Recent Advances in Functional Polymer Materials for Energy, Water, and Biomedical Applications: A Review
Abstract
1. Introduction
2. Energy Applications of Polymers
2.1. Batteries as an Energy Storage Application of Polymers
2.2. Solar and Fuel Cells as an Energy Production Application of Polymers
3. Oil and Gas Applications
3.1. Polyacrylamides
3.2. Xanthan Gum/Biopolymer
3.3. Superabsorbent Polymer Composites for Enhanced Oil Recovery
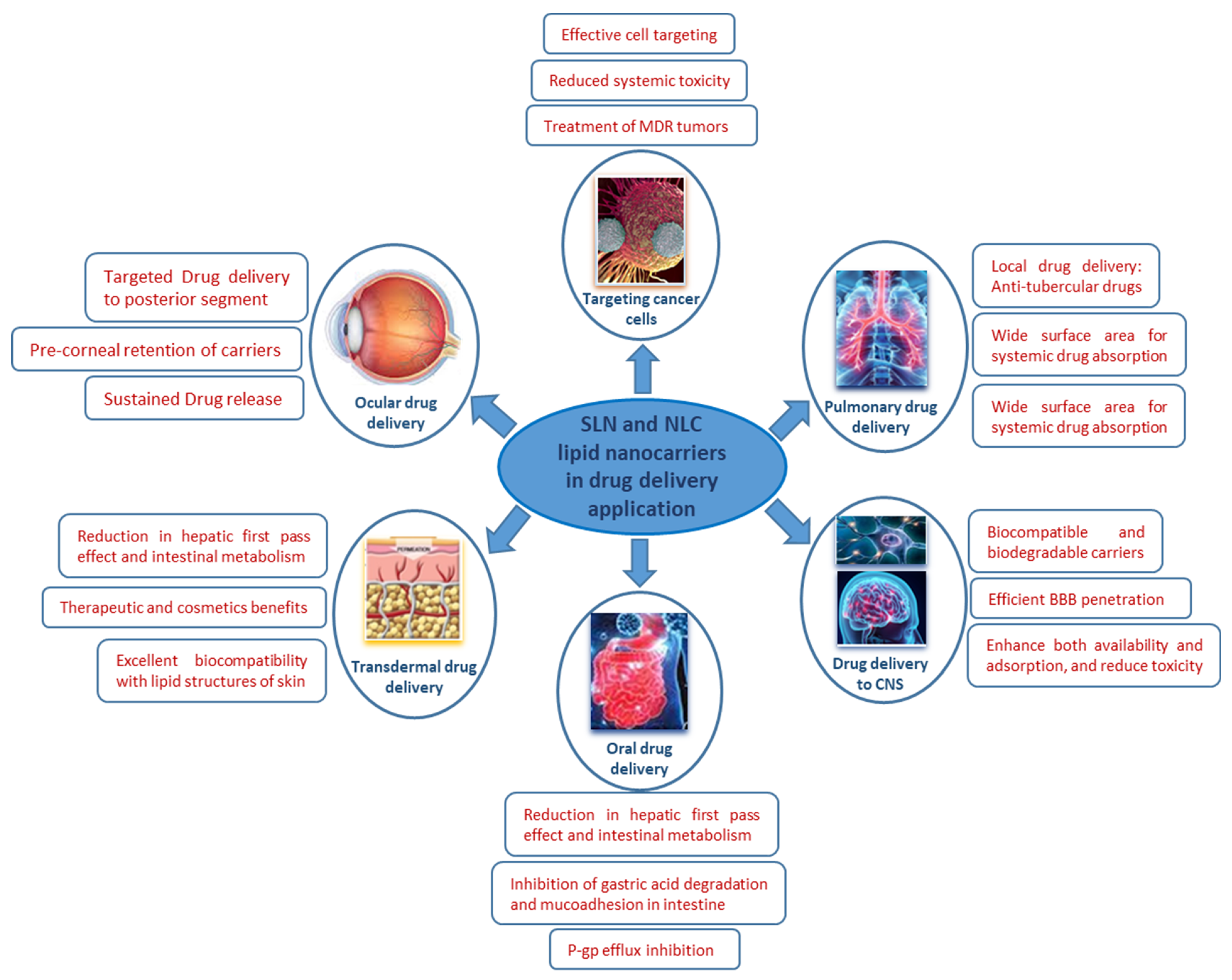
4. Advances in Biomedical Applications

5. Industrial Water Treatment Applications
5.1. Chemical Additives
5.2. Polymeric Membranes
5.3. Polymers in the Treatment of Industrial Wastewaters
5.4. Other Applications in Water Sector
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, L.; Weaver, J.L.; Groenenboom, M.; Nakamura, N.; Rus, E.; Anand, P.; Jha, S.K.; Okasinski, J.S.; Dura, J.A.; Reeja-Jayan, B. Tailoring Electrode–Electrolyte Interfaces in Lithium-Ion Batteries Using Molecularly Engineered Functional Polymers. ACS Appl. Mater. Interfaces 2021, 13, 9919–9931. [Google Scholar] [CrossRef]
- Arreguin-Campos, R.; Jiménez-Monroy, K.L.; Diliën, H.; Cleij, T.J.; van Grinsven, B.; Eersels, K. Imprinted Polymers as Synthetic Receptors in Sensors for Food Safety. Biosensors 2021, 11, 46. [Google Scholar] [CrossRef]
- Alashrah, S.; El-Ghoul, Y.; Omer, M.A.A. Synthesis and Characterization of a New Nanocomposite Film Based on Polyvinyl Alcohol Polymer and Nitro Blue Tetrazolium Dye as a Low Radiation Dosimeter in Medical Diagnostics Application. Polymers 2021, 13, 1815. [Google Scholar] [CrossRef]
- D’Agata, R.; Bellassai, N.; Jungbluth, V.; Spoto, G. Recent Advances in Antifouling Materials for Surface Plasmon Resonance Biosensing in Clinical Diagnostics and Food Safety. Polymers 2021, 13, 1929. [Google Scholar] [CrossRef] [PubMed]
- Alashrah, S.; El-Ghoul, Y.; Almutairi, F.M.; Omer, M.A.A. Development, Characterization and Valuable Use of Novel Dosimeter Film Based on PVA Polymer Doped Nitro Blue Tetrazolium Dye and AgNO3 for the Accurate Detection of Low X-ray Doses. Polymers 2021, 13, 3140. [Google Scholar] [CrossRef]
- Wu, D.; Shi, W.; Ding, S.; Xie, X. Diverse functional groups decorated, bifunctional polyesteramide as efficient Pb(II) electrochemical probe and methylene blue adsorbent. Eur. Polym. J. 2021, 160, 110810. [Google Scholar] [CrossRef]
- Shi, H.; Dai, Z.; Sheng, X.; Xia, D.; Shao, P.; Yang, L.; Luo, X. Conducting polymer hydrogels as a sustainable platform for advanced energy, biomedical and environmental applications. Sci. Total Environ. 2021, 786, 147430. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Mohd Nurazzi, N.; Asyraf, M.R.M.; Khalina, A.; Abdullah, N.; Sabaruddin, F.A.; Kamarudin, S.H.; Ahmad, S.; Mahat, A.M.; Lee, C.L.; Aisyah, H.A.; et al. Fabrication, Functionalization, and Application of Carbon Nanotube-Reinforced Polymer Composite: An Overview. Polymers 2021, 13, 1047. [Google Scholar] [CrossRef] [PubMed]
- Wieszczycka, K.; Staszak, K.; Woźniak-Budych, M.J.; Litowczenko, J.; Maciejewska, B.M.; Jurga, S. Surface functionalization–The way for advanced applications of smart materials. Coord. Chem. Rev. 2021, 436, 213846. [Google Scholar] [CrossRef]
- Liyanage, S.; Acharya, S.; Parajuli, P.; Shamshina, J.L.; Abidi, N. Production and Surface Modification of Cellulose Bioproducts. Polymers 2021, 13, 3433. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.-T.; Hong, K.V.T.; Nguyen, T.-B. Surface Modification by the DBD Plasma to Improve the Flame-Retardant Treatment for Dyed Polyester Fabric. Polymers 2021, 13, 3011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ouyang, Q.; Hu, Z.; Lu, S.; Quan, W.; Li, P.; Chen, Y.; Li, S. Catechol functionalized chitosan/active peptide microsphere hydrogel for skin wound healing. Int. J. Biol. Macromol. 2021, 173, 591–606. [Google Scholar] [CrossRef]
- Basinska, T.; Gadzinowski, M.; Mickiewicz, D.; Slomkowski, S. Functionalized Particles Designed for Targeted Delivery. Polymers 2021, 13, 2022. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Niu, W.; Lei, B.; Boccaccini, A.R. Immunomodulatory bioactive glasses for tissue regeneration. Acta Biomater. 2021, 133, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.G.D.; Reis, B.; Costas, B.; Lima, S.A.C.; Reis, S. Modulation of Macrophages M1/M2 Polarization Using Carbohydrate-Functionalized Polymeric Nanoparticles. Polymers 2021, 13, 88. [Google Scholar] [CrossRef]
- Zhou, Z.; Ren, L.; Zhang, L.; Zhong, J.; Xiao, Y.; Jia, Z.; Guo, L.; Yang, J.; Wang, C.; Jiang, S.; et al. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell Host Microbe 2020, 27, 883–890. [Google Scholar] [CrossRef]
- Yoo, S.; Seok, D.; Jung, Y.; Lee, K. Hydrophilic Surface Treatment of Carbon Powder Using CO2 Plasma Activated Gas. Coatings 2021, 11, 925. [Google Scholar] [CrossRef]
- Yamada, S.; Yassin, M.A.; Weigel, T.; Schmitz, T.; Hansmann, J.; Mustafa, K. Surface activation with oxygen plasma promotes osteogenesis with enhanced extracellular matrix formation in three-dimensional microporous scaffolds. J. Biomed. Mater. Res. Part A 2021, 109, 1560–1574. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, Y.-S.; Kuk, Y.-S.; Kwac, L.K.; Choi, S.-H.; Park, J.; Shin, H.K. Preparation and Characterization of Carbon Fibers from Lyocell Precursors Grafted with Polyacrylamide via Electron-Beam Irradiation. Molecules 2021, 26, 2459. [Google Scholar] [CrossRef]
- Gatto, M.L.; Groppo, R.; Bloise, N.; Fassina, L.; Visai, L.; Galati, M.; Iuliano, L.; Mengucci, P. Topological, Mechanical and Biological Properties of Ti6Al4V Scaffolds for Bone Tissue Regeneration Fabricated with Reused Powders via Electron Beam Melting. Materials 2021, 14, 224. [Google Scholar] [CrossRef] [PubMed]
- Szustakiewicz, K.; Kryszak, B.; Dzienny, P.; Poźniak, B.; Tikhomirov, M.; Hoppe, V.; Szymczyk-Ziółkowska, P.; Tylus, W.; Grzymajło, M.; Gadomska-Gajadhur, A.; et al. Cytotoxicity Study of UV-Laser-Irradiated PLLA Surfaces Subjected to Bio-Ceramisation: A New Way towards Implant Surface Modification. Int. J. Mol. Sci. 2021, 22, 8436. [Google Scholar] [CrossRef]
- Arroyo-Lamas, N.; Arteagoitia, I.; Ugalde, U. Surface Activation of Titanium Dental Implants by Using UVC-LED Irradiation. Int. J. Mol. Sci. 2021, 22, 2597. [Google Scholar] [CrossRef] [PubMed]
- Gabardo, R.S.; de Carvalho Cotre, D.S.; Arias, M.J.L.; Moisés, M.P.; Ferreira, B.T.M.; Samulewski, R.B.; Hinestroza, J.P.; Bezerra, F.M. Surface Modification of Polyester Fabrics by Ozone and Its Effect on Coloration Using Disperse Dyes. Materials 2021, 14, 3492. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Z.; Gao, Y.; Gao, W.; Hou, Z.; Zhu, Y. Facile Method for Surface-Grafted Chitooligosaccharide on Medical Segmented Poly(ester-urethane) Film to Improve Surface Biocompatibility. Membranes 2021, 11, 37. [Google Scholar] [CrossRef]
- Chen, J.-C.; Chen, C.-H.; Chang, K.-C.; Liu, S.-M.; Ko, C.-L.; Shih, C.-J.; Sun, Y.-S.; Chen, W.-C. Evaluation of the Grafting Efficacy of Active Biomolecules of Phosphatidylcholine and Type I Collagen on Polyether Ether Ketone: In Vitro and In Vivo. Polymers 2021, 13, 2081. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M. Chemical Functionalization of Carbon Nanotubes with Polymers: A Brief Overview. Macromolecules 2021, 1, 64–83. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Tsai, M.-Y.; Wang, C.-F.; Huang, C.-F.; Danko, M.; Dai, L.; Chen, T.; Kuo, S.-W. Multifunctional Polyhedral Oligomeric Silsesquioxane (POSS) Based Hybrid Porous Materials for CO2 Uptake and Iodine Adsorption. Polymers 2021, 13, 221. [Google Scholar] [CrossRef]
- Khan, M.A.; Govindasamy, R.; Ahmad, A.; Siddiqui, M.R.; Alshareef, S.A.; Hakami, A.A.H.; Rafatullah, M. Carbon Based Polymeric Nanocomposites for Dye Adsorption: Synthesis, Characterization, and Application. Polymers 2021, 13, 419. [Google Scholar] [CrossRef]
- Pereira, A.M.; Gomes, D.; da Costa, A.; Dias, S.C.; Casal, M.; Machado, R. Protein-Engineered Polymers Functionalized with Antimicrobial Peptides for the Development of Active Surfaces. Appl. Sci. 2021, 11, 5352. [Google Scholar] [CrossRef]
- Charoensri, K.; Rodwihok, C.; Wongratanaphisan, D.; Ko, J.A.; Chung, J.S.; Park, H.J. Investigation of Functionalized Surface Charges of Thermoplastic Starch/Zinc Oxide Nanocomposite Films Using Polyaniline: The Potential of Improved Antibacterial Properties. Polymers 2021, 13, 425. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Zamuner, A.; Battocchio, C.; Cassari, L.; Todesco, M.; Graziani, V.; Iucci, G.; Marsotto, M.; Tortora, L.; Secchi, V.; et al. Bio-Functionalized Chitosan for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 5916. [Google Scholar] [CrossRef]
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules 2021, 26, 272. [Google Scholar] [CrossRef]
- Beagan, A.M.; Alghamdi, A.A.; Lahmadi, S.S.; Halwani, M.A.; Almeataq, M.S.; Alhazaa, A.N.; Alotaibi, K.M.; Alswieleh, A.M. Folic Acid-Terminated Poly(2-Diethyl Amino Ethyl Methacrylate) Brush-Gated Magnetic Mesoporous Nanoparticles as a Smart Drug Delivery System. Polymers 2021, 13, 59. [Google Scholar] [CrossRef]
- Donoso-González, O.; Lodeiro, L.; Aliaga, Á.E.; Laguna-Bercero, M.A.; Bollo, S.; Kogan, M.J.; Yutronic, N.; Sierpe, R. Functionalization of Gold Nanostars with Cationic β-Cyclodextrin-Based Polymer for Drug Co-Loading and SERS Monitoring. Pharmaceutics 2021, 13, 261. [Google Scholar] [CrossRef] [PubMed]
- Deyab, M.A.; El Bali, B.; Mohsen, Q.; Essehli, R. Design new epoxy nanocomposite coatings based on metal vanadium oxy-phosphate M0.5VOPO4 for anti-corrosion applications. Sci. Rep. 2021, 11, 8182. [Google Scholar] [CrossRef]
