Nanoarchitectonics of BN/AgNWs/Epoxy Composites with High Thermal Conductivity and Electrical Insulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
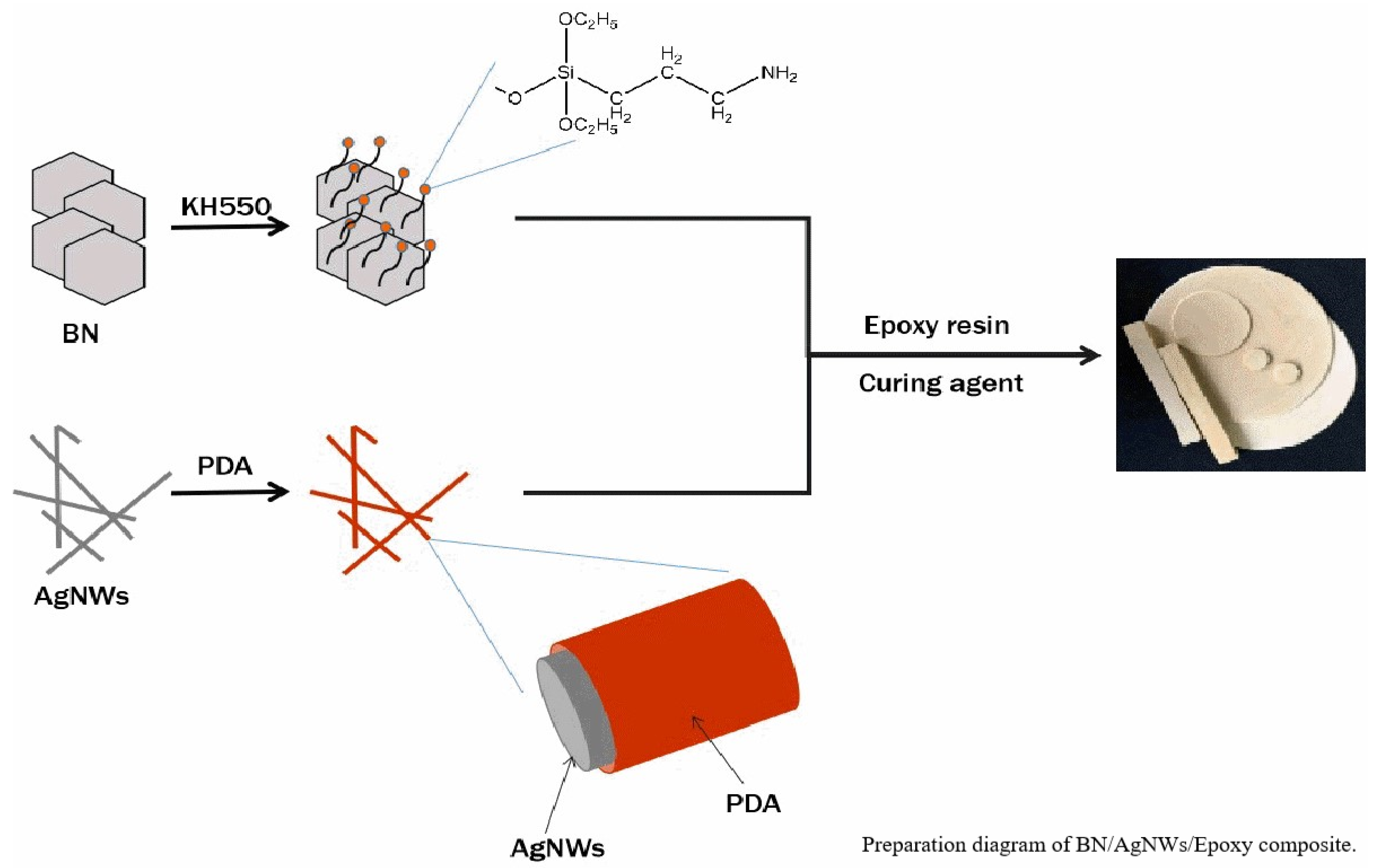
2.2. Composites Preparation
2.3. Measurements
3. Results
3.1. Surface Treatment and Characterization of AgNWs
3.2. Surface Treatment and Characterization of BN
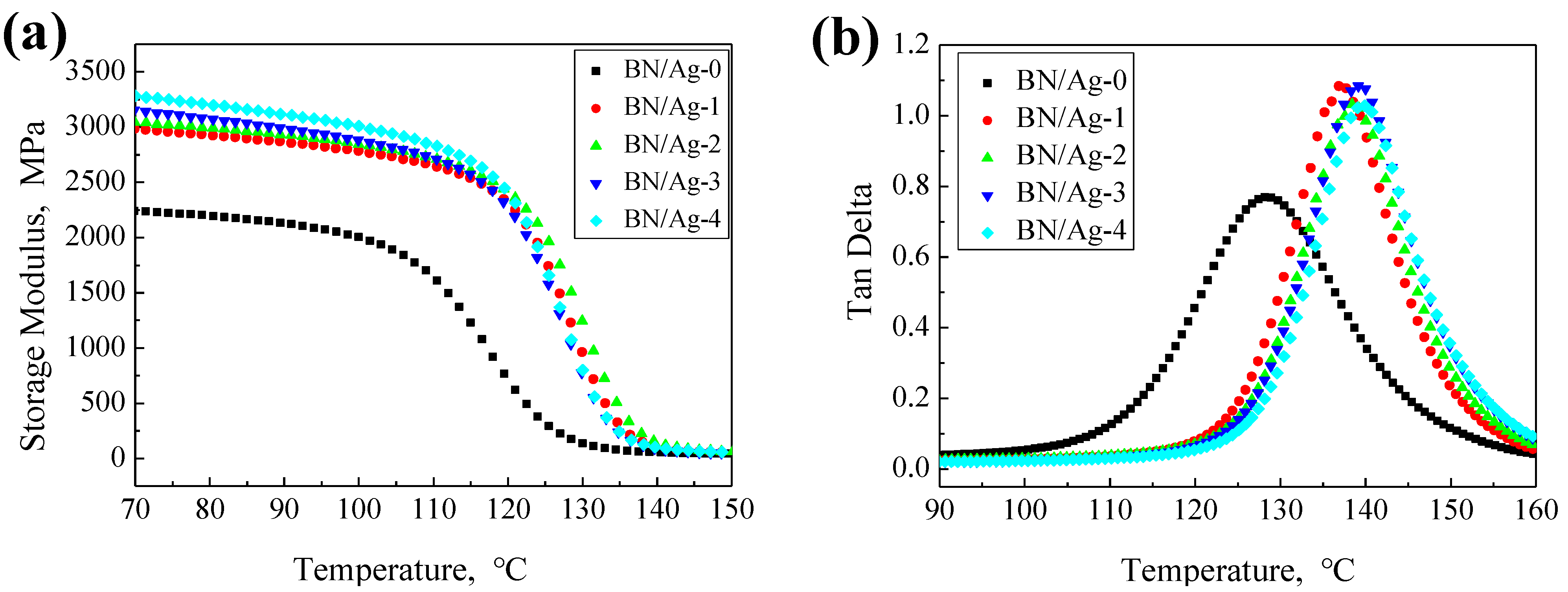
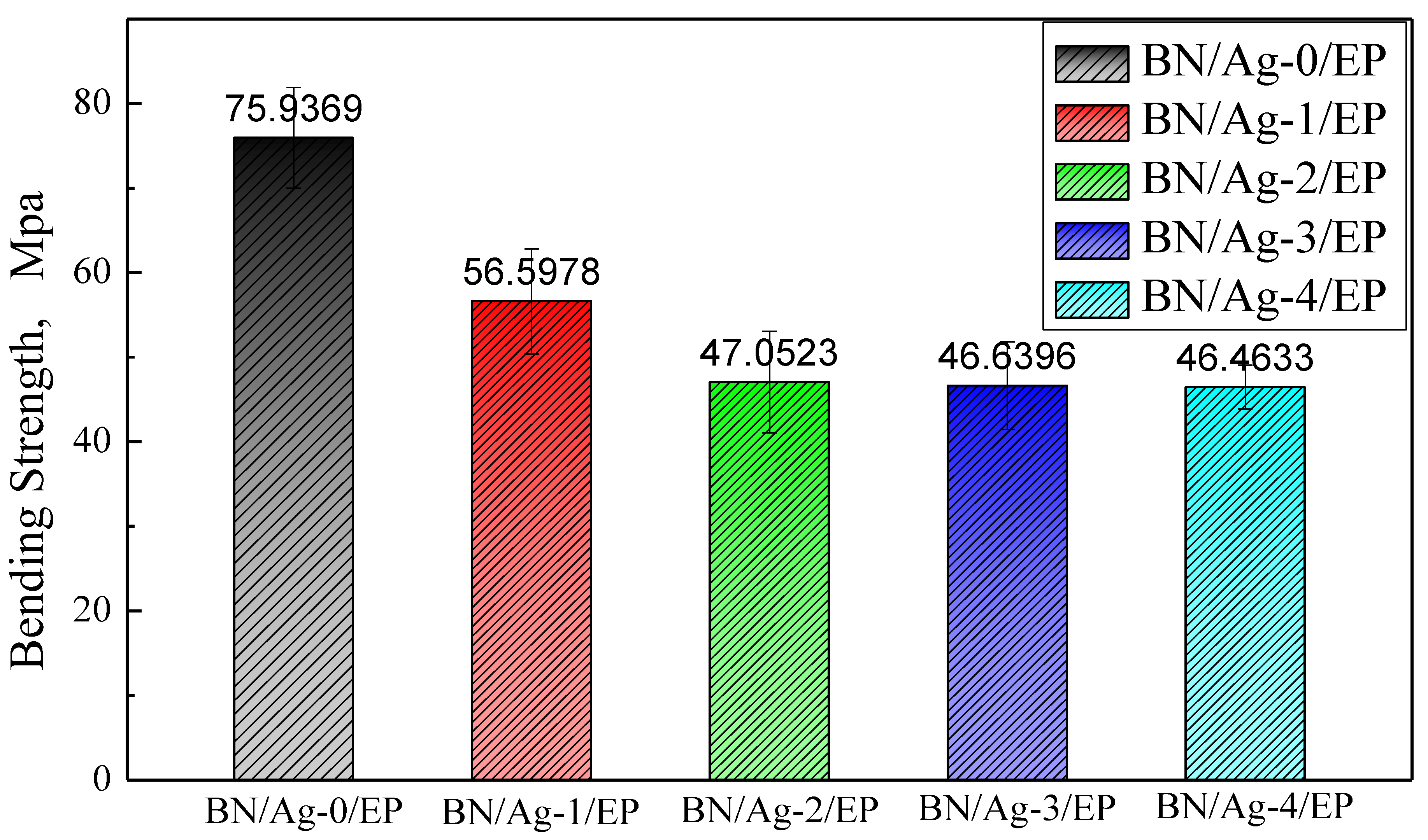
3.3. Mechanical Properties of BN/AgNWs/EP Composites
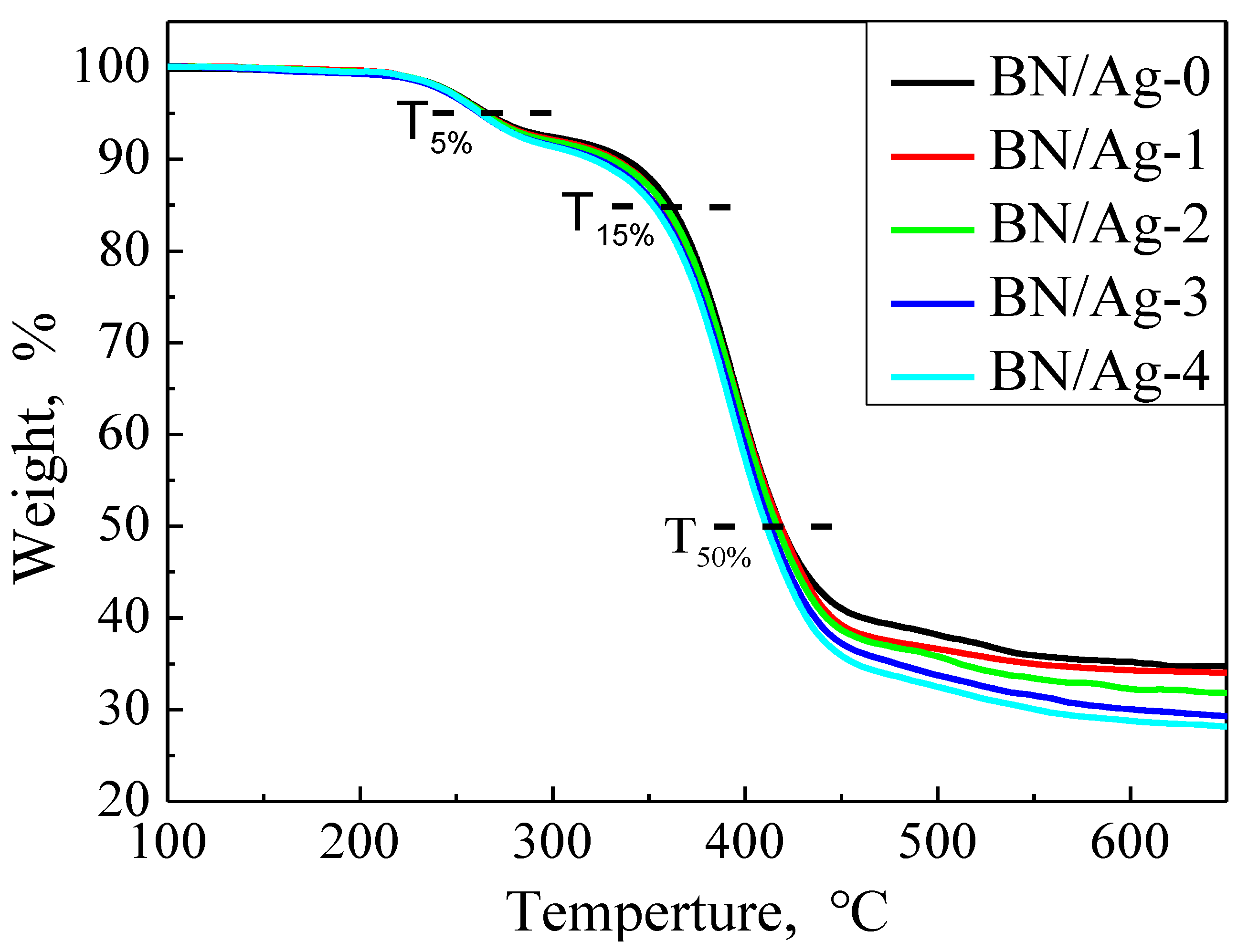
3.4. Thermo Gravimetric Properties of BN/AgNWs/EP Composites
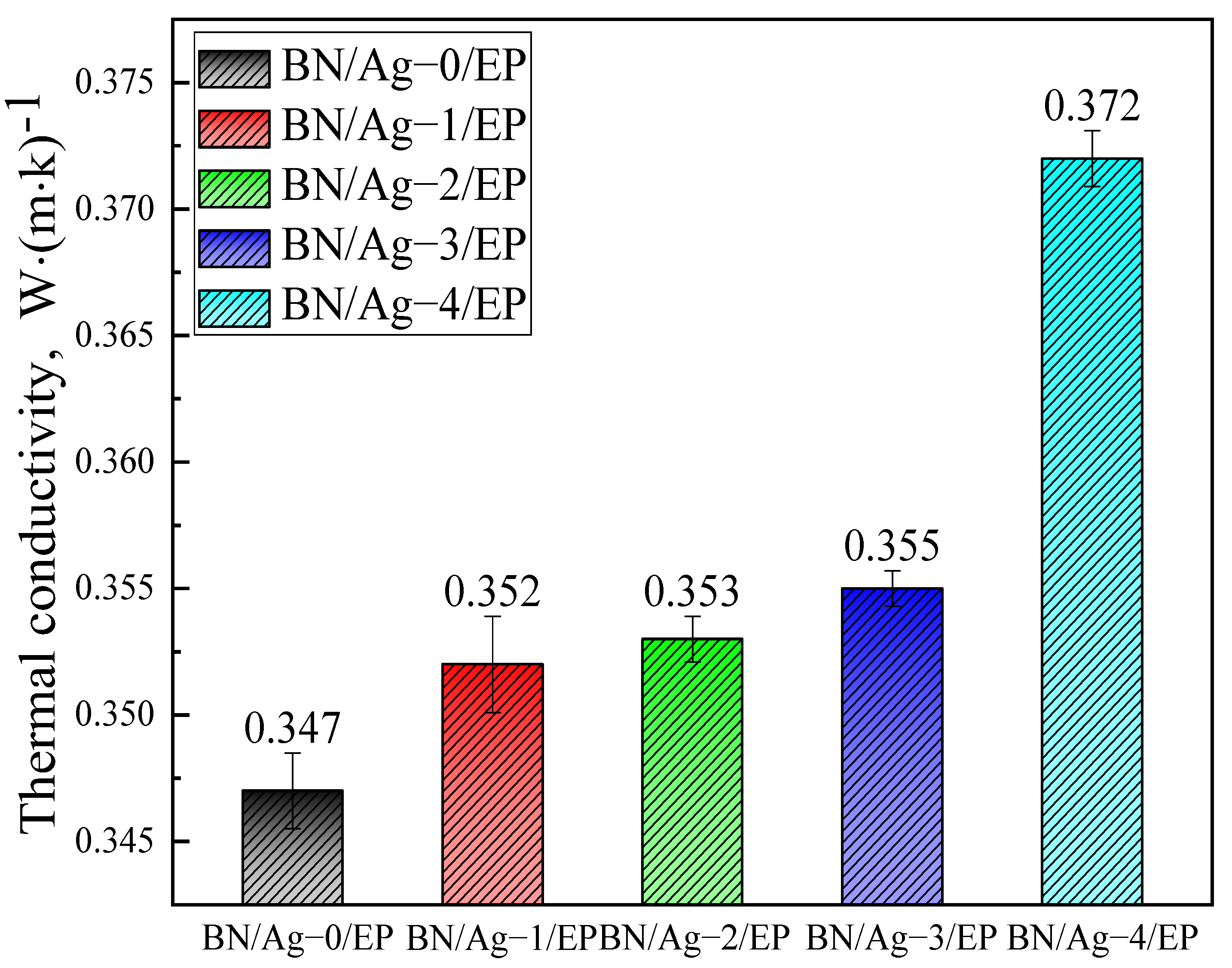
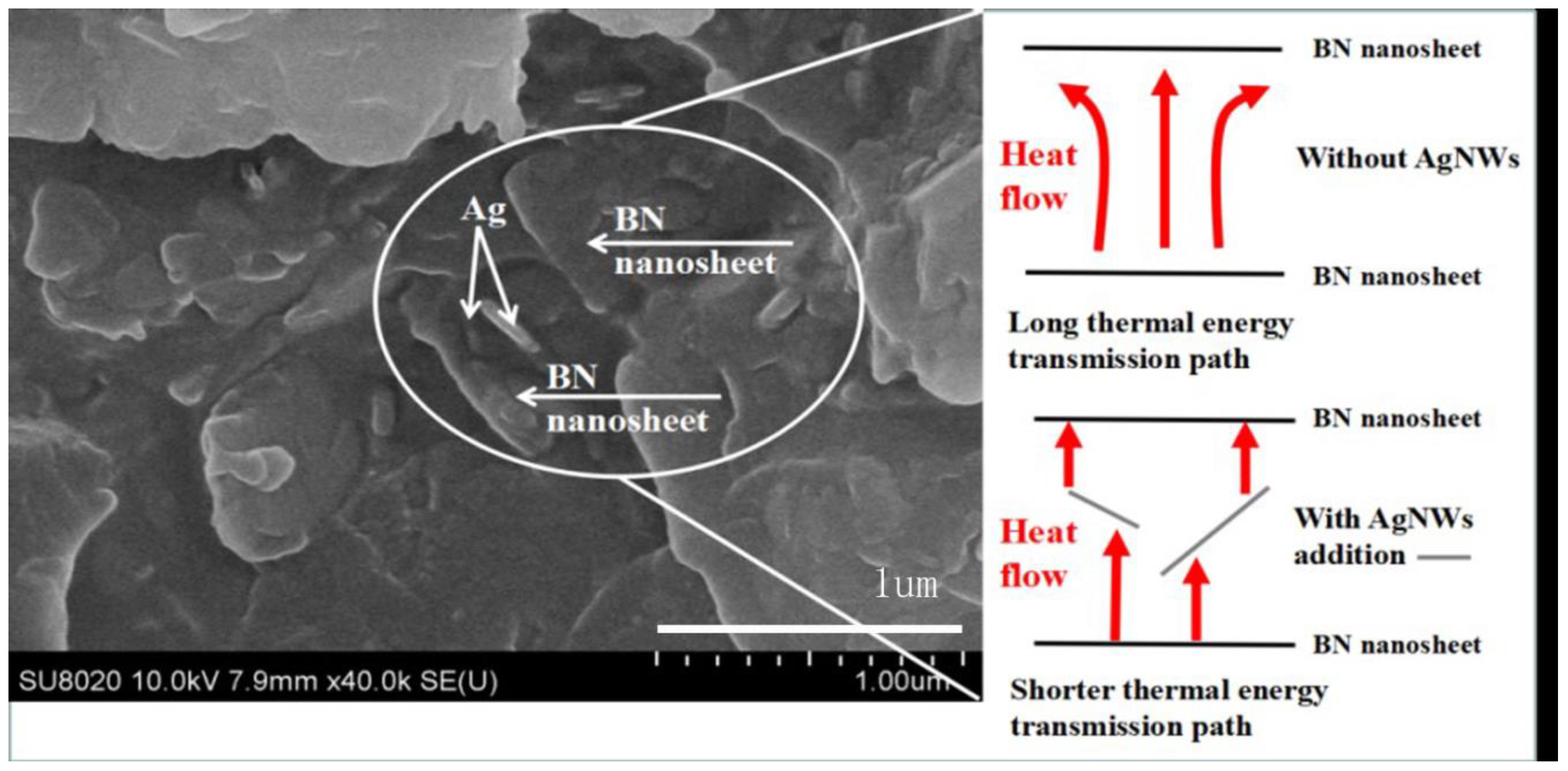
3.5. Thermal Conductivity of BN/AgNWs/EP Composites
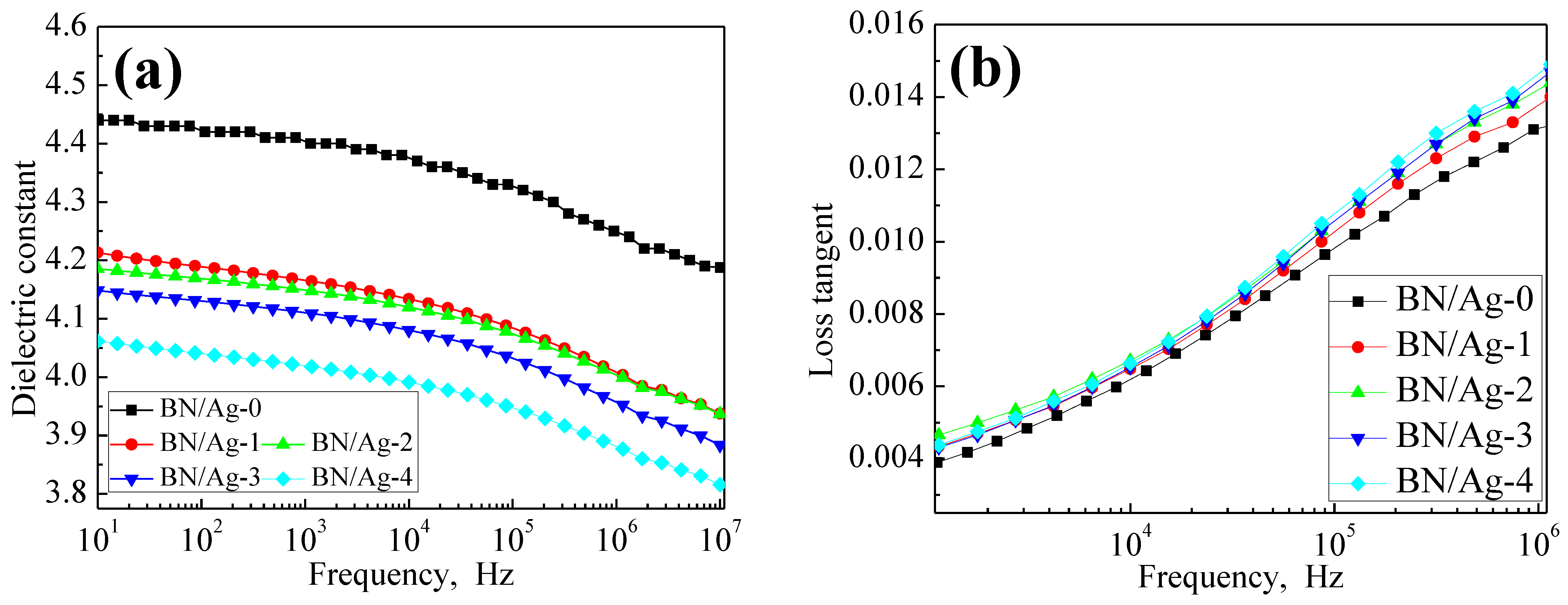
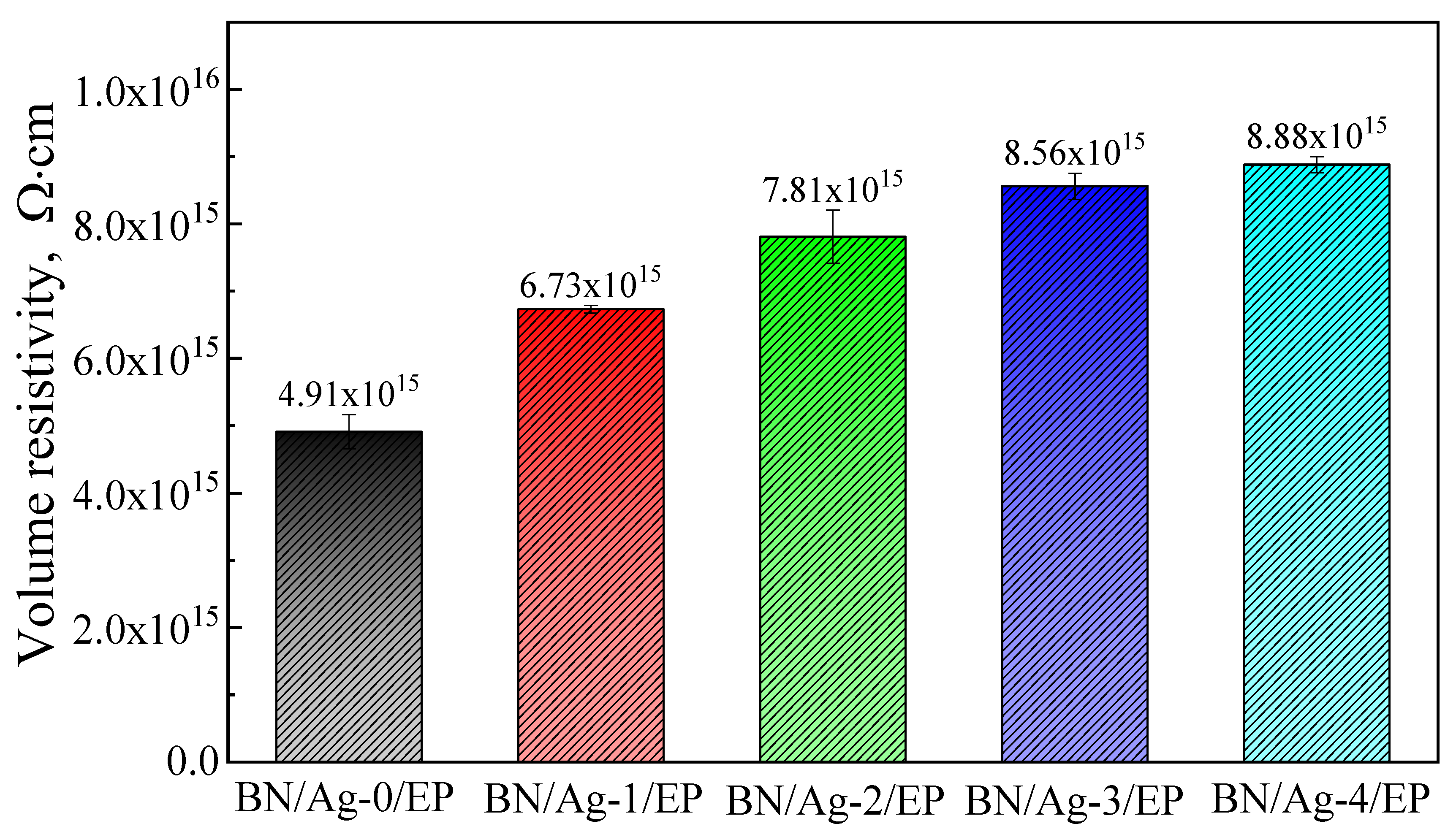
3.6. Dielectric Properties of BN/AgNWs/EP Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Fan, D. Improving Heat Dissipation and Temperature Uniformity in Radiative Cooling Coating. Energy Technol. 2020, 8, 1901362. [Google Scholar] [CrossRef]
- Snead, L.L.; Balden, M.; Causey, R.A.; Atsumi, H. High thermal conductivity of graphite fiber silicon carbide composites for fusion reactor application. J. Nucl. Mater. 2002, 307, 1200–1204. [Google Scholar] [CrossRef]
- Zhong, J.; Liu, D.; Zhuo, L.; Sun, X. High thermal conductivity materials and their application on the electronic products. In Proceedings of the 2012 IEEE Asia-Pacific Conference on Antennas and Propagation, Singapore, 27–29 August 2012; pp. 173–175. [Google Scholar] [CrossRef]
