An Investigative Study on the Progress of Nanoclay-Reinforced Polymers: Preparation, Properties, and Applications: A Review
Abstract
:1. Introduction
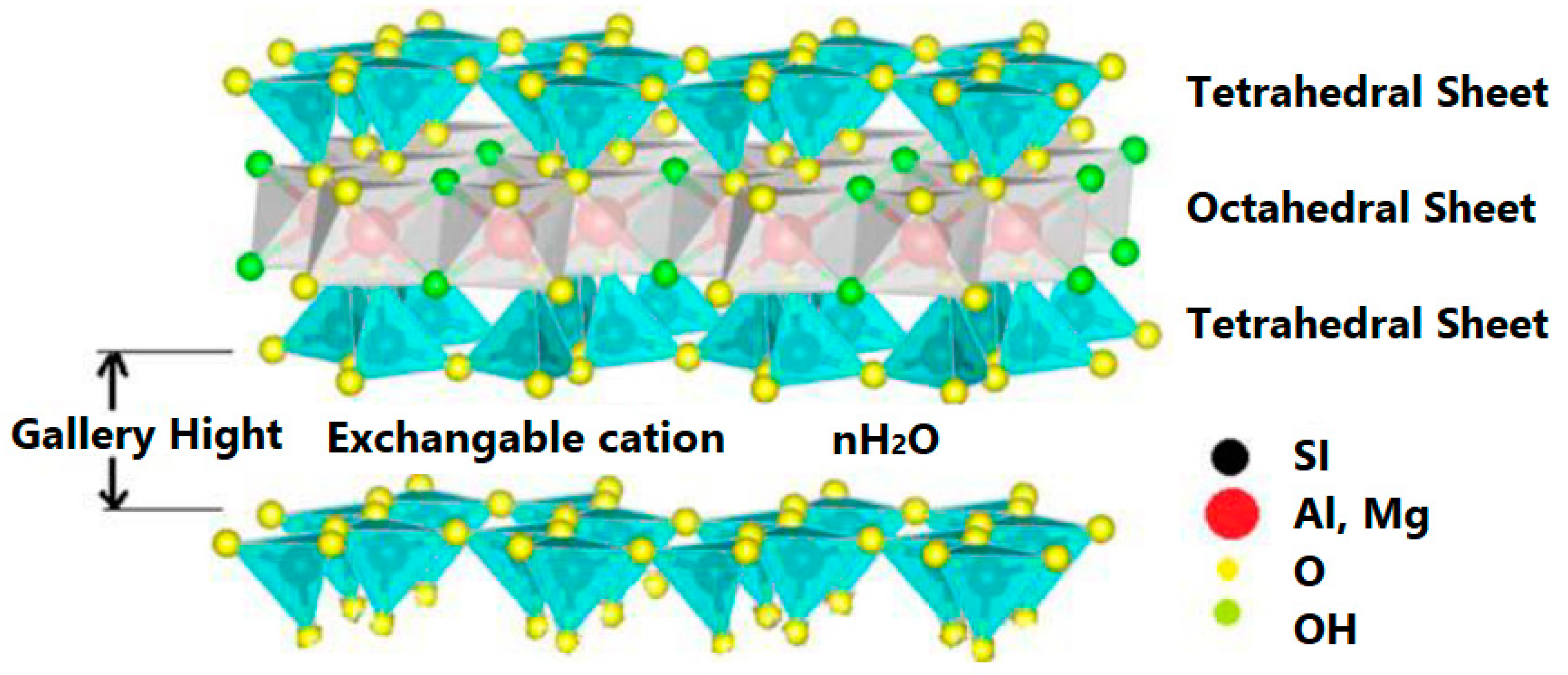
2. Structure of Clay (2:1 Phyllosilicate)
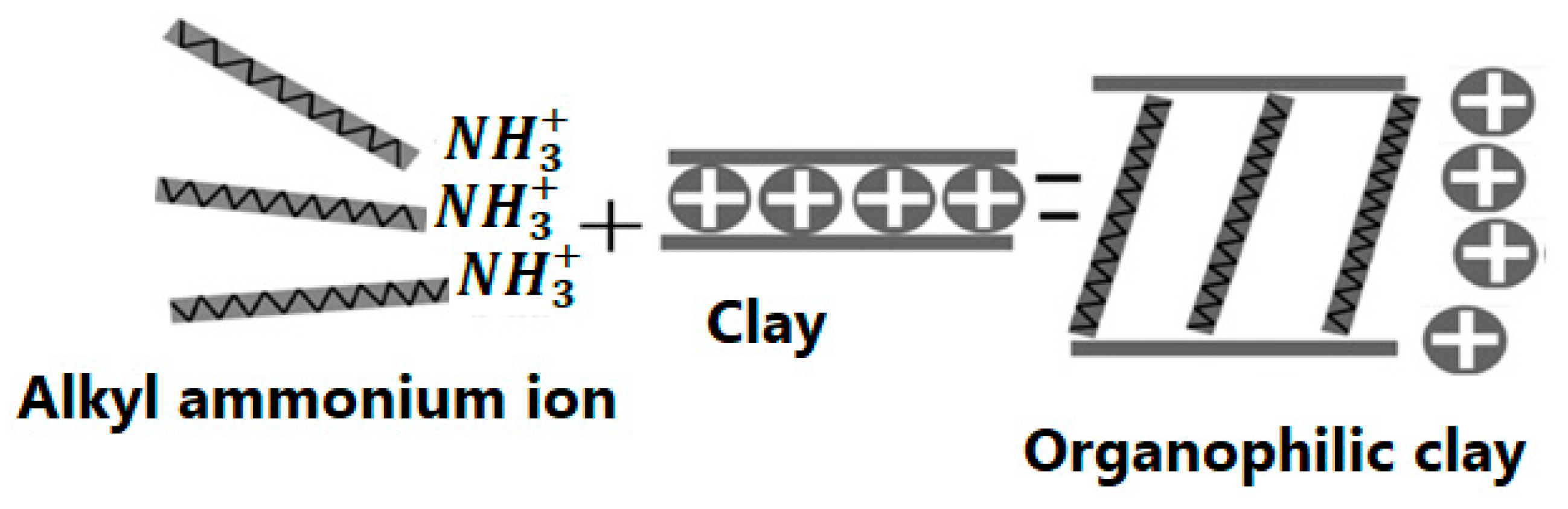
3. Modification of Clay Particles
4. Processing of Clay/Polymer Nanocomposites
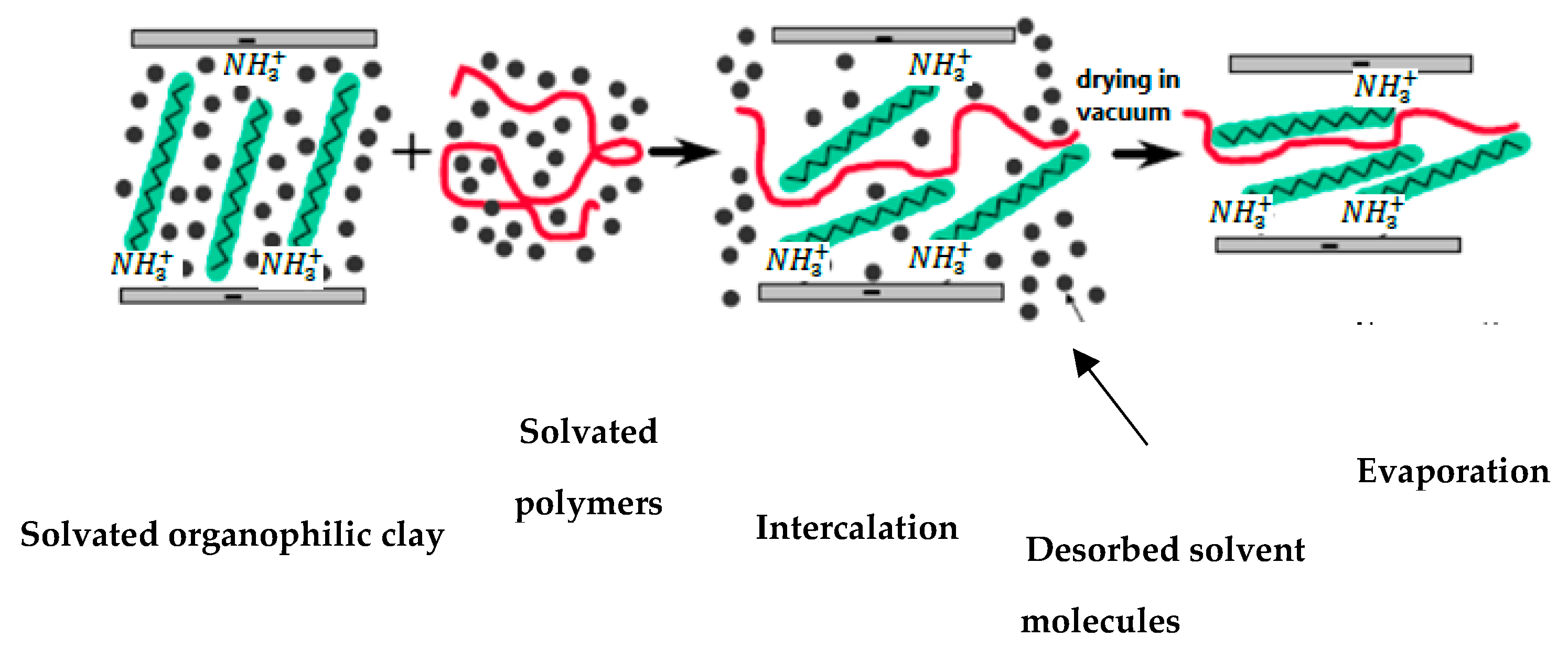
4.1. Solution Blending Method
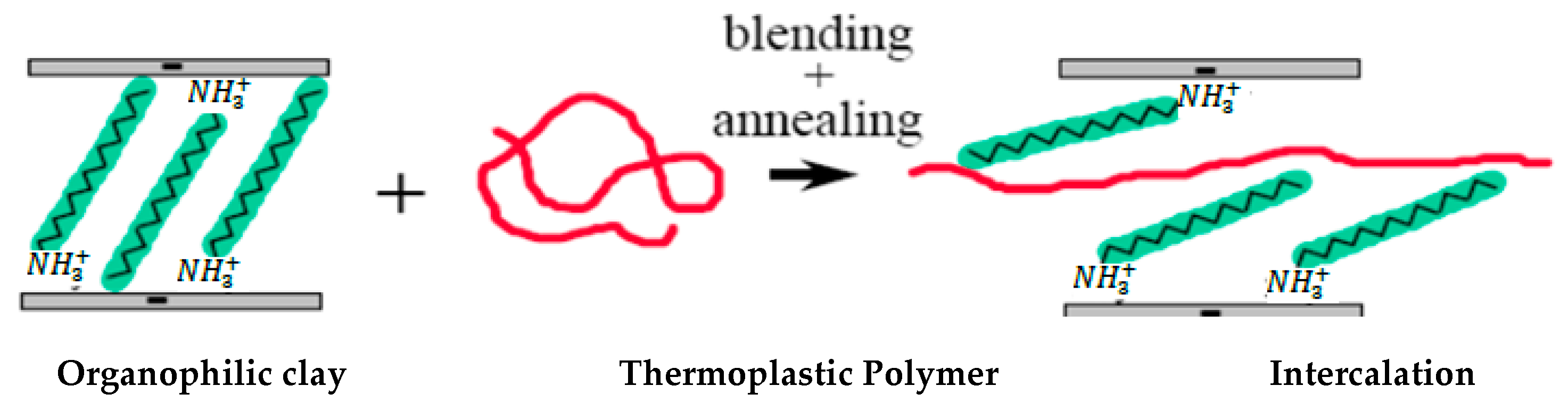
4.2. Melt Blending Method
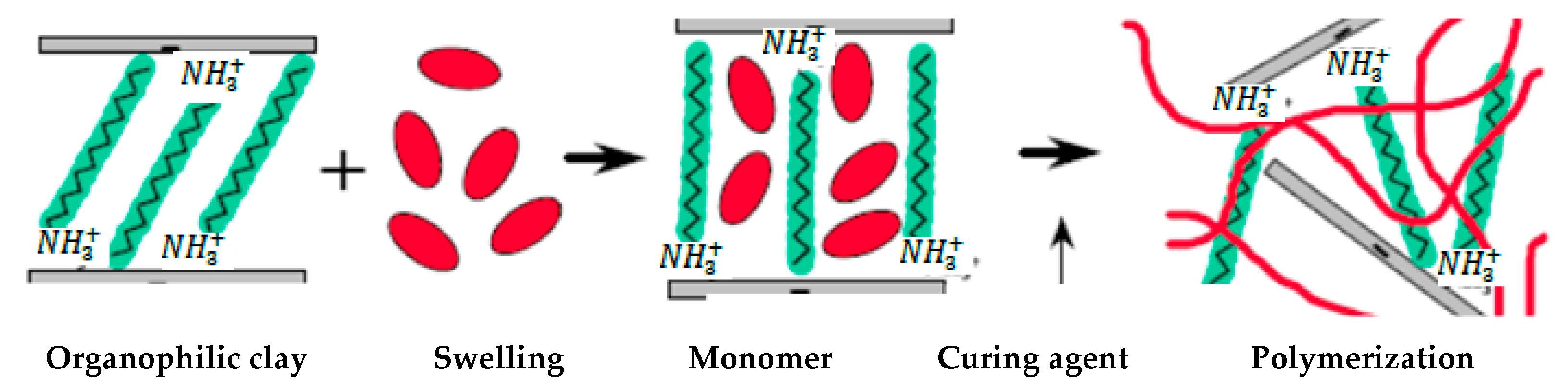
4.3. In Situ Polymerization Method
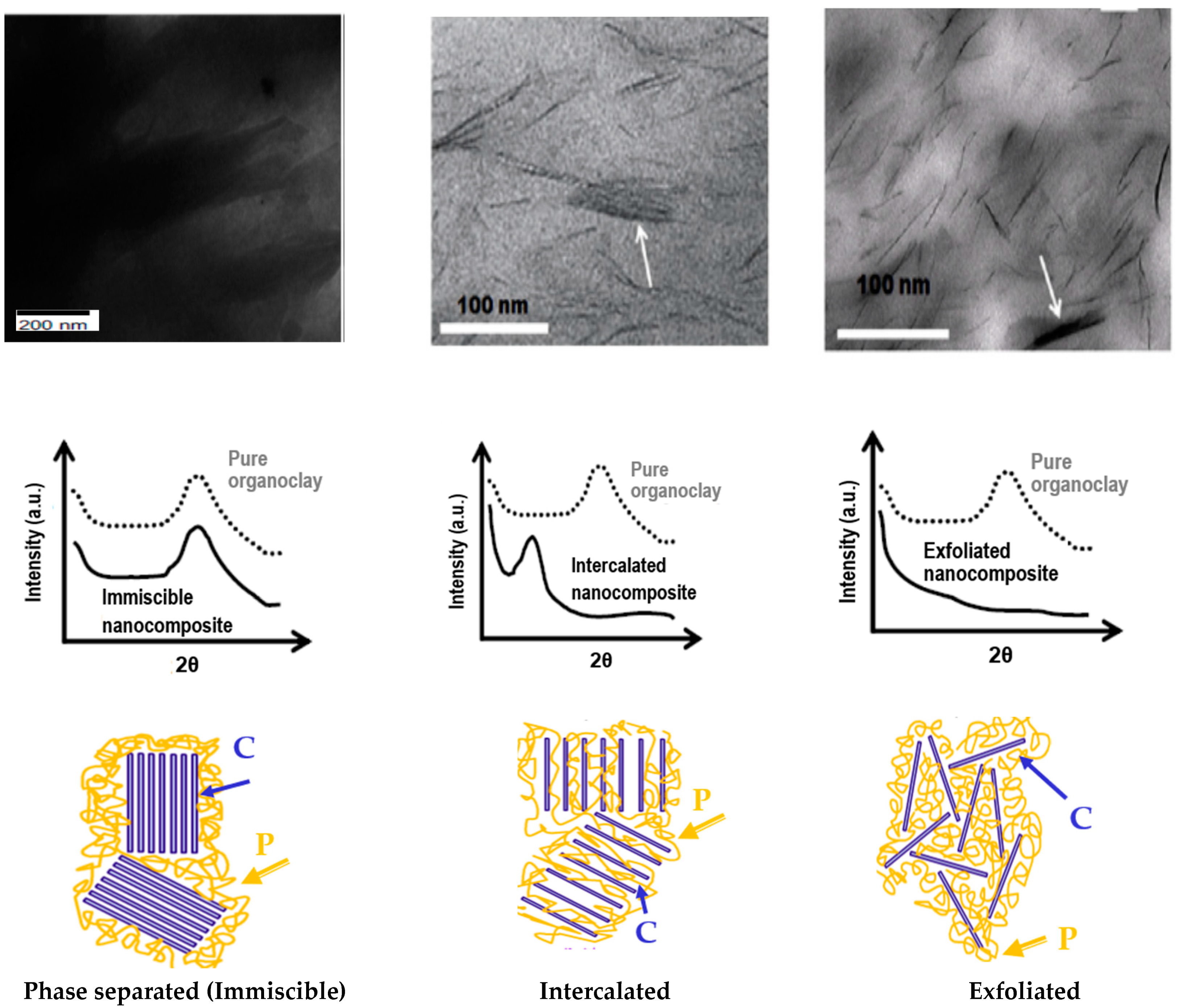
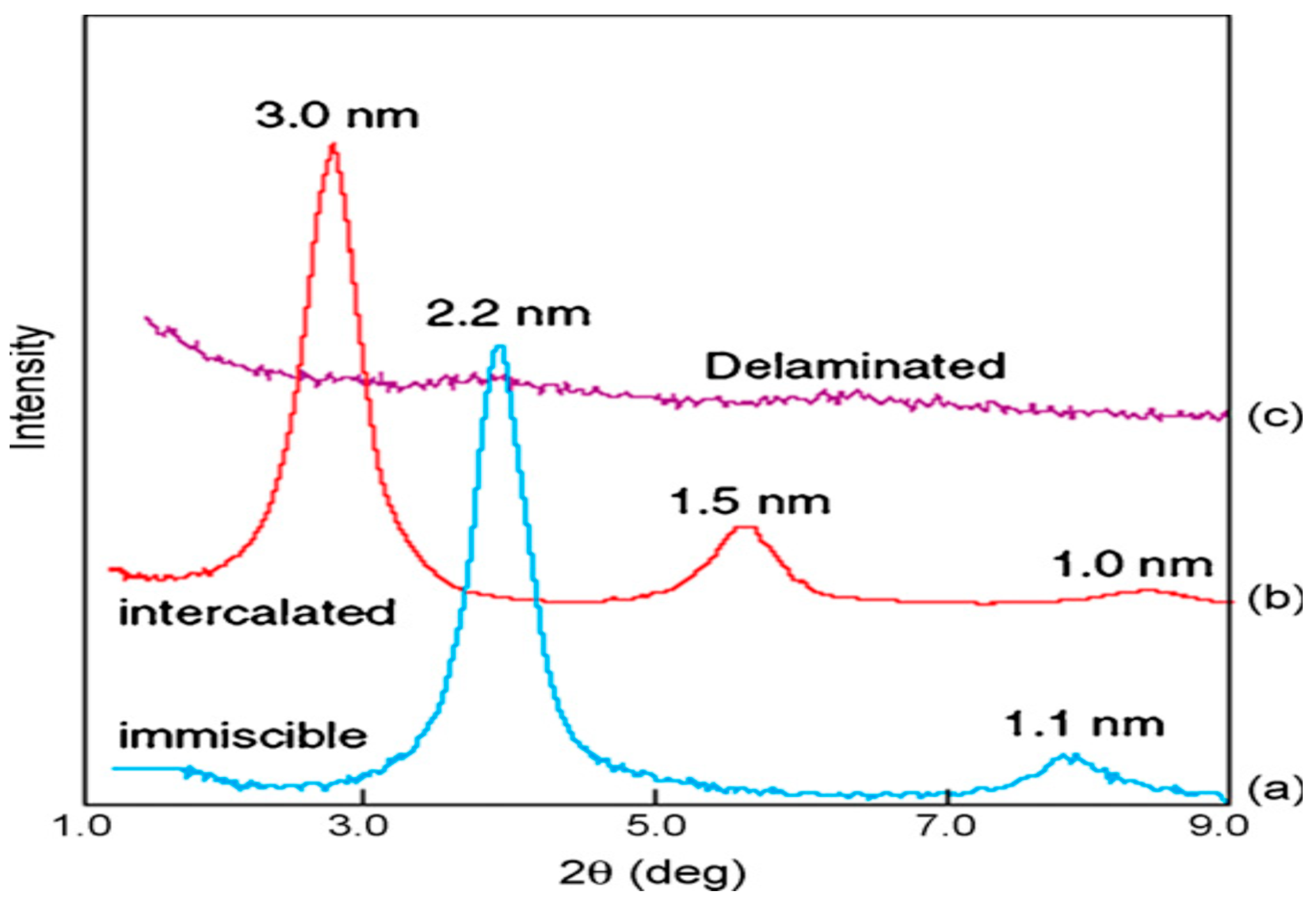
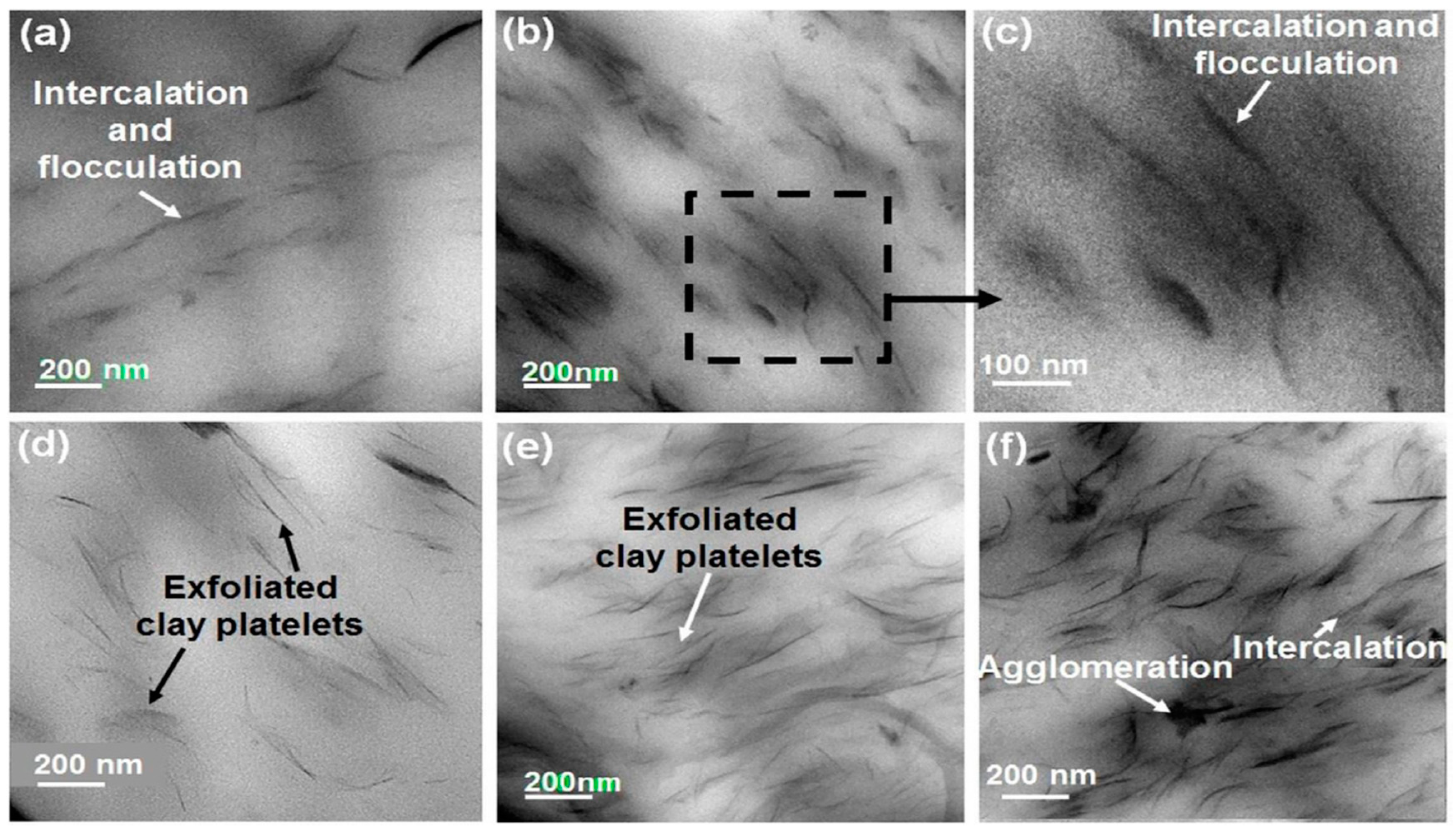
5. Structures of the Nanocomposites PCN
5.1. Phase-Separated Structure
5.2. Intercalated Structure
5.3. Exfoliated Structure
6. Morphological Characterization of PCN
7. Properties of PCN
7.1. Mechanical Properties
7.2. Thermal Properties
7.3. Gas Barrier Properties
7.4. Non-Flammability
8. Applications of PCNs
8.1. Applications in Automotive Field
8.2. Applications in Packaging
8.3. Flame Retardant Applications
8.4. Drug Delivery Applications
8.5. Other Applications
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, H.H. Overview—Clay mineral applications. Appl. Clay Sci. 1991, 5, 379–395. [Google Scholar] [CrossRef]
- Abulyazied, D.E.; Abomostafa, H.M. Magnetic structured nickel core-shell@ silica/PMMA nanocomposites from synthesis to applications. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2335–2346. [Google Scholar] [CrossRef]
- Eyssa, H.M.; Abulyazied, D.E.; Abo-State, M.A.M. Application of polyurethane/gamma-irradiated carbon nanotubes composites as antifouling coat. Polym. Compos. 2018, 39, E1196–E1207. [Google Scholar] [CrossRef]
- Chan, J.X.; Wong, J.F.; Petrů, M.; Hassan, A.; Nirmal, U.; Othman, N.; Ilyas, R.A. Effect of Nanofillers on Tribological Properties of Polymer Nanocomposites: A Review on Recent Development. Polymers 2021, 13, 2867. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.H.; Zhang, Y.; Mahmoodi, A.; Xu, G.; Yu, J.; Wu, J.; Shi, X. Nanocomposite organic coatings for corrosion protection of metals: A review of recent advances. Prog. Org. Coat. 2022, 162, 106573. [Google Scholar] [CrossRef]
- Saba, N.; Tahir, P.M.; Jawaid, M. A review on potentiality of nano filler/natural fiber filled polymer hybrid composites. Polymers 2014, 6, 2247–2273. [Google Scholar] [CrossRef]
- Motawie, A.M.; Madany, M.M.; El-Dakrory, A.Z.; Osman, H.M.; Ismail, E.A.; Badr, M.M.; El-Komy, D.A.; Abulyazied, D.E. Physico-chemical characteristics of nano-organo bentonite prepared using different organo-modifiers. Egypt. J. Pet. 2014, 23, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Albdiry, M.T.; Yousif, B.F.; Ku, H.; Lau, K.T. A critical review on the manufacturing processes in relation to the properties of nanoclay/polymer composites. J. Compos. Mater. 2013, 47, 1093–1115. [Google Scholar] [CrossRef]
- Du, J.; Cheng, H. The fabrication, properties, and uses of graphene/polymer composites. Macromol. Chem. Phys. 2012, 213, 1060–1077. [Google Scholar] [CrossRef]
- Calvert, P. A recipe for strength. Nature 1999, 399, 210–211. [Google Scholar] [CrossRef]
- Reynaud, E.; Gauthier, C.; Perez, J. Nanophases in polymers. Rev. Metall. 1999, 96, 169–176. [Google Scholar] [CrossRef]
- Mark, J.E. Ceramic-reinforced polymers and polymer-modified ceramics. Polym. Eng. Sci. 1996, 36, 2905–2920. [Google Scholar] [CrossRef]