- Platnieks, O.; Gaidukovs, S.; Barkane, A.; Gaidukova, G.; Grase, L.; Thakur, V.K.; Filipova, I.; Fridrihsone, V.; Skute, M.; Laka, M. Highly Loaded Cellulose/Poly (butylene succinate) Sustainable Composites for Woody-Like Advanced Materials Application. Molecules 2020, 25, 121. [Google Scholar] [CrossRef]
- Saska, S.; Pilatti, L.; Blay, A.; Shibli, J.A. Bioresorbable Polymers: Advanced Materials and 4D Printing for Tissue Engineering. Polymers 2021, 13, 563. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Imran, R.; Arif, Z.U.; Akram, N.; Arshad, H.; Al Rashid, A.; Márquez, F.P.G. Developments in Chemical Treatments, Manufacturing Techniques and Potential Applications of Natural-Fibers-Based Biodegradable Composites. Coatings 2021, 11, 293. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, T.; Cheng, C.; Wang, Q.; Lin, S.; Liu, C.; Han, X. Research Progress on Synthesis and Application of Cyclodextrin Polymers. Molecules 2021, 26, 1090. [Google Scholar] [CrossRef]
- Suriani, M.J.; Zainudin, H.A.; Ilyas, R.A.; Petrů, M.; Sapuan, S.M.; Ruzaidi, C.M.; Mustapha, R. Kenaf Fiber/Pet Yarn Reinforced Epoxy Hybrid Polymer Composites: Morphological, Tensile, and Flammability Properties. Polymers 2021, 13, 1532. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Khan, M.U.A.; Abdullah, A.M.; Razak, S.I.A. A Review on Current Trends of Polymers in Orthodontics: BPA-Free and Smart Materials. Polymers 2021, 13, 1409. [Google Scholar] [CrossRef]
- Salmi, M. Additive Manufacturing Processes in Medical Applications. Materials 2021, 14, 191. [Google Scholar] [CrossRef]
- Saleh Alghamdi, S.; John, S.; Roy Choudhury, N.; Dutta, N.K. Additive Manufacturing of Polymer Materials: Progress, Promise and Challenges. Polymers 2021, 13, 753. [Google Scholar] [CrossRef] [PubMed]
- Soares, B.G.; Barra, G.M.O.; Indrusiak, T. Conducting Polymeric Composites Based on Intrinsically Conducting Polymers as Electromagnetic Interference Shielding/Microwave Absorbing Materials—A Review. J. Compos. Sci. 2021, 5, 173. [Google Scholar] [CrossRef]
- Duburg, J.C.; Azizi, K.; Primdahl, S.; Hjuler, H.A.; Zanzola, E.; Schmidt, T.J.; Gubler, L. Composite Polybenzimidazole Membrane with High Capacity Retention for Vanadium Redox Flow Batteries. Molecules 2021, 26, 1679. [Google Scholar] [CrossRef]
- Sánchez-Vergara, M.E.; Hamui, L.; Gómez, E.; Chans, G.M.; Galván-Hidalgo, J.M. Design of Promising Heptacoordinated Organotin (IV) Complexes-PEDOT: PSS-Based Composite for New-Generation Optoelectronic Devices Applications. Polymers 2021, 13, 1023. [Google Scholar] [CrossRef]
- Azizighannad, S.; Wang, Z.; Siddiqui, Z.; Kumar, V.; Mitra, S. Nano Carbon Doped Polyacrylamide Gel Electrolytes for High Performance Supercapacitors. Molecules 2021, 26, 2631. [Google Scholar] [CrossRef]
- Sung, Y.-S.; Lin, L.-Y. Systematic Design of Polypyrrole/Carbon Fiber Electrodes for Efficient Flexible Fiber-Type Solid-State Supercapacitors. Nanomaterials 2020, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Deepali, K.; Sapana, J.; Rizwan, A.; Shagufta, J. Functionalization of conducting polymers and their applications in optoelectronics. Polym.-Plast. Technol. Mater. 2021, 60, 465–487. [Google Scholar] [CrossRef]
- Aziz, S.B.; Nofal, M.M.; Kadir, M.F.Z.; Dannoun, E.M.A.; Brza, M.A.; Hadi, J.M.; Abdullah, R.M. Bio-Based Plasticized PVA Based Polymer Blend Electrolytes for Energy Storage EDLC Devices: Ion Transport Parameters and Electrochemical Properties. Materials 2021, 14, 1994. [Google Scholar] [CrossRef] [PubMed]
- Begum, B.; Bilal, S.; Shah, A.u.H.A.; Röse, P. Physical, Chemical, and Electrochemical Properties of Redox-Responsive Polybenzopyrrole as Electrode Material for Faradaic Energy Storage. Polymers 2021, 13, 2883. [Google Scholar] [CrossRef]
- Aziz, S.B.; Nofal, M.M.; Abdulwahid, R.T.; Ghareeb, H.O.; Dannoun, E.M.A.; Abdullah, R.M.; Hamsan, M.H.; Kadir, M.F.Z. Plasticized Sodium-Ion Conducting PVA Based Polymer Electrolyte for Electrochemical Energy Storage—EEC Modeling, Transport Properties, and Charge-Discharge Characteristics. Polymers 2021, 13, 803. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P.; Omran, A.A.B.; Singh, J.; Ilyas, R.A. Recent Trends and Developments in Conducting Polymer Nanocomposites for Multifunctional Applications. Polymers 2021, 13, 2898. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.U.; Röse, P.; Shah, A.U.H.A.; Krewer, U.; Bilal, S.; Farooq, S. Exploring the Functional Properties of Sodium Phytate Doped Polyaniline Nanofibers Modified FTO Electrodes for High-Performance Binder Free Symmetric Supercapacitors. Polymers 2021, 13, 2329. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, H.; Lu, Y.; Wei, S.; Wujcik, E.K.; Guo, Z. Multifunctional Carbon Nanostructures for Advanced Energy Storage Applications. Nanomaterials 2015, 5, 755–777. [Google Scholar] [CrossRef]
- Kausar, A. Green Nanocomposites for Energy Storage. J. Compos. Sci. 2021, 5, 202. [Google Scholar] [CrossRef]
- Siwal, S.S.; Zhang, Q.; Devi, N.; Thakur, V.K. Carbon-Based Polymer Nanocomposite for High-Performance Energy Storage Applications. Polymers 2020, 12, 505. [Google Scholar] [CrossRef]
- Yang, S.; An, X.; Qian, X. Integrated Conductive Hybrid Electrode Materials Based on PPy@ZIF-67-Derived Oxyhydroxide@CFs Composites for Energy Storage. Polymers 2021, 13, 1082. [Google Scholar] [CrossRef]
- Bi, X.; Yang, L.; Wang, Z.; Zhan, Y.; Wang, S.; Zhang, C.; Li, Y.; Miao, Y.; Zha, J. Construction of a Three-Dimensional BaTiO3 Network for Enhanced Permittivity and Energy Storage of PVDF Composites. Materials 2021, 14, 3585. [Google Scholar] [CrossRef]
- Kondratiev, V.V.; Holze, R. Intrinsically conducting polymers and their combinations with redox-active molecules for rechargeable battery electrodes: An update. Chem. Pap. 2021, 75, 4981–5007. [Google Scholar] [CrossRef]
- Meng, N.; Lian, F.; Cui, G. Macromolecular Design of Lithium Conductive Polymer as Electrolyte for Solid-State Lithium Batteries. Small 2021, 17, 2005762. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Z.; Zhao, S.; Westover, A.S.; Belharouak, I.; Cao, P. Single-Ion Conducting Polymer Electrolytes for Solid-State Lithium–Metal Batteries: Design, Performance, and Challenges. Adv. Energy Mater. 2021, 11, 2003836. [Google Scholar] [CrossRef]
- Yu, L.M.; Man, J.X.; Chen, T.; Luo, D.; Wang, J.; Yang, H.; Zhao, Y.B.; Wang, H.; Yang, Y.; Lu, Z.H. Colorful conducting polymers for vivid solar panels. Nano Energy 2021, 85, 105937. [Google Scholar] [CrossRef]
- Chae, J.E.; Lee, S.Y.; Yoo, S.J.; Kim, J.Y.; Jang, J.H.; Park, H.-Y.; Park, H.S.; Seo, B.; Henkensmeier, D.; Song, K.H.; et al. Polystyrene-Based Hydroxide-Ion-Conducting Ionomer: Binder Characteristics and Performance in Anion-Exchange Membrane Fuel Cells. Polymers 2021, 13, 690. [Google Scholar] [CrossRef]
- Zhou, Z.; Zholobko, O.; Wu, X.-F.; Aulich, T.; Thakare, J.; Hurley, J. Polybenzimidazole-Based Polymer Electrolyte Membranes for High-Temperature Fuel Cells: Current Status and Prospects. Energies 2021, 14, 135. [Google Scholar] [CrossRef]
- Tan, S.; Li, J.; Zhou, L.; Chen, P.; Shi, J.; Xu, Z. Modified Carbon Fiber Paper-Based Electrodes Wrapped by Conducting Polymers with Enhanced Electrochemical Performance for Supercapacitors. Polymers 2018, 10, 1072. [Google Scholar] [CrossRef]
- Ruano, G.; Molina, B.G.; Torras, J.; Alemán, C. Free-Standing, Flexible Nanofeatured Polymeric Films Prepared by Spin-Coating and Anodic Polymerization as Electrodes for Supercapacitors. Molecules 2021, 26, 4345. [Google Scholar] [CrossRef] [PubMed]
- Stempien, Z.; Khalid, M.; Kozanecki, M.; Filipczak, P.; Wrzesińska, A.; Korzeniewska, E.; Sąsiadek, E. Inkjet Printing of Polypyrrole Electroconductive Layers Based on Direct Inks Freezing and Their Use in Textile Solid-State Supercapacitors. Materials 2021, 14, 3577. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, S.; Guo, L.; Zhang, G.; Zhang, H.; Cao, M.; Wu, L.; Xiang, T.; Peng, Y. A Self-Healing Ionic Liquid-Based Ionically Cross-Linked Gel Polymer Electrolyte for Electrochromic Devices. Polymers 2021, 13, 742. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-W.; Chang, J.-C.; Chang, J.-K.; Huang, S.-W.; Lee, P.-Y.; Wu, T.-Y. Electrodeposited Copolymers Based on 9,9′-(5-Bromo-1,3-phenylene)biscarbazole and Dithiophene Derivatives for High-Performance Electrochromic Devices. Polymers 2021, 13, 1136. [Google Scholar] [CrossRef] [PubMed]
- Alsultan, M.; Ameen, A.M.; Al-keisy, A.; Swiegers, G.F. Conducting-Polymer Nanocomposites as Synergistic Supports That Accelerate Electro-Catalysis: PEDOT/Nano Co3O4/rGO as a Photo Catalyst of Oxygen Production from Water. J. Compos. Sci. 2021, 5, 245. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Conducting Polymers in the Design of Biosensors and Biofuel Cells. Polymers 2021, 13, 49. [Google Scholar] [CrossRef]
- Shimoga, G.; Palem, R.R.; Choi, D.-S.; Shin, E.-J.; Ganesh, P.-S.; Saratale, G.D.; Saratale, R.G.; Lee, S.-H.; Kim, S.-Y. Polypyrrole-Based Metal Nanocomposite Electrode Materials for High-Performance Supercapacitors. Metals 2021, 11, 905. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Umar, K.; Bhawani, S.A.; Khan, A.; Asiri, A.M.; Khan, M.R.; Azam, M.; AlAmmari, A.M. Cellulose Derived Graphene/Polyaniline Nanocomposite Anode for Energy Generation and Bioremediation of Toxic Metals via Benthic Microbial Fuel Cells. Polymers 2021, 13, 135. [Google Scholar] [CrossRef]