- Mimura, K.; Nakamura, Y.; Masaki, M.; Nishimura, T. Development of Resin Insulated Material with High Thermal Conductivity and Application to the Power Module. J. Photopolym. Sci. Technol. 2015, 28, 169–173. [Google Scholar] [CrossRef]
- Tanaka, T.; Montanari, G.C.; Mulhaupt, R. Polymer nanocomposites as dielectrics and electrical insulation-perspectives for processing technologies, material characterization and future applications. Dielectr. Electr. Insul. IEEE Trans. 2004, 11, 763–784. [Google Scholar] [CrossRef]
- Zhang, D.L.; Zha, J.W.; Li, C.Q.; Li, W.K.; Wang, S.J.; Wen, Y.; Dang, Z.M. High thermal conductivity and excellent electrical insulation performance in double-percolated three-phase polymer nanocomposites. Compos. Sci. Technol. 2017, 144, 36–42. [Google Scholar] [CrossRef]
- Ozel, A.; Mimaroglu, A.; Unal, H. Tribological Performance of Polymer Composites in Use in Electrical Insulation Applications. Soc. Sci. Electron. Publ. 2015, 1, 20–24. [Google Scholar]
- Izzati, W.A.; Arief, Y.Z.; Adzis, Z.; Shafanizam, M. Partial Discharge Characteristics of Polymer Nanocomposite Materials in Electrical Insulation: A Review of Sample Preparation Techniques, Analysis Methods, Potential Applications, and Future Trends. Sci. World J. 2014, 2014, 735070. [Google Scholar] [CrossRef] [PubMed]
- Crosby, A.J.; Hageman, M.; Duncan, A. Controlling Polymer Adhesion with “Pancakes”. Langmuir 2005, 21, 11738–11743. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, P.; Westedt, U.; Wombacher, R.; Kissel, T.; Schaper, A.; Wendorff, J.H.; Greiner, A. Coating of poly(p-xylylene) by PLA-PEO-PLA triblock copolymers with excellent polymer-polymer adhesion for stent applications. Biomacromolecules 2006, 7, 2086–2090. [Google Scholar] [CrossRef] [PubMed]