- Valapa, R.B.; Loganathan, S.; Pugazhenthi, G.; Thomas, S.; Varghese, T.O. Chapter 2—An Overview of Polymer–Clay Nanocomposites. In Clay-Polymer Nanocomposites; Jlassi, K., Chehimi, M.M., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 29–81. ISBN 9780323461535. [Google Scholar]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-Y.; Aitomäki, Y.; Berglund, L.A.; Oksman, K.; Bismarck, A. On the use of nanocellulose as reinforcement in polymer matrix composites. Compos. Sci. Technol. 2014, 105, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Barus, S.; Zanetti, M.; Lazzari, M.; Costa, L. Preparation of polymeric hybrid nanocomposites based on PE and nanosilica. Polymer 2009, 50, 2595–2600. [Google Scholar] [CrossRef]
- Zhang, J.; Manias, E.; Wilkie, C.A. Polymerically modified layered silicates: An effective route to nanocomposites. J. Nanosci. Nanotechnol. 2008, 8, 1597–1615. [Google Scholar] [CrossRef] [Green Version]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Echegoyen Sanz, Y.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U. Review on the processing and properties of polymer nanocomposites and nanocoatings and their applications in the packaging, automotive and solar energy fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [Green Version]
- Mrah, L.; Meghabar, R. In situ polymerization of styrene–clay nanocomposites and their properties. Polym. Bull. 2021, 78, 3509–3526. [Google Scholar] [CrossRef]
- Chiu, F.-C.; Behera, K.; Cai, H.-J.; Chang, Y.-H. Polycarbonate/Poly (vinylidene fluoride)-Blend-Based Nanocomposites—Effect of Adding Different Carbon Nanofillers/Organoclay. Polymers 2021, 13, 2626. [Google Scholar] [CrossRef]
- Krasinskyi, V.; Suberlyak, O.; Sikora, J.; Zemke, V. Nanocomposites based on polyamide-6 and montmorillonite intercalated with polyvinylpyrrolidone. Polym. Technol. Mater. 2021, 60, 1641–1655. [Google Scholar] [CrossRef]
- Sabbagh, F.; Khatir, N.M.; Karim, A.K.; Omidvar, A.; Nazari, Z.; Jaberi, R. Mechanical properties and swelling behavior of acrylamide hydrogels using montmorillonite and kaolinite as clays. J. Environ. Treat. Tech. 2019, 7, 211–219. [Google Scholar]
- Chandio, A.D.; Channa, I.A.; Rizwan, M.; Akram, S.; Javed, M.S.; Siyal, S.H.; Saleem, M.; Makhdoom, M.A.; Ashfaq, T.; Khan, S. Polyvinyl Alcohol and Nano-Clay Based Solution Processed Packaging Coatings. Coatings 2021, 11, 942. [Google Scholar] [CrossRef]
- Kotal, M.; Bhowmick, A.K. Polymer nanocomposites from modified clays: Recent advances and challenges. Prog. Polym. Sci. 2015, 51, 127–187. [Google Scholar] [CrossRef] [Green Version]
- Sabbagh, F. A comparative study on the clays incorporated with acrylamide-based hydrogels. Adv. Appl. NanoBio-Technol. 2021, 2, 15–23. [Google Scholar]
- Essomba, J.S.; Jaya, P.A.; Placide, D.; Belibi, B.; Ketcha, J.M.; Nishter, N.F. Clay/polymer nanocomposites as filler materials for leather. J. Clean. Prod. 2019, 237, 117837. [Google Scholar]
- Ramos Filho, F.G.; Mélo, T.J.A.; Rabello, M.S.; Silva, S.M.L. Thermal stability of nanocomposites based on polypropylene and bentonite. Polym. Degrad. Stab. 2005, 89, 383–392. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C.D. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 2010, 35, 902–958. [Google Scholar] [CrossRef]
- Wang, R.; Li, H.; Ge, G.; Dai, N.; Rao, J.; Ran, H.; Zhang, Y. Montmorillonite-Based Two-Dimensional Nanocomposites: Preparation and Applications. Molecules 2021, 26, 2521. [Google Scholar] [CrossRef]
- Kausar, A. A review of fundamental principles and applications of polymer nanocomposites filled with both nanoclay and nano-sized carbon allotropes–graphene and carbon nanotubes. J. Plast. Film Sheeting 2020, 36, 209–228. [Google Scholar] [CrossRef]
