Polydopamine-Bi2WO6-Decorated Gauzes as Dual-Functional Membranes for Solar Steam Generation and Photocatalytic Degradation Applications
Abstract
:1. Introduction
2. Experimental
2.1. Nomenclature
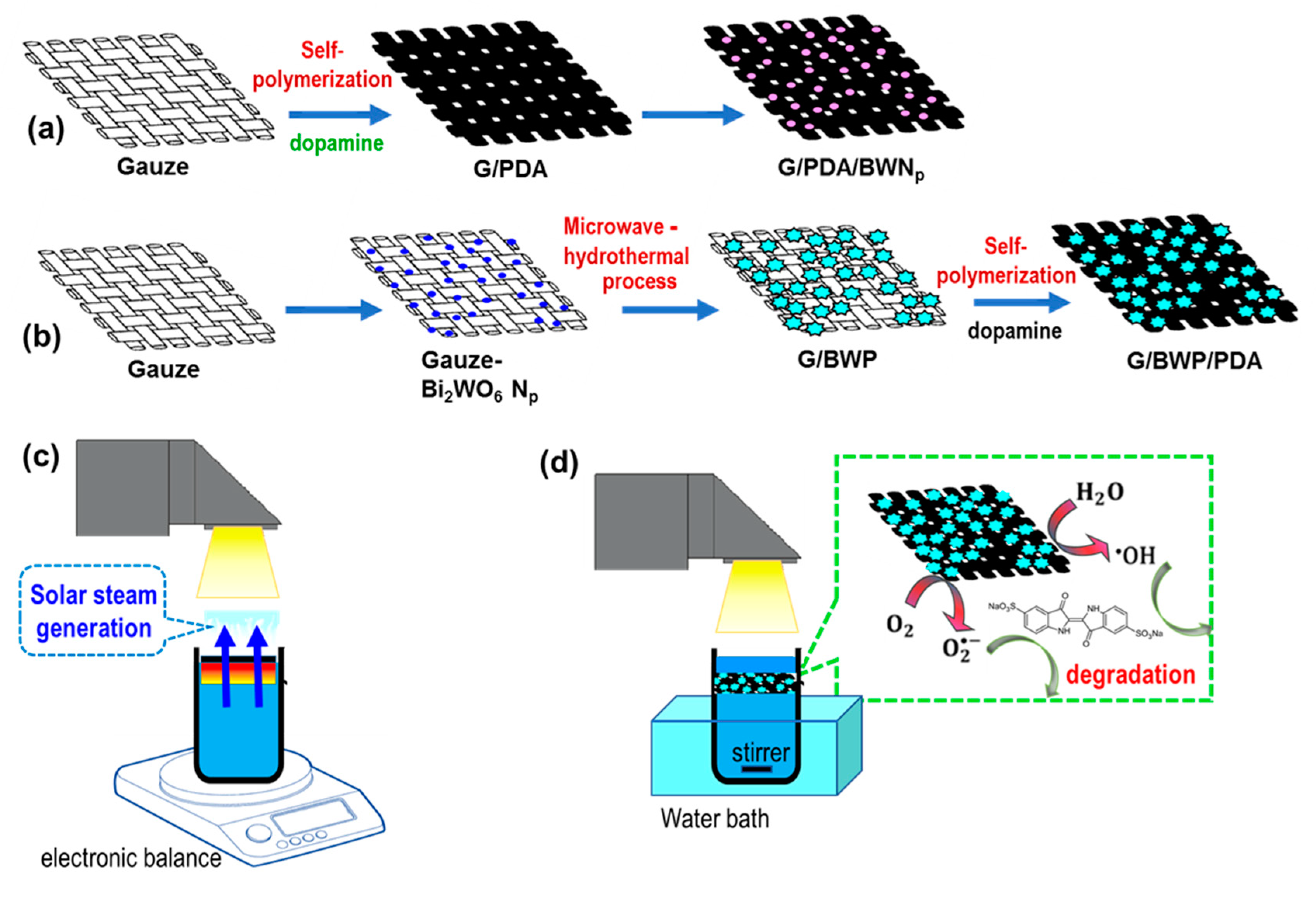
2.2. Preparation of Dual-Functional Membrane
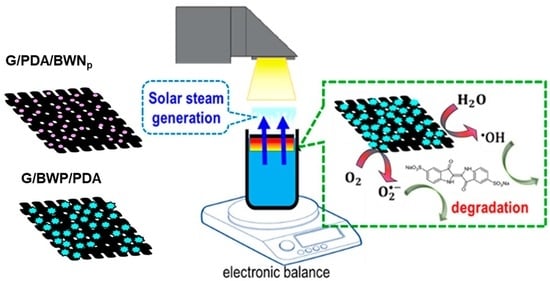
2.3. Solar Steam Generation
2.4. Photocatalytic Degradation
2.5. Characterization
3. Results and Discussion
3.1. Morphology
3.2. Chemical Compositions
3.2.1. Transmission Electron Microscopy Energy-Dispersive X-ray (TEM EDX)
3.2.2. X-ray Photoelectron Spectra (XPS) Analysis
3.2.3. Raman Spectra
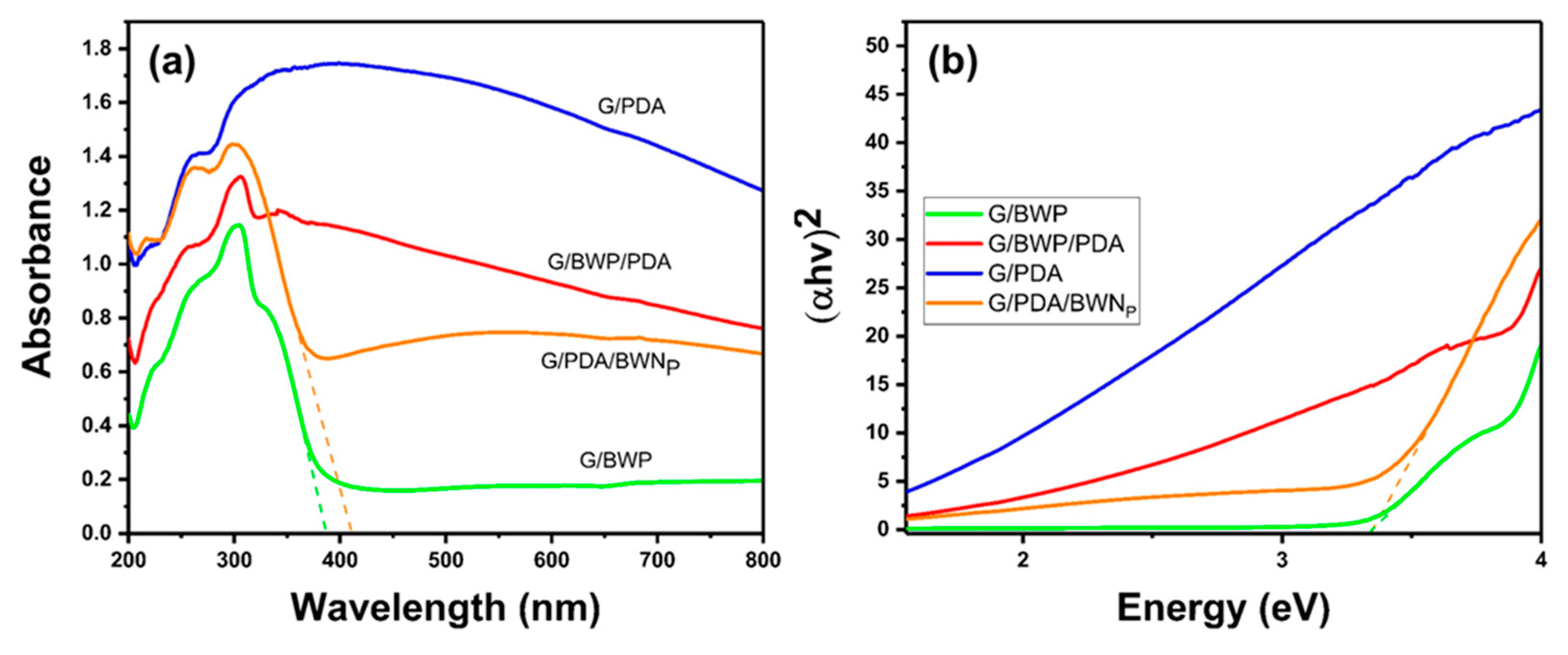
3.3. Diffuse Reflection Spectra (DRS)
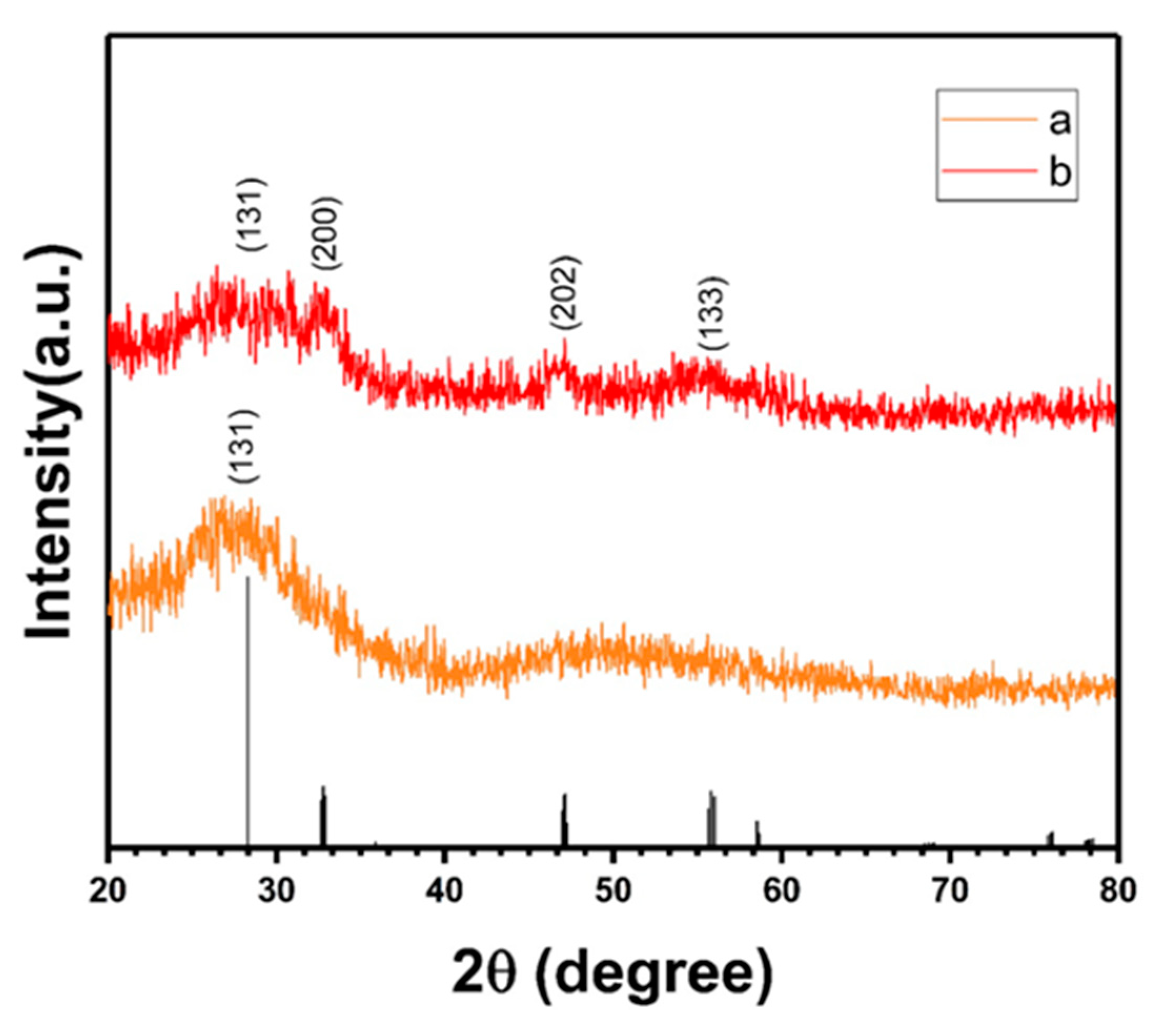
3.4. X-ray Diffraction (XRD) Spectra
3.5. Surface Hydrophilicity
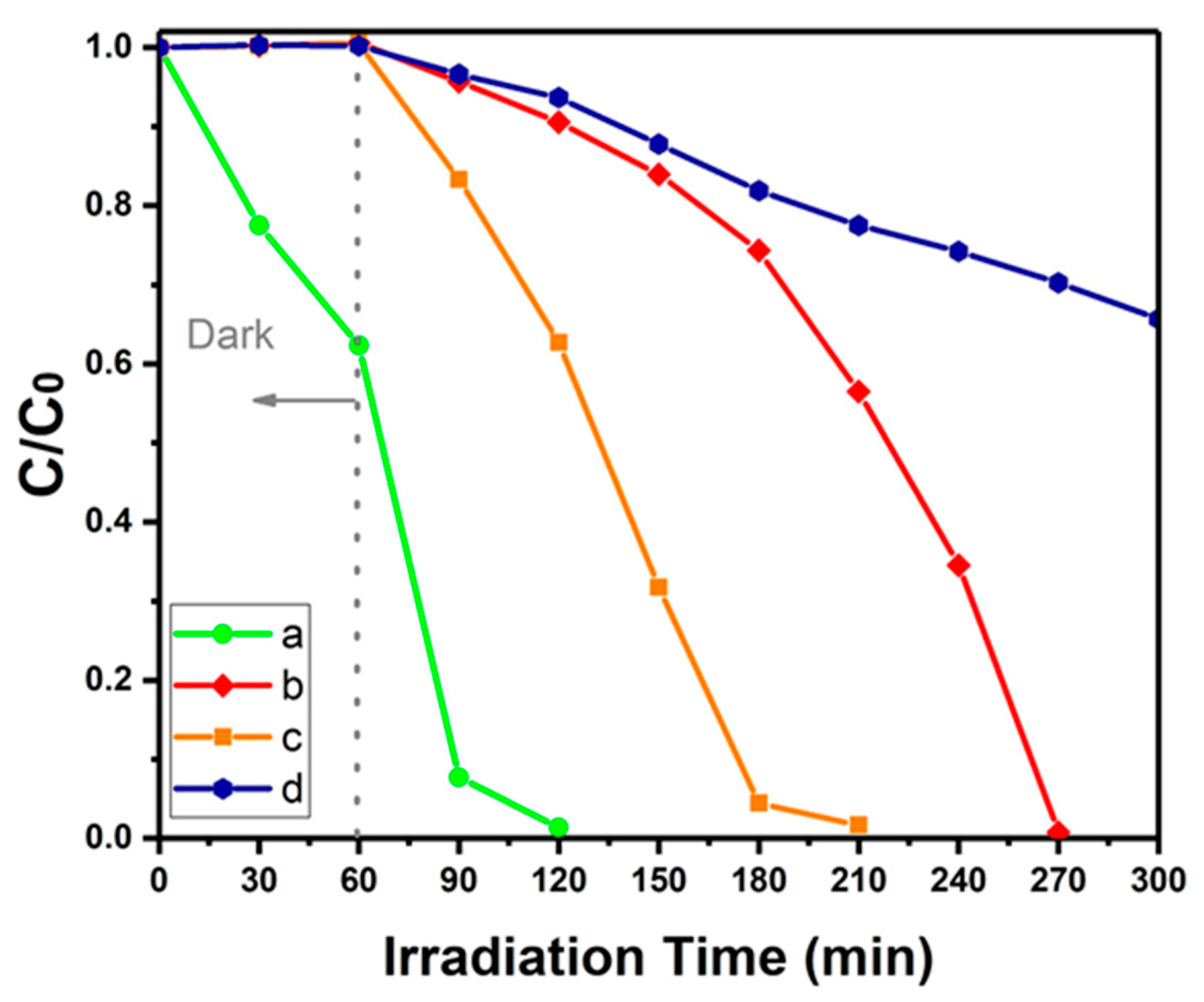
3.6. Photocatalytic Property
3.7. Solar-Steam Generation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Wang, X.; Huang, J.; Cheng, G.; He, Y. Volumetric solar steam generation enhanced by reduced graphene oxide nanofluid. Appl. Energy 2018, 220, 302–312. [Google Scholar] [CrossRef]
- Ha, J.W.; Park, J.B.; Park, H.J.; Hwang, D.H. Novel Conjugated Polymers Containing 3-(2-Octyldodecyl) thieno [3, 2-b] thiophene as a π-Bridge for Organic Photovoltaic Applications. Polymers 2020, 12, 2121. [Google Scholar] [CrossRef]
- Barretta, C.; Oreski, G.; Feldbacher, S.; Resch-Fauster, K.; Pantani, R. Comparison of Degradation Behavior of Newly Developed Encapsulation Materials for Photovoltaic Applications under Different Artificial Ageing Tests. Polymers 2021, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.F.; Wu, C.L.; Kuo, S.W.; Hung, W.S.; Lee, K.J.; Tsai, H.C.; Chang, C.J.; Lai, J.Y. Preparation of Efficient Photothermal Materials from Waste Coffee Grounds for Solar Desalination and Water Purification. Sci. Rep. 2020, 10, 1–10. [Google Scholar]
- Hu, N.; Xu, Y.; Liu, Z.; Liu, M.; Shao, X.; Wang, J. Double-layer cellulose hydrogel solar steam generation for high-efficiency desalination. Carbohydr. Polym. 2020, 243, 116480. [Google Scholar] [CrossRef]
- Mohsenpour, M.; Motahari, S.; Tajabadi, F.; Najafi, M. Preparation and application of sunlight absorbing ultra-black carbon aerogel/graphene oxide membrane for solar steam generation systems. RSC Adv. 2020, 10, 41780–41790. [Google Scholar] [CrossRef]
- Chang, C.J.; Lee, Z.; Chu, K.W.; Wei, Y.H. CoFe2O4@ZnS core–shell spheres as magnetically recyclable photocatalysts for hydrogen production. J. Taiwan Inst. Chem. Eng. 2016, 66, 386–393. [Google Scholar] [CrossRef]
