Synthesis and Spectroscopic Analyses of New Polycarbonates Based on Bisphenol A-Free Components
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Methods
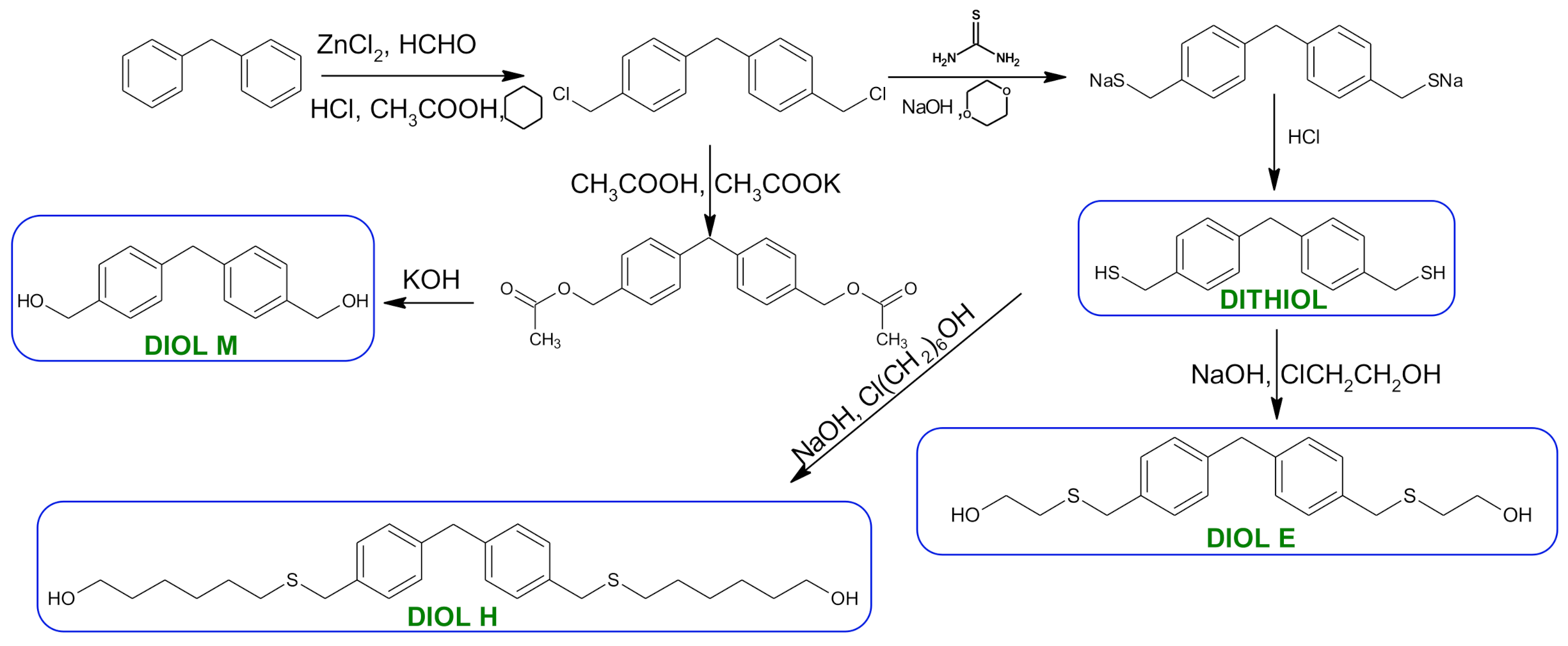
2.3. Synthesis of Monomers
Synthesis of (methanediyldibenzene-4,1-diyl)dimethanol (diol M)
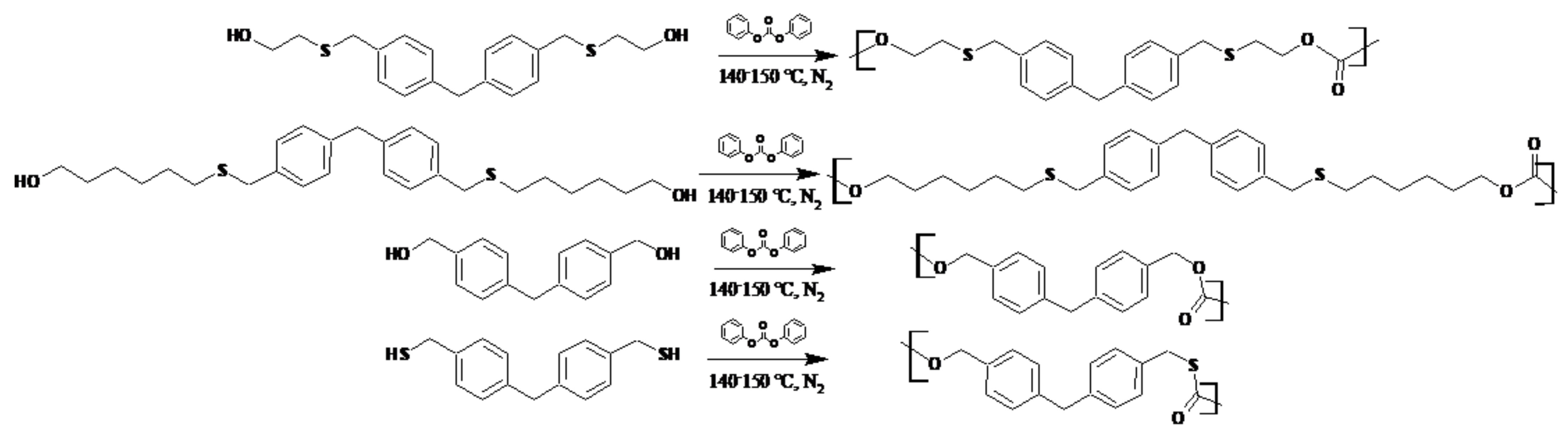
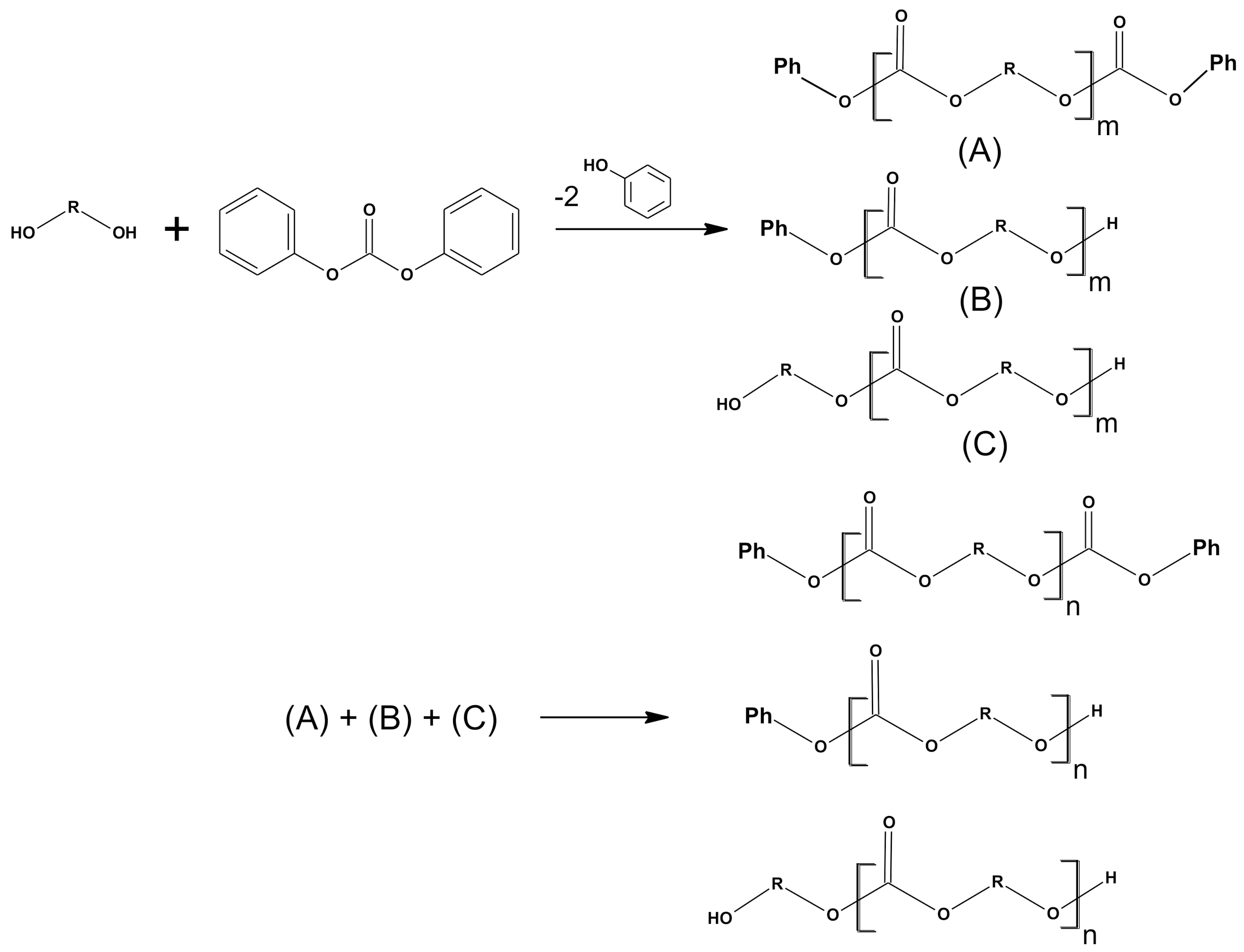