- Fischer, H. Polymer nanocomposites: From fundamental research to specific applications. Mater. Sci. Eng. C 2003, 23, 763–772. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Hong, S.-I.; Ha, C.-S. Tensile, water vapor barrier and antimicrobial properties of PLA/nanoclay composite films. LWT-Food Sci. Technol. 2009, 42, 612–617. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Akhtar, M.J.; Cleymand, F.; Desobry, S. Structural, mechanical and barrier properties of active PLA–antioxidant films. J. Food Eng. 2012, 110, 380–389. [Google Scholar] [CrossRef]
- Gong, X.; Pan, L.; Tang, C.Y.; Chen, L.; Hao, Z.; Law, W.-C.; Wang, X.; Tsui, C.P.; Wu, C. Preparation, optical and thermal properties of CdSe–ZnS/poly (lactic acid)(PLA) nanocomposites. Compos. Part B Eng. 2014, 66, 494–499. [Google Scholar] [CrossRef]
- Pesetskii, S.S.; Bogdanovich, S.P.; Aderikha, V.N. Polymer/clay nanocomposites produced by dispersing layered silicates in thermoplastic melts. In Research Anthology on Synthesis, Characterization, and Applications of Nanomaterials; IGI Global: Hershey, PA, USA, 2021; pp. 1002–1030. [Google Scholar]
- Najafi, N.; Heuzey, M.C.; Carreau, P.J. Polylactide (PLA)-clay nanocomposites prepared by melt compounding in the presence of a chain extender. Compos. Sci. Technol. 2012, 72, 608–615. [Google Scholar] [CrossRef]
- Tawakkal, I.S.M.A.; Cran, M.J.; Bigger, S.W. Effect of kenaf fibre loading and thymol concentration on the mechanical and thermal properties of PLA/kenaf/thymol composites. Ind. Crops Prod. 2014, 61, 74–83. [Google Scholar] [CrossRef]
- Ray, S.S. Clay-Containing Polymer Nanocomposites: From Fundamentals to Real Applications; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780444594372. [Google Scholar]
- Derdar, H.; Meghabar, R.; Benachour, M.; Mitchell, G.R.; Bachari, K.; Belbachir, M.; Cherifi, Z.; Baghdadli, M.C.; Harrane, A. Polymer-Clay Nanocomposites: Exfoliation and Intercalation of Organophilic Montmorillonite Nanofillers in Styrene–Limonene Copolymer. Polym. Sci. Ser. A 2021, 63, 568–575. [Google Scholar] [CrossRef]
- Gao, F. Clay/polymer composites: The story. Mater. Today 2004, 7, 50–55. [Google Scholar] [CrossRef]
- Tan, B.; Thomas, N.L. A review of the water barrier properties of polymer/clay and polymer/graphene nanocomposites. J. Memb. Sci. 2016, 514, 595–612. [Google Scholar] [CrossRef] [Green Version]
- Mittal, V. Polymer layered silicate nanocomposites: A review. Materials 2009, 2, 992–1057. [Google Scholar] [CrossRef] [Green Version]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymer–layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Yeh, J.-M.; Chang, K.-C. Polymer/layered silicate nanocomposite anticorrosive coatings. J. Ind. Eng. Chem. 2008, 14, 275–291. [Google Scholar] [CrossRef]
- Anadão, P. Clay-containing polysulfone nanocomposites. In Advances in Nanocomposite Technology; Hashim, A., Ed.; In Tech: Rijeka, Croatia, 2011; pp. 133–146. ISBN 978-953-307-347-7. [Google Scholar]
- Ke, Y.C.; Stroeve, P. Polymer-Layered Silicate and Silica Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2005; ISBN 0080457584. [Google Scholar]
- Kumar, A.; Zhang, R.; Venkatesan, M.; Stamenov, P.; Coey, J.M.D. Exfoliation of hematite: Morphological, structural and magnetic investigations. J. Magn. Magn. Mater. 2022, 542, 168507. [Google Scholar] [CrossRef]
- Ali, F.; Ullah, H.; Ali, Z.; Rahim, F.; Khan, F.; Rehman, Z.U. Polymer-clay Nanocomposites, Preparations and Current Applications: A Review. Curr. Nanomater. 2016, 1, 83–95. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar]
