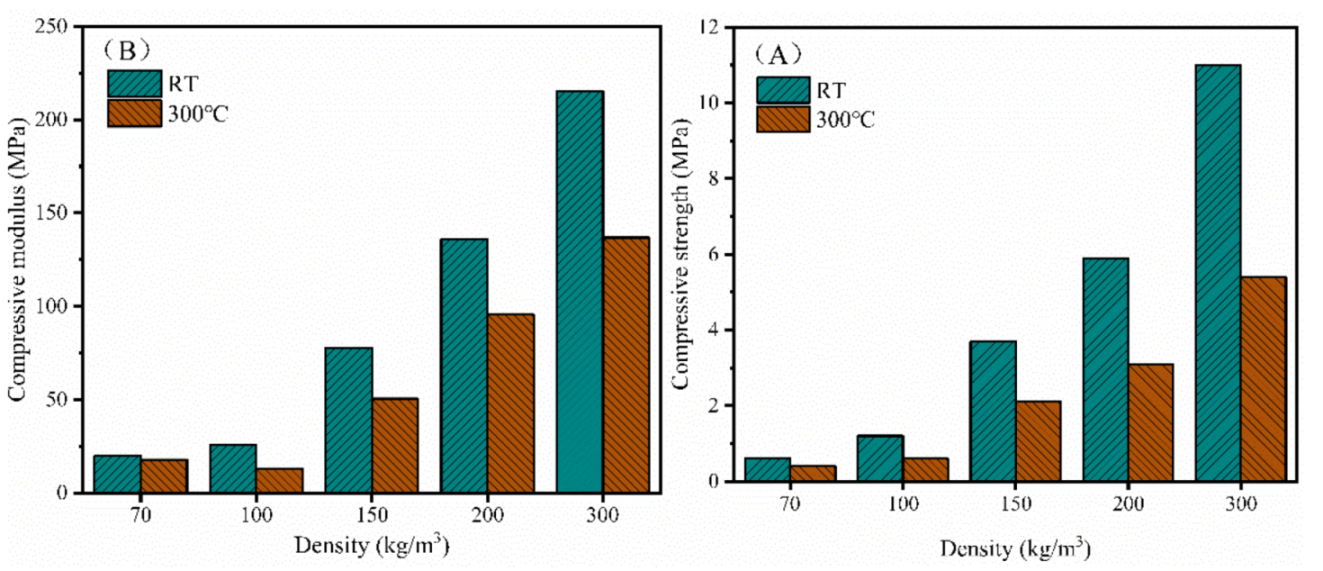
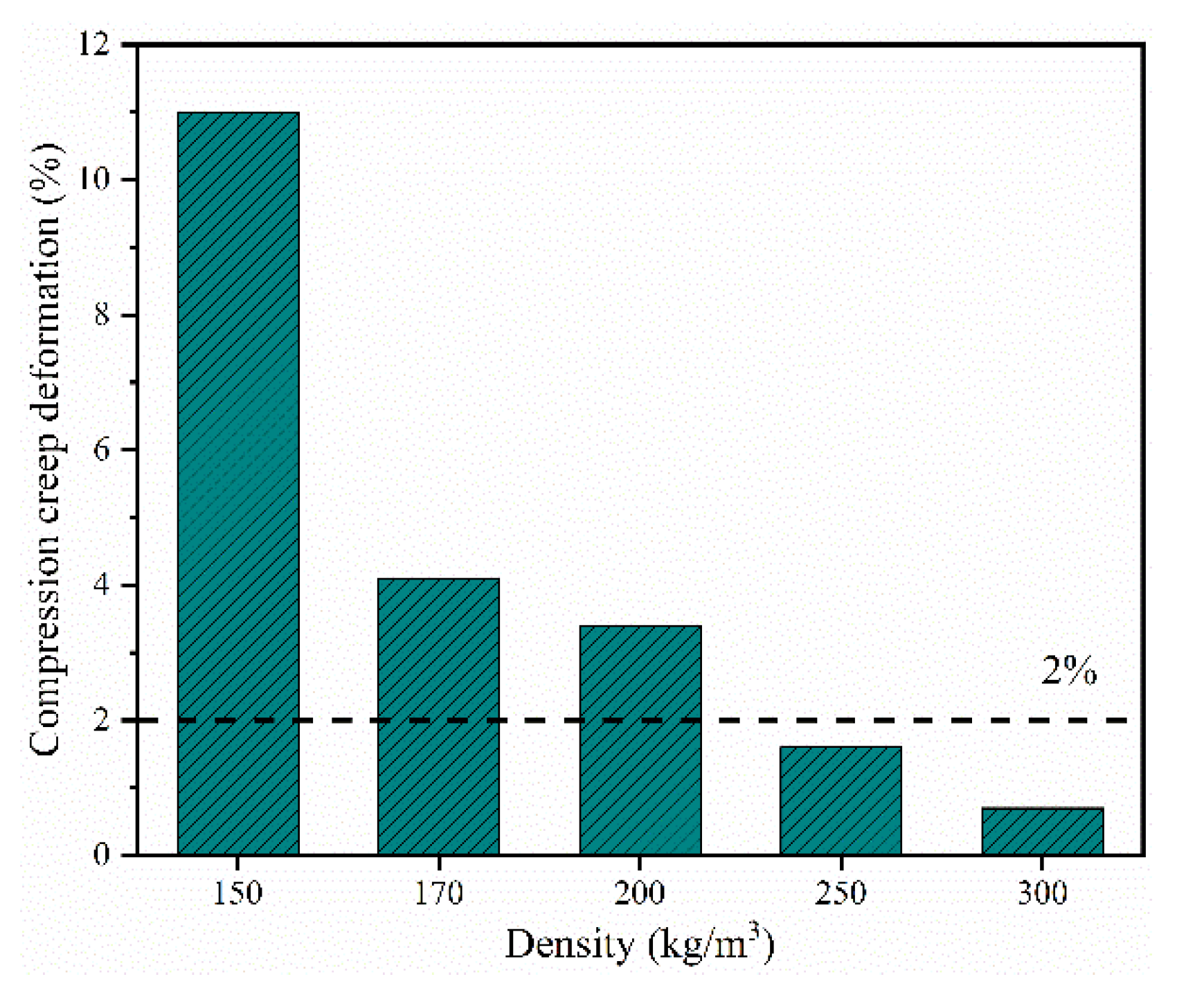
3.5. Mechanical Properties of the Closed-Cell Rigid PI Foams with Different Densities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhai, W.; Feng, W.; Ling, J.; Zheng, W. Fabrication of Lightweight Microcellular Polyimide Foams with Three-Dimensional Shape by CO2 Foaming and Compression Molding. Ind. Eng. Chem. Res. (ACS) 2012, 51, 12827–12834. [Google Scholar] [CrossRef]
- Edward, A.L.; Paul, W.W. Cellular Polyimide Product. U.S. Patent 3310506, 21 March 1967. [Google Scholar]
- Caps, R.; Heinemann, U.; Fricke, J.; Keller, K. Thermal conductivity of polyimide foams. Int. J. Heat Mass Transf. 1997, 40, 269–280. [Google Scholar] [CrossRef]
- Cook, B.A.; Yudin, V.E.; Otaigbe, J.U. Thermal properties of polyimide foam composites. J. Mater. Sci. Lett. 2000, 19, 1971–1973. [Google Scholar] [CrossRef]
- Hower, R.T.; Hoang, S.V. Polyimide Foam-Containing Radomes. U.S. Patent 5662293, 2 September 1997. [Google Scholar]
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in Polyimide---Based Materials for Space Applications. Adv. Mater. 2019, 31, 1807738. [Google Scholar] [CrossRef] [PubMed]
- Indyke, D.M. Polyimide Foams and Their Production. U.S. Patent 4952611, 28 August 1990. [Google Scholar]
- Yang, J.Y.; Ye, Y.S.; Li, X.P.; Lu, X.Z.; Chen, R.J. Flexible, conductive, and highly pressure-sensitive graphene-polyimide foam for pressure sensor application. Compos. Sci. Technol. 2018, 164, 187–194. [Google Scholar] [CrossRef]
- Weiser, E.S.; Grimsley, B.W.; Pipes, R.B.; Williams, M.K. Polyimide Foams from Friable Balloons; Society for the Advancement of Material and Process Engineering Conference; SAMPE: Long Beach, CA, USA, 2002. [Google Scholar]
- Weiser, E.S.; Baillif, F.F.; Grimsley, B.W.; Marchello, J.M. High temperature structural foam. In Proceedings of the 43rd International SAMPE Symposium, Hampton, VA, USA, 31 May 1998; pp. 730–744. [Google Scholar]
- McConnell, V.P. NASA gets hands-on with X-33 design. High Perf. Comp. 1997, 5, 56–58. [Google Scholar]
- Fisher, K. Resin flow control is the key to RTM success. High Perf. Comp. 1997, 5, 34–38. [Google Scholar]