2.4. Synthesis of Polymers
3. Results and Discussion
3.1. 1H and 13C NMR Analysis
- (a)
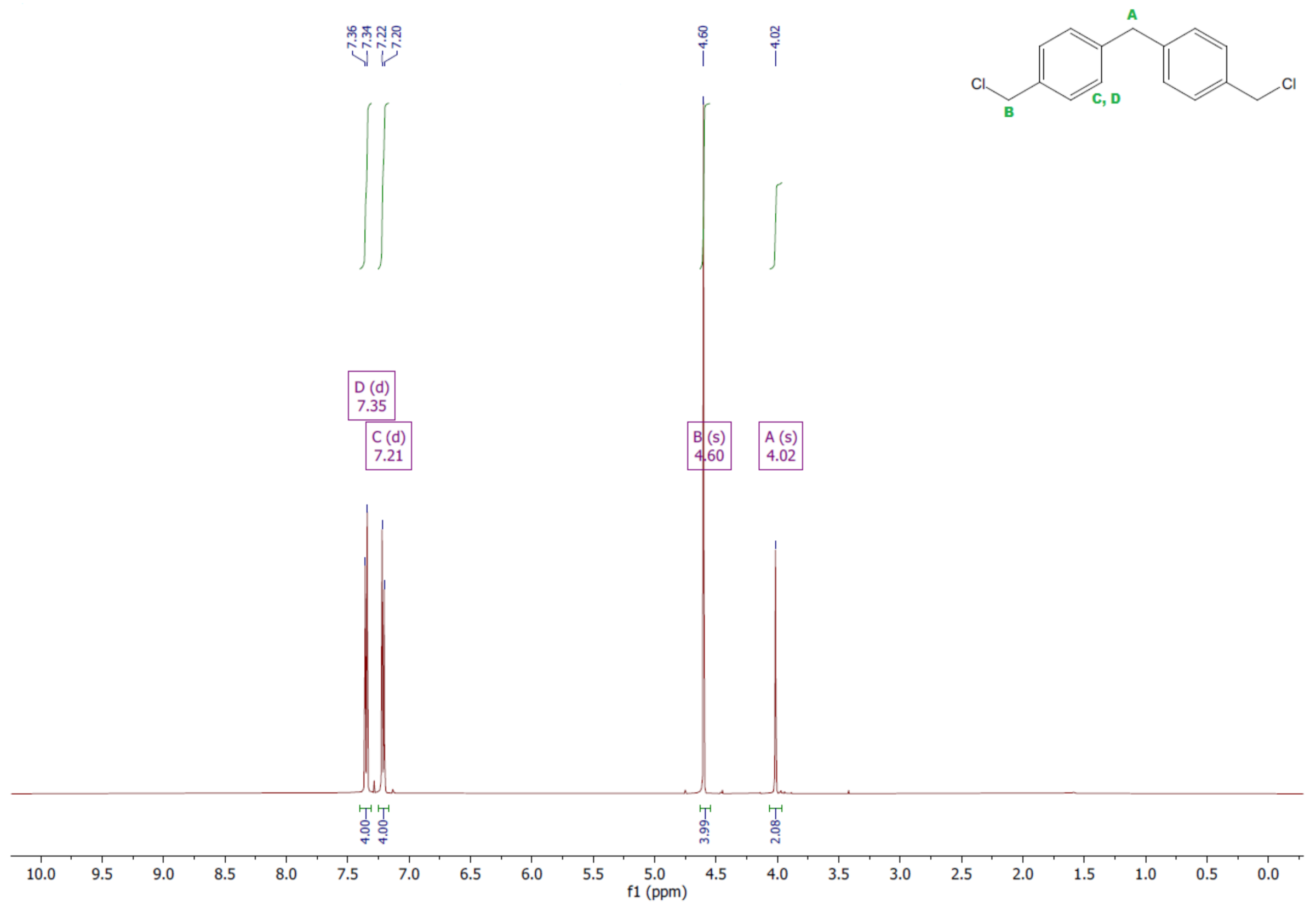
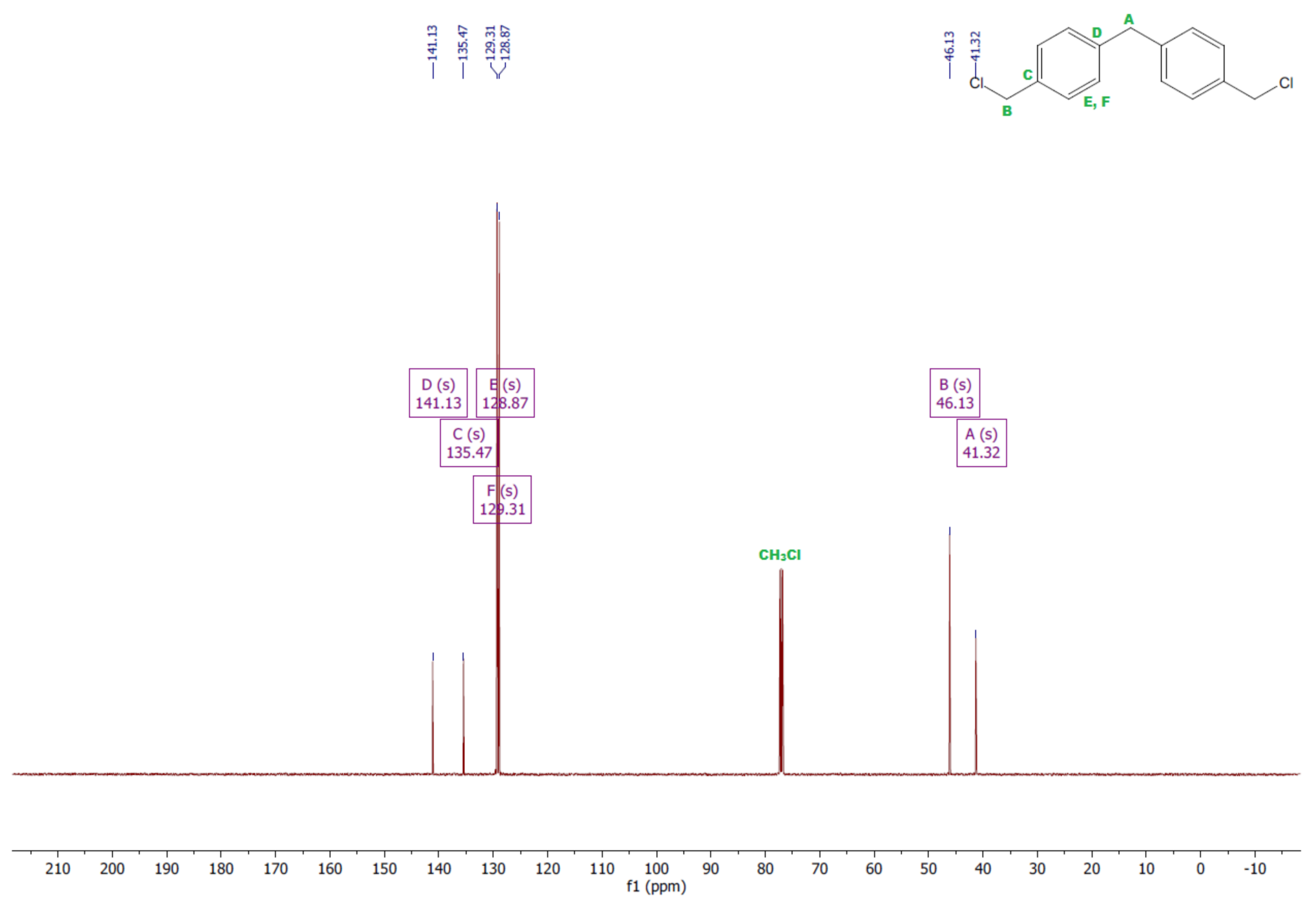
- 1,1′-methanediylbis[4-(chloromethyl)benzene]
- 1HNMR (500 MHz, CDCl3—d, δ ppm): 7.35 (d, J = 8.1 Hz, 4H), 7.21 (d, J = 8.21 Hz, 4H), 4.60 (s, 4H), 4.02 (s, 2H).