- Porter, D.; Metcalfe, E.; Thomas, M.J.K. Nanocomposite fire retardants—A review. Fire Mater. 2000, 24, 45–52. [Google Scholar] [CrossRef]
- Vaia, R.A.; Giannelis, E.P. Polymer melt intercalation in organically-modified layered silicates: Model predictions and experiment. Macromolecules 1997, 30, 8000–8009. [Google Scholar] [CrossRef]
- Morgan, A.B.; Gilman, J.W. Characterization of polymer-layered silicate (clay) nanocomposites by transmission electron microscopy and X-ray diffraction: A comparative study. J. Appl. Polym. Sci. 2003, 87, 1329–1338. [Google Scholar] [CrossRef]
- Ma, J.; Xu, J.; Ren, J.-H.; Yu, Z.-Z.; Mai, Y.-W. A new approach to polymer/montmorillonite nanocomposites. Polymer 2003, 44, 4619–4624. [Google Scholar] [CrossRef]
- Adak, B.; Butola, B.S.; Joshi, M. Effect of organoclay-type and clay-polyurethane interaction chemistry for tuning the morphology, gas barrier and mechanical properties of clay/polyurethane nanocomposites. Appl. Clay Sci. 2018, 161, 343–353. [Google Scholar] [CrossRef]
- Motawie, A.M.; Madani, M.; Esmail, E.A.; Dacrorry, A.Z.; Othman, H.M.; Badr, M.M.; Abulyazied, D.E. Electrophysical characteristics of polyurethane/organo-bentonite nanocomposites. Egypt. J. Pet. 2014, 23, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Chen, B. Polymer–clay nanocomposites: An overview with emphasis on interaction mechanisms. Br. Ceram. Trans. 2004, 103, 241–249. [Google Scholar] [CrossRef]
- Shokrieh, M.M.; Kefayati, A.R.; Chitsazzadeh, M. Fabrication and mechanical properties of clay/epoxy nanocomposite and its polymer concrete. Mater. Des. 2012, 40, 443–452. [Google Scholar] [CrossRef]
- Penaloza, D.P., Jr. Enhanced mechanical, thermal and barrier properties of clay-based polymer nanocomposite systems. Építöanyag (Online) 2019, 71, 74–79. [Google Scholar] [CrossRef]
- Kornmann, X.; Berglund, L.A.; Sterte, J.; Giannelis, E.P. Nanocomposites based on montmorillonite and unsaturated polyester. Polym. Eng. Sci. 1998, 38, 1351–1358. [Google Scholar] [CrossRef]
- Tyan, H.; Wei, K.; Hsieh, T. Mechanical properties of clay–polyimide (BTDA–ODA) nanocomposites via ODA-modified organoclay. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 2873–2878. [Google Scholar] [CrossRef]
- Shelley, J.S.; Mather, P.T.; DeVries, K.L. Reinforcement and environmental degradation of nylon-6/clay nanocomposites. Polymer 2001, 42, 5849–5858. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.-J.; Daniel, I.M. Characterization and modeling of mechanical behavior of polymer/clay nanocomposites. Compos. Sci. Technol. 2003, 63, 1607–1616. [Google Scholar] [CrossRef]
- Kim, D.H.; Fasulo, P.D.; Rodgers, W.R.; Paul, D.R. Structure and properties of polypropylene-based nanocomposites: Effect of PP-g-MA to organoclay ratio. Polymer 2007, 48, 5308–5323. [Google Scholar] [CrossRef]
- Kaushik, A.K.; Podsiadlo, P.; Qin, M.; Shaw, C.M.; Waas, A.M.; Kotov, N.A.; Arruda, E.M. The role of nanoparticle layer separation in the finite deformation response of layered polyurethane-clay nanocomposites. Macromolecules 2009, 42, 6588–6595. [Google Scholar] [CrossRef]
- Gelineau, P.; Stepień, M.; Weigand, S.; Cauvin, L.; Bédoui, F. Elastic properties prediction of nano-clay reinforced polymer using multi-scale modeling based on a multi-scale characterization. Mech. Mater. 2015, 89, 12–22. [Google Scholar] [CrossRef]