- Senasu, T.; Nijpanich, S.; Juabrum, S.; Chanlek, N.; Nanan, S. CdS/BiOBr heterojunction photocatalyst with high performance for solar-light-driven degradation of ciprofloxacin and norfloxacin antibiotics. Appl. Surf. Sci. 2021, 567, 150850. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, D.; Xiang, H.; Ren, S.; Zhu, Z.; Liu, D.; Xu, H.; Cui, F.; Wang, W. Easily scaled-up photo-thermal membrane with structure-dependent auto-cleaning feature for high-efficient solar desalination. J. Membr. Sci. 2019, 586, 222–230. [Google Scholar] [CrossRef]
- Wilson, H.M.; Ahirrao, D.J.; Ar, S.R.; Jha, N. Biomass-derived porous carbon for excellent low intensity solar steam generation and seawater desalination. Sol. Energy Mater. Sol. Cells 2020, 215, 110604. [Google Scholar] [CrossRef]
- Wilson, H.M.; Ar, S.R.; Jha, N. Plant-derived carbon nanospheres for high efficiency solar-driven steam generation and seawater desalination at low solar intensities. Sol. Energy Mater. Sol. Cells 2020, 210, 110489. [Google Scholar] [CrossRef]
- Higgins, M.W.; Rahmaan, A.S.; Devarapalli, R.R.; Shelke, M.V.; Jha, N. Carbon fabric based solar steam generation for waste water treatment. Sol. Energy 2018, 159, 800–810. [Google Scholar] [CrossRef]
- Ibrahim, I.; Seo, D.H.; McDonagh, A.M.; Shon, H.K.; Tijing, L. Semiconductor photothermal materials enabling efficient solar steam generation toward desalination and wastewater treatment. Desalination 2021, 500, 114853. [Google Scholar] [CrossRef]
- Abinaya, M.; Govindan, K.; Kalpana, M.; Saravanakumar, K.; Prabavathi, S.L.; Muthuraj, V.; Jang, A. Reduction of hexavalent chromium and degradation of tetracycline using a novel indium-doped Mn2O3 nanorod photocatalyst. J. Hazard. Mater. 2020, 397, 122885. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Chao, P.Y.; Lin, K.S. Flower-like BiOBr decorated stainless steel wire-mesh as immobilized photocatalysts for photocatalytic degradation applications. Appl. Surf. Sci. 2019, 494, 492–500. [Google Scholar] [CrossRef]
- Bellardita, M.; Fiorenza, R.; D’Urso, L.; Spitaleri, L.; Gulino, A.; Compagnini, G.; Compagnini, G.; Scirè, S.; Palmisano, L. Exploring the photothermo-catalytic performance of Brookite TiO2-CeO2 composites. Catalysts 2020, 10, 765. [Google Scholar] [CrossRef]
- Fan, D.; Lu, Y.; Zhang, H.; Xu, H.; Lu, C.; Tang, Y.; Yang, X. Synergy of photocatalysis and photothermal effect in integrated 0D perovskite oxide/2D MXene heterostructures for simultaneous water purification and solar steam generation. Appl. Catal. B 2021, 295, 120285. [Google Scholar] [CrossRef]