- 13CNMR (126 MHz, CDCl3—d, δ ppm): 141.13 (PhC), 135.47 (CPh), 129.31 (Ph), 128.87 (Ph), 46.13 (Cl-CH2-Ph), 41.32 (Ph-CH2-Ph).
- (b)
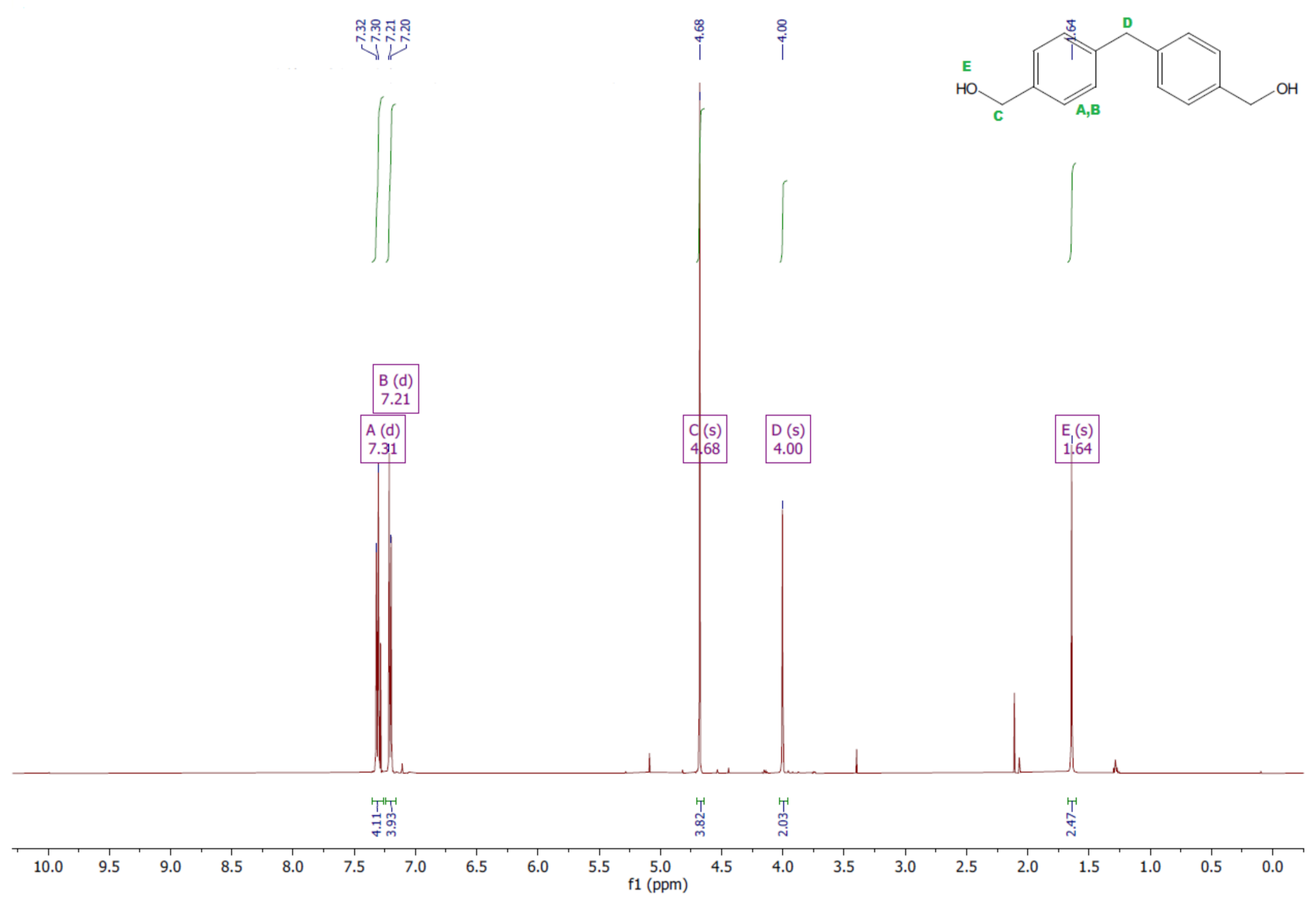
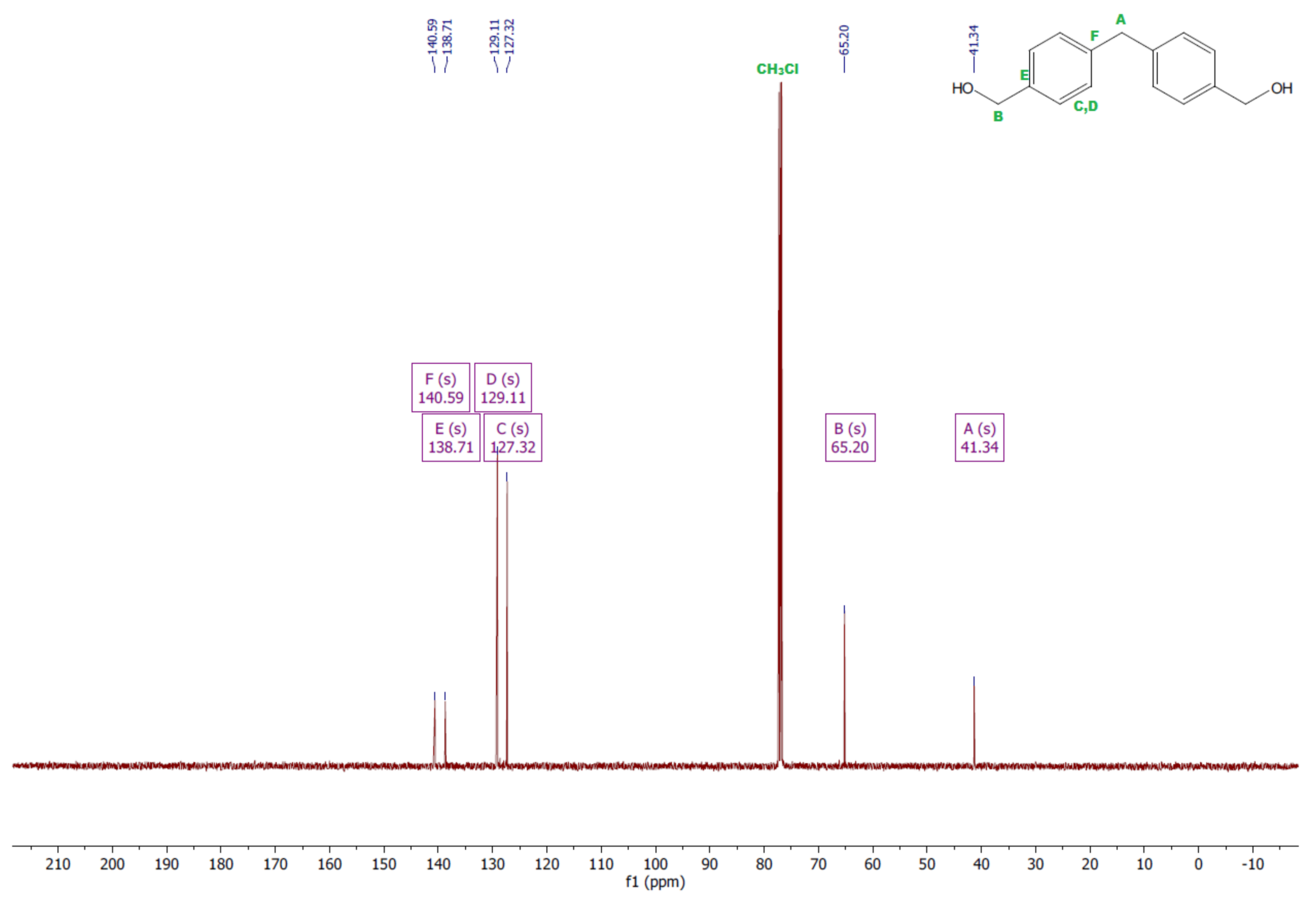
- Diol M
- 1HNMR (500 MHz, CDCl3—d, δ ppm): 7.31 (d, J = 8.1 Hz, 4H), 7.21 (d, H = 8.21 Hz, 4H), 4.68 (s, 4H), 4.00 (s, 2H), 1.64 (s, 2H).