- Kabalan, L.; Zagho, M.M.; Al-Marri, M.J.; Khader, M.M. Experimental and theoretical studies on the mechanical and structural changes imposed by the variation of clay loading on poly (vinyl alcohol)/cloisite® 93A nanocomposites. J. Vinyl Addit. Technol. 2019, 25, 172–181. [Google Scholar] [CrossRef]
- Yas, M.H.; Shahrani Korani, H.; Zare Jouneghani, F. Studying the Mechanical and Thermal Properties of Polymer Nanocomposites Reinforced with Montmorillonite Nanoparticles Using Micromechanics Method. J. Solid Mech. 2020, 12, 90–101. [Google Scholar]
- Burmistr, M.V.; Sukhyy, K.M.; Shilov, V.V.; Pissis, P.; Spanoudaki, A.; Sukha, I.V.; Tomilo, V.I.; Gomza, Y.P. Synthesis, structure, thermal and mechanical properties of nanocomposites based on linear polymers and layered silicates modified by polymeric quaternary ammonium salts (ionenes). Polymer 2005, 46, 12226–12232. [Google Scholar] [CrossRef]
- Ray, S.S.; Bousmina, M. Biodegradable polymers and their layered silicate nanocomposites: In greening the 21st century materials world. Prog. Mater. Sci. 2005, 50, 962–1079. [Google Scholar]
- Becker, O.; Varley, R.J.; Simon, G.P. Thermal stability and water uptake of high performance epoxy layered silicate nanocomposites. Eur. Polym. J. 2004, 40, 187–195. [Google Scholar] [CrossRef]
- Zhu, J.; Uhl, F.M.; Morgan, A.B.; Wilkie, C.A. Studies on the mechanism by which the formation of nanocomposites enhances thermal stability. Chem. Mater. 2001, 13, 4649–4654. [Google Scholar] [CrossRef]
- Cherifi, Z.; Boukoussa, B.; Zaoui, A.; Belbachir, M.; Meghabar, R. Structural, morphological and thermal properties of nanocomposites poly (GMA)/clay prepared by ultrasound and in-situ polymerization. Ultrason. Sonochem. 2018, 48, 188–198. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Dranca, I.; Fan, X.; Advincula, R. Kinetics of the thermal and thermo-oxidative degradation of a polystyrene–clay nanocomposite. Macromol. Rapid Commun. 2004, 25, 498–503. [Google Scholar] [CrossRef]
- Baniasadi, H.; Trifol, J.; Ranta, A.; Seppälä, J. Exfoliated clay nanocomposites of renewable long-chain aliphatic polyamide through in-situ polymerization. Compos. Part B Eng. 2021, 211, 108655. [Google Scholar] [CrossRef]
- Fu, X.; Qutubuddin, S. Polymer–clay nanocomposites: Exfoliation of organophilic montmorillonite nanolayers in polystyrene. Polymer 2001, 42, 807–813. [Google Scholar] [CrossRef]
- Zhang, W.A.; Chen, D.Z.; Xu, H.Y.; Shen, X.F.; Fang, Y.E. Influence of four different types of organophilic clay on the morphology and thermal properties of polystyrene/clay nanocomposites prepared by using the γ-ray irradiation technique. Eur. Polym. J. 2003, 39, 2323–2328. [Google Scholar] [CrossRef]
- Naderi-Samani, H.; Loghman-Estarki, M.R.; Razavi, R.S.; Ramazani, M. The Effects of organoclay on the morphology, thermal stability, transparence and hydrophobicity properties of polyamide—Imide/nanoclay nanocomposite coatings. Prog. Org. Coat. 2017, 112, 162–168. [Google Scholar] [CrossRef]
- Fekry, M.; Mazrouaa, A.M.; Mohamed, M.G.; Abulyazied, D.E. Properties of polyethylcyanoacrylate/modified Mt composites with highly exfoliated montmorillonite. Polym. Bull. 2021, 78, 5685–5711. [Google Scholar] [CrossRef]
- Abdallah, W.; Yilmazer, U. Novel thermally stable organo-montmorillonites from phosphonium and imidazolium surfactants. Thermochim. Acta 2011, 525, 129–140. [Google Scholar] [CrossRef]
- Yano, K.; Usuki, A.; Okada, A.; Kurauchi, T.; Kamigaito, O. Synthesis and properties of polyimide–clay hybrid. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 2493–2498. [Google Scholar] [CrossRef]