- Parteli, E.J.R.; Schmidt, J.; Blümel, C.; Wirth, K.-E.; Peukert, W.; Pöschel, T. Attractive particle interaction forces and packing density of fine glass powders. Sci. Rep. 2014, 4, 6227. [Google Scholar] [CrossRef]
- Li, Y.; Li, N.; Gao, J. Tooling design and microwave curing technologies for the manufacturing of fiber-reinforced polymer composites in aerospace applications. Int. J. Adv. Manuf. Technol. 2014, 70, 591–606. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Xiong, L.; Zhao, L. Phosphorus-containing liquid cycloaliphatic epoxy resins for reworkable environment-friendly electronic packaging materials. Polymer 2010, 51, 4776–4783. [Google Scholar] [CrossRef]
- Onishi, Y.; Shiga, T.; Ohkawa, Y.; Katoh, H.; Nagasawa, K.; Okada, T.; Saimen, K.; Itano, F.; Murano, Y.; Tsujita, Y.; et al. Study on Polymer Materials for Development of the Super 100 MGy-Radiation Resistant Motor. Polym. J. 2004, 36, 617–622. [Google Scholar] [CrossRef][Green Version]
- Lee, D.; Lee, S.; Byun, S.; Paik, K.-W.; Song, S.H. Novel dielectric BN/epoxy nanocomposites with enhanced heat dissipation performance for electronic packaging. Compos. Part A Appl. Sci. Manuf. 2018, 107, 217–223. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, F.; Wang, H. Novel organic–inorganic composites with high thermal conductivity for electronic packaging applications: A key issue review. Polym. Compos. 2017, 38, 803–813. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, H.; Hong, H.; Liu, H.; Zhang, X. A Thermally Conductive Composite with a Silica Gel Matrix and Carbon-Encapsulated Copper Nanoparticles as Filler. J. Electron. Mater. 2014, 43, 2759–2769. [Google Scholar] [CrossRef]