- 13CNMR (126 MHz, CDCl3—d, δ ppm): 140.59 (PhC), 138.71 (CPh), 129.11 (Ph), 127.32 (Ph), 65.20 (OH-CH2-Ph), 41.34 (Ph-CH2-Ph).
- (c)
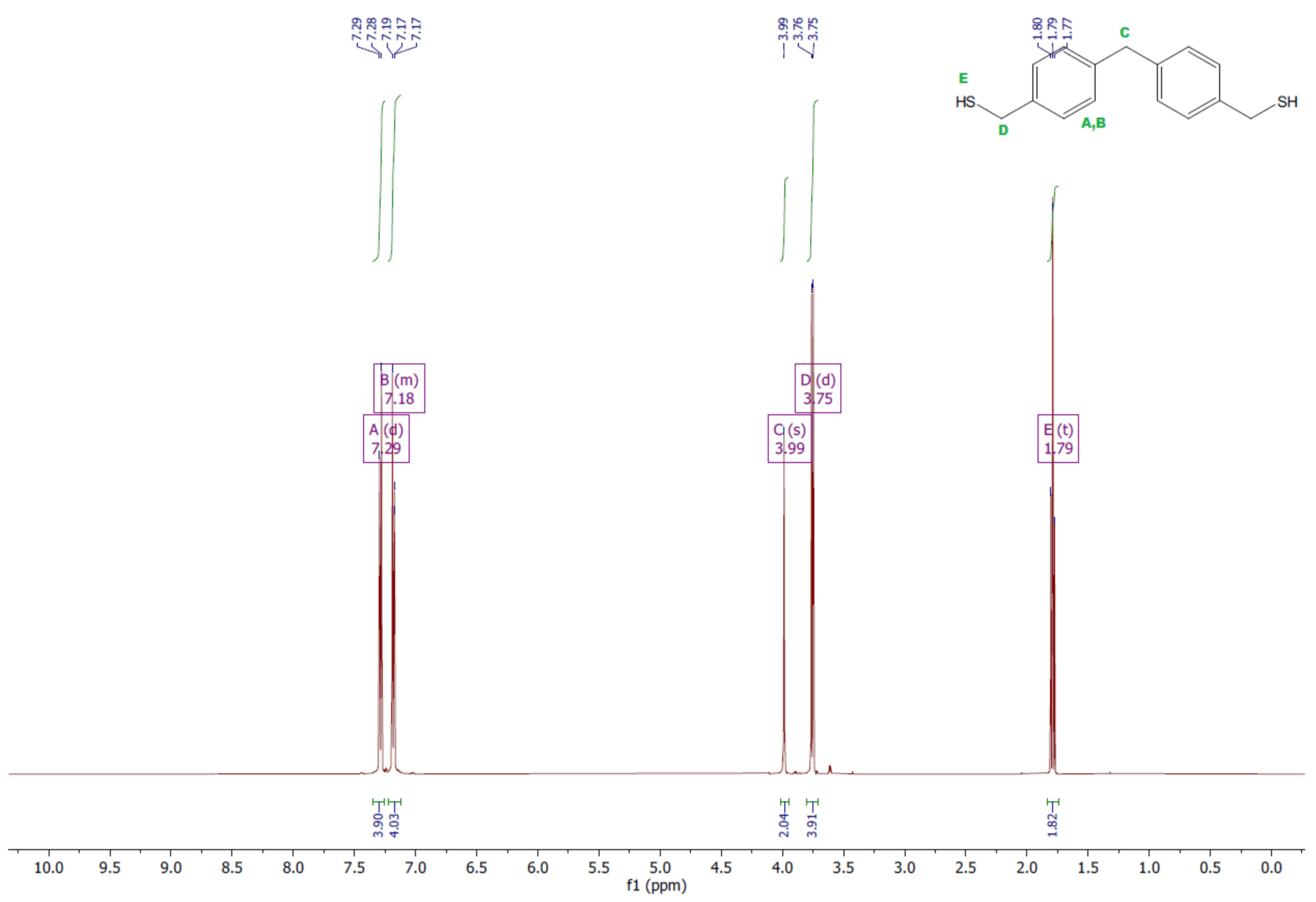
- Dithiol
- 1HNMR (500 MHz, CDCl3—d, δ ppm): 7.29 (d, J = 8.1 Hz, 4H), 7.22–7.00 (m, 4H), 3.99 (s, 2H), 3.75 (d, J = 7.5 Hz, 4H), 1.79 (t, J = 7.5 Hz, 2H).
- 13CNMR (126 MHz, CDCl3—d, δ ppm): 139.89 (PhC), 138.99 (CPh), 129.23 (Ph), 128.22 (Ph), 41.23 (-CH2), 28.70 (SH-CH2-Ph).
- (d)
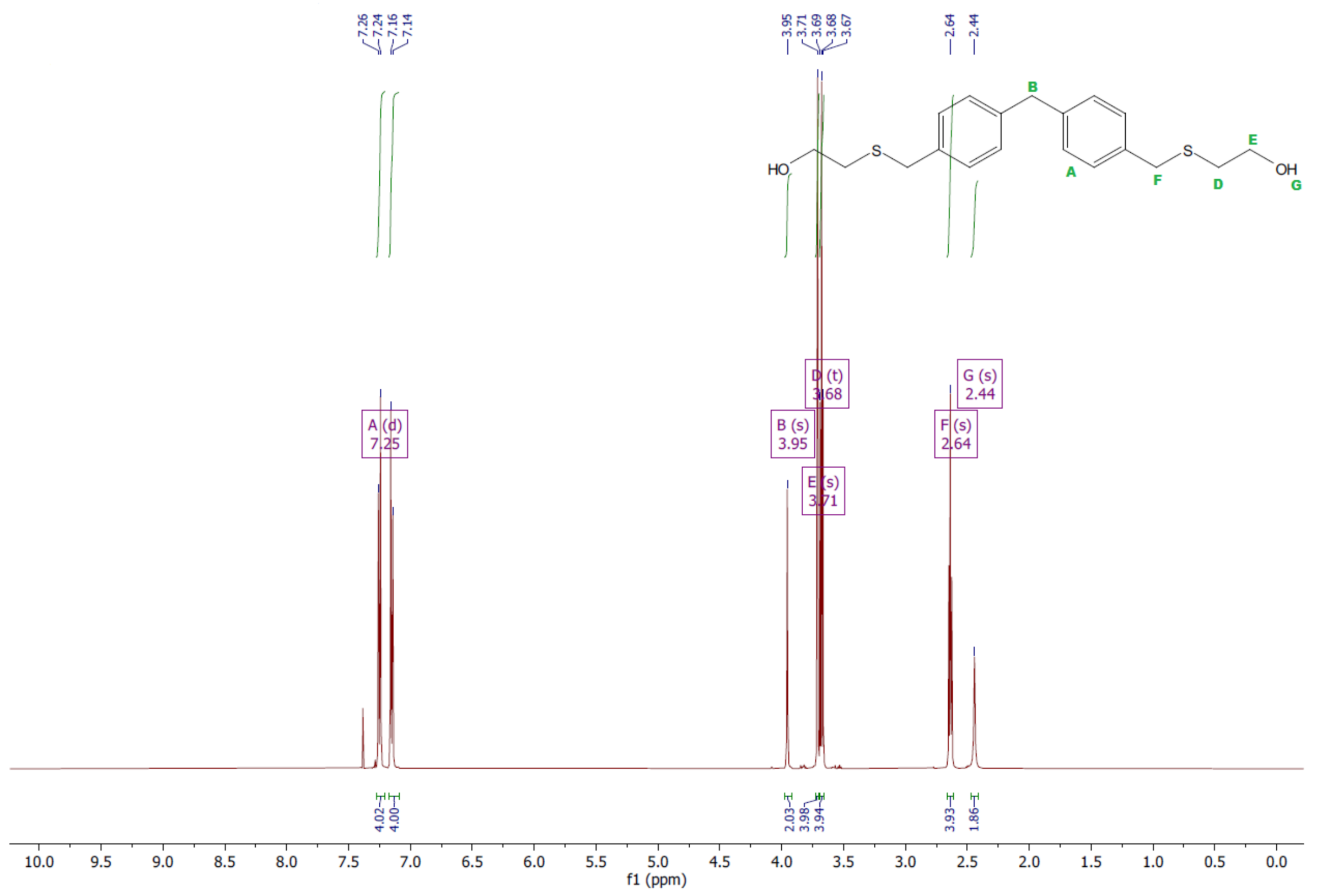
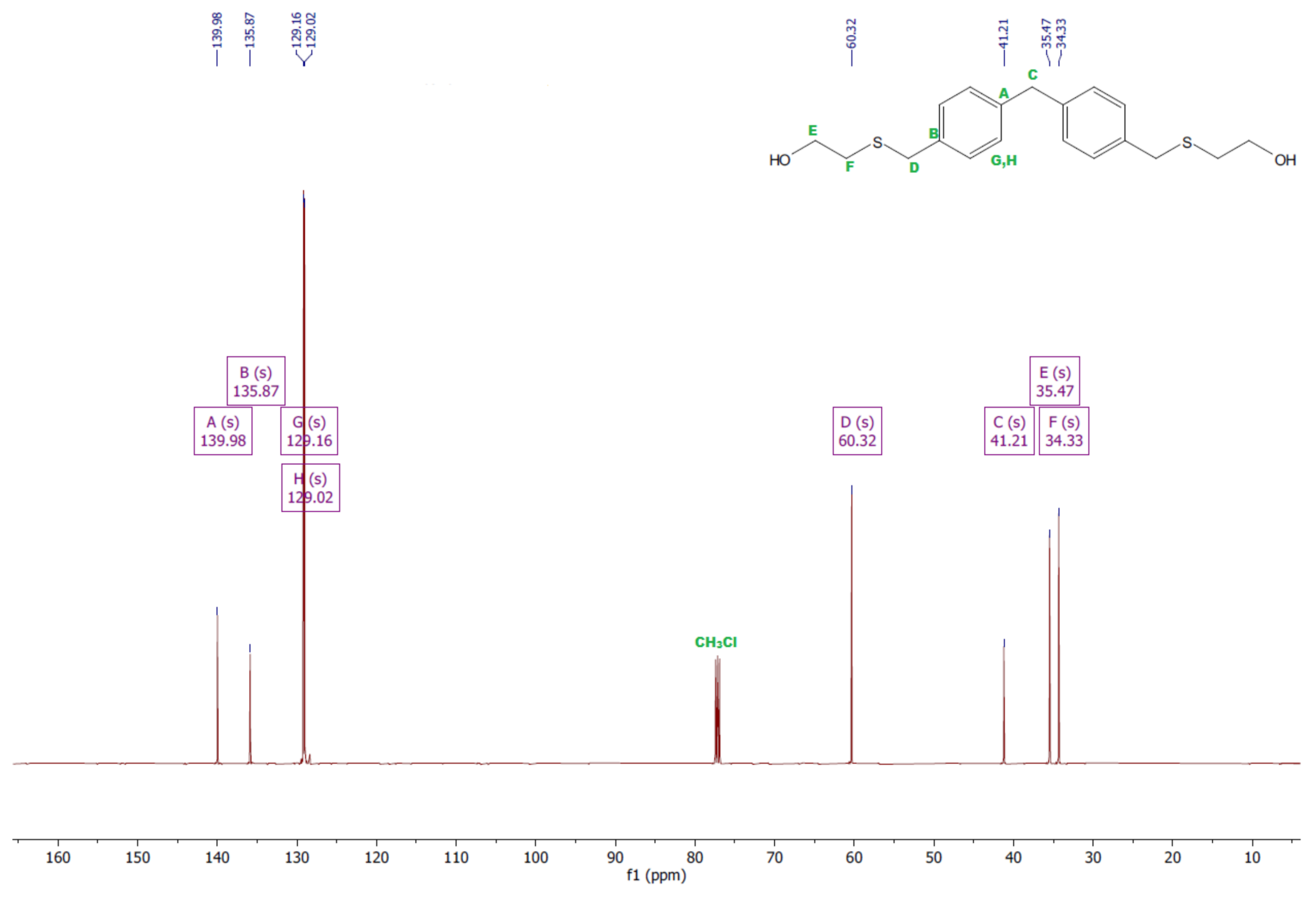
- Diol E
- 1HNMR (500 MHz, CDCl3—d, δ ppm): 7.25 (d, J = 8.1 Hz, 8H), 3.95 (s, 2H), 3.71 (s, 4H), 3.68 (t, J = 6.1 Hz, 4H), 2.64 (s, 4H), 2.44 (s, 2H).
- 13CNMR (126 MHz, CDCl3—d, δ ppm): 139.98 (PhC), 135.87 (CPh), 129.16 (Ph), 129.02 (Ph), 60.32 (-S-CH2-Ph), 41.21 (Ph-CH2-Ph), 35.47 (OH-CH2-), 34.33 (-CH2-S-).
- (e)
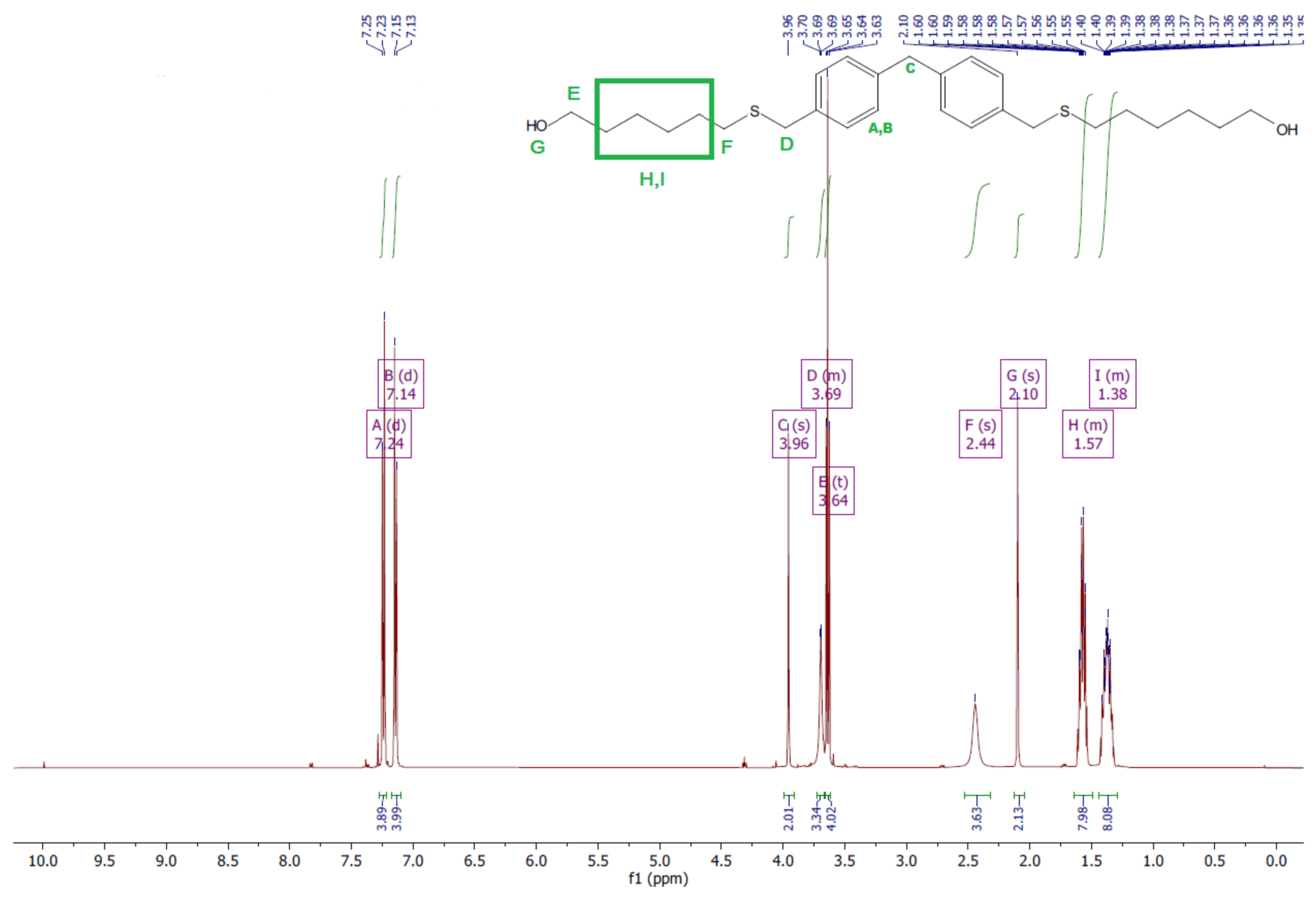
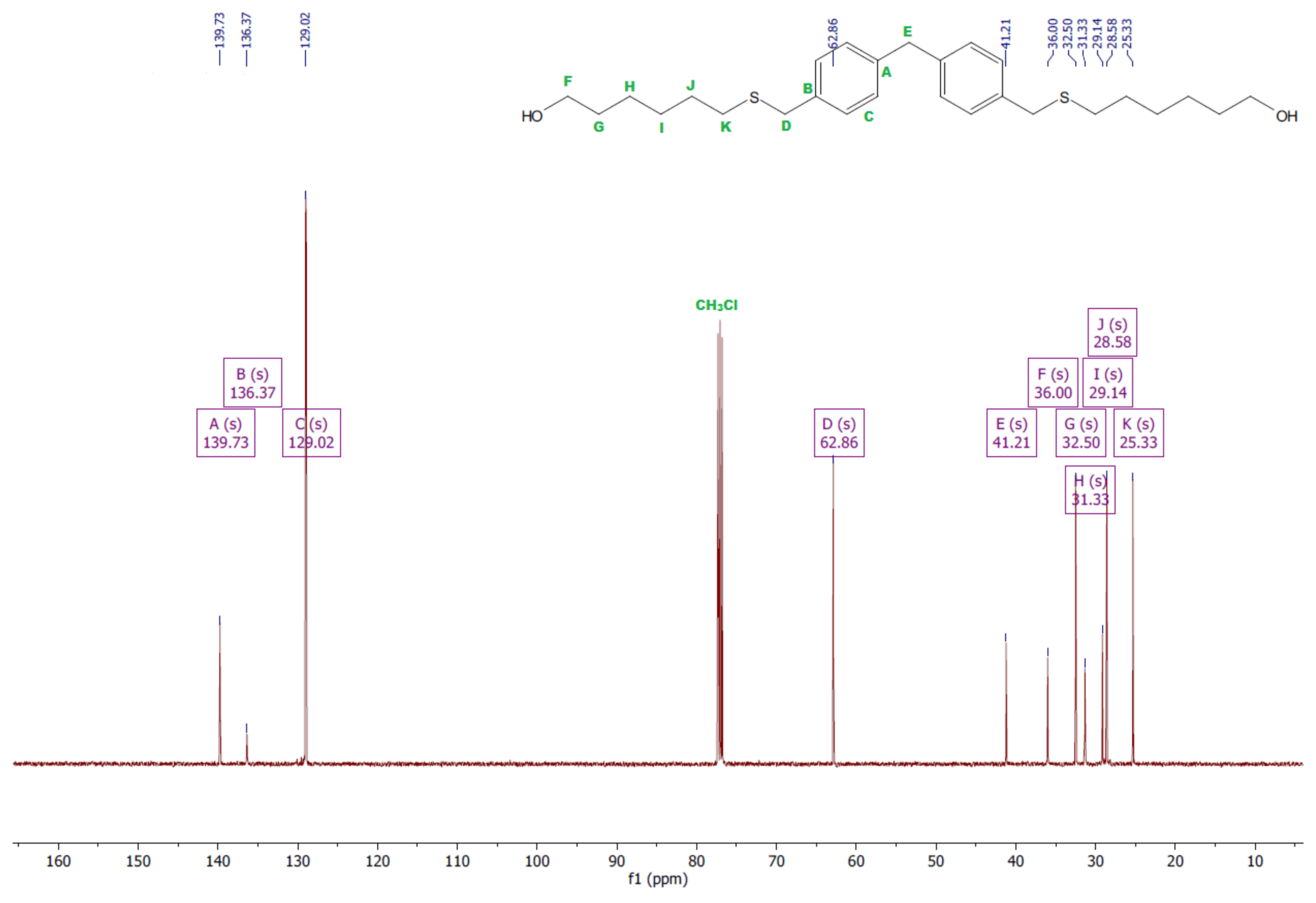
- Diol H
- 1HNMR (500 MHz, CDCl3—d, δ ppm): 7.24 (d, J = 8.1 Hz, 4H), 7.14 (d, J = 8.1 Hz, 4H), 3.96 (s, 2H), 3.69 (m, 4H), 3.64 (t, J = 6.6 Hz, 4H), 2.44 (s, 4H), 2.10 (s, 2H), 1.68–1.31 (m,16H).
- 13CNMR (126 MHz, CDCl3—d, δ ppm): 139.73 (PhC), 136.37 (CPh), 129.02 (Ph), 62.86 (-S-CH2-Ph), 41.21 (Ph-CH2-Ph), 36.00 (OH-CH2-), 32.50 (-CH2(II)-), 31.33 (-CH2(III)-), 29.14 (-CH2(IV)-), 28.58 (-CH2(V)-), 25.33 (-CH2-S-).
3.2. ATR–FTIR Analysis
3.3. DSC Analysis (Differential Scanning Calorimetry)
3.4. GPC Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hammani, S.; Moulai-Mostefa, N.; Benyahia, L.; Tassin, J.F. Effects of composition and extrusion parameters on the morphological development and rheological properties of PP/PC blends. Co-continuity investigation. J. Polym. Res. 2012, 19, 994. [Google Scholar] [CrossRef]