- Ghafoor, U.; Aqeel, A.B.; Zaman, U.K.u.; Zahid, T.; Noman, M.; Ahmad, M.S. Effect of Molybdenum Disulfide on the Performance of Polyaniline Based Counter Electrode for Dye-Sensitized Solar Cell Applications. Energies 2021, 14, 3786. [Google Scholar] [CrossRef]
- Noh, S.; Gong, H.Y.; Lee, H.J.; Koh, W.G. Electrically Conductive Micropatterned Polyaniline-Poly(ethylene glycol) Composite Hydrogel. Materials 2021, 14, 308. [Google Scholar] [CrossRef]
- Muench, S.; Wild, A.; Friebe, C.; Häupler, B.; Janoschka, T.; Schubert, U.S. Polymer-Based Organic Batteries. Chem. Rev. 2016, 116, 9438–9484. [Google Scholar] [CrossRef]
- Jyothibasu, J.P.; Chen, M.-Z.; Tien, Y.-C.; Kuo, C.-C.; Chen, E.-C.; Lin, Y.-C.; Chiang, T.-C.; Lee, R.-H. V2O5/Carbon Nanotube/Polypyrrole Based Freestanding Negative Electrodes for High-Performance Supercapacitors. Catalysts 2021, 11, 980. [Google Scholar] [CrossRef]
- Hong, X.; Liu, Y.; Li, Y.; Wang, X.; Fu, J.; Wang, X. Application Progress of Polyaniline, Polypyrrole and Polythiophene in Lithium-Sulfur Batteries. Polymers 2020, 12, 331. [Google Scholar] [CrossRef]
- Zhu, L.; Lei, A.; Cao, Y.L.; Ai, X.P.; Yang, H.X. An all-organic rechargeable battery using bipolar polyparaphenylene as a redox-active cathode and anode. Chem. Commun. 2013, 49, 567–569. [Google Scholar] [CrossRef]
- Seol, M.-L.; Nam, I.; Sadatian, E.; Dutta, N.; Han, J.-W.; Meyyappan, M. Printable Gel Polymer Electrolytes for Solid-State Printed Supercapacitors. Materials 2021, 14, 316. [Google Scholar] [CrossRef]
- Nawaz, B.; Ali, G.; Ullah, M.O.; Rehman, S.; Abbas, F. Investigation of the Electrochemical Properties of Ni0.5Zn0.5Fe2O4 as Binder-Based and Binder-Free Electrodes of Supercapacitors. Energies 2021, 14, 3297. [Google Scholar] [CrossRef]
- Azimov, F.; Kim, J.; Choi, S.M.; Jung, H.M. Synergistic Effects of Fe2O3 Nanotube/Polyaniline Composites for an Electrochemical Supercapacitor with Enhanced Capacitance. Nanomaterials 2021, 11, 1557. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Ahmed, M.M.M.; Du, W.-T.; Kuo, S.-W. Meso/Microporous Carbons from Conjugated Hyper-Crosslinked Polymers Based on Tetraphenylethene for High-Performance CO2 Capture and Supercapacitor. Molecules 2021, 26, 738. [Google Scholar] [CrossRef] [PubMed]
- Bekhoukh, A.; Moulefera, I.; Sabantina, L.; Abdelghani, B. Development, Investigation, and Comparative Study of the Effects of Various Metal Oxides on Optical Electrochemical Properties Using a Doped PANI Matrix. Polymers 2021, 13, 3344. [Google Scholar] [CrossRef]
- Cai, X.; Sun, K.; Qiu, Y.; Jiao, X. Recent Advances in Graphene and Conductive Polymer Composites for Supercapacitor Electrodes: A Review. Crystals 2021, 11, 947. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, F.; Lu, C.; Zhu, J.; Ke, C.; Han, S.; Zhuang, X. A Terpyridine-Fe2+-Based Coordination Polymer Film for On-Chip Micro-Supercapacitor with AC Line-Filtering Performance. Polymers 2021, 13, 1002. [Google Scholar] [CrossRef]
- Ruano, G.; Iribarren, J.I.; Pérez-Madrigal, M.M.; Torras, J.; Alemán, C. Electrical and Capacitive Response of Hydrogel Solid-Like Electrolytes for Supercapacitors. Polymers 2021, 13, 1337. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, S.; Sun, J.; Zhou, J.; Wang, Y.; Tao, K.; Xiao, X.; Han, L. Enhanced Capacitance Performance by Coupling 2D Conductive Metal–Organic Frameworks and Conducting Polymers for Hybrid Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 9534–9541. [Google Scholar] [CrossRef]
- Olean-Oliveira, A.; Brito, G.A.O.; Cardoso, C.X.; Teixeira, M.F.S. Nanocomposite Materials Based on Electrochemically Synthesized Graphene Polymers: Molecular Architecture Strategies for Sensor Applications. Chemosensors 2021, 9, 149. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Development of Graphene-Based Polymeric Nanocomposites: A Brief Overview. Polymers 2021, 13, 2978. [Google Scholar] [CrossRef]
- Gul, H.; Shah, A.-U.-H.A.; Bilal, S. Achieving Ultrahigh Cycling Stability and Extended Potential Window for Supercapacitors through Asymmetric Combination of Conductive Polymer Nanocomposite and Activated Carbon. Polymers 2019, 11, 1678. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Chai, L.; Wang, Y.; Di, J. Enhanced efficiency and stability of perovskite solar cell by adding polymer mixture in perovskite photoactive layer. J. Alloy. Compd. 2021, 864, 158793. [Google Scholar] [CrossRef]
- Peng, J.; Walter, D.; Ren, Y.; Tebyetekerwa, M.; Wu, Y.; Duong, T.; Lin, Q.; Li, J.; Lu, T.; Mahmud, M.A.; et al. Nanoscale localized contacts for high fill factors in polymer-passivated perovskite solar cells. Science 2021, 371, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Han, T.H.; Lee, J.W.; Choi, C.; Tan, S.; Lee, C.; Zhao, Y.; Dai, Z.; De Marco, N.; Lee, S.J.; Bae, S.H.; et al. Perovskite-polymer composite cross-linker approach for highly-stable and efficient perovskite solar cells. Nat. Commun. 2019, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Shaik, S.; Zhou, Z.; Ouyang, Z.; Han, R.; Li, D. Polymer Additive Assisted Fabrication of Compact and Ultra-Smooth Perovskite Thin Films with Fast Lamp Annealing. Energies 2021, 14, 2656. [Google Scholar] [CrossRef]
- Bonardd, S.; Moreno-Serna, V.; Kortaberria, G.; Díaz, D.D.; Leiva, A.; Saldías, C. Dipolar Glass Polymers Containing Polarizable Groups as Dielectric Materials for Energy Storage Applications. A Minireview. Polymers 2019, 11, 317. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Wang, S.; He, J.; Zhang, T.; Wang, J.; Wu, G. Simultaneously Enhanced Thermal Conductivity and Dielectric Breakdown Strength in Sandwich AlN/Epoxy Composites. Nanomaterials 2021, 11, 1898. [Google Scholar] [CrossRef]
- Rajalakshmi, N.; Gopalan, R. Recent Trends in Science and Technology of Hydrogen and Polymer Electrolyte Membrane Fuel Cells. Trans. Indian Natl. Acad. Eng. 2021, 6, 189–218. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, G.; Zhang, X.; Sun, C.; Jiao, K.; Wang, Y. Thermal management of polymer electrolyte membrane fuel cells: A review of cooling methods, material properties, and durability. Appl. Energy 2021, 286, 116496. [Google Scholar] [CrossRef]
- Shih, K.-Y.; Wei, J.-J.; Tsai, M.-C. One-Step Microwave-Assisted Synthesis of PtNiCo/rGO Electrocatalysts with High Electrochemical Performance for Direct Methanol Fuel Cells. Nanomaterials 2021, 11, 2206. [Google Scholar] [CrossRef]
- Cheng, G.; Li, Z.; Ren, S.; Han, D.; Xiao, M.; Wang, S.; Meng, Y. A Robust Composite Proton Exchange Membrane of Sulfonated Poly (Fluorenyl Ether Ketone) with an Electrospun Polyimide Mat for Direct Methanol Fuel Cells Application. Polymers 2021, 13, 523. [Google Scholar] [CrossRef]
- Simari, C.; Nicotera, I.; Aricò, A.S.; Baglio, V.; Lufrano, F. New Insights into Properties of Methanol Transport in Sulfonated Polysulfone Composite Membranes for Direct Methanol Fuel Cells. Polymers 2021, 13, 1386. [Google Scholar] [CrossRef]
- Sanij, F.D.; Balakrishnan, P.; Leung, P.; Shah, A.; Su, H.; Xu, Q. Advanced Pd-based nanomaterials for electro-catalytic oxygen reduction in fuel cells: A review. Int. J. Hydrog. Energy 2021, 46, 14596–14627. [Google Scholar] [CrossRef]
- Wang, R.; Lou, M.; Zhang, J.; Sun, Z.; Li, Z.; Wen, P. ZIF-8@ZIF-67-Derived Co Embedded into Nitrogen-Doped Carbon Nanotube Hollow Porous Carbon Supported Pt as an Efficient Electrocatalyst for Methanol Oxidation. Nanomaterials 2021, 11, 2491. [Google Scholar] [CrossRef]
- Kehoe, K.D.; Romeral, L.; Lundy, R.; Morris, M.A.; Lyons, M.G.; Gun’ko, Y.K. One Dimensional AuAg Nanostructures as Anodic Catalysts in the Ethylene Glycol Oxidation. Nanomaterials 2020, 10, 719. [Google Scholar] [CrossRef]
- Ferreira, P.; Abreu, B.; Freire, C.; Fernandes, D.M.; Marques, E.F. Nanocomposites Prepared from Carbon Nanotubes and the Transition Metal Dichalcogenides WS2 and MoS2 via Surfactant-Assisted Dispersions as Electrocatalysts for Oxygen Reactions. Materials 2021, 14, 896. [Google Scholar] [CrossRef]
- Yaqoob, L.; Noor, T.; Iqbal, N. Recent progress in development of efficient electrocatalyst for methanol oxidation reaction in direct methanol fuel cell. Int. J. Energy Res. 2021, 45, 6550–6583. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, J.; Yuan, J. Porous PtIr bimetallic nanotubes with core shell structure for enhanced electrocatalysis on methanol oxidation. Nanotechnology 2021, 32, 365402. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, Z.; Dong, H.; Mikhailova, M.V.; Davarpanah, A. Hybrid Application of Nanoparticles and Polymer in Enhanced Oil Recovery Processes. Polymers 2021, 13, 1414. [Google Scholar] [CrossRef]
- Hu, X.; Li, M.; Peng, C.; Davarpanah, A. Hybrid Thermal-Chemical Enhanced Oil Recovery Methods; An Experimental Study for Tight Reservoirs. Symmetry 2020, 12, 947. [Google Scholar] [CrossRef]
- Saberi, H.; Esmaeilnezhad, E.; Choi, H.J. Artificial Neural Network to Forecast Enhanced Oil Recovery Using Hydrolyzed Polyacrylamide in Sandstone and Carbonate Reservoirs. Polymers 2021, 13, 2606. [Google Scholar] [CrossRef]
- Liang, S.; Liu, Y.; Hu, S.; Shen, A.; Yu, Q.; Yan, H.; Bai, M. Experimental Study on the Physical Performance and Flow Behavior of Decorated Polyacrylamide for Enhanced Oil Recovery. Energies 2019, 12, 562. [Google Scholar] [CrossRef]
- Ahsani, T.; Tamsilian, Y.; Rezaei, A. Molecular dynamic simulation and experimental study of wettability alteration by hydrolyzed polyacrylamide for enhanced oil recovery: A new finding for polymer flooding process. J. Pet. Sci. Eng. 2021, 196, 108029. [Google Scholar] [CrossRef]
- Mohan, A.; Rao, A.; Vancso, J.; Mugele, F. Towards enhanced oil recovery: Effects of ionic valency and pH on the adsorption of hydrolyzed polyacrylamide at model surfaces using QCM-D. Appl. Surf. Sci. 2021, 560, 149995. [Google Scholar] [CrossRef]