- Wang, M.; Wang, P.; Zhang, J.; Li, C.; Jin, Y. A Ternary Pt/Au/TiO2-Decorated Plasmonic Wood Carbon for High-Efficiency Interfacial Solar Steam Generation and Photodegradation of Tetracycline. ChemSusChem 2019, 12, 467–472. [Google Scholar] [CrossRef]
- Ding, Y.; Feng, K.; He, P.; Liu, N.; Hao, L.; Gong, J.; Niu, R.; Qu, J. A synergistic photothermal and photocatalytic membrane for efficient solar-driven contaminated water treatment. Sustain. Energy Fuels 2021, 5, 5627–5637. [Google Scholar] [CrossRef]
- Shao, B.; Wang, Y.; Wu, X.; Lu, Y.; Yang, X.; Chen, G.Y.; Owens, G.; Xu, H. Stackable nickel–cobalt@ polydopamine nanosheet based photothermal sponges for highly efficient solar steam generation. J. Mater. Chem. A 2020, 8, 11665–11673. [Google Scholar] [CrossRef]
- Tsai, M.H.; Chang, C.J.; Lu, H.H.; Liao, Y.F.; Tseng, I.H. Properties of magnetron-sputtered moisture barrier layer on transparent polyimide/graphene nanocomposite film. Thin Solid Film. 2013, 544, 324–330. [Google Scholar] [CrossRef]
- Nosrati, R.; Olad, A.; Maramifar, R. Degradation of ampicillin antibiotic in aqueous solution by ZnO/polyaniline nanocomposite as photocatalyst under sunlight irradiation. Environ. Sci. Pollut. Res. 2012, 19, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zang, Y.; Xu, L.; Wang, J.; Jia, H.; Miao, F. Synthesis of functional conjugated microporous polymer/TiO2 nanocomposites and the mechanism of the photocatalytic degradation of organic pollutants. J. Mater. Sci. 2021, 56, 7936–7950. [Google Scholar] [CrossRef]
- Chang, C.J.; Hung, S.T. Electrochemical deposition of ZnO pore-array structures and photoconductivity of ZnO/polymer hybrid films. Thin Solid Film. 2008, 517, 1279–1283. [Google Scholar] [CrossRef]
- Chang, C.J.; Tsai, M.H.; Hsu, Y.H.; Tuan, C.S. Morphology and optoelectronic properties of ZnO rod array/conjugated polymer hybrid films. Thin Solid Film. 2008, 516, 5523–5526. [Google Scholar] [CrossRef]
- Jian, K.S.; Chang, C.J.; Wu, J.J.; Chang, Y.C.; Tsay, C.Y.; Chen, J.H.; Horng, T.L.; Lee, G.J.; Karuppasamy, L.; Anandan, S.; et al. High response CO sensor based on a polyaniline/SnO2 nanocomposite. Polymers 2019, 11, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.W.; Sun, Y.J.; Wang, J.L.; Yu, B.R. Antimicrobial and antifouling surfaces through polydopamine bio-inspired coating. Rare Met. 2021, 40, 1–20. [Google Scholar] [CrossRef]