- Fleck, N.A.; Stronge, W.J.; Liu, J.H. High strain rate shear response of polycarbonate and polymethyl methacrylate. Proc. R. Soc. Lond. A Math. Phys. Sci. 1990, 429, 459–479. [Google Scholar]
- Yan, Y.; Mao, Y.; Li, B.; Zhou, P. Machinability of the Thermoplastic Polymers: PEEK, PI, and PMMA. Polymers 2021, 13, 69. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Liu, S.; Wang, Q.; Zhang, H.; Wang, G. Synthesis of Poly (isosorbide carbonate) via Melt Polycondensation Catalyzed by a KF/MgO Catalyst. Chem. Res. Chin. Univ. 2019, 35, 721–728. [Google Scholar] [CrossRef]
- Pivnenko, K.; Pedersen, G.A.; Eriksson, E.; Astrup, T.F. Waste material recycling: Assessment of contaminants limiting recycling. Waste Manag. 2015, 44, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 2009, 30, 75–95. [Google Scholar] [CrossRef]
- Geens, T.; Aerts, D.; Berthot, C.; Bourguignon, J.P.; Goeyens, L.; Lecomte, P. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef]
- Geens, T.; Goeyens, L.; Covaci, A. Are potential sources for human exposure to bisphenol-A overlooked? Int. J. Hyg. Environ. Health 2011, 214, 339–347. [Google Scholar] [CrossRef]
- Sun, A.F.; Kang, L.; Xiang, X.; Lil, H.; Luol, C.; Luo1, R.; Lu1, C.; Peng, X. Recent advances and progress in the detection of bisphenol A. Anal. Bioanal. Chem. 2016, 408, 6913–6927. [Google Scholar] [CrossRef]
- Bair, H.E.; Falcone, D.R.; Hellman, M.Y.; Johnson, G.E.; Kelleher, P.G. Hydrolysis of polycarbonate to yield BPA. J. Appl. Polym. Sci. 1981, 26, 1777. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, B.; Wang, G.Y.; Wu, J. Synthesis of polycarbonate diol catalyzed by metal-organic framework Zn4O[CO2-C6H4-CO2]3. Sci. China Chem. 2011, 54, 1468–1473. [Google Scholar] [CrossRef]
- Hirotoshi, I.; Takeuchi, K.; Michihiko, A.; Mitsuru, U. Oxidative carbonylation of phenol to diphenyl carbonate catalyzed by Pd-pyridyl complexes tethered on polymer support. Catal. Commun. 2001, 2, 145–150. [Google Scholar]