- Lu, X.; Cao, B.; Xie, K.; Cao, W.; Liu, Y.; Zhang, Y.; Wang, X.; Zhang, J. Enhanced oil recovery mechanisms of polymer flooding in a heterogeneous oil reservoir. Pet. Explor. Dev. 2021, 48, 169–178. [Google Scholar] [CrossRef]
- Han, X.; Li, C.; Pan, F.; Li, Y.; Feng, Y. A comparative study on enhancing oil recovery with partially hydrolyzed polyacrylamide: Emulsion versus powder. Can. J. Chem. Eng. 2021. [Google Scholar] [CrossRef]
- Kang, P.S.; Lim, J.S.; Huh, C. Temperature Dependence of the Shear-Thinning Behavior of Partially Hydrolyzed Polyacrylamide Solution for Enhanced Oil Recovery. J. Energy Resour. Technol. 2021, 143, 063002. [Google Scholar] [CrossRef]
- Jang, H.Y.; Zhang, K.; Chon, B.H.; Choi, H.J. Enhanced oil recovery performance and viscosity characteristics of polysaccharide xanthan gum solution. J. Ind. Eng. Chem. 2015, 21, 741–745. [Google Scholar] [CrossRef]
- Romero-Zerón, L.; Espinosa, C. Advantageous supramolecular system through self-association of xanthan gum/cationic surfactant via β-cyclodextrin host-guest complexations for Enhanced Oil Recovery Applications. J. Pet. Sci. Eng. 2020, 185, 106644. [Google Scholar] [CrossRef]
- Shimaa, M.E.; Elsayed, G.Z.; Walaa, A.E.O. Guar Gum-Based Hydrogels as Potent Green Polymers for Enhanced Oil Recovery in High-Salinity Reservoirs. ACS Omega 2021, 6, 23421–23431. [Google Scholar]
- Malmir, P.; Hashemi, A.; Soulgani, B.S. Mechanistic study of the wettability alteration induced by preformed particle gel (PPG) in carbonate reservoirs. J. Mol. Liq. 2021, 328, 115422. [Google Scholar] [CrossRef]
- Cao, M.; Cai, Q.; Guo, G.; Guo, H.; Chen, Y.; Zhang, Y. The preparation and displacement performances of a hollow structure microsphere with swelling–deswelling properties for enhanced oil recovery (EOR). Polym. Bull. 2021, 1–5. [Google Scholar] [CrossRef]
- Jamali, A.; Moghbeli, M.R.; Ameli, F.; Roayaie, E.; Karambeigi, M.S. Synthesis and characterization of pH-sensitive poly(acrylamide-co-methylenebisacrylamide-co-acrylic acid) hydrogel microspheres containing silica nanoparticles: Application in enhanced oil recovery processes. J. Appl. Polym. Sci. 2020, 137, 48491. [Google Scholar] [CrossRef]
- Riswati, S.S.; Setiati, R.; Kasmungin, S.; Prakoso, S.; Fathaddin, M.T. Sugarcane bagasse for environmentally friendly super-absorbent polymer: Synthesis methods and potential applications in oil industry. IOP Conf. Ser. Earth Environ. Sci. 2021, 819, 012017. [Google Scholar] [CrossRef]
- Vallejos, S.; Hernando, E.; Trigo, M.; García, F.C.; García-Valverde, M.; Iturbe, D.; Cabero, M.J.; Quesada, R.; García, J.M. Polymeric chemosensor for the detection and quantification of chloride in human sweat. Application to the diagnosis of cystic fibrosis. J. Mater. Chem. B 2018, 6, 3735–3741. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Park, H.; Ha, D.H.; Yun, H.S.; Yi, H.G.; Lee, H. 3D Bioprinting of In Vitro Models Using Hydrogel-Based Bioinks. Polymers 2021, 13, 366. [Google Scholar] [CrossRef]
- Campbell, S.; Wu, Q.; Yazbeck, J.; Liu, C.; Okhovatian, S.; Radisic, M. Beyond polydimethylsiloxane: Alternative materials for fabrication of organ on a chip devices and microphysiological systems. ACS Biomater. Sci. Eng. 2021, 7, 2880–2899. [Google Scholar] [CrossRef]
- Cui, X.; Li, J.; Hartanto, Y.; Durham, M.; Tang, J.; Zhang, H.; Hooper, G.; Lim, K.; Woodfield, T. Advances in Extrusion 3D Bioprinting: A Focus on Multicomponent Hydrogel-Based Bioinks. Adv. Health Mater. 2020, 9, e1901648. [Google Scholar] [CrossRef]
- Gebeyehu, A.; Surapaneni, S.K.; Huang, J.; Mondal, A.; Wang, V.Z.; Haruna, N.F.; Bagde, A.; Arthur, P.; Kutlehria, S.; Patel, N.; et al. Polysaccharide hydrogel based 3D printed tumor models for chemotherapeutic drug screening. Sci. Rep. 2021, 11, 372. [Google Scholar] [CrossRef]
- Lin, N.Y.C.; Homan, K.A.; Robinson, S.S.; Kolesky, D.B.; Duarte, N.; Moisan, A.; Lewis, J.A. Renal reabsorption in 3D vascularized proximal tubule models. Proc. Natl. Acad. Sci. USA 2019, 116, 5399–5404. [Google Scholar] [CrossRef]
- Sun, C.-K.; Ke, C.-J.; Lin, Y.-W.; Lin, F.-H.; Tsai, T.-H.; Sun, J.-S. Transglutaminase Cross-Linked Gelatin-Alginate-Antibacterial Hydrogel as the Drug Delivery-Coatings for Implant-Related Infections. Polymers 2021, 13, 414. [Google Scholar] [CrossRef]
- Buxadera-Palomero, J.; Fricke, K.; Reuter, S.; Gil, F.J.; Rodriguez, D.; Canal, C. One-Step Liquid Phase Polymerization of HEMA by Atmospheric-Pressure Plasma Discharges for Ti Dental Implants. Appl. Sci. 2021, 11, 662. [Google Scholar] [CrossRef]
- Cornejo-Bravo, J.M.; Palomino, K.; Palomino-Vizcaino, G.; Pérez-Landeros, O.M.; Curiel-Alvarez, M.; Valdez-Salas, B.; Bucio, E.; Magaña, H. Poly(N-vinylcaprolactam) and Salicylic Acid Polymeric Prodrug Grafted onto Medical Silicone to Obtain a Novel Thermo- and pH-Responsive Drug Delivery System for Potential Medical Devices. Materials 2021, 14, 1065. [Google Scholar] [CrossRef]
- De Leo, V.; Milano, F.; Agostiano, A.; Catucci, L. Recent Advancements in Polymer/Liposome Assembly for Drug Delivery: From Surface Modifications to Hybrid Vesicles. Polymers 2021, 13, 1027. [Google Scholar] [CrossRef]
- Neves, A.R.; Albuquerque, T.; Faria, R.; Paul, M.; Biswas, S.; Sousa, Â.; Costa, D. Development of Tailor-Made Dendrimer Ternary Complexes for Drug/Gene Co-Delivery in Cancer. Pharmaceutics 2021, 13, 1256. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, X.; Jing, X.; Hu, H. PAMAM-Functionalized Cellulose Nanocrystals with Needle-Like Morphology for Effective Cancer Treatment. Nanomaterials 2021, 11, 1640. [Google Scholar] [CrossRef]
- Tarach, P.; Janaszewska, A. Recent Advances in Preclinical Research Using PAMAM Dendrimers for Cancer Gene Therapy. Int. J. Mol. Sci. 2021, 22, 2912. [Google Scholar] [CrossRef]
- Hanurry, E.Y.; Mekonnen, T.W.; Andrgie, A.T.; Darge, H.F.; Birhan, Y.S.; Hsu, W.-H.; Chou, H.-Y.; Cheng, C.-C.; Lai, J.-Y.; Tsai, H.-C. Biotin-Decorated PAMAM G4.5 Dendrimer Nanoparticles to Enhance the Delivery, Anti-Proliferative, and Apoptotic Effects of Chemotherapeutic Drug in Cancer Cells. Pharmaceutics 2020, 12, 443. [Google Scholar] [CrossRef]
- Mandal, A.K. Dendrimers in targeted drug delivery applications: A review of diseases and cancer. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 287–297. [Google Scholar] [CrossRef]
- Chittasupho, C.; Anuchapreeda, S.; Sarisuta, N. CXCR4 targeted dendrimer for anti-cancer drug delivery and breast cancer cell migration inhibition. Eur. J. Pharm. Biopharm. 2017, 119, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Valente, J.F.A.; Pereira, P.; Sousa, A.; Queiroz, J.A.; Sousa, F. Effect of Plasmid DNA Size on Chitosan or Polyethyleneimine Polyplexes Formulation. Polymers 2021, 13, 793. [Google Scholar] [CrossRef]
- Maity, S.; Choudhary, P.; Manjunath, M.; Kulkarni, A.; Murthy, N. A biodegradable adamantane polymer with ketal linkages in its backbone for gene therapy. Chem. Commun. 2015, 51, 1–4. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, G.; Gong, Y.; Zhang, Y.; Liang, X.; Yang, L. Functionalized Folate-Modified Graphene Oxide/PEI siRNA Nanocomplexes for Targeted Ovarian Cancer Gene Therapy. Nanoscale Res. Lett. 2020, 15, 1–11. [Google Scholar] [CrossRef]
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B 2019, 7, 709–729. [Google Scholar] [CrossRef]
- Zhang, G.; Yi, H.; Bao, C. Stimuli-Responsive Poly(aspartamide) Derivatives and Their Applications as Drug Carriers. Int. J. Mol. Sci. 2021, 22, 8817. [Google Scholar] [CrossRef]
- McCarthy, P.C.; Zhang, Y.; Abebe, F. Recent Applications of Dual-Stimuli Responsive Chitosan Hydrogel Nanocomposites as Drug Delivery Tools. Molecules 2021, 26, 4735. [Google Scholar] [CrossRef]
- Atanasova, D.; Staneva, D.; Grabchev, I. Textile Materials Modified with Stimuli-Responsive Drug Carrier for Skin Topical and Transdermal Delivery. Materials 2021, 14, 930. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Wang, H. Temperature-responsive polymers: Synthesis, properties, and biomedical applications. Nano Res. 2018, 11, 5400–5423. [Google Scholar] [CrossRef]
- Xian, S.; Webber, M.J. Temperature-responsive supramolecular hydrogels. J. Mater. Chem. B 2020, 8, 9197–9211. [Google Scholar] [CrossRef]
- Kotsuchibashi, Y.; Ebara, M.; Aoyagi, T.; Narain, R. Recent Advances in Dual Temperature Responsive Block Copolymers and Their Potential as Biomedical Applications. Polymers 2016, 8, 380. [Google Scholar] [CrossRef]
- Nastyshyn, S.; Raczkowska, J.; Stetsyshyn, Y.; Orzechowska, B.; Bernasik, A.; Shymborska, Y.; Brzychczy-Włoch, M.; Gosiewski, T.; Lishchynskyi, O.; Ohar, H.; et al. Non-cytotoxic, temperature-responsive and antibacterial POEGMA based nanocomposite coatings with silver nanoparticles. RSC Adv. 2020, 10, 10155–10166. [Google Scholar] [CrossRef]
- Gajos, K.; Szafraniec, K.; Petrou, P.; Budkowski, A. Surface density dependent orientation and immunological recognition of antibody on silicon: TOF-SIMS and surface analysis of two covalent immobilization methods. Appl. Surf. Sci. 2020, 518, 146269. [Google Scholar] [CrossRef]
- Raczkowska, J.; Stetsyshyn, Y.; Awsiuk, K.; Brzychczy-Włoch, M.; Gosiewski, T.; Jany, B.; Lishchynskyi, O.; Shymborska, Y.; Nastyshyn, S.; Bernasik, A.; et al. Command surfaces with thermo-switchable antibacterial activity. Mater. Sci. Eng. C 2019, 103, 109806. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Layered composite based on halloysite and natural polymers: A carrier for pH controlled release of drugs. New J. Chem. 2019, 43, 10887–10893. [Google Scholar] [CrossRef]