- Gao, H.; Sun, Y.; Zhou, J.; Xu, R.; Duan, H. Mussel-Inspired Synthesis of Polydopamine-Functionalized Graphene Hydrogel as Reusable Adsorbents for Water Purification. ACS Appl. Mater. Interfaces 2013, 5, 425–432. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, H.C.; Wan, L.S.; Liang, H.Q.; Li, H.; Xu, Z.K. Polydopamine-Coated Porous Substrates as a Platform for Mineralized β-FeOOH Nanorods with Photocatalysis under Sunlight. ACS Appl. Mater. Interfaces 2015, 7, 11567–11574. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-Melanin Colloidal Nanospheres: An Efficient Near-Infrared Photothermal Therapeutic Agent for in vivo Cancer Therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef]
- Zhu, Z.; Su, M. Polydopamine nanoparticles for combined chemo-and photothermal cancer therapy. Nanomaterials 2017, 7, 160. [Google Scholar] [CrossRef]
- Liu, M.; Peng, Y.; Nie, Y.; Liu, P.; Hu, S.; Ding, J.; Zhou, W. Co-delivery of doxorubicin and DNAzyme using ZnO@ polydopamine core-shell nanocomposites for chemo/gene/photothermal therapy. Acta Biomater. 2020, 110, 242–253. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, P.; Yang, L.; Li, N.; Duan, G.; Liu, X.; Li, Y. Boosting solar steam generation by photothermal enhanced polydopamine/wood composites. Polymer 2021, 217, 123464. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Chen, Z.; Zhang, Y.; Xia, D.; Wang, Q. Flexible cotton fabrics/PDA/BiOBr composite photocatalyst using bioinspired polydopamine as electron transfer mediators for dye degradation and Cr (VI) reduction under visible light. Colloids Surf. A Physicochem. Eng. Asp. 2020, 593, 124623. [Google Scholar] [CrossRef]
- Yu, Z.; Li, F.; Yang, Q.; Shi, H.; Chen, Q.; Xu, M. Nature-mimic method to fabricate polydopamine/graphitic carbon nitride for enhancing photocatalytic degradation performance. ACS Sustain. Chem. Eng. 2017, 5, 7840–7850. [Google Scholar]
- Nie, N.; He, F.; Zhang, L.; Cheng, B. Direct Z-scheme PDA-modified ZnO hierarchical microspheres with enhanced photocatalytic CO2 reduction performance. Appl. Surf. Sci. 2018, 457, 1096–1102. [Google Scholar] [CrossRef]
- Sun, X.; Yan, L.; Xu, R.; Xu, M.; Zhu, Y. Surface modification of TiO2 with polydopamine and its effect on photocatalytic degradation mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 199–209. [Google Scholar] [CrossRef]
- Huang, X.; Niu, Y.; Peng, Z.; Hu, W. Core-shell structured BiOCl@polydopamine hierarchical hollow microsphere for highly efficient photocatalysis. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123747. [Google Scholar] [CrossRef]