- Xu, J.; Munari, A.; Dalton, E.; Mathewson, A.; Razeeb, K.M. Silver nanowire array-polymer composite as thermal interface material. J. Appl. Phys. 2009, 106, 124310. [Google Scholar] [CrossRef]
- Rivière, L.; Lonjon, A.; Dantras, E.; Lacabanne, C.; Olivier, P.; Gleizes, N.R. Silver fillers aspect ratio influence on electrical and thermal conductivity in PEEK/Ag nanocomposites. Eur. Polym. J. 2016, 85, 115–125. [Google Scholar] [CrossRef]
- Teng, C.-C.; Ma, C.-C.M.; Lu, C.-H.; Yang, S.-Y.; Lee, S.-H.; Hsiao, M.-C.; Yen, M.-Y.; Chiou, K.-C.; Lee, T.-M. Thermal conductivity and structure of non-covalent functionalized graphene/epoxy composites. Carbon 2011, 49, 5107–5116. [Google Scholar] [CrossRef]
- Yan, H.; Tang, Y.; Long, W.; Li, Y. Enhanced thermal conductivity in polymer composites with aligned graphene nanosheets. J. Mater. Sci. 2014, 49, 5256–5264. [Google Scholar] [CrossRef]
- Wang, F.; Drzal, L.T.; Qin, Y.; Huang, Z. Mechanical properties and thermal conductivity of graphene nanoplatelet/epoxy composites. J. Mater. Sci. 2015, 50, 1082–1093. [Google Scholar] [CrossRef]
- Haddadi, M.; Agoudjil, B.; Boudenne, A. Thermal conductivity of Polymer/Carbon nanotube composites. Mater. Sci. Forum 2012, 714, 99–113. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Z.; Gong, X.; Yao, E.; Zou, H. Thermal conductivity of Graphene-polymer composites: Implications for thermal management. Heat Mass Transf. 2020, 56, 1931–1945. [Google Scholar] [CrossRef]
- Cai, D.; Song, M. Latex technology as a simple route to improve the thermal conductivity of a carbon nanotube/polymer composite. Carbon 2008, 46, 2107–2112. [Google Scholar] [CrossRef]