- Tortora, M.; Vittoria, V.; Galli, G.; Ritrovati, S.; Chiellini, E. Transport properties of modified montmorillonite-poly (e-caprolactone) nanocomposites. Macromol. Mater. Eng. 2002, 287, 243–249. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Yamada, K.; Okamoto, M.; Ogami, A.; Ueda, K. New polylactide/layered silicate nanocomposites. 3. High-performance biodegradable materials. Chem. Mater. 2003, 15, 1456–1465. [Google Scholar] [CrossRef]
- Lange, J.; Wyser, Y. Recent innovations in barrier technologies for plastic packaging—A review. Packag. Technol. Sci. 2003, 16, 149–158. [Google Scholar] [CrossRef]
- Koh, H.C.; Park, J.S.; Jeong, M.A.; Hwang, H.Y.; Hong, Y.T.; Ha, S.Y.; Nam, S.Y. Preparation and gas permeation properties of biodegradable polymer/layered silicate nanocomposite membranes. Desalination 2008, 233, 201–209. [Google Scholar] [CrossRef]
- Żenkiewicz, M.; Richert, J. Permeability of polylactide nanocomposite films for water vapour, oxygen and carbon dioxide. Polym. Test. 2008, 27, 835–840. [Google Scholar] [CrossRef]
- Żenkiewicz, M.; Richert, J.; Różański, A. Effect of blow moulding ratio on barrier properties of polylactide nanocomposite films. Polym. Test. 2010, 29, 251–257. [Google Scholar] [CrossRef]
- Duan, Z.; Thomas, N.L.; Huang, W. Water vapour permeability of poly (lactic acid) nanocomposites. J. Memb. Sci. 2013, 445, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Lilichenko, N.; Maksimov, R.D.; Zicans, J.; Meri, R.M.; Plume, E. A biodegradable polymer nanocomposite: Mechanical and barrier properties. Mech. Compos. Mater. 2008, 44, 45–56. [Google Scholar] [CrossRef]
- Giannakas, A.; Spanos, C.G.; Kourkoumelis, N.; Vaimakis, T.; Ladavos, A. Preparation, characterization and water barrier properties of PS/organo-montmorillonite nanocomposites. Eur. Polym. J. 2008, 44, 3915–3921. [Google Scholar] [CrossRef]
- Tortora, M.; Gorrasi, G.; Vittoria, V.; Galli, G.; Ritrovati, S.; Chiellini, E. Structural characterization and transport properties of organically modified montmorillonite/polyurethane nanocomposites. Polymer 2002, 43, 6147–6157. [Google Scholar] [CrossRef]
- Alix, S.; Follain, N.; Tenn, N.; Alexandre, B.; Bourbigot, S.; Soulestin, J.; Marais, S. Effect of highly exfoliated and oriented organoclays on the barrier properties of polyamide 6 based nanocomposites. J. Phys. Chem. C 2012, 116, 4937–4947. [Google Scholar] [CrossRef]
- Sanchez-Olivares, G.; Sanchez-Solis, A.; Calderas, F.; Medina-Torres, L.; Herrera-Valencia, E.E.; Rivera-Gonzaga, A.; Manero, O. Extrusion with ultrasound applied on intumescent flame-retardant polypropylene. Polym. Eng. Sci. 2013, 53, 2018–2026. [Google Scholar] [CrossRef]
- Hiujian, L.; Jinwei, Y.; Ling, X. The synthesis and application of a high performance amino resin nanocomposite as a leather flame retardant. J. Soc. Leather Technol. Chem. 2012, 96, 5–10. [Google Scholar]
- Sanchez-Olivares, G.; Sanchez-Solis, A.; Calderas, F.; Medina-Torres, L.; Manero, O.; Di Blasio, A.; Alongi, J. Sodium montmorillonite effect on the morphology, thermal, flame retardant and mechanical properties of semi-finished leather. Appl. Clay Sci. 2014, 102, 254–260. [Google Scholar] [CrossRef]
- Zeng, Q.H.; Yu, A.B.; Lu, G.Q.; Paul, D.R. Clay-based polymer nanocomposites: Research and commercial development. J. Nanosci. Nanotechnol. 2005, 5, 1574–1592. [Google Scholar] [CrossRef] [PubMed]
- Panchuk, M.; Kryshtopa, S.; Sładkowski, A.; Panchuk, A. Environmental aspects of the production and use of biofuels in transport. In Ecology in Transport: Problems and Solutions. Lecture Notes in Networks and Systems (LNNS, Volume 124); Springer Nature: Cham, Switzerland, 2020; pp. 115–168. [Google Scholar]