- Ding, K.; Wang, W.; Yu, D.; Gao, P.; Liu, B. Facile formation of flexible Ag/AgCl/polydopamine/cotton fabric composite photocatalysts as an efficient visible-light photocatalysts. Appl. Surf. Sci. 2018, 454, 101–111. [Google Scholar] [CrossRef]
- Ran, J.; He, M.; Li, W.; Cheng, D.; Wang, X. Growing ZnO nanoparticles on polydopamine-templated cotton fabrics for durable antimicrobial activity and UV protection. Polymers 2018, 10, 495. [Google Scholar] [CrossRef] [Green Version]
- Ran, J.; Chen, H.; Bai, X.; Bi, S.; Jiang, H.; Cai, G.; Cheng, D.; Wang, X. Immobilizing CuO/BiVO4 nanocomposite on PDA-templated cotton fabric for visible light photocatalysis, antimicrobial activity and UV protection. Appl. Surf. Sci. 2019, 493, 1167–1176. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Yu, D.; Zhou, X.; Lu, W. Enhancement in the photocatalytic and photoelectrochemical properties of visible-light driven BiVO4 photocatalyst. Rare Met. 2011, 30, 192–198. [Google Scholar] [CrossRef]
- Lai, K.; Wei, W.; Dai, Y.; Zhang, R.; Huang, B. DFT calculations on structural and electronic properties of Bi2MO6 (M = Cr, Mo, W). Rare Met. 2011, 30, 166–172. [Google Scholar] [CrossRef]
- Chang, C.J.; Chao, P.Y.; Chou, C.Y.; Chen, Y.J.; Huang, C.F. Polymer/BiOBr-modified gauze as a dual-functional membrane for heavy metal removal and photocatalytic dye decolorization. Polymers 2020, 12, 2082. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhou, Q. Novel Bi2WO6 modified by N-doped graphitic carbon nitride photocatalyst for efficient photocatalytic degradation of phenol under visible light. Appl. Catal. B 2020, 268, 118426. [Google Scholar] [CrossRef]
- Wang, J.; Tang, L.; Zeng, G.; Deng, Y.; Dong, H.; Liu, Y.; Wang, L.; Peng, B.; Zhang, C.; Chen, F. 0D/2D interface engineering of carbon quantum dots modified Bi2WO6 ultrathin nanosheets with enhanced photoactivity for full spectrum light utilization and mechanism insight. Appl. Catal. B 2018, 222, 115–123. [Google Scholar] [CrossRef]
- Chang, C.J.; Chao, P.Y. Efficient photocatalytic hydrogen production by doped ZnS grown on Ni foam as porous immobilized photocatalysts. Int. J. Hydrogen Energy 2019, 44, 20805–20814. [Google Scholar] [CrossRef]
- Chang, C.J.; Wei, Y.H.; Kuo, W.S. Free-standing CuS–ZnS decorated carbon nanotube films as immobilized photocatalysts for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 30553–30562. [Google Scholar] [CrossRef]