- Bobrowska, J.A.; Awsiuk, K.; Pabijan, J.; Bobrowski, P.; Lekki, J.; Sowa, K.M.; Rysz, J.; Budkowski, A.; Lekka, M. Biophysical and biochemical characteristics as complementary indicators of melanoma progression. Anal. Chem. 2019, 91, 9885–9892. [Google Scholar] [CrossRef]
- Lishchynskyi, O.; Stetsyshyn, Y.; Raczkowska, J.; Awsiuk, K.; Orzechowska, B.; Abalymov, A.; Skirtach, A.G.; Bernasik, A.; Nastyshyn, S.; Budkowski, A. Fabrication and Impact of Fouling-Reducing Temperature-Responsive POEGMA Coatings with Embedded CaCO3 Nanoparticles on Different Cell Lines. Materials 2021, 14, 1417. [Google Scholar] [CrossRef]
- Lazzara, G.; Cavallaro, G.; Panchal, A.; Fakhrullin, R.; Stavitskaya, A.; Vinokurov, V.; Lvov, Y. An assembly of organic-inorganic composites using halloysite clay nanotubes. Curr. Opin. Colloid Interface Sci. 2018, 35, 42–50. [Google Scholar] [CrossRef]
- Stetsyshyn, Y.; Raczkowska, J.; Harhay, K.; Gajos, K.; Melnyk, Y.; Dąbczyński, P.; Shevtsova, T.; Budkowski, A. Temperature-responsive and multi-responsive grafted polymer brushes with transitions based on critical solution temperature: Synthesis, properties, and applications. Colloid Polym. Sci. 2021, 299, 363–383. [Google Scholar] [CrossRef]
- Park, Y.; Kim, M.; Chung, H.j.; Woo, A.H.; Noda, I.; Jung, Y.M. The Study of pH Effects on Phase Transition of Multi-Stimuli Responsive P(NiPAAm-co-AAc) Hydrogel Using 2D-COS. Polymers 2021, 13, 1447. [Google Scholar] [CrossRef]
- Longo, R.; Gorrasi, G.; Guadagno, L. Electromagnetically Stimuli-Responsive Nanoparticles-Based Systems for Biomedical Applications: Recent Advances and Future Perspectives. Nanomaterials 2021, 11, 848. [Google Scholar] [CrossRef]
- Andrade, F.; Roca-Melendres, M.M.; Durán-Lara, E.F.; Rafael, D.; Schwartz, S., Jr. Stimuli-Responsive Hydrogels for Cancer Treatment: The Role of pH, Light, Ionic Strength and Magnetic Field. Cancers 2021, 13, 1164. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.-l. Review of Applications and Future Prospects of Stimuli-Responsive Hydrogel Based on Thermo-Responsive Biopolymers in Drug Delivery Systems. Polymers 2021, 13, 2086. [Google Scholar] [CrossRef]
- Alwattar, J.K.; Mneimneh, A.T.; Abla, K.K.; Mehanna, M.M.; Allam, A.N. Smart Stimuli-Responsive Liposomal Nanohybrid Systems: A Critical Review of Theranostic Behavior in Cancer. Pharmaceutics 2021, 13, 355. [Google Scholar] [CrossRef]
- Wu, G.; Wang, J.; Liu, Q.; Lu, R.; Wei, Y.; Cheng, F.; Han, J.; Xing, W.; Huang, Y. Surface Permeability of Membrane and Catalytic Performance Based on Redox-Responsive of Hybrid Hollow Polymeric Microcapsules. Molecules 2021, 26, 633. [Google Scholar] [CrossRef]
- Turanlı, Y.; Acartürk, F. Fabrication and characterization of budesonide loaded colon-specific nanofiber drug delivery systems using anionic and cationic polymethacrylate polymers. J. Drug Deliv. Sci. Technol. 2021, 63, 102511. [Google Scholar] [CrossRef]
- Sengupta, I.; Kumar, S.S.; Gupta, K.; Chakraborty, S. In-vitro Release Study through Novel Graphene Oxide Aided Alginate Based pH-Sensitive Drug Carrier for Gastrointestinal Tract. Mater. Today Commun. 2021, 26, 101737. [Google Scholar] [CrossRef]
- Xue, S.; Wu, Y.; Wang, J.; Guo, M.; Liu, D.; Lei, W. Boron Nitride Nanosheets/PNIPAM Hydrogels with Improved Thermo-Responsive Performance. Materials 2018, 11, 1069. [Google Scholar] [CrossRef]
- Thiele, S.; Andersson, J.; Dahlin, A. Tuning the Thermoresponsive Behavior of Surface-Attached PNIPAM Networks: Varying the Crosslinker Content in SI-ATRP. Langmuir 2021, 11, 3391–3398. [Google Scholar] [CrossRef]
- Giaouzi, D.; Pispas, S. Complexation behavior of PNIPAM-b-QPDMAEA copolymer aggregates with linear DNAs of different lengths. Eur. Polym. J. 2021, 155, 110575. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Alqahtani, A.; Al-Thabit, A.; Roni, M.; Syed, R. Novel lignin nanoparticles for oral drug delivery. J. Mater. Chem. B 2019, 7, 4461–4473. [Google Scholar] [CrossRef]
- Ni, S. Nanoparticles carrying natural product for drug delivery. J. Drug Deliv. Ther. 2017, 7, 73–75. [Google Scholar] [CrossRef][Green Version]
- Lin, C.H.; Chen, C.H.; Lin, Z.C.; Fang, J.Y. Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. J. Food Drug Anal. 2017, 25, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.F.; Yuan, M.; Chen, M.S.; Liu, S.M.; Zhu, L.; Huang, B.Y.; Liu, X.W.; Xiong, W. Pharmacokinetics, tissue distribution and relative bioavailability of puerarin solid lipid nanoparticles following oral administration. Int. J. Pharm. 2011, 410, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.F.; Hou, N.; Tian, J.; Yuan, M.; Liu, S.M.; Xiong, L.G.; Luo, J.D.; Chen, M.S. Metabolic profile of puerarin in rats after intragastric administration of puerarin solid lipid nanoparticles. Int. J. Nanomed. 2013, 8, 933–940. [Google Scholar] [CrossRef]
- Zhang, C.; Gu, C.; Peng, F.; Liu, W.; Wan, J.; Xu, H.; Lam, C.W.; Yang, X. Preparation and optimization of triptolide-loaded solid lipid nanoparticles for oral delivery with reduced gastric irritation. Molecules 2013, 18, 13340–13356. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Zaki, M.A.; Garg, A.; Ahmad, J. Advancement in design of nanostructured lipid carriers for cancer targeting and theranostic application. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2021, 1865, 129936. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, B.; Arshad, N.M.; Malagobadan, S.; Misran, M.; Nyamathulla, S.; Mun, K.S.; Nagoor, N.H. Development and Evaluation of 1′-Acetoxychavicol Acetate (ACA)-Loaded Nanostructured Lipid Carriers for Prostate Cancer Therapy. Pharmaceutics 2021, 13, 439. [Google Scholar] [CrossRef]
- Elmowafy, M.; Al-Sanea, M.M. Nanostructured lipid carriers (NLCs) as drug delivery platform: Advances in formulation and delivery strategies. Saudi Pharm. J. 2021, 29, 999–1012. [Google Scholar] [CrossRef]
- Nordin, N.; Yeap, S.K.; Rahman, H.S.; Zamberi, N.R.; Mohamad, N.E.; Abu, N.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. Antitumor and Anti-Metastatic Effects of Citral-Loaded Nanostructured Lipid Carrier in 4T1-Induced Breast Cancer Mouse Model. Molecules 2020, 25, 2670. [Google Scholar] [CrossRef]
- Ong, Y.S.; Yazan, L.S.; Ng, W.K.; Abdullah, R.; Mustapha, N.M.; Sapuan, S.; Foo, J.B.; Tor, Y.S.; How, C.W.; Abd Rahman, N.; et al. Thymoquinone loaded in nanostructured lipid carrier showed enhanced anticancer activity in 4T1 tumor-bearing mice. Nanomedicine 2018, 13, 1567–1582. [Google Scholar] [CrossRef]
- Nordin, N.; Yeap, S.K.; Zamberi, N.R.; Abu, N.; Mohamad, N.E.; Rahman, H.S.; How, C.W.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. Characterization and toxicity of citral incorporated with nanostructured lipid carrier. PeerJ 2018, 6, e3916. [Google Scholar] [CrossRef]
- Rahman, H.S.; Rasedee, A.; Abdul, A.B.; Zeenathul, N.A.; Othman, H.H.; Yeap, S.K.; How, C.W.; Hafiza, W.A.G.W.N. Zerumbone-loaded nanostructured lipid carrier induces G2/M cell cycle arrest and apoptosis via mitochondrial pathway in a human lymphoblastic leukemia cell line. Int. J. Nanomed. 2014, 9, 527–538. [Google Scholar] [CrossRef]
- Butowska, K.; Woziwodzka, A.; Borowik, A.; Piosik, J. Polymeric Nanocarriers: A Transformation in Doxorubicin Therapies. Materials 2021, 14, 2135. [Google Scholar] [CrossRef]
- Mulvihill, J.J.; Cunnane, E.M.; Ross, A.M.; Duskey, J.T.; Tosi, G.; Grabrucker, A.M. Drug delivery across the blood–brain barrier: Recent advances in the use of nanocarriers. Nanomedicine 2020, 15, 205–214. [Google Scholar] [CrossRef]
- Oddone, N.; Pederzoli, F.; Duskey, J.T.; De Benedictis, C.A.; Grabrucker, A.M.; Forni, F.; Vandelli, M.A.; Ruozi, B.; Tosi, G. ROS-responsive “smart” polymeric conjugate: Synthesis, characterization and proof-of-concept study. Int. J. Pharm. 2019, 570, 118655. [Google Scholar] [CrossRef]
- Zhu, F.D.; Hu, Y.J.; Yu, L.; Zhou, X.G.; Wu, J.M.; Tang, Y.; Qin, D.L.; Fan, Q.Z.; Wu, A.G. Nanoparticles: A Hope for the Treatment of Inflammation in CNS. Front. Pharmacol. 2021, 12, 683935. [Google Scholar] [CrossRef]
- Gupta, J.; Fatima, M.T.; Islam, Z.; Khan, R.H.; Uversky, V.N.; Salahuddin, P. Nanoparticle formulations in the diagnosis and therapy of Alzheimer’s disease. Int. J. Biol. Macromol. 2019, 130, 515–526. [Google Scholar] [CrossRef]
- Boridy, S.; Takahashi, H.; Akiyoshi, K.; Maysinger, D. The binding of pullulan modified cholesteryl nanogels to Abeta oligomers and their suppression of cytotoxicity. Biomaterials 2009, 30, 5583–5591. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Ahmed, S.; Sharma, N.; Kumar, N.; Debas, A.; Matsumura, K. Review of the current state of protein aggregation inhibition from a materials chemistry perspective: Special focus on polymeric materials. Mater. Adv. 2021, 2, 1139–1176. [Google Scholar] [CrossRef]
- Cano, A.; Sánchez-López, E.; Ettcheto, M. Current advances in the development of novel polymeric nanoparticles for the treatment of neurodegenerative diseases. Nanomedecine 2020, 15, 1239–1261. [Google Scholar] [CrossRef]
- Bittner, A.; Gosselet, F.; Sevin, E.; Dehouck, L.; Ducray, A.D.; Gaschen, V.; Stoffel, M.H.; Cho, H.; Mevissen, M. Time-Dependent Internalization of Polymer-Coated Silica Nanoparticles in Brain Endothelial Cells and Morphological and Functional Effects on the Blood-Brain Barrier. Int. J. Mol. Sci. 2021, 22, 1657. [Google Scholar] [CrossRef]
- Chan, M.H.; Chen, W.; Li, C.H.; Fang, C.Y.; Chang, Y.C.; Wei, D.H.; Liu, R.S.; Hsiao, M. An Advanced In Situ Magnetic Resonance Imaging and Ultrasonic Theranostics Nanocomposite Platform: Crossing the Blood–Brain Barrier and Improving the Suppression of Glioblastoma Using Iron-Platinum Nanoparticles in Nanobubbles. ACS Appl. Mater. Interfaces 2021, 13, 26759–26769. [Google Scholar] [CrossRef]
