Biocompatible Diselenide-Containing Protein Hydrogels with Effective Visible-Light-Initiated Self-Healing Properties
Abstract
:1. Introduction
2. Materials and Methods
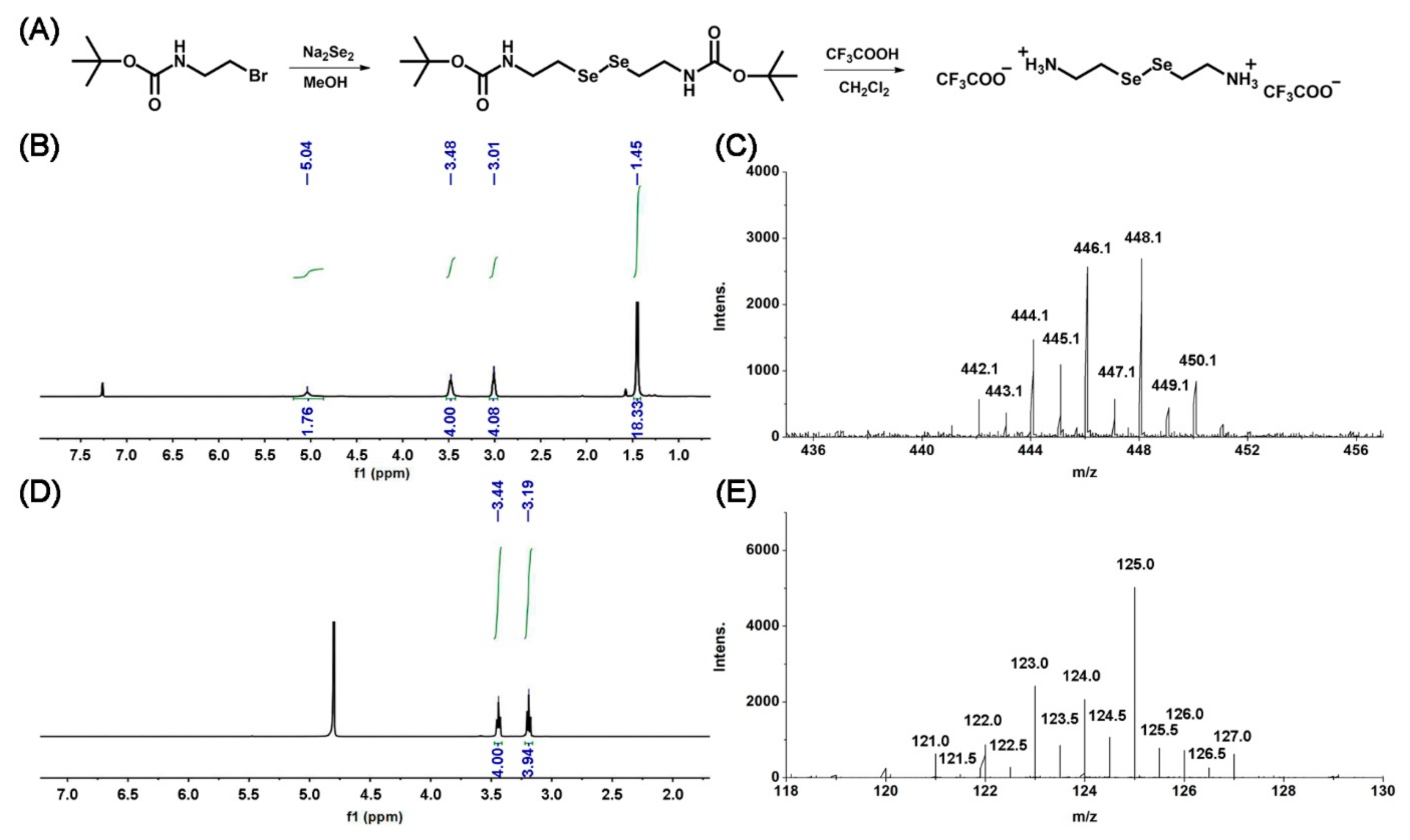
2.1. Synthesis of the Gelator SeCy
2.2. Formation of the Diselenide-Containing Protein Hydrogels
2.3. Scanning Electron Microscopy Characterization
2.4. Rheological and Tensile Tests
2.5. Self-Healing Tests of the Diselenide-Containing Protein Hydrogels
2.6. Degradation Tests of the Diselenide-Containing Protein Hydrogels
3. Results and Discussion
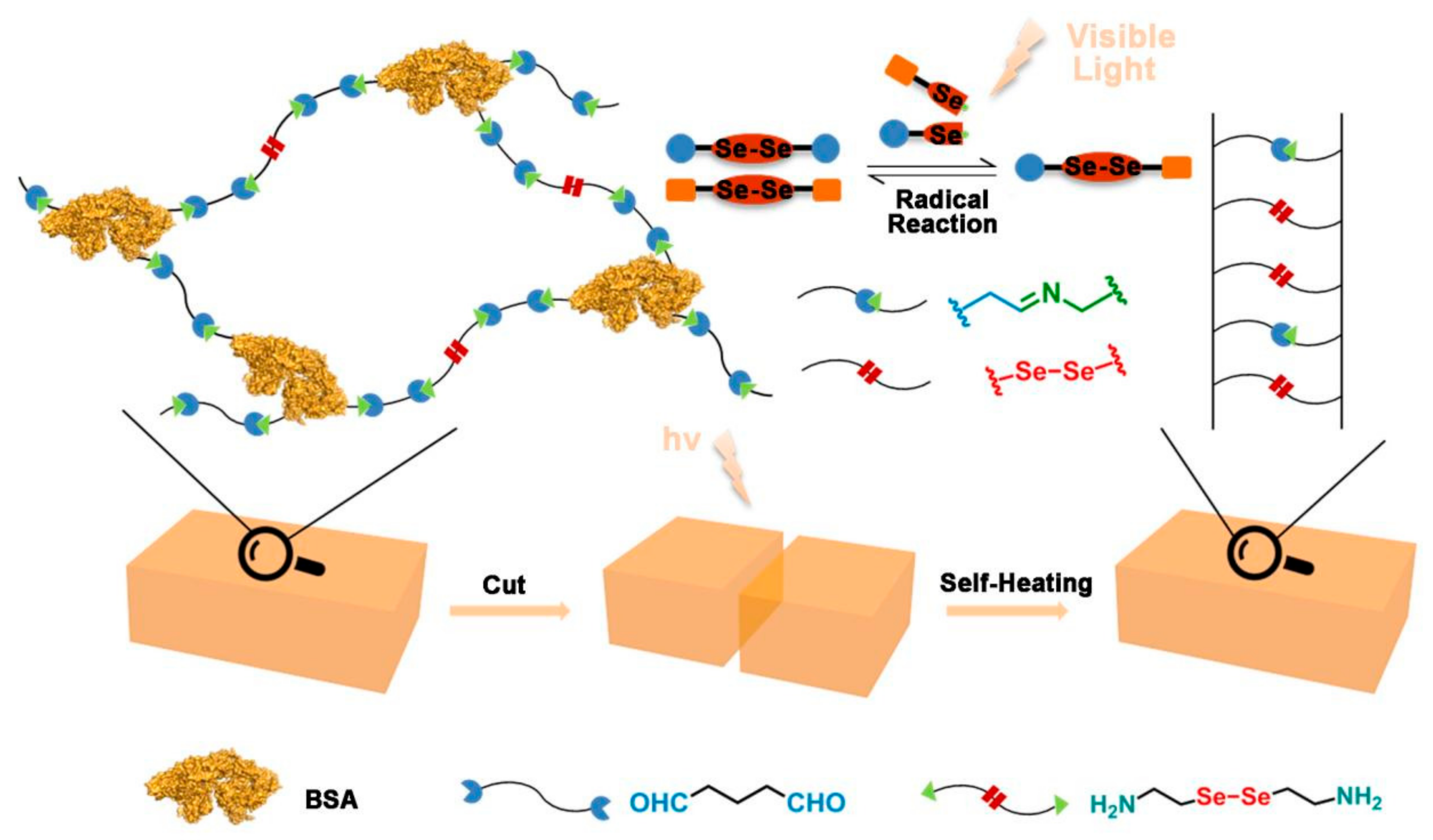
3.1. Synthesis and Characterization of the Gelator
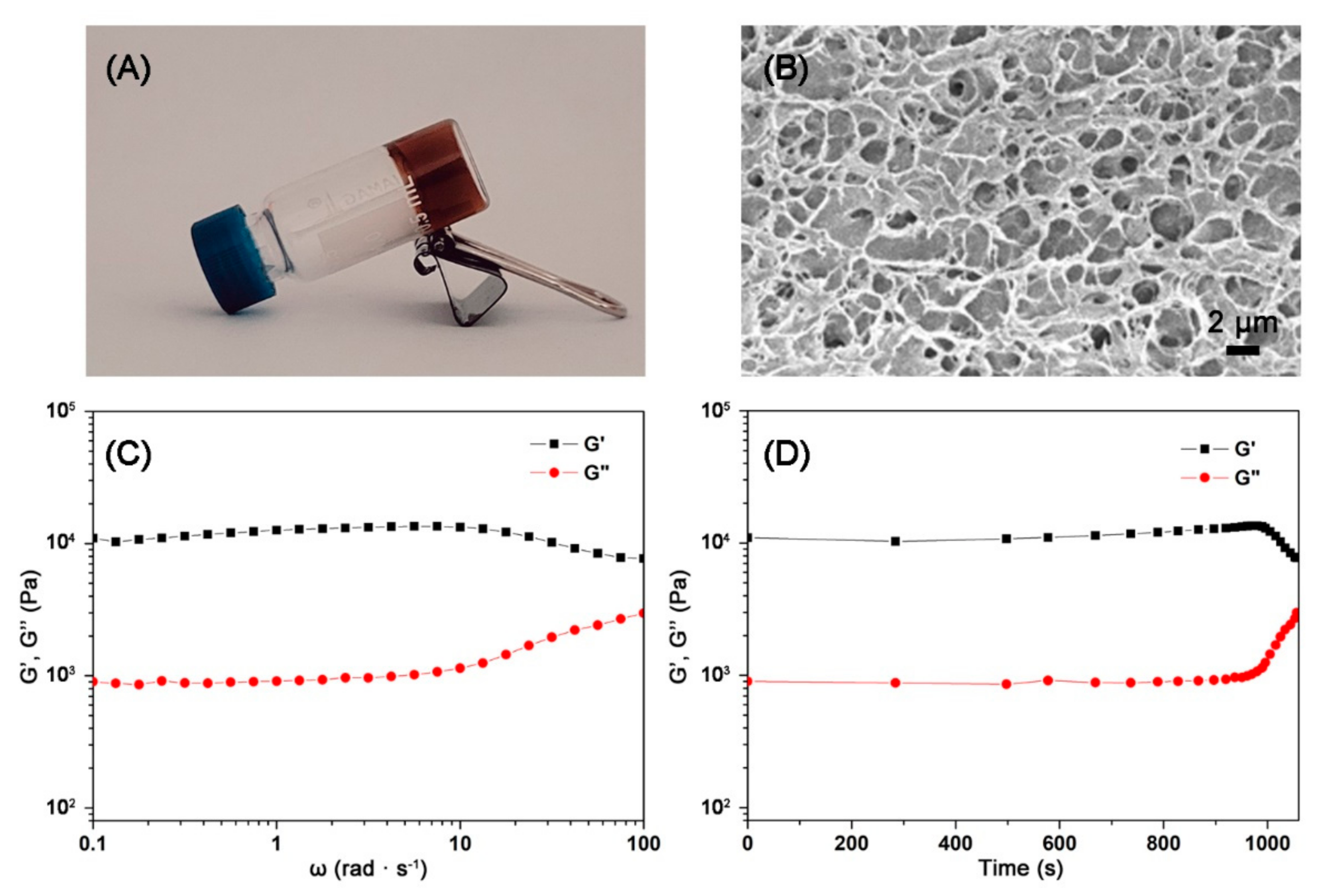
3.2. Fabrication and Characterization of the Diselenide-Containing Protein Hydrogels
3.3. Self-Healing Ability of the Diselenide-Containing Protein Hydrogels
3.4. Biocompatibility Assessment and Stimulus Response of the Diselenide-Containing Protein Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oliva, N.; Conde, J.; Wang, K.; Artzi, N. Designing hydrogels for on-demand therapy. Acc. Chem. Res. 2017, 50, 669–679. [Google Scholar] [CrossRef]
- Huang, Q.; Zou, Y.; Arno, M.C.; Chen, S.; Wang, T.; Gao, J.; Dove, A.P.; Du, J. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem. Soc. Rev. 2017, 46, 6255–6275. [Google Scholar] [CrossRef] [PubMed]
- Devi V. K., A.; Shyam, R.; Palaniappan, A.; Jaiswal, A.K.; Oh, T.-H.; Nathanael, A.J. Self-healing hydrogels: Preparation, mechanism and advancement in biomedical applications. Polymers 2021, 13, 3782. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Razaq, S.I.A.; Mehboob, H.; Rehman, S.; Al-Arjan, W.S.; Amin, R. Antibacterial and hemocompatible pH-responsive hydrogel for skin wound healing application: In vitro drug release. Polymers 2021, 13, 3703. [Google Scholar] [CrossRef]