- Lee, S.L.; Chang, C.J. Recent progress on metal sulfide composite nanomaterials for photocatalytic hydrogen production. Catalysts 2019, 9, 457. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.J.; Chu, K.W. ZnS/polyaniline composites with improved dispersing stability and high photocatalytic hydrogen production activity. Int. J. Hydrogen Energy 2016, 41, 21764–21773. [Google Scholar] [CrossRef]
- Ratova, M.; West, G.T.; Kelly, P.J. Photocatalytic visible-light active bismuth tungstate coatings deposited by reactive magnetron sputtering. Vacuum 2015, 115, 66–69. [Google Scholar] [CrossRef]
- Xu, J.H.; Wang, W.Z.; Shang, M.; Sun, S.M.; Ren, J.; Zhang, L. Efficient visible light induced degradation of organic contaminants by Bi2WO6 film on SiO2 modified reticular substrate. Appl. Catal. B 2010, 93, 227–232. [Google Scholar] [CrossRef]
- Chang, C.J.; Chen, J.K.; Lin, K.S.; Wei, Y.H.; Chao, P.Y.; Huang, C.Y. Enhanced visible-light-driven photocatalytic degradation by metal wire-mesh supported Ag/flower-like Bi2WO6 photocatalysts. J. Alloys Compd. 2020, 813, 152186. [Google Scholar] [CrossRef]
- Du, Z.; Cheng, C.; Tan, L.; Lan, J.; Jiang, S.; Zhao, L.; Guo, R. Enhanced photocatalytic activity of Bi2WO6/TiO2 composite coated polyester fabric under visible light irradiation. Appl. Surf. Sci. 2018, 435, 626–634. [Google Scholar] [CrossRef]
- Du, Z.; Guo, R.; Lan, J.; Jiang, S.; Cheng, C.; Zhao, L.; Peng, L. Preparation and photocatalytic activity of bismuth tungstate coated polyester fabric. Fibers Polym. 2017, 18, 2212–2218. [Google Scholar] [CrossRef]
- Vautier, M.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation of dyes in water: Case study of indigo and of indigo carmine. J. Catal. 2001, 201, 46–59. [Google Scholar] [CrossRef]
- Palma-Goyes, R.E.; Silva-Agredo, J.; González, I.; Torres-Palma, R.A. Comparative degradation of indigo carmine by electrochemical oxidation and advanced oxidation processes, Electrochim. Acta 2014, 140, 427–433. [Google Scholar]
- Silva, T.A.; Pereira, G.F.; Fatibello-Filho, O.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Electroanalytical sensing of indigo carmine dye in water samples using a cathodically pretreated boron-doped diamond electrode. J. Electroanal. Chem. 2016, 769, 28–34. [Google Scholar] [CrossRef]
- Zhang, X.; John, S. Enhanced photocatalysis by light-trapping optimization in inverse opals. J. Mater. Chem. A 2020, 8, 18974–18986. [Google Scholar] [CrossRef]