- El-Ghoul, Y.; Alminderej, F.M. Bioactive and superabsorbent cellulosic dressing grafted alginate and Carthamus tinctorius polysaccharide extract for the treatment of chronic wounds. Text. Res. J. 2021, 91, 235–248. [Google Scholar] [CrossRef]
- El-Ghoul, Y. Biological and microbiological performance of new polymer-based chitosan and synthesized aminocyclodextrin finished polypropylene abdominal wall prosthesis biomaterial. Text. Res. J. 2020, 90, 2690–2702. [Google Scholar] [CrossRef]
- Alminderej, M.F.; El-Ghoul, Y. Synthesis and study of a new biopolymer-based chitosan/hematoxylin grafted to cotton wound dressings. J. Appl. Polym. Sci. 2019, 136, 47625. [Google Scholar] [CrossRef]
- Salah, F.; El Ghoul, Y.; Alminderej, F.M.; El-Golli-Bennour, E.; Ouanes, Z.; Maciejak, O.; Jarroux, N.; Majdoub, H.; Sakli, F. Development, characterization, and biological assessment of biocompatible cellulosic wound dressing grafted Aloe vera bioactive polysaccharide. Cellulose 2019, 26, 4957–4970. [Google Scholar] [CrossRef]
- Salah, F.; El-Ghoul, Y.; Mahdhi, A.; Majdoub, H.; Jarroux, N.; Sakli, F. Effect of the deacetylation degree on the antibacterial and antibiofilm activity of acemannan from Aloe vera. Ind. Crop. Prod. 2017, 103, 13–18. [Google Scholar] [CrossRef]
- El-Ghoul, Y.; Salah, F.; Majdoub, H.; Sakli, F. Synthesis and study of drug delivery system obtained via β-cyclodextrin functionalization of viscose/polyester dressings. J. Ind. Text. 2017, 47, 489–504. [Google Scholar] [CrossRef]
- El-Ghoul, Y.; Ammar, C.; El-Achari, A. New polymer based modified cyclodextrins grafted to textile fibers; characterization and application to cotton wound dressings. Int. J. Appl. Res. Text. 2014, 2, 11–21. [Google Scholar]
- Ammar, C.; El Ghoul, Y.; El Achari, A. Finishing of polypropylene fibers with cyclodextrins and polyacrylic acid as a crosslinking agent. Text. Res. J. 2015, 85, 171–179. [Google Scholar] [CrossRef]
- El Ghoul, Y.; Blanchemain, N.; Laurent, T.; Campagne, C.; El Achari, A.; Roudesli, S.; Morcellet, M.; Martel, B.; Hildebrand, H.F. Chemical, biological and microbiological evaluation of cyclodextrin finished polyamide inguinal meshes. Acta Biomater. 2008, 4, 1392–1400. [Google Scholar] [CrossRef]
- Alminderej, F. Study of new cellulosic dressing with enhanced antibacterial performance grafted with a biopolymer of chitosan and myrrh polysaccharide extract. Arab. J. Chem. 2020, 13, 3672–3681. [Google Scholar] [CrossRef]
- Salah, F.; El-Ghoul, Y.; Roudesli, S. Bacteriological effects of functionalized cotton dressings. J. Text. Inst. 2016, 107, 171–181. [Google Scholar] [CrossRef]
- Alminderej, F.M.; Ammar, C.; El-Ghoul, Y. Functionalization, characterization and microbiological performance of new biocompatible cellulosic dressing grafted chitosan and Suaeda fruticosa polysaccharide extract. Cellulose 2021, 28, 9821–9835. [Google Scholar] [CrossRef]
- Shen, S.; Chen, X.; Shen, Z.; Chen, H. Marine Polysaccharides for Wound Dressings Application: An Overview. Pharmaceutics 2021, 13, 1666. [Google Scholar] [CrossRef]
- Mokhtari, H.; Tavakoli, S.; Safarpour, F.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Ramakrishna, S.; Berto, F. Recent Advances in Chemically-Modified and Hybrid Carrageenan-Based Platforms for Drug Delivery, Wound Healing, and Tissue Engineering. Polymers 2021, 13, 1744. [Google Scholar] [CrossRef]
- Jung, H.S.; Kim, M.H.; Shin, J.Y.; Park, S.R.; Jung, J.Y.; Park, W.H. Electrospinning and wound healing activity of β-chitin extracted from cuttlefish bone. Carbohydr. Polym. 2018, 193, 205–211. [Google Scholar] [CrossRef]
- Moradkhannejhad, L.; Abdouss, M.; Nikfarjam, N.; Mazinani, S.; Heydari, V. Electrospinning of zein/propolis nanofibers; antimicrobial properties and morphology investigation. J. Mater. Sci. Mater. Med. 2018, 29, 165. [Google Scholar] [CrossRef]
- D’Amato, A.R.; Puhl, D.L.; Ellman, S.A.T.; Balouch, B.; Gilbert, R.J.; Palermo, E.F. Vastly extended drug release from poly(pro-17-β-estradiol) materials facilitates in vitro neurotrophism and neuroprotection. Nat. Commun. 2019, 10, 4830. [Google Scholar] [CrossRef]
- Cleeton, C.; Keirouz, A.; Chen, X.; Radacsi, N. Electrospun Nanofibers for Drug Delivery and Biosensing. ACS Biomater. Sci. Eng. 2019, 5, 4183–4205. [Google Scholar] [CrossRef]
- Chen, S.; Li, R.; Li, X.; Xie, J. Electrospinning: An Enabling Nanotechnology Platform for Drug Delivery and Regenerative Medicine. Adv. Drug Deliv. Rev. 2018, 132, 188–213. [Google Scholar] [CrossRef]
- Horne, J.; McLoughlin, L.; Bridgers, B.; Wujcik, E.K. Recent Developments in Nanofiber-Based Sensors for Disease Detection, Immunosensing, and Monitoring. Sens. Actuators Rep. 2020, 2, 100005. [Google Scholar] [CrossRef]
- Yang, T.; Zhan, L.; Huang, C.Z. Recent Insights into Functionalized Electrospun Nanofibrous Films for Chemo-/Bio-Sensors. TrAC Trends Anal. Chem. 2020, 124, 115813. [Google Scholar] [CrossRef]
- Liu, S.H.; Zhang, H.G.; Hu, Q.X.; Wang, B.; Li, S.; Zhang, C. Development and Evaluation of Biomimetic 3D Coated Composite Scaffold for Application as Skin Substitutes. Macromol. Mater. Eng. 2020, 305, 1900848. [Google Scholar] [CrossRef]
- Yang, X.P.; Li, L.F.; Yang, D.Z.; Nie, J.; Ma, G.P. Electrospun Core–Shell Fibrous 2D Scaffold with Biocompatible Poly(Glycerol Sebacate) and Poly-L-Lactic Acid for Wound Healing. Adv. Fiber Mater. 2020, 2, 105–117. [Google Scholar] [CrossRef]
- Huang, R.; Chen, X.; Dong, Y.; Zhang, X.; Wei, Y.; Yang, Z.; Li, W.; Guo, Y.; Liu, J.; Yang, Z.; et al. MXene Composite Nanofibers for Cell Culture and Tissue Engineering. ACS Appl. Bio Mater. 2020, 3, 2125–2131. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Haggerty, A.E.; Yan, J.; Lan, M.; Seu, M.; Yang, M.; Marlow, M.M.; Maldonado-Lasunción, I.; Cho, B.; et al. The Effect of a Nanofiber-Hydrogel Composite on Neural Tissue Repair and Regeneration in the Contused Spinal Cord. Biomaterials 2020, 245, 119978. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial Biohybrid Nanofibers for Wound Dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef]
- Lv, D.; Zhu, M.M.; Jiang, Z.C.; Jiang, S.H.; Zhang, Q.L.; Xiong, R.H.; Huang, C.B. Green Electrospun Nanofibers and Their Application in Air Filtration. Macromol. Mater. Eng. 2018, 303, 1800336. [Google Scholar] [CrossRef]
- Ding, X.X.; Li, Y.Y.; Si, Y.; Yin, X.; Yu, J.Y.; Ding, B. Electrospun polyvinylidene fluoride/SiO2 nanofibrous membranes with enhanced electret property for efficient air filtration. Chem. Commun. 2019, 13, 57–62. [Google Scholar] [CrossRef]
- Birhanu, G.; Tanha, S.; Javar, H.A.; Seyedjafari, E.; Zandi-Karimi, A.; Dehkordi, B.K. Dexamethasone Loaded Multi-Layer Poly-L-Lactic Acid/Pluronic P123 Composite Electrospun Nanofiber Scaffolds for Bone Tissue Engineering and Drug Delivery. Pharm. Dev. Technol. 2019, 24, 338–347. [Google Scholar] [CrossRef]
- Chan, A.H.P.; Filipe, E.C.; Tan, R.P.; Miguel, S.; Yang, N.J.; Hung, J.; Feng, J.Y.; Nazir, S.; Benn, J.A.; Ng, C.K.M.; et al. Altered processing enhances the efficacy of small-diameter silk fibroin vascular grafts. Sci. Rep. 2019, 9, 17461. [Google Scholar] [CrossRef]
- Nagiah, N.; Murdock, C.J.; Bhattacharjee, M.; Nair, L.; Laurencin, C.T. Development of Tripolymeric Triaxial Electrospun Fibrous Matrices for Dual Drug Delivery Applications. Sci. Rep. 2020, 10, 609. [Google Scholar] [CrossRef]
- Dos Santos, D.M.; Chagas, P.A.M.; Leite, I.S.; Inada, N.M.; de Annunzio, S.R.; Fontana, C.R.; Campana-Filho, S.P.; Correa, D.S. Core-Sheath Nanostructured Chitosan-Based Nonwovens as a Potential Drug Delivery System for Periodontitis Treatment. Int. J. Biol. Macromol. 2020, 142, 521–534. [Google Scholar] [CrossRef]
- Yang, J.; Wang, K.; Yu, D.-G.; Yang, Y.; Bligh, S.W.A.; Williams, G.R. Electrospun Janus Nanofibers Loaded with a Drug and Inorganic Nanoparticles as an Effective Antibacterial Wound Dressing. Mater. Sci. Eng. C 2020, 111, 110805. [Google Scholar] [CrossRef]
- Niiyama, E.; Uto, K.; Lee, C.M.; Sakura, K.; Ebara, M. Hyperthermia Nanofiber Platform Synergized by Sustained Release of Paclitaxel to Improve Antitumor Efficiency. Adv. Health Mater. 2019, 8, 1900102. [Google Scholar] [CrossRef]
- Altinbasak, I.; Jijie, R.; Barras, A.; Golba, B.; Sanyal, R.; Bouckaert, J.; Drider, D.; Bilyy, R.; Dumych, T.; Paryzhak, S.; et al. Reduced Graphene-Oxide-Embedded Polymeric Nanofiber Mats: An “on-Demand” Photothermally Triggered Antibiotic Release Platform. ACS Appl. Mater. Interfaces 2018, 10, 41098–41106. [Google Scholar] [CrossRef]
- Pinese, C.; Lin, J.; Milbreta, U.; Li, M.; Wang, Y.; Leong, K.W.; Chew, S.Y. Sustained Delivery of SiRNA/Mesoporous Silica Nanoparticle Complexes from Nanofiber Scaffolds for Long-Term Gene Silencing. Acta Biomater. 2018, 76, 164–177. [Google Scholar] [CrossRef]
- Baek, J.; Lee, E.; Lotz, M.K.; D’Lima, D.D. Bioactive Proteins Delivery through Core-Shell Nanofibers for Meniscal Tissue Regeneration. Nanomed. Nanotechnol. Biol. Med. 2020, 23, 102090. [Google Scholar] [CrossRef]
- Li, J.; Xu, W.; Li, D.; Liu, T.; Zhang, Y.S.; Ding, J.; Chen, X. Locally Deployable Nanofiber Patch for Sequential Drug Delivery in Treatment of Primary and Advanced Orthotopic Hepatomas. ACS Nano 2018, 12, 6685–6699. [Google Scholar] [CrossRef]
- Yu, D.G.; Li, X.Y.; Wang, X.; Yang, J.H.; Bligh, S.W.A.; Williams, G.R. Nanofibers Fabricated Using Triaxial Electrospinning as Zero Order Drug Delivery Systems. ACS Appl. Mater. Interfaces 2015, 7, 18891–18897. [Google Scholar] [CrossRef]