- Mortazavi, B.; Pereira, L.F.C.; Jiang, J.-W.; Rabczuk, T. Modelling heat conduction in polycrystalline hexagonal boron-nitride films. Sci. Rep. 2015, 5, 13228. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Bao, J.; Mu, W.; Fu, Y.; Zhang, Y.; Ye, L.; Liu, J. Cooling Hot Spots by Hexagonal Boron Nitride Heat Spreaders. Proc.-Electron. Compon. Technol. Conf. 2015, 1658–1663. [Google Scholar] [CrossRef]
- Barnard, H.R.; Zossimova, E.; Mahlmeister, N.H.; Lawton, L.M.; Luxmoore, I.J.; Nash, G.R. Boron nitride encapsulated graphene infrared emitters. Appl. Phys. Lett. 2016, 108, 131110. [Google Scholar] [CrossRef]
- Loeblein, M.; Tsang, S.H.; Han, Y.; Zhang, X.; Teo, E.H.T. Heat Dissipation Enhancement of 2.5D Package with 3D Graphene and 3D Boron Nitride Networks as Thermal Interface Material (TIM). In Proceedings of the 2016 IEEE 66th Electronic Components and Technology Conference (ECTC), Las Vegas, NV, USA, 31 May–3 June 2016; pp. 707–713. [Google Scholar] [CrossRef]
- Kargar, F.; Barani, Z.; Salgado, R.; Debnath, B.; Lewis, J.S.; Aytan, E.; Lake, R.K.; Balandin, A.A. Thermal Percolation Threshold and Thermal Properties of Composites with High Loading of Graphene and Boron Nitride Fillers. ACS Appl. Mater. Interfaces 2018, 10, 37555–37565. [Google Scholar] [CrossRef]
- Deng, J.; Wang, Y.; Zhang, X.; Zhou, T.; Wang, X.; Yao, Q.; Chen, G. IR Spectra Characteristics of Boron Nitride Thin Films. J. Light Scatt. 2008, 56–59. [Google Scholar] [CrossRef]