- Robert, H.W. Forming a Foamed Polyimide Article. U.S. Patent 3249561 A, 3 May 1966. [Google Scholar]
- Zhan, M.S.; Wang, K. Polyimide Foam Materials; National Defense Industry Press: Beijing, China, 2018; p. 5. [Google Scholar]
- Weiser, E.S.; Johnson, T.F.; St Clair, T.L.; Echigo, Y.; Kaneshiro, H.; Grimsley, B.W. Polyimide foams for aerospace vehicles. High Perform. Polym. 2000, 12, 1–12. [Google Scholar] [CrossRef]
- Li, J.W.; Zhang, G.C.; Li, J.T.; Zhou, L.S.; Jing, Z.X.; Ma, Z.L. Preparation and properties of polyimide/chopped carbon fiber composite foams. Polym. Adv. Technol. 2017, 28, 28–34. [Google Scholar] [CrossRef]
- Weng, L.; Wang, T.; Ju, P.H.; Liu, L.Z. Preparation and properties of polyimide/Fe3O4 composite foams. Pigm Resin Technol. 2018, 47, 173–179. [Google Scholar] [CrossRef]
- Ma, J.J.; Wang, K.; Zhan, M.S. A comparative study of structure and electromagnetic interference shielding performance for silver nanostructure hybrid polyimide foams. RSC Adv. 2015, 5, 65283–65296. [Google Scholar] [CrossRef]
- Pan, L.Y.; Zhan, M.S.; Wang, K. High-Temperature-Resistant Polyimide/Montmorillonite Nanocomposite Foams by Solid Blending. Polym. Eng. Sci. 2011, 51, 1397–1403. [Google Scholar] [CrossRef]
- Yan, L.; Fu, L.W.; Chen, Y.J.; Tian, H.F.; Xiang, A.M.; Rajulu, A.V. Improved thermal stability and flame resistance of flexible polyimide foams by vermiculite reinforcement. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, S.; Li, B.; Hou, W.; Li, G.; Memon, M.A.; Huang, Y.; Geng, J. Graphene Oxide: A Versatile Agent for Polyimide Foams with Improved Foaming Capability and Enhanced Flexibility. ACS Chem. Mater. 2015, 27, 4358–4367. [Google Scholar] [CrossRef]
- Qi, K.L.; Zhang, G.C. Effect of Organoclay on the Morphology, Mechanical, and Thermal Properties of Polyimide/Organoclay Nanocomposite Foams. Polym. Compos. 2014, 35, 2311–2317. [Google Scholar] [CrossRef]
- Chu, H.J.; Zhu, B.K.; Xu, Y.Y. Polyimide foams with ultralow dielectric constants. J. Appl. Polym. Sci. 2006, 102, 1734–1740. [Google Scholar] [CrossRef]
- Chu, H.J.; Zhu, B.K.; Xu, Y.Y. Preparation and dielectric properties of polyimide foams containing crosslinked structures. Polym. Adv. Technol. 2006, 17, 366–371. [Google Scholar] [CrossRef]