- Komiya, K.; Fukuoka, S.; Aminaka, M.; Hasegawa, K.; Hachiya, H.; Okamato, H.; Watanabe, T.; Yoneda, H.; Fukawa, I.; Dozono, T. New Process for Producing Polycarbonate Without Phosgene and Methylene Chloride. In ACS Symposium Series; Anastas, T., Williamson, T.C., Eds.; ACS: Washington, DC, USA, 1996; Volume 626, pp. 20–32. [Google Scholar]
- Hsu, J.P.; Wong, J.J. Kinetic modeling of melt transesterification of diphenyl carbonate and bisphenol-A. Polymer 2003, 44, 5851–5857. [Google Scholar] [CrossRef]
- Kreye, O.; Meier, M.A.R. Base catalyzed sustainable synthesis of phenyl esters from carboxylic acids using diphenyl carbonate. RSC Adv. 2015, 5, 53155–53160. [Google Scholar] [CrossRef]
- Brunelle, D.J.; Sannon, T.G. Preparation and polymerization of bisphenol A cyclic oligomeric carbonates. Macromolecules 1991, 24, 3035. [Google Scholar] [CrossRef]
- Haba, O.; Itakura, I.; Ueda, M.; Kuze, S. Synthesis of Polycarbonate from Dimethyl Carbonate and Bisphenol-A Through a Non-Phosgene Process. Polym. Chem. 1999, 37, 2087–2093. [Google Scholar] [CrossRef]
- Kim, W.B.; Lee, J.S. Comparison of Polycarbonate Precursors Synthesized from Catalytic Reactions of Bisphenol-A with Diphenyl Carbonate, Dimethyl Carbonate, or Carbon Monoxide. J. Appl. Polym. Sci. 2002, 86, 937–947. [Google Scholar] [CrossRef]
- Park, J.H.; Jeon, J.Y.; Lee, J.J.; Jang, Y.; Varghese, J.K.; Lee, B.Y. Preparation of High-Molecular-Weight Aliphatic Polycarbonates by Condensation Polymerization of Diols and Dimethyl Carbonate. Macromolecules 2013, 46, 3301–3308. [Google Scholar] [CrossRef]
- Sun, J.; Kuckling, D. Synthesis of high-molecular-weight aliphatic polycarbonates by organo-catalysis. Polym. Chem. 2016, 7, 1642–1649. [Google Scholar]