- Feng, A.; Jia, Z.; Yu, Q.; Zhang, H.; Wu, G. Preparation and Characterization of Carbon Nanotubes/Carbon Fiber/Phenolic Composites on Mechanical and Thermal Conductivity Properties. NANO 2018, 13, 1850037. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, X.; Yan, T. Effects of Surface Modification on Thermal and Mechanical Properties of EP/BN Composites. Plast. Sci. Technol. 2018, 46, 49–53. [Google Scholar] [CrossRef]
- Wang, N.; Hu, C.; Guo, S.; Liao, J.; Huo, J. Impact of Dopamine Modified Boron Nitride on the Properties of Epoxy Resin Composites. Mater. Rep. 2019, 33, 3837–3841. [Google Scholar]
- Zhang, Y.; Choi, J.R.; Park, S.-J. Enhancing the heat and load transfer efficiency by optimizing the interface of hexagonal boron nitride/elastomer nanocomposites for thermal management applications. Polymer 2018, 143, 1–9. [Google Scholar] [CrossRef]
- Guo, Y.; Ruan, K.; Gu, J. Controllable thermal conductivity in composites by constructing thermal conduction networks. Mater. Today Phys. 2021, 20, 100449. [Google Scholar] [CrossRef]
- Ryu, S.H.; Cho, H.B.; Kwon, Y.T.; Song, Y.; Choa, Y.H. Quasi-Isotropic Thermal Conduction in Percolation Networks: Using the Pore-Filling Effect to Enhance Thermal Conductivity in Polymer Nanocomposites. ACS Appl. Polym. Mater. 2020, 3, 1293–1305. [Google Scholar] [CrossRef]
- Liu, H. Electrocaloric effect enhanced thermal conduction of a multilayer ceramic structure. Chin. Phys. B 2020, 29, 534–537. [Google Scholar] [CrossRef]
- Xiong, K.; Liu, Z.; Zeng, C.; Li, B. Thermal-siphon phenomenon and thermal/electric conduction in complex networks. Natl. Sci. Rev. 2020, 7, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.F. Theoretical analysis of cross-plane lattice thermal conduction in graphite. Chin. Phys. B 2019, 28, 066301. [Google Scholar] [CrossRef]