- Ou, A.; Huang, Z.; Qin, R.; Chen, X.; Li, Y.; Liu, Y.; Liu, X.; Wang, X. Preparation of Thermosetting/Thermoplastic Polyimide Foam with Pleated Cellular Structure via In Situ Simultaneous Orthogonal Polymerization. ACS Appl. Polym. Mater. 2019, 1, 2430–2440. [Google Scholar] [CrossRef]
- Wang, L.L.; Hu, A.J.; Fan, L.; Yang, S.Y. Structures and properties of closed-cell polyimide rigid foams. J. Appl. Polym. Sci. 2013, 130, 3282–3291. [Google Scholar] [CrossRef]
- Wang, L.L.; Hu, A.J.; Fan, L.; Yang, S.Y. Approach to produce rigid closed-cell polyimide foams. High Perform. Polym. 2013, 25, 956–965. [Google Scholar] [CrossRef]
- Li, J.; Yu, N.; Ding, Y.; Xu, T.; Zhang, G.; Jing, Z.; Shi, X. Fabrication of rigid polyimide foams with overall enhancement of thermal and mechanical properties. J. Cell. Plast. 2020, 57, 717–731. [Google Scholar] [CrossRef]
- Li, J.; Yu, N.; Jing, Z.; He, X.; Shi, X.; Zhang, G. Fabrication of rigid polyimide foams via thermal foaming of nadimide-end-capped polyester-amine precursor. Polym. Bull. 2020, 77, 5899–5912. [Google Scholar] [CrossRef]
- Wang, X.; Ou, P.A. Polyimide Foam and Preparation Method and Application Thereof. CN Patent 109880096 A, 21 April 2019. [Google Scholar]
- Li, J.W.; Zhang, G.C.; Yao, Y.; Jing, Z.X.; Zhou, L.S.; Ma, Z.L. Synthesis and properties of polyimide foams containing benzimidazole units. RSC Adv. 2016, 6, 60094–60100. [Google Scholar] [CrossRef]
- Liu, J.N.; Wu, D.Y.; Liang, W.H.; Cao, J.H. Effect of DAPBO segment on the structure and performance enhancement of powder foamed BTDA---ODA polyimide. J. Appl. Polym. Sci. 2021, 138, 49911. [Google Scholar] [CrossRef]
- Dong, J.; Yin, C.; Zhang, Z.; Wang, X.; Li, H.; Zhang, Q. Hydrogen---bonding interactions and molecular packing in polyimide fibers containing benzimidazole units. Macromol. Mater. Eng. 2014, 299, 1170–1179. [Google Scholar] [CrossRef]
- Ahn, T.-K.; Kim, M.; Choe, S. Hydrogen-bonding strength in the blends of polybenzimidazole with BTDA-and DSDA-based polyimides. Macromolecules 1997, 30, 3369–3374. [Google Scholar] [CrossRef]
- Dynes, P.; Panos, R.; Hamermesh, C. Investigation of the crosslinking efficiency of some additional curing polyimides. J. Appl. Polym. Sci. 1980, 25, 1059–1070. [Google Scholar] [CrossRef]