- Chittasupho, C.; Angklomklew, J.; Thongnopkoon, T.; Senavongse, W.; Jantrawut, P.; Ruksiriwanich, W. Biopolymer hydrogel scaffolds containing doxorubicin as a localized drug delivery system for inhibiting lung cancer cell proliferation. Polymers 2021, 13, 3580. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Yuan, J.; Huang, F. A pillar[5]arene/imidazolium [2]rotaxane: Solvent- and thermo-driven molecular motions and supramolecular gel formation. Chem. Sci. 2014, 5, 247–252. [Google Scholar] [CrossRef]
- Blaiszik, B.J.; Kramer, S.L.B.; Olugebefola, S.C.; Moore, J.S.; Sottos, N.R.; White, S.R. Self-healing polymers and composites. Annu. Rev. Mater. Res. 2010, 40, 179–211. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, D.; Yan, X.; Chen, J.; Dong, S.; Zheng, B.; Huang, F. Self-healing supramolecular gels formed by crown ether based host-guest interactions. Angew. Chem. Int. Ed. 2012, 51, 7011–7015. [Google Scholar] [CrossRef]
- Yan, X.; Xu, D.; Chen, J.; Zhang, M.; Hu, B.; Yu, Y.; Huang, F. A self-healing supramolecular polymer gel with stimuli-responsiveness constructed by crown ether based molecular recognition. Polym. Chem. 2013, 4, 3312–3322. [Google Scholar] [CrossRef]
- Dawn, A. Supramolecular gel as the template for catalysis, inorganic superstructure, and pharmaceutical crystallization. Int. J. Mol. Sci. 2019, 20, 781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehn, J.M. Dynamic combinatorial chemistry and virtual combinatorial libraries. Chem. Eur. J. 1999, 5, 2455–2463. [Google Scholar] [CrossRef]