- Kim, W.B.; Joshi, U.A.; Lee, J.S. Making Polycarbonates without Employing Phosgene: An Overview on Catalytic Chemistry of Intermediate and Precursor Syntheses for Polycarbonate. Ind. Eng. Chem. Res. 2004, 43, 1897–1914. [Google Scholar] [CrossRef]
- Puszka, A.; Kultys, A.; Rogulska, M. Influence of DMPA content on the properties of new thermoplastic poly(ether-urethane) elastomers. J. Elastomers Plast. 2018, 50, 140–150. [Google Scholar] [CrossRef]
- Kultys, A.; Puszka, A. New thermoplastic polyurethane elastomers based on sulfur-containing chain extenders. Pol. J. Chem. Tech. 2013, 15, 4. [Google Scholar] [CrossRef]
- Song, M.; Yang, X.; Wang, G. Synthesis of polycarbonate diols (PCDLs) via two-step process using CH3COONa as an effective catalyst. Chem. Res. Chin. Univ. 2018, 34, 578–583. [Google Scholar] [CrossRef]
- Wnuczek, K.; Puszka, A.; Klapiszewski, Ł.; Podkościelna, B. Preparation, thermal and thermo-mechanical characterization of polymeric blends based on di(meth)acrylate monomers. Polymers 2021, 13, 878. [Google Scholar] [CrossRef]
- Fila, K.; Podkościelna, B.; Podgórski, M. Cross-linked polythiomethacrylate esters based on naphthalene—synthesis, properties and reprocessing. Materials 2020, 13, 3021. [Google Scholar] [CrossRef]
- Fila, K.; Goliszek, M.; Podkościelna, B.; Podgórski, M. Polymer side-chain modification in methacrylate and styrene copolymers through thiol-thioester dynamic exchange. Eur. Polym. J. 2020, 136, 109918. [Google Scholar] [CrossRef]



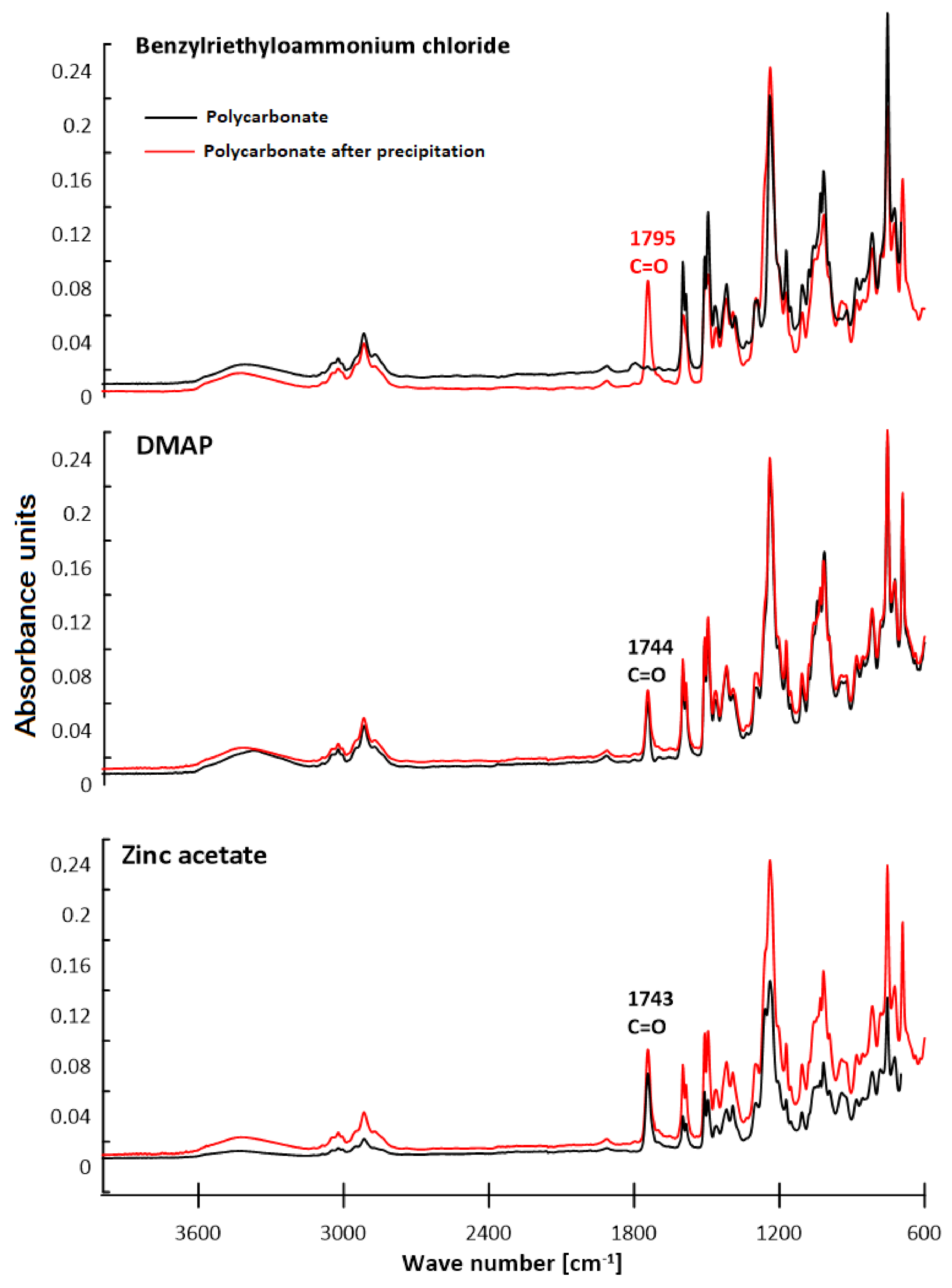
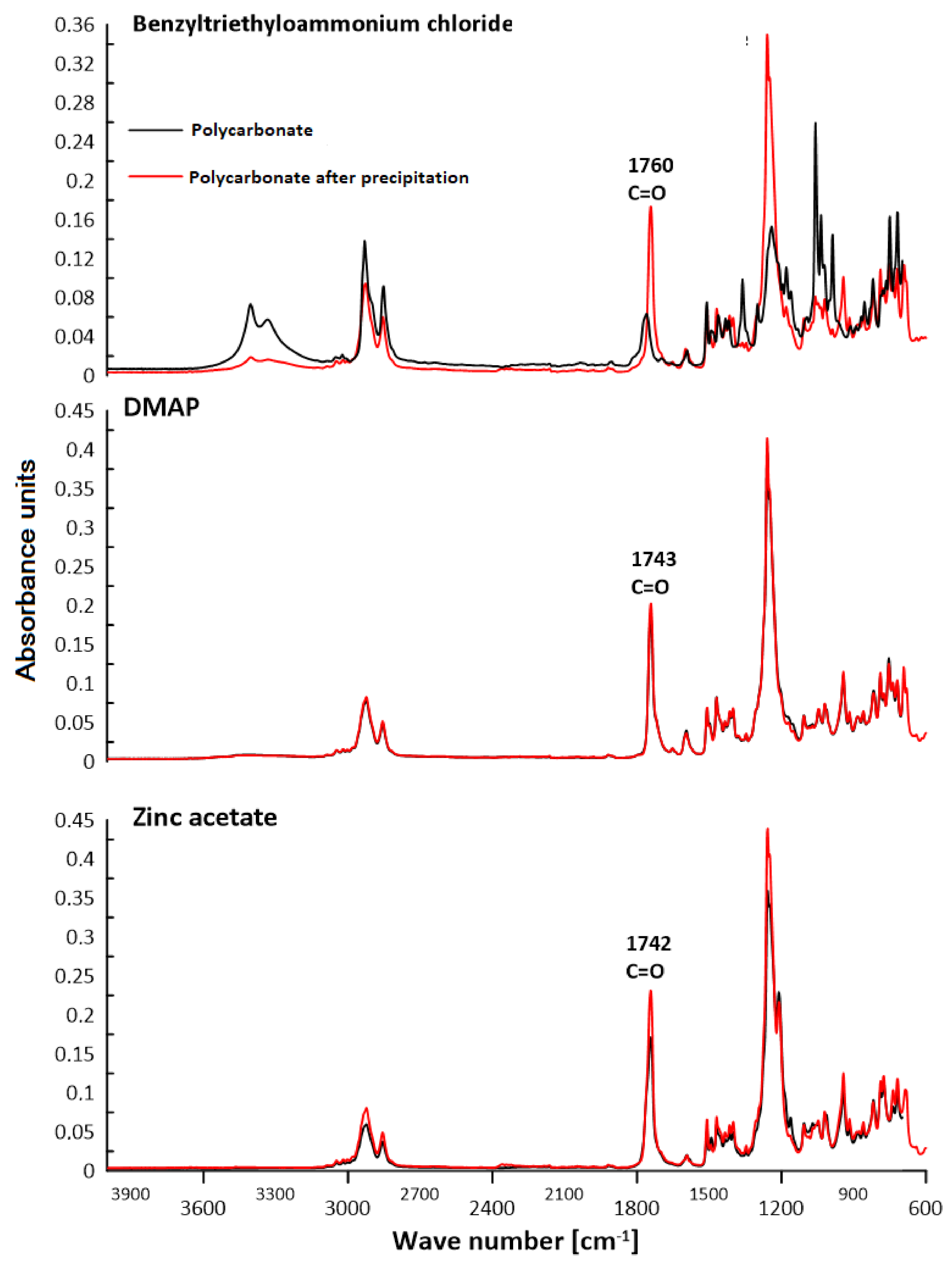

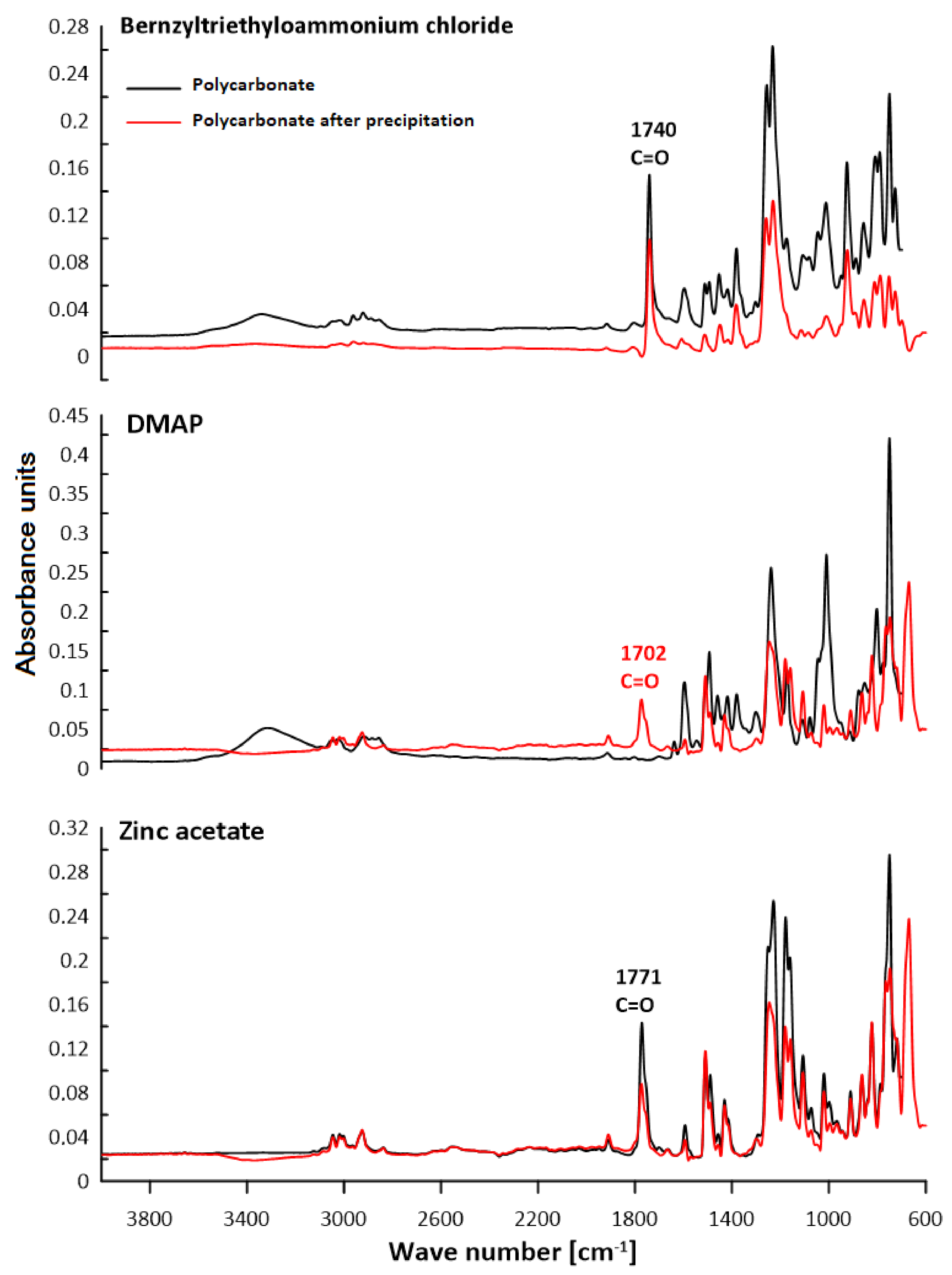


| Polymer | C–H Aliph. | C–H Arom. | C=C Arom. | C–O | C=O | –OH |
|---|---|---|---|---|---|---|
| Diol E + DPC + Zinc acetate | 2916 | 881 | 1596 1463 | 1239 1172 | 1743 | 3426 |
| Diol E + DPC + DMAP | 2915 | 816 | 1598 | 1239 1171 | 1744 | - |
| Diol E + DPC + Benzyltriethylammonium chloride | 2916 | 880 | 1597 | 1295 1238 1104 | - | 3458 |