- Kuang, G.; Zhang, Z.; Liu, S.; Zhou, D.; Lu, X.; Jing, X.; Huang, Y. Biphasic Drug Release from Electrospun Polyblend Nanofibers for Optimized Local Cancer Treatment. Biomater. Sci. 2018, 6, 324–331. [Google Scholar] [CrossRef]
- Bonani, W.; Motta, A.; Migliaresi, C.; Tan, W. Biomolecule Gradient in Micropatterned Nanofibrous Scaffold for Spatiotemporal Release. Langmuir 2012, 28, 13675–13687. [Google Scholar] [CrossRef]
- Qu, M.; Jiang, X.; Zhou, X.; Wang, C.; Wu, Q.; Ren, L.; Zhu, J.; Zhu, S.; Tebon, P.; Sun, W.; et al. Stimuli-Responsive Delivery of Growth Factors for Tissue Engineering. Adv. Health Mater. 2020, 9, 1901714. [Google Scholar] [CrossRef]
- Li, F.; Qin, Y.; Lee, J.; Liao, H.; Wang, N.; Davis, T.P.; Qiao, R.; Ling, D. Stimuli-responsive nano-assemblies for remotely controlled drug delivery. J. Control. Release 2020, 322, 566–592. [Google Scholar] [CrossRef]
- Ballesteros, C.A.S.; Correa, D.S.; Zucolotto, V. Polycaprolactone Nanofiber Mats Decorated with Photoresponsive Nanogels and Silver Nanoparticles: Slow Release for Antibacterial Control. Mater. Sci. Eng. C 2020, 107, 110334. [Google Scholar] [CrossRef]
- Singh, B.; Shukla, N.; Kim, J.; Kim, K.; Park, M.-H. Stimuli-Responsive Nanofibers Containing Gold Nanorods for On-Demand Drug Delivery Platforms. Pharmaceutics 2021, 13, 1319. [Google Scholar] [CrossRef]
- Mamidi, N.; Zuníga, A.E.; Villela-Castrejón, J. Engineering and Evaluation of Forcespun Functionalized Carbon Nano-Onions Reinforced Poly (ε-Caprolactone) Composite Nanofibers for PH-Responsive Drug Release. Mater. Sci. Eng. C 2020, 112, 110928. [Google Scholar] [CrossRef]
- Banimohamad-Shotorbani, B.; Rahmani Del Bakhshayesh, A.; Mehdipour, A.; Jarolmasjed, S.; Shafaei, H. The efficiency of PCL/HAp electrospun nanofibers in bone regeneration: A review. J. Med. Eng. Technol. 2021, 45, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Malysheva, K.; Kwaśniak, K.; Gnilitskyi, I.; Barylyak, A.; Zinchenko, V.; Fahmi, A.; Korchynskyi, O.; Bobitski, Y. Functionalization of Polycaprolactone Electrospun Osteoplastic Scaffolds with Fluorapatite and Hydroxyapatite Nanoparticles: Biocompatibility Comparison of Human Versus Mouse Mesenchymal Stem Cells. Materials 2021, 14, 1333. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zou, Q.; Wang, C.; Lin, M.; Li, Y.; Zhang, R.; Li, Y. Electrospinning and 3D printed hybrid bi-layer scaffold for guided bone regeneration. Mater. Des. 2021, 210, 110047. [Google Scholar] [CrossRef]
- Hashemi, S.F.; Mehrabi, M.; Ehterami, A.; Gharravi, A.M.; Bitaraf, F.S.; Salehi, M. In-vitro and in-vivo studies of PLA/PCL/gelatin composite scaffold containing ascorbic acid for bone regeneration. J. Drug Deliv. Sci. Technol. 2021, 61, 102077. [Google Scholar] [CrossRef]
- Semitela, A.; Girao, A.F.; Fernandes, C.; Ramalho, G.; Bdikin, I.; Completo, A.; Marques, P.A. Electrospinning of bioactive polycaprolactone-gelatin nanofibres with increased pore size for cartilage tissue engineering applications. J. Biomater. Appl. 2020, 35, 459–470. [Google Scholar] [CrossRef]
- Rofiqoh, N.; Putri, E.; Wang, X.; Chen, Y.; Li, X.; Kawazoe, N.; Chen, G. Preparation of PLGA-collagen hybrid scaffolds with controlled pore structures for cartilage tissue engineering. Prog. Nat. Sci. Mater. Int. 2020, 30, 642–650. [Google Scholar] [CrossRef]
- Kadir, N.D.; Yang, Z.; Hassan, A.; Denslin, V.; Lee, E.H. Electrospun fibers enhanced the paracrine signaling of mesenchymal stem cells for cartilage regeneration. Stem Cell Res. Ther. 2021, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, W.; Chen, Y.; Mo, X.; Fan, C. Chondroitin sulfate modified 3D porous electrospun nano fi ber scaffolds promote cartilage regeneration. Mater. Sci. Eng. C 2021, 118, 111312. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, W.; Shafiq, M.; Tang, J.; Hao, J.; Xie, X.; Yuan, Z.; Xiao, X.; Liu, Y.; Mo, X. Three-dimensional porous gas-foamed electrospun nanofiber scaffold for cartilage regeneration. J. Colloid Interface Sci. 2021, 603, 94–109. [Google Scholar] [CrossRef]
- Eom, S.; Park, S.M.; Hwang, D.G.; Kim, H.W.; Jang, J.; Kim, D.S. Fabrication of an align-random distinct, heterogeneous nanofiber mat endowed with bifunctional properties for engineered 3D cardiac anisotropy. Compos. Part B Eng. 2021, 226, 109336. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Haghighi, V.; Mirhaj, M.; Tavafoghi, M.; Shams, F.; Darabi, A. Highly osteogenic and mechanically strong nanofibrous scaffolds based on functionalized multi-walled carbon nanotubes-reinforced electrospun keratin/poly(ε-caprolactone). Mater. Today Commun. 2021, 27, 102401. [Google Scholar] [CrossRef]
- Haldar, S.; Sharma, A.; Gupta, S.; Chauhan, S.; Roy, P.; Lahiri, D. Bioengineered smart trilayer skin tissue substitute for efficient deep wound healing. Mater. Sci. Eng. C 2019, 105, 110140. [Google Scholar] [CrossRef] [PubMed]
- Ranjbarvan, P.; Golchin, A.; Azari, A.; Niknam, Z. The bilayer skin substitute based on human adipose-derived mesenchymal stem cells and neonate keratinocytes on the 3D nanofibrous PCL-platelet gel scaffold. Polym. Bull. 2021, 1–18. [Google Scholar] [CrossRef]
- Choi, W.S.; Kim, J.H.; Ahn, C.B.; Lee, J.H.; Kim, Y.J.; Son, K.H.; Lee, J.W. Development of a Multi-Layer Skin Substitute Using Human Hair Keratinic Extract-Based Hybrid 3D Printing. Polymers 2021, 13, 2584. [Google Scholar] [CrossRef]
- Hivechi, A.; Milan, P.B.; Modabberi, K.; Amoupour, M.; Ebrahimzadeh, K.; Gholipour, A.R.; Sedighi, F.; Amini, N.; Bahrami, S.H.; Rezapour, A.; et al. Synthesis and Characterization of Exopolysaccharide Encapsulated PCL/Gelatin Skin Substitute for Full-Thickness Wound Regeneration. Polymers 2021, 13, 854. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, G.; Dey, K.; Ghosh, P.; Sharma, B.K.; Erhan, S.Z. A Short Review on Polymeric Biomaterials as Additives for Lubricants. Polymers 2021, 13, 1333. [Google Scholar] [CrossRef]
- Antiscalants/Scale Inhibitors Market by Type (Phosphonates, Carboxylates/Acrylic, Sulfonates, and Others), Application (Power and Construction, Mining, Oil and Gas, Water and Wastewater Treatment, Food and Beverages, and Others) and by Region—Global Forecast to 2020. Available online: https://www.marketsandmarkets.com/Market-Reports/scale-inhibitors-market-482.html (accessed on 1 December 2021).
- Mpelwa, M.; Tang, S.F. State of the art of synthetic threshold scale inhibitors for mineral scaling in the petroleum industry: A review. Pet. Sci. 2019, 16, 830–849. [Google Scholar] [CrossRef]
- Al-Hamzah, A.A.; Fellows, C.M. A comparative study of novel scale inhibitors with commercial scale inhibitors used in seawater desalination. Desalination 2015, 359, 22–25. [Google Scholar] [CrossRef]
- Chen, J.Q.; Liu, T.R.; Sun, M.M.; Zhao, Y.Z.; Ge, H.H. Inhibition of Poly(ethylenediaminetetraacetic acid-diethanolamine) on Deposition of Calcium Sulfate Crystal in Simulated Industrial Water. Crystals 2020, 10, 544. [Google Scholar] [CrossRef]
- Moya, S.M.; Botella, N.B. Review of Techniques to Reduce and Prevent Carbonate Scale. Prospecting in Water Treatment by Magnetism and Electromagnetism. Water 2021, 13, 2365. [Google Scholar] [CrossRef]
- Hou, L.; Kumar, D.; Yoo, C.G.; Gitsov, I.; Majumder, E.L.W. Conversion and removal strategies for microplastics in wastewater treatment plants and landfills. Chem. Eng. J. 2021, 406, 126715. [Google Scholar] [CrossRef]
- Zeino, A.; Albakri, M.; Khaled, M.; Zarzour, M. Comparative study of the synergistic effect of ATMP and DTPMPA on CaSO4 scale inhibition and evaluation of induction time effect. J. Water Process. Eng. 2018, 21, 1–8. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, W.; Yin, X.; Liu, Y.; Yin, P.; Wang, J. Investigation of CaCO3 scale inhibition by PAA, ATMP and PAPEMP. Desalination 2008, 228, 55–60. [Google Scholar] [CrossRef]
- Li, C.; Zhang, C.; Zhang, W. The inhibition effect mechanisms of four scale inhibitors on the formation and crystal growth of CaCO3 in solution. Sci. Rep. 2019, 9, 13366. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yin, X.; Chen, Z.; Yang, W.; Chen, Y.; Liu, Y.; Zuo, Y.; Li, L. Use of polyaminoamide dendrimers starting from different core-initial molecules for inhibition of silica scale: Experiment and theory. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126095. [Google Scholar] [CrossRef]
- Blanco, M.; Monteserín, C.; Angulo, A.; Pérez-Márquez, A.; Maudes, J.; Murillo, N.; Aranzabe, E.; Ruiz-Rubio, L.; Vilas, J.L. TiO2-Doped Electrospun Nanofibrous Membrane for Photocatalytic Water Treatment. Polymers 2019, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Gontarek-Castro, E.; Rybarczyk, M.K.; Castro-Muñoz, R.; Morales-Jiménez, M.; Barragán-Huerta, B.; Lieder, M. Characterization of PVDF/Graphene Nanocomposite Membranes for Water Desalination with Enhanced Antifungal Activity. Water 2021, 13, 1279. [Google Scholar] [CrossRef]
- Babaei-Ghazvini, A.; Acharya, B.; Korber, D.R. Antimicrobial Biodegradable Food Packaging Based on Chitosan and Metal/Metal-Oxide Bio-Nanocomposites: A Review. Polymers 2021, 13, 2790. [Google Scholar] [CrossRef] [PubMed]
- Membrane Filtration Market by Type (RO, UF, MF, NF), Application (Water, Dairy, Drinks & Concentrates, Wine & Beer), Module Design (Spiral, Tubular, Plate & Frame), Membrane Material (Polymeric & Ceramic), and Region—Global Forecast to 2025. Available online: https://www.marketwatch.com/press-release/membrane-filtration-market-size-is-expected-to-reach-usd-244-billion-by-2026-2021-01-28 (accessed on 1 December 2021).