- Sauer, J.; Sustmann, R. Mechanistic aspects of Diels-Alder reactions: A critical survey. Angew. Chem. Int. Ed. 1980, 19, 779–807. [Google Scholar] [CrossRef]
- Huc, I.; Lehn, J.M. Virtual combinatorial libraries: Dynamic generation of molecular and supramolecular diversity by self-assembly. Proc. Natl. Acad. Sci. USA 1997, 94, 2106–2110. [Google Scholar] [CrossRef] [Green Version]
- Otto, S.; Furlan, R.L.E.; Sanders, J.K.M. Dynamic combinatorial libraries of macrocyclic disulfides in water. J. Am. Chem. Soc. 2000, 122, 12063–12064. [Google Scholar] [CrossRef]
- Ramström, O.; Lehn, J.M. In situ generation and screening of a dynamic combinatorial carbohydrate library against concanavalin A. ChemBioChem 2000, 1, 41–48. [Google Scholar] [CrossRef]
- Imato, K.; Nishihara, M.; Kanehara, T.; Amamoto, Y.; Takahara, A.; Otsuka, H. Self-healing of chemical gels cross-linked by diarylbibenzofuranone-based trigger-free dynamic covalent bonds at room temperature. Angew. Chem. Int. Ed. 2012, 51, 1138–1142. [Google Scholar] [CrossRef]
- Deng, G.; Tang, C.; Li, F.; Liu, C.; Sun, W.; Jiang, H.; Chen, Y. Dynamic hydrogels with an environmental adaptive self-healing ability and dual responsive sol-gel transitions. ACS Macro Lett. 2012, 1, 275–279. [Google Scholar] [CrossRef]
- Deng, G.; Li, F.; Jiang, H.; Chen, Y. Covalent cross-linked polymer gels with reversible sol-gel transition and self-healing properties. Macromolecules 2010, 43, 1191–1194. [Google Scholar] [CrossRef]