- Chang, C.J.; Kuo, E.H. Light-trapping effects and dye adsorption of ZnO hemisphere-array surface containing growth-hindered nanorods. Colloids Surf. A Physicochem. Eng. Asp. 2010, 363, 22–29. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Zhao, X.; Dai, J.; Mo, S. Synthesis of uniform Bi2WO6-reduced graphene oxide nanocomposites with significantly enhanced photocatalytic reduction activity. J. Phys. Chem. C 2015, 119, 3068–3078. [Google Scholar] [CrossRef]
- Zhang, F.J.; Zhu, S.F.; Xie, F.Z.; Zhang, J.; Meng, Z.D. Plate-on-plate structured Bi2MoO6/Bi2WO6 heterojunction with high-efficiently gradient charge transfer for decolorization of MB. Sep. Purif. Technol. 2013, 113, 1–8. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of polydopamine thin films deposited at short times by autoxidation of dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef] [PubMed]
- Bernsmann, F.; Ponche, A.; Ringwald, C.; Hemmerle, J.; Raya, J.; Bechinger, B.; Voegel, J.C.; Schaaf, P.; Ball, V. Characterization of dopamine-melanin growth on silicon oxide. J. Phys. Chem. C 2009, 113, 8234–8242. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the structure of poly(dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef]
- Wang, R.; Jiang, Z.; Xu, L.; Liu, C. Synthesis of Dy (III) doped Bi2WO6 photocatalyst with highly efficient photocatalytic performance under simulated sunlight. J. Mater. Sci.-Mater. Electron. 2021, 32, 6931–6941. [Google Scholar] [CrossRef]
- Huang, H.; Chen, H.; Xia, Y.; Tao, X.; Gan, Y.; Weng, X.; Zhang, W. Controllable synthesis and visible-light-responsive photocatalytic activity of Bi2WO6 fluffy microsphere with hierarchical architecture. J. Colloid Interface Sci. 2012, 370, 132–138. [Google Scholar] [CrossRef]
- Tejido-Rastrilla, R.; Baldi, G.; Boccaccini, A.R. Ag containing polydopamine coating on a melt-derived bioactive glass-ceramic: Effect on surface reactivity. Ceram. Int. 2018, 44, 16083–16087. [Google Scholar] [CrossRef]
- Ku, S.H.; Lee, J.S.; Park, C.B. Spatial control of cell adhesion and patterning through mussel-inspired surface modification by polydopamine. Langmuir 2010, 26, 15104–15108. [Google Scholar] [CrossRef]
- Shalev, T.; Gopin, A.; Bauer, M.; Stark, R.W.; Rahimipour, S. Non-leaching antimicrobial surfaces through polydopamine bio-inspired coating of quaternary ammonium salts or an ultrashort antimicrobial lipopeptide. J. Mater. Chem. 2012, 22, 2026–2032. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Dai, Z.; Li, J.; Tong, X.; Zhao, H. Polydopamine-decorated boron nitride as nano-reinforcing fillers for epoxy resin with enhanced thermomechanical and tribological properties. Mater. Res. Express 2018, 5, 075029. [Google Scholar] [CrossRef]