- Gul, S.; Kausar, A.; Muhammad, B.; Jabeen, S. Research progress on properties and applications of polymer/clay nanocomposite. Polym. Plast. Technol. Eng. 2016, 55, 684–703. [Google Scholar] [CrossRef]
- Galimberti, M.; Cipolletti, V.R.; Coombs, M. Applications of clay–polymer nanocomposites. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 539–586. ISBN 1572-4352. [Google Scholar]
- Bandyopadhyay, J.; Ray, S.S. Applications of nanoclay-containing polymer nanocomposites. In Inorganic Nanosheets and Nanosheet-Based Materials; Springer: Tokyo, Japan, 2017; pp. 501–521. ISBN 978-4-431-56496-6. [Google Scholar]
- Cogen, J.M.; Lin, T.S.; Morgan, A.B.; Garcés, J.M. Novel synthetic nanocomposite materials and their application in polyolefin-based wire and cable compounds. In Proceedings of the 52nd International Wire and Cable Symposium, Philadelphia, PA, USA, 17–20 November 2003; Volume 732, pp. 638–644. [Google Scholar]
- Lee, Y.H.; Lee, J.H.; An, I.-G.; Kim, C.; Lee, D.S.; Lee, Y.K.; Nam, J.-D. Electrospun dual-porosity structure and biodegradation morphology of Montmorillonite reinforced PLLA nanocomposite scaffolds. Biomaterials 2005, 26, 3165–3172. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Manias, E.; Snyder, A.J.; Runt, J. New biomedical poly (urethane urea)− layered silicate nanocomposites. Macromolecules 2001, 34, 337–339. [Google Scholar] [CrossRef]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Li, H.; Zuo, J.; An, Y. Effect of copolymer structure on micellar behavior of acrylamide-octylphenylpoly (oxyethylene) acrylate surfactant. Colloid Polym. Sci. 2001, 279, 279–282. [Google Scholar] [CrossRef]
- Unuabonah, E.I.; Taubert, A. Clay–polymer nanocomposites (CPNs): Adsorbents of the future for water treatment. Appl. Clay Sci. 2014, 99, 83–92. [Google Scholar] [CrossRef]
- Guo, F.; Aryana, S.; Han, Y.; Jiao, Y. A review of the synthesis and applications of polymer–nanoclay composites. Appl. Sci. 2018, 8, 1696. [Google Scholar] [CrossRef] [Green Version]
- Kara, A.; Tekin, N.; Alan, A.; Şafaklı, A. Physicochemical parameters of Hg (II) ions adsorption from aqueous solution by sepiolite/poly (vinylimidazole). J. Environ. Chem. Eng. 2016, 4, 1642–1652. [Google Scholar] [CrossRef]
- Yildiz, G.; Senkal, B.F. Formation of composites between polyvinylimidazole and bentonites and their use for removal of remazol black B from water. Sep. Sci. Technol. 2016, 51, 2596–2603. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, C.; Xiao, C.; Shao, D.; Xu, Z.; Wang, J.; Hu, S.; Li, X.; Wang, W. Polyaniline (PANI) modified bentonite by plasma technique for U (VI) removal from aqueous solution. Appl. Surf. Sci. 2017, 411, 331–337. [Google Scholar] [CrossRef]
- El-Korashy, S.A.; Elwakeel, K.Z.; Abd El-Hafeiz, A. Fabrication of bentonite/thiourea-formaldehyde composite material for Pb (II), Mn (VII) and Cr (VI) sorption: A combined basic study and industrial application. J. Clean. Prod. 2016, 137, 40–50. [Google Scholar] [CrossRef]
- Amari, A.; Alzahrani, F.M.; Mohammedsaleh Katubi, K.; Alsaiari, N.S.; Tahoon, M.A.; Rebah, F. Ben Clay-polymer nanocomposites: Preparations and utilization for pollutants removal. Materials 2021, 14, 1365. [Google Scholar] [CrossRef]
- Prasanth, R.; Shubha, N.; Hng, H.H.; Srinivasan, M. Effect of nano-clay on ionic conductivity and electrochemical properties of poly (vinylidene fluoride) based nanocomposite porous polymer membranes and their application as polymer electrolyte in lithium ion batteries. Eur. Polym. J. 2013, 49, 307–318. [Google Scholar] [CrossRef]