- Zhao, J.; Diaz-Dussan, D.; Wu, M.; Peng, Y.Y.; Wang, J.; Zeng, H.; Duan, W.; Kong, L.; Hao, X.; Narain, R. Dual-cross-linked network hydrogels with multiresponsive, self-healing, and shear strengthening properties. Biomacromolecules 2021, 22, 800–810. [Google Scholar] [CrossRef]
- Li, S.; Pei, M.; Wan, T.; Yang, H.; Gu, S.; Tao, Y.; Liu, X.; Zhou, Y.; Xu, W.; Xiao, P. Self-healing hyaluronic acid hydrogels based on dynamic Schiff base linkages as biomaterials. Carbohydr. Polym. 2020, 250, 116922. [Google Scholar] [CrossRef]
- Amamoto, Y.; Kamada, J.; Otsuka, H.; Takahara, A.; Matyjaszewski, K. Repeatable photoinduced self-healing of covalently cross-linked polymers through reshuffling of trithiocarbonate units. Angew. Chem. Int. Ed. 2011, 50, 1660–1663. [Google Scholar] [CrossRef] [PubMed]
- Canadell, J.; Goossens, H.; Klumperman, B. Self-healing materials based on disulfide links. Macromolecules 2011, 44, 2536–2541. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Singh, S.P.; Bowman, C.N.; Anseth, K.S. Photodegradable, photoadaptable hydrogels via radical-mediated disulfide fragmentation reaction. Macromolecules 2011, 44, 2444–2450. [Google Scholar] [CrossRef] [PubMed]
- Amamoto, Y.; Otsuka, H.; Takahara, A.; Matyjaszewski, K. Self-healing of covalently cross-linked polymers by reshuffling thiuram disulfide moieties in air under visible light. Adv. Mater. 2012, 24, 3975–3980. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.Q.; Xiang, H.P.; Yuan, Y.J.; Rong, M.Z.; Zhang, M.Q. Room-temperature self-healable and remoldable cross-linked polymer based on the dynamic exchange of disulfide bonds. Chem. Mater. 2014, 26, 2038–2046. [Google Scholar] [CrossRef]