- Wilson, D. PMR---15 processing, properties and problems—A review. Br. Polym. J. 1988, 20, 405–416. [Google Scholar] [CrossRef]
- Luo, L.; Yao, J.; Wang, X.; Li, K.; Huang, J.; Li, B.; Wang, H.; Liu, X. The evolution of macromolecular packing and sudden crystallization in rigid-rod polyimide via effect of multiple H-bonding on charge transfer (CT) interactions. Polymer 2014, 55, 4258–4269. [Google Scholar] [CrossRef]
- Williams, M.K.; Weiser, E.S.; Fesmire, J.E.; Grimsley, B.W.; Smith, T.M.; Brenner, J.R.; Nelson, G.L. Effects of cell structure and density on the properties of high performance polyimide foams. Polym. Adv. Technol. 2005, 16, 167–174. [Google Scholar] [CrossRef]
- Swellam, M.; Yi, S.; Ahmad, M.F.; Huber, L.M. Mechanical properties of cellular materials. I. Linear analysis of hexagonal honeycombs. J. Appl. Polym. Sci. 1997, 63, 383–393. [Google Scholar] [CrossRef]







| Calcd Mw (g/mol) | Mn (g/mol) | Mw (g/mol) | Polydispersity |
|---|---|---|---|
| 1000 | 1644 | 2385 | 1.45 |
| 1250 | 1662 | 2490 | 1.50 |
| 1500 | 1686 | 2508 | 1.49 |
| 1750 | 1688 | 2528 | 1.50 |
| 2000 | 1718 | 2626 | 1.53 |
| NPO | Minimum Melt Viscosity (Pa·s) | Temperature (°C) | n(BIA): n(BIA+PDA) |
|---|---|---|---|
| NPO-0 | 233 | 317.3 | 0% |
| NPO-1 | 826 | 324.1 | 10% |
| NPO-2 | 5134 | 328.9 | 20% |
| NPO-3 | 45,490 | 304.6 | 30% |
| PIFs | TGA | DMA | n(BIA): n(BIA+PDA) | Calcd Mw (g/mol) | ||
|---|---|---|---|---|---|---|
| T5 (°C) | T10 (°C) | E′ (°C) | tan δ (°C) | |||
| PIF-0 | 474.1 | 546.8 | 339.2 | 371.4 | 0% | 1500 |
| PIF-1 | 489.0 | 557.5 | 347.2 | 381.8 | 10% | 1500 |
| PIF-2 | 492.1 | 556.9 | 365.3 | 395.0 | 20% | 1500 |
| PIF-3 | 492.1 | 553.9 | 376.8 | 402.9 | 30% | 1500 |
| PIF-2-1000 | 479.1 | 521.8 | 378.2 | 409.3 | 20% | 1000 |
| PIF-2-1250 | 488.0 | 542.5 | 369.8 | 397.5 | 20% | 1250 |
| PIF-2-1500 | 492.1 | 556.9 | 365.3 | 395.0 | 20% | 1500 |
| PIF-2-1750 | 508.4 | 574.0 | 363.5 | 389.8 | 20% | 1750 |
| PIF-2-2000 | 512.9 | 578.5 | 362.8 | 387.0 | 20% | 2000 |
| PIFs | Compressive Properties | Tensile Properties | |||
|---|---|---|---|---|---|
| Strength (MPa) | Modulus (MPa) | Strength (MPa) | Modulus (MPa) | Elongation at Breakage (%) | |