- Jeon, Y.M.; Kim, S.; Lee, M.; Lee, W.B.; Park, J.H. Polymer-Clay Nanocomposite Solid-State Electrolyte with Selective Cation Transport Boosting and Retarded Lithium Dendrite Formation. Adv. Energy Mater. 2020, 10, 2003114. [Google Scholar] [CrossRef]






| Polymer Type | Pure Polymer Modulus, GPa | Nano-Clay Wt.% | PCN Modulus GPa | Ref. |
|---|---|---|---|---|
| Polyester | 2.87 | 5 | 3.79 | [60] |
| Polyamide | 2.45 | 7 | 3.46 | [61] |
| Nylon 6 | 1.2 | 5 | 2.43 | [62] |
| Epoxy | 2.05 | 5 | 3 | [63] |
| Polypropylene | 1.76 | 7 | 2.7 | [64] |
| Polyurethane | 0.025 | 13 | 0.45 | [65] |
| Polycarbonate | 2.3 | 4 | 3 | [66] |
| Polylactic acid | 3.6 | 5 | 4.8 | [66] |
| Polyvinyl chloride | 0.209 | 4 | 0.54 | [67] |
| LDPE | 1.05 | 5 | 1.09 | [68] |
| Polymer | Content (Wt.%) | Unmodified Clay | Organo-Clay | ||||
|---|---|---|---|---|---|---|---|
| Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) | ||
| Polyamide 6 | 0 | 30.86 | 28.75 | 1.07 | 30.86 | 28.75 | 1.07 |
| 1 | 39.84 | 22.15 | 1.79 | 46.17 | 35.00 | 1.61 | |
| 2 | 36.76 | 18.31 | 2.00 | 76.12 | 34.61 | 2.10 | |
| 5 | 32.71 | 10.81 | 3.02 | 32.71 | 10.81 | 3.02 | |
| Polypropylene | 0 | 14.48 | 61.33 | 0.23 | 14.48 | 61.33 | 0.23 |
| 1 | 12.77 | 80.00 | 0.159 | 19.36 | 133.00 | 0.14 | |
| 2 | 15.50 | 90.67 | 0.178 | 21.08 | 157.21 | 0.13 | |
| 5 | 6.73 | 40.00 | 0.168 | 20.27 | 26.67 | 0.76 | |
| Polystyrene | 0 | 17.50 | 13.53 | 1.29 | 17.50 | 13.53 | 1.29 |
| 1 | 18.80 | 25.00 | 0.75 | 21.15 | 39.51 | 0.53 | |
| 2 | 18.45 | 19.75 | 0.93 | 22.34 | 42.15 | 0.53 | |
| 5 | 16.50 | 8.17 | 2.03 | 17.72 | 12.34 | 1.43 | |
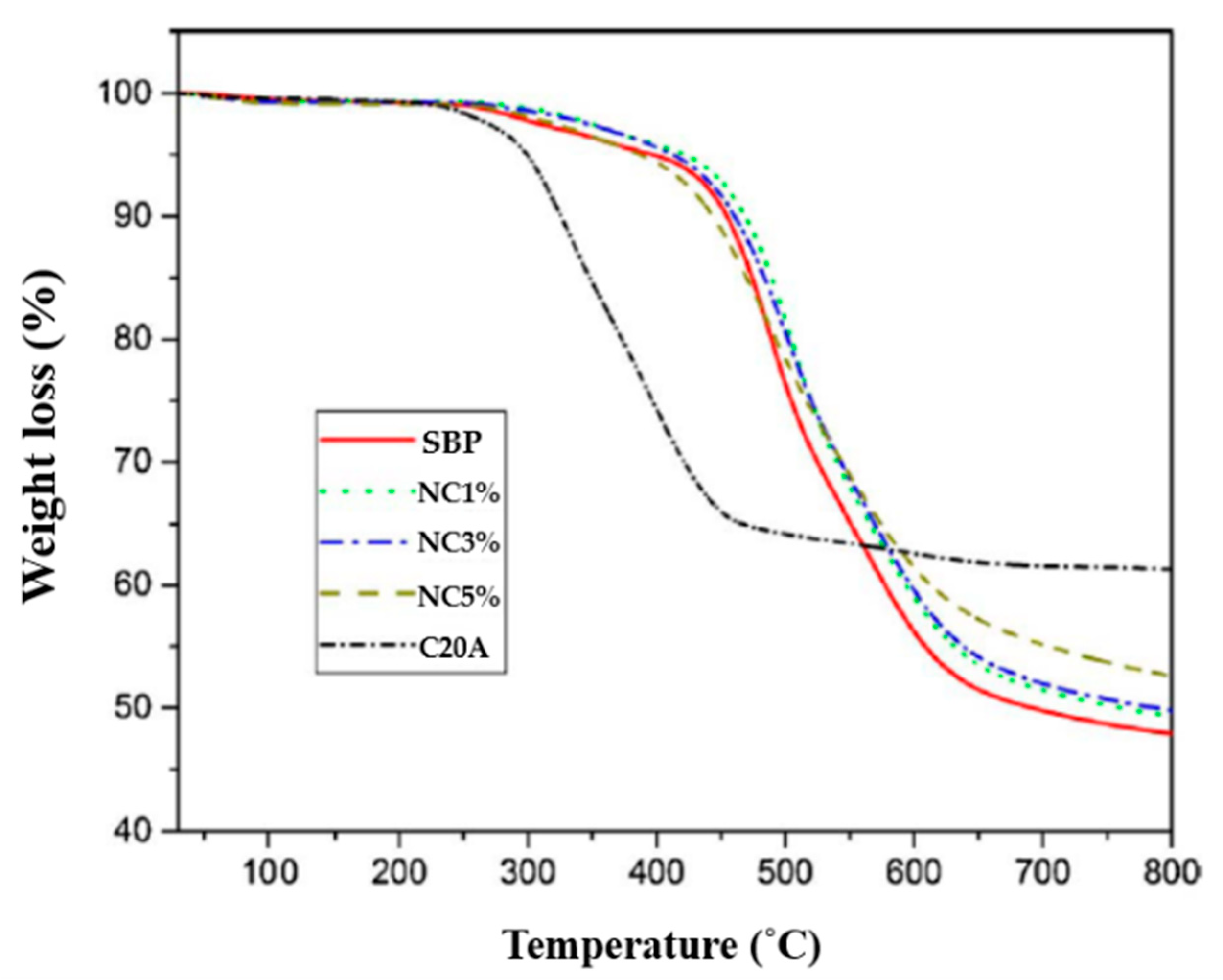
| Organoclay wt.% | T0 (°C) | T5 (°C) | T10 (°C) |
|---|---|---|---|
| Neat SBP | 249 | 397 | 454 |
| 1% | 285 | 422 | 469 |
| 3% | 272 | 414 | 461 |
| 5% | 254 | 387 | 433 |
| Wt.% of Modified MMT | T10 (°C) | T50 (°C) | Char Residue at 350 °C |
|---|---|---|---|
| Pure PECA | 160 | 210 | 0.94 |
| 1% | 169 | 221 | 1.39 |
| 3% | 162 | 227 | 1.24 |
| 5% | 166 | 222 | 4.37 |
| 7% | 167 | 224 | 3.85 |
| Polymer | Filler | Clay Content | Maximum Reduction in Permeability% | Refs |
|---|---|---|---|---|
| Polylactic Acid PLA | Closite 30B | 5 wt% | 60% | [86] |
| 5 wt% | 58% | [87] | ||
| 15 wt% | 95% | [87] | ||
| MMT | 6 wt% | 43% | [88] | |
| Polystyrene PS | MMT | 6 wt% | 70% | [89] |
| 10 wt% | 54% | [90] | ||
| Polyurethane PU | MMT | 40 wt% | 90% | [91] |
| Polyacrylamide PA6 | MMT | 4 vol% | 30% | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abulyazied, D.E.; Ene, A. An Investigative Study on the Progress of Nanoclay-Reinforced Polymers: Preparation, Properties, and Applications: A Review. Polymers 2021, 13, 4401. https://doi.org/10.3390/polym13244401
Abulyazied DE, Ene A. An Investigative Study on the Progress of Nanoclay-Reinforced Polymers: Preparation, Properties, and Applications: A Review. Polymers. 2021; 13(24):4401. https://doi.org/10.3390/polym13244401
Chicago/Turabian StyleAbulyazied, Dalia E., and Antoaneta Ene. 2021. "An Investigative Study on the Progress of Nanoclay-Reinforced Polymers: Preparation, Properties, and Applications: A Review" Polymers 13, no. 24: 4401. https://doi.org/10.3390/polym13244401
APA StyleAbulyazied, D. E., & Ene, A. (2021). An Investigative Study on the Progress of Nanoclay-Reinforced Polymers: Preparation, Properties, and Applications: A Review. Polymers, 13(24), 4401. https://doi.org/10.3390/polym13244401