- Ji, S.; Cao, W.; Yu, Y.; Xu, H. Dynamic diselenide bonds: Exchange reaction induced by visible light without catalysis. Angew. Chem. Int. Ed. 2014, 53, 6781–6785. [Google Scholar] [CrossRef]
- Xia, J.; Ji, S.; Xu, H. Diselenide covalent chemistry at the interface: Stabilizing asymmetric diselenide-containing polymer via micelle formation. Polym. Chem. 2016, 7, 6708–6713. [Google Scholar] [CrossRef]
- Fan, F.; Ji, S.; Sun, C.; Liu, C.; Yu, Y.; Fu, Y.; Xu, H. Wavelength-controlled dynamic metathesis: A light-driven exchange reaction between disulfide and diselenide bonds. Angew. Chem. Int. Ed. 2018, 57, 16426–16430. [Google Scholar] [CrossRef]
- Xia, J.; Zhao, P.; Pan, S.; Xu, H. Diselenide-containing polymeric vesicles with osmotic pressure response. ACS Macro Lett. 2019, 8, 629–633. [Google Scholar] [CrossRef]
- Xia, J.; Tan, Y.; Xu, H. Selenium-containing dynamic covalent polymers. Acta Polym. Sin. 2020, 51, 1190. [Google Scholar]
- Zhang, X.; Xu, J.; Lang, C.; Qiao, S.; An, G.; Fan, X.; Zhao, L.; Hou, C.; Liu, J. Enzyme-regulated fast self-healing of a pillararene-based hydrogel. Biomacromolecules 2017, 18, 1885–1892. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, S.; Yan, T.; Fan, X.; Li, F.; Yang, X.; Ren, B.; Xu, J.; Liu, J. Injectable and fast self-healing protein hydrogels. Soft Matter 2019, 15, 7583–7589. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Huang, Z.; Yang, H.; Xu, H.; Zhang, X. Controlling the reactivity of the Se-Se bond by the supramolecular chemistry of cucurbituril. Chemphyschem 2015, 16, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Li, F.; Zang, M.; Zhang, Z.; Ji, Y.; Yu, X.; Luo, Q.; Guan, S.; Xu, J.; Liu, J. Diselenium-containing ultrathin polymer nanocapsules for highly efficient targeted drug delivery and combined anticancer effect. J. Mater. Chem. B 2019, 7, 4927–4932. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; Validi, M.; Gholipour, A.; Makvandi, P.; Sharifi, E. Chitosan nanofiber biocomposites for potential wound healing applications: Antioxidant activity with synergic antibacterial effect. Bioeng. Transl. Med. 2021, e10254. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Deng, S.; Yan, T.; Zhang, X.; Tian, R.; Xu, J.; Sun, H.; Yu, S.; Liu, J. Biocompatible Diselenide-Containing Protein Hydrogels with Effective Visible-Light-Initiated Self-Healing Properties. Polymers 2021, 13, 4360. https://doi.org/10.3390/polym13244360
Liu S, Deng S, Yan T, Zhang X, Tian R, Xu J, Sun H, Yu S, Liu J. Biocompatible Diselenide-Containing Protein Hydrogels with Effective Visible-Light-Initiated Self-Healing Properties. Polymers. 2021; 13(24):4360. https://doi.org/10.3390/polym13244360
Chicago/Turabian StyleLiu, Shengda, Shengchao Deng, Tengfei Yan, Xin Zhang, Ruizhen Tian, Jiayun Xu, Hongcheng Sun, Shuangjiang Yu, and Junqiu Liu. 2021. "Biocompatible Diselenide-Containing Protein Hydrogels with Effective Visible-Light-Initiated Self-Healing Properties" Polymers 13, no. 24: 4360. https://doi.org/10.3390/polym13244360
APA StyleLiu, S., Deng, S., Yan, T., Zhang, X., Tian, R., Xu, J., Sun, H., Yu, S., & Liu, J. (2021). Biocompatible Diselenide-Containing Protein Hydrogels with Effective Visible-Light-Initiated Self-Healing Properties. Polymers, 13(24), 4360. https://doi.org/10.3390/polym13244360