| PIF-0 | 1.0 | 31.0 | 0.6 | 21.5 | 3.0 |
| PIF-1 | 1.0 | 35.7 | 0.6 | 15.3 | 5.4 |
| PIF-2 | 1.1 | 24.7 | 0.9 | 17.0 | 6.6 |
| PIF-3 | 0.6 | 9.8 | 0.6 | 11.2 | 6.4 |
| PIF-2-1000 | 1.3 | 27.8 | 0.6 | 26.1 | 2.7 |
| PIF-2-1250 | 1.1 | 28.2 | 1.0 | 28.6 | 4.0 |
| PIF-2-1500 | 1.1 | 24.7 | 0.9 | 17.0 | 6.6 |
| PIF-2-1750 | 1.4 | 35.9 | 0.6 | 15.7 | 4.8 |
| PIF-2-2000 | 1.0 | 22.5 | 0.6 | 11.9 | 6.5 |
| PI Foams | Density (kg/m3) | Closed-Cell Rate (%) | Tg (°C) | Compressive Strength (MPa) | Compressive Modulus (MPa) |
|---|---|---|---|---|---|
| PIF-2 (this work) α-BPDA-PDA/BIA | 100 | 91 | 395 | 1.10 | 25.7 |
| 150 | 95 | 395 | 3.70 | 77.6 | |
| α-BPDA-PDA [27] | 100 | 89 | 374 | 1.34 | 37.1 |
| α-BPDA-3,4′-ODA [27] | 100 | 82 | 318 | 0.96 | 21.1 |
| α-BPDA-4,4′-ODA [27] | 100 | 86 | 364 | 0.70 | 16.3 |
| BTDA-4,4′-ODA [15] | 32 | 32 | 300 | 0.30 | 11.03 |
| ODPA-3,4′-ODA [15] | 80 | - | 237 | 0.84 | 6.13 |
| 32 | 32 | 237 | 0.19 | 3.89 | |
| BTDA-MDA/BDM [25] | 101 | - | 285 | 0.86 | 10.79 |
| BTDA-4,4′-ODA/BIA [28] | 243 | - | 345 | 7.45 a | 23.8 a |
| ODPA-4,4′-ODA/BIA [31] | 54 | - | 306 | 0.71 | - |
| BTDA-4,4′-ODA/DAPBO [32] | 77 | - | 368 | 1.03 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Hu, A.; Wang, Z.; Li, K.; Yang, S. Closed-Cell Rigid Polyimide Foams for High-Temperature Applications: The Effect of Structure on Combined Properties. Polymers 2021, 13, 4434. https://doi.org/10.3390/polym13244434
Shi Y, Hu A, Wang Z, Li K, Yang S. Closed-Cell Rigid Polyimide Foams for High-Temperature Applications: The Effect of Structure on Combined Properties. Polymers. 2021; 13(24):4434. https://doi.org/10.3390/polym13244434
Chicago/Turabian StyleShi, Yawei, Aijun Hu, Zhiyuan Wang, Kedi Li, and Shiyong Yang. 2021. "Closed-Cell Rigid Polyimide Foams for High-Temperature Applications: The Effect of Structure on Combined Properties" Polymers 13, no. 24: 4434. https://doi.org/10.3390/polym13244434
APA StyleShi, Y., Hu, A., Wang, Z., Li, K., & Yang, S. (2021). Closed-Cell Rigid Polyimide Foams for High-Temperature Applications: The Effect of Structure on Combined Properties. Polymers, 13(24), 4434. https://doi.org/10.3390/polym13244434