- Yu, J.; Huang, X.; Chao, W.; Wu, X.; Wang, G.; Jiang, P. Interfacial modification of boron nitride nanoplatelets for epoxy composites with improved thermal properties. Polymer 2012, 53, 471–480. [Google Scholar] [CrossRef]
- Jakubinek, M.B.; Niven, J.F.; Johnson, M.B.; Ashrafi, B.; Kim, K.S.; Simard, B.; White, M.A. Thermal conductivity of bulk boron nitride nanotube sheets and their epoxy-impregnated composites: Thermal conductivity of bulk boron nitride nanotube sheets. Phys. Status Solidi A 2016, 213, 2237–2242. [Google Scholar] [CrossRef]
- Wu, J.; Song, X.; Gong, Y.; Yang, W.; Bian, X. Analysis of the Heat Conduction Mechanism for Al2O3/Silicone Rubber Composite Material with FEM Based on Experiment Observations. Compos. Sci. Technol. 2021, 210, 108809. [Google Scholar] [CrossRef]
- Matsubara, H.; Ohara, T. Effect of In-Plane Aspect Ratio of Graphene Filler on Anisotropic Heat Conduction in Paraffin/Graphene Composite. Phys. Chem. Chem. Phys. 2021, 23, 12082–12092. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, J.; Liu, J.; Wu, W.; Deng, H. Thermal conduction mechanism based on microstructural transformations of molten slag: The role of calcium oxide. Int. J. Heat Mass Transf. 2020, 160, 120167. [Google Scholar] [CrossRef]

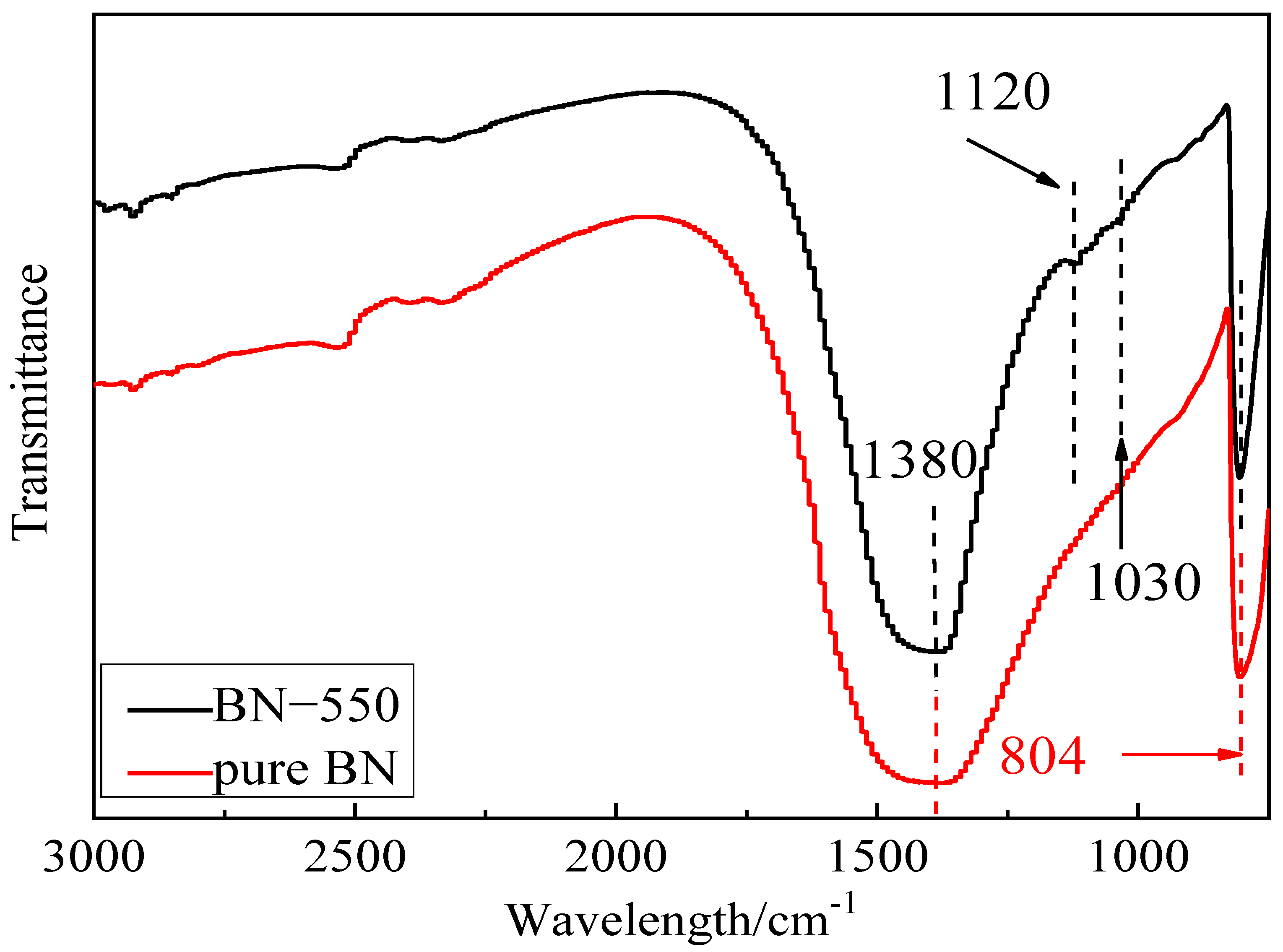
| Group | Band Position (cm−1) | Pure BN | BN-550 | Vibration Mode |
|---|---|---|---|---|
| Si-O | 1030, 1120 | No | Yes | Telescopic vibration |
| h-BN sp2 | 804 | Yes | Yes | Bending vibration |
| h-BN sp2 | 1380 | Yes | Yes | Telescopic vibration |