- Jiang, Z.; Liang, X.; Zheng, H.; Liu, Y.; Wang, Z.; Wang, P.; Zhang, X.; Qin, X.; Dai, Y.; Whangbo, M.H.; et al. Photocatalytic reduction of CO2 to methanol by three-dimensional hollow structures of Bi2WO6 quantum dots. Appl. Catal. B Environ. 2017, 219, 209–215. [Google Scholar] [CrossRef]
- Chao, P.Y.; Chang, C.J.; Lin, K.S.; Wang, C.F. Synergistic effects of morphology control and calcination on the activity of flower-like Bi2WO6-Bi2O3 photocatalysts prepared by an ionic liquid-assisted solvothermal method. J. Alloys Compd. 2021, 883, 160920. [Google Scholar] [CrossRef]
- De Andrade, F.V.; De Lima, G.M.; Augusti, R.; Coelho, M.G.; Ardisson, J.D.; Romero, O.B. A versatile approach to treat aqueous residues of textile industry: The photocatalytic degradation of Indigo Carmine dye employing the autoclaved cellular concrete/Fe2O3 system. Chem. Eng. J. 2012, 180, 25–31. [Google Scholar] [CrossRef]
- Han, D.L.; Yu, P.L.; Liu, X.M.; Xu, Y.D.; Wu, S.L. Polydopamine modified CuS@HKUST for rapid sterilization through enhanced photothermal property and photocatalytic ability. Rare Met. 2021, 40, 1–10. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, H.; Shan, X.; Di, Y.; Zhao, A.; Hu, Y.; Gan, Z. Solar steam generation based on the photothermal effect: From designs to applications, and beyond. J. Mater. Chem. A 2019, 7, 19203–19227. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-C.; Chang, C.-J.; Huang, C.-F.; Zhang, H.-C.; Kang, C.-W. Polydopamine-Bi2WO6-Decorated Gauzes as Dual-Functional Membranes for Solar Steam Generation and Photocatalytic Degradation Applications. Polymers 2021, 13, 4335. https://doi.org/10.3390/polym13244335
Wang Y-C, Chang C-J, Huang C-F, Zhang H-C, Kang C-W. Polydopamine-Bi2WO6-Decorated Gauzes as Dual-Functional Membranes for Solar Steam Generation and Photocatalytic Degradation Applications. Polymers. 2021; 13(24):4335. https://doi.org/10.3390/polym13244335
Chicago/Turabian StyleWang, Yea-Chin, Chi-Jung Chang, Chih-Feng Huang, Hao-Cheng Zhang, and Chun-Wen Kang. 2021. "Polydopamine-Bi2WO6-Decorated Gauzes as Dual-Functional Membranes for Solar Steam Generation and Photocatalytic Degradation Applications" Polymers 13, no. 24: 4335. https://doi.org/10.3390/polym13244335
APA StyleWang, Y.-C., Chang, C.-J., Huang, C.-F., Zhang, H.-C., & Kang, C.-W. (2021). Polydopamine-Bi2WO6-Decorated Gauzes as Dual-Functional Membranes for Solar Steam Generation and Photocatalytic Degradation Applications. Polymers, 13(24), 4335. https://doi.org/10.3390/polym13244335