| Diol H + DPC + Zinc acetate | 2923 | 943 | 1449 | 1257 1210 | 1742 | - |
| Diol H + DPC + DMAP | 2929 | 943 | 1598 1494 | 1259 | 1743 | - |
| Diol H + DPC + Benzyltriethylammonium chloride | 2931 | 988 819 | 1452 | 1239 1180 1100 | 1760 | 3408 |
| Diol M + DPC + Zinc acetate | 2961 | 924 854 | 1469 | 1228 | 1738 | - |
| Diol M + DPC + DMAP | 2917 | 915 860 | 1469 | 1235 1172 1109 | - | 3319 |
| Diol M + DPC + Benzyltriethylammonium chloride | 2929 | 915 860 | 1587 1490 | 1253 1230 1021 | 1740 | - |
| Dithiol + DPC + Zinc acetate | 2958 | 920 863 | 1593 | 1178 1106 | 1771 | - |
| Dithiol + DPC + DMAP | 2960 | 914 825 | 1595 | 1238 1172 1119 | - | 3315 |
| Dithiol + DPC + Benzyltriethylammonium chloride | 2962 | 925 811 | 1596 | 1256 1231 1174 | 1740 | 3370 |
| Monomer | Tm [°C] | ΔHm [J/g] |
|---|---|---|
| Diol E | 85 | 143 |
| Diol H | 68 | 161 |
| Diol M | 119 | 154 |
| Dithiol | 84 | 101 |
| DPC | 84 | 134 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wnuczek, K.; Puszka, A.; Podkościelna, B. Synthesis and Spectroscopic Analyses of New Polycarbonates Based on Bisphenol A-Free Components. Polymers 2021, 13, 4437. https://doi.org/10.3390/polym13244437
Wnuczek K, Puszka A, Podkościelna B. Synthesis and Spectroscopic Analyses of New Polycarbonates Based on Bisphenol A-Free Components. Polymers. 2021; 13(24):4437. https://doi.org/10.3390/polym13244437
Chicago/Turabian StyleWnuczek, Krystyna, Andrzej Puszka, and Beata Podkościelna. 2021. "Synthesis and Spectroscopic Analyses of New Polycarbonates Based on Bisphenol A-Free Components" Polymers 13, no. 24: 4437. https://doi.org/10.3390/polym13244437
APA StyleWnuczek, K., Puszka, A., & Podkościelna, B. (2021). Synthesis and Spectroscopic Analyses of New Polycarbonates Based on Bisphenol A-Free Components. Polymers, 13(24), 4437. https://doi.org/10.3390/polym13244437