| Case 1 [34] | Tensile Strength | Case 2 [35] | Tensile Strength | This Research | Bending Strength |
|---|---|---|---|---|---|
| BN/EP | 14.6 | EP | 46.1 | BN/EP | 75.94 |
| KH550/BN/EP | 15.1 | BN/EP | 35.9 | BN/Ag-1/EP | 56.60 |
| KH550-BN/EP | 14.8 | DA-BN/EP | 58.7 | BN/Ag-4/EP | 46.46 |
| Samples | T5% (°C) | T10% (°C) | T50% (°C) |
|---|---|---|---|
| BN/Ag-0/EP | 2.66 ± 0.022 × 102 | 3.38 ± 0.028 × 102 | 4.19 ± 0.005 × 102 |
| BN/Ag-1/EP | 2.65 ± 0.021 × 102 | 3.32 ± 0.030 × 102 | 4.19 ± 0.005 × 102 |
| BN/Ag-2/EP | 2.64 ± 0.020 × 102 | 3.31 ± 0.034 × 102 | 4.17 ± 0.005 × 102 |
| BN/Ag-3/EP | 2.62 ± 0.020 × 102 | 3.23 ± 0.042 × 102 | 4.13 ± 0.005 × 102 |
| BN/Ag-4/EP | 2.73 ± 0.019 × 102 | 3.21 ± 0.040 × 102 | 4.11 ± 0.005 × 102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Weng, L.; Wang, H.; Wang, X. Nanoarchitectonics of BN/AgNWs/Epoxy Composites with High Thermal Conductivity and Electrical Insulation. Polymers 2021, 13, 4417. https://doi.org/10.3390/polym13244417
Li X, Weng L, Wang H, Wang X. Nanoarchitectonics of BN/AgNWs/Epoxy Composites with High Thermal Conductivity and Electrical Insulation. Polymers. 2021; 13(24):4417. https://doi.org/10.3390/polym13244417
Chicago/Turabian StyleLi, Xue, Ling Weng, Hebing Wang, and Xiaoming Wang. 2021. "Nanoarchitectonics of BN/AgNWs/Epoxy Composites with High Thermal Conductivity and Electrical Insulation" Polymers 13, no. 24: 4417. https://doi.org/10.3390/polym13244417
APA StyleLi, X., Weng, L., Wang, H., & Wang, X. (2021). Nanoarchitectonics of BN/AgNWs/Epoxy Composites with High Thermal Conductivity and Electrical Insulation. Polymers, 13(24), 4417. https://doi.org/10.3390/polym13244417