- Rabajczyk, A.; Zielecka, M.; Cygańczuk, K.; Pastuszka, Ł.; Jurecki, L. Nanometals-Containing Polymeric Membranes for Purification Processes. Materials 2021, 14, 513. [Google Scholar] [CrossRef]
- Mozia, S.; Grylewicz, A.; Zgrzebnicki, M.; Darowna, D.; Czyżewski, A. Investigations on the Properties and Performance of Mixed-Matrix Polyethersulfone Membranes Modified with Halloysite Nanotubes. Polymers 2019, 11, 671. [Google Scholar] [CrossRef]
- Kim, H.; Shim, I.; Zhan, M. Chemical Enhanced Backwashing for Controlling Organic Fouling in Drinking Water Treatment Using a Novel Hollow-Fiber Polyacrylonitrile Nanofiltration Membrane. Appl. Sci. 2021, 11, 6764. [Google Scholar] [CrossRef]
- Liu, L.-F.; Gu, X.-L.; Xie, X.; Li, R.-H.; Yu, C.-Y.; Song, X.-X.; Gao, C.-J. Modification of PSf/SPSf Blended Porous Support for Improving the Reverse Osmosis Performance of Aromatic Polyamide Thin Film Composite Membranes. Polymers 2018, 10, 686. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef]
- Yu, J.; Gu, K.; Yang, B.; Wang, K.; Zhou, Y.; Gao, C. The Permeability and Selectivity of the Polyamide Reverse Osmosis Membrane were Significantly Enhanced by PhSiCl3. Membranes 2021, 11, 142. [Google Scholar] [CrossRef]
- Yahagi, T.; Degawa, M.; Seino, Y.; Matsushima, T.; Nagao, M.; Sugimura, T.; Hashimoto, Y. Mutagenicity of carcinogenic azo dyes and their derivatives. Cancer Lett. 1975, 1, 91–96. [Google Scholar] [CrossRef]
- Golka, K.; Kopps, S.; Myslak, Z.W. Carcinogenicity of azo colorants: Influence of solubility and bioavailability. Toxicol. Lett. 2004, 151, 203–210. [Google Scholar] [CrossRef]
- Pagga, U.; Brown, D. The degradation of dyestuffs: Part II Behaviour of dyestuffs in aerobic biodegradation tests. Chemosphere 1986, 15, 479–491. [Google Scholar] [CrossRef]
- Ishak, S.A.; Murshed, M.F.; Akil, H.M.; Ismail, N.; Rasib, S.Z.M.; Al-Gheethi, A.A.S. The Application of Modified Natural Polymers in Toxicant Dye Compounds Wastewater: A Review. Water 2020, 12, 2032. [Google Scholar] [CrossRef]
- Bahrpaima, K.; Fatehi, P. Preparation and Coagulation Performance of Carboxypropylated and Carboxypentylated Lignosulfonates for Dye Removal. Biomolecules 2019, 9, 383. [Google Scholar] [CrossRef]
- El-Shamy, A.G. An efficient removal of methylene blue dye by adsorption onto carbon dot @ zinc peroxide embedded poly vinyl alcohol (PVA/CZnO2) nano-composite: A novel Reusable adsorbent. Polymer 2020, 202, 122565. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Aly, K.M.; Khan, Z.A.; Meky, A.E.; Saleh, T.S.; Elbogamy, A.S. Development of novel acid–base ions exchanger for basic dye removal: Phosphoric acid doped pyrazole-g-polyglycidyl methacrylate. Desalination Water Treat. 2016, 57, 24047–24055. [Google Scholar] [CrossRef]
- Moghaddam, R.H.; Shabani, A.M.H.; Dadfarnia, S. Synthesis of new hydrogels based on pectin by electron beam irradiation with and without surface modification for methylene blue removal. J. Environ. Chem. Eng. 2019, 7, 102919. [Google Scholar] [CrossRef]
- Mokhtari, N.; Afshari, M.; Dinari, M. Synthesis and characterization of a novel fluorene-based covalent triazine framework as a chemical adsorbent for highly efficient dye removal. Polymer 2020, 195, 122430. [Google Scholar] [CrossRef]
- Khan, A.; Momina, M.; Siddiqui, M.R.; Otero, M.; Alshareef, S.A.; Rafatullah, M. Removal of Rhodamine B from Water Using a Solvent Impregnated Polymeric Dowex 5WX8 Resin: Statistical Optimization and Batch Adsorption Studies. Polymers 2020, 12, 500. [Google Scholar] [CrossRef]
- Saleh, T.A. Protocols for synthesis of nanomaterials, polymers, and green materials as adsorbents for water treatment technologies. Environ. Technol. Innov. 2021, 24, 101821. [Google Scholar] [CrossRef]
- Mok, C.F.; Ching, Y.C.; Muhamad, F.; Osman, N.A.A.; Dai Hai, N.; Hassan, C.R.C. Adsorption of Dyes Using Poly(vinyl alcohol) (PVA) and PVA-Based Polymer Composite Adsorbents: A Review. J. Polym. Environ. 2020, 28, 775–793. [Google Scholar] [CrossRef]
- Kong, Y.; Zhuang, Y.; Han, Z.; Yu, J.; Shi, B.; Han, K.; Hao, H. Dye removal by eco-friendly physically cross-linked double network polymer hydrogel beads and their functionalized composites. J. Environ. Sci. 2019, 78, 81–91. [Google Scholar] [CrossRef]
- Abid, Z.; Hakiki, A.; Boukoussa, B.; Launay, F.; Hamaizi, H.; Bengueddach, A.; Hamacha, R. Preparation of highly hydrophilic PVA/SBA-15 composite materials and their adsorption behavior toward cationic dye: Effect of PVA content. J. Mater. Sci. 2019, 54, 7679–7691. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Ollier, R.P.; Alvarez, V.A. Sorption behavior of polyvinyl alcohol/bentonite hydrogels for dyes removal. J. Polym. Res. 2019, 26, 142. [Google Scholar] [CrossRef]
- Sabarish, R.; Unnikrishnan, G. Polyvinyl alcohol/carboxymethyl cellulose/ZSM-5 zeolite biocomposite membranes for dye adsorption applications. Carbohydr. Polym. 2018, 199, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Lakouraj, M.M.; Kasirian, N. Development of effective nano-biosorbent based on poly-m-phenylenediamine grafted dextrin for removal of Pb(II) and methylene blue from water. Carbohydr. Polym. 2018, 201, 539–548. [Google Scholar] [CrossRef]
- Stejskal, J. Interaction of conducting polymers, polyaniline and polypyrrole, with organic dyes: Polymer morphology control, dye adsorption and photocatalytic decomposition. Chem. Pap. 2020, 74, 1–5. [Google Scholar] [CrossRef]
- Zeng, S.; Yang, J.; Qiu, X.Y.; Liang, Z.Y.; Zhang, Y.M. Magnetically recyclable MnFe2O4/polyaniline composite with enhanced visible light photocatalytic activity for rhodamine B degradation. J. Ceram. Soc. Jpn. 2016, 124, 1152–1156. [Google Scholar] [CrossRef]
- Cova, T.F.; Murtinho, D.; Aguado, R.; Pais, A.A.C.C.; Valente, A.J.M. Cyclodextrin Polymers and Cyclodextrin-Containing Polysaccharides for Water Remediation. Polysaccharides 2021, 2, 16–38. [Google Scholar] [CrossRef]
- Crini, G. Kinetic and equilibrium studies on the removal of cationic dyes from aqueous solution by adsorption onto a cyclodextrin polymer. Dye. Pigment. 2008, 77, 415–426. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, G.; Lu, J.; Chen, H.; Zhou, Y. PDA-cross-linked beta-cyclodextrin: A novel adsorbent for the removal of BPA and cationic dyes. Water Sci. Technol. 2020, 81, 2337–2350. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Li, M.M.; Ye, H.; Zhao, B.X. Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles. J. Hazard. Mater. 2012, 211, 366–372. [Google Scholar] [CrossRef]
- Ge, F.; Ye, H.; Li, M.M.; Zhao, B.X. Efficient removal of cationic dyes from aqueous solution by polymer-modified magnetic nanoparticles. Chem. Eng. J. 2012, 198, 11–17. [Google Scholar] [CrossRef]
- Liu, A.; Wang, C.C.; Wang, C.Z.; Fu, H.F.; Peng, W.; Cao, Y.L.; Chu, H.Y.; Du, A.F. Selective adsorption activities toward organic dyes and antibacterial performance of silver-based coordination polymers. J. Colloid Interface Sci. 2018, 512, 730–739. [Google Scholar] [CrossRef]
- Yeamin, M.B.; Islam, M.M.; Chowdhury, A.N.; Awual, M.R. Efficient encapsulation of toxic dyes from wastewater using several biodegradable natural polymers and their composites. J. Clean. Prod. 2021, 291, 125920. [Google Scholar] [CrossRef]
- Ramazani, A.; Oveisi, M.; Sheikhi, M.; Gouranlou, F. Natural Polymers as environmental Friendly Adsorbents for Organic Pollutants such as Dyes Removal from Colored Wastewater. Curr. Org. Chem. 2018, 22, 1297–1306. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, N.; Du, M.; Zhu, H.; Ke, T. Preparation of bio-absorbents by modifying licorice residue via chemical methods and removal of copper ions from wastewater. Water Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Vidovix, T.B.; Quesada, H.B.; Bergamasco, R.; Vieira, M.F.; Vieira, A.M.S. Adsorption of Safranin-O dye by copper oxide nanoparticles synthesized from Punica granatum leaf extract. Environ. Technol. 2021, 2021, 1–17. [Google Scholar] [CrossRef]
- Sebeia, N.; Jabli, M.; Ghith, A.; El-Ghoul, Y.; Alminderej, F.M. Production of cellulose from Aegagropila Linnaei macro-algae: Chemical modification, characterization and application for the bio-sorption of cationic and anionic dyes from water. Int. J. Biol. Macromol. 2019, 135, 152–162. [Google Scholar] [CrossRef]
- Sebeia, N.; Jabli, M.; Ghith, A.; El-Ghoul, Y.; Alminderej, F.M. Populus tremula, Nerium oleander and Pergularia tomentosa seed fibers as sources of cellulose and lignin for the bio-sorption of methylene blue. Int. J. Biol. Macromol. 2019, 121, 655–665. [Google Scholar] [CrossRef]
- Ammar, C.; El-Ghoul, Y.; Jabli, M. Characterization and valuable use of Calotropis gigantea seedpods as a biosorbent of methylene blue. Int. J. Phytoremediation 2021, 23, 1085–1094. [Google Scholar] [CrossRef]
- Kayan, G.Ö.; Kayan, A. Composite of Natural Polymers and Their Adsorbent Properties on the Dyes and Heavy Metal Ions. J. Polym. Environ. 2021. [Google Scholar] [CrossRef]
- Almutairi, F.M.; El-Ghoul, Y.; Jabli, M. Extraction of Cellulose Polymeric Material from Populus tremula Fibers: Characterization and Application to the Adsorption of Methylene Blue and Crystal Violet. Polymers 2021, 13, 3334. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, M.; Ahmadpoor, F.; Nasrollahzadeh, M.; Ghafuri, H. Lignin-derived (nano) materials for environmental pollution remediation: Current challenges and future perspectives. Int. J. Biol. Macromol. 2021, 178, 394–423. [Google Scholar] [CrossRef]
- Yadav, S.; Asthana, A.; Chakraborty, R.; Jain, B.; Singh, A.K.; Carabineiro, S.A.C.; Susan, M.A.B.H. Cationic Dye Removal Using Novel Magnetic/Activated Charcoal/β-Cyclodextrin/Alginate Polymer Nanocomposite. Nanomaterials 2020, 10, 170. [Google Scholar] [CrossRef]
- Bhangi, B.K.; Ray, S.K. Nano silver chloride and alginate incorporated composite copolymer adsorbent for adsorption of a synthetic dye from water in a fixed bed column and its photocatalytic reduction. Int. J. Biol. Macromol. 2020, 144, 801–812. [Google Scholar] [CrossRef]
- Agougui, H.; Sebeia, N.; Jabli, M.; El-Ghoul, Y.; Boughzala, B. Synthesis of hydroxyapatite-sodium metasilicate via double decomposition method: Characterization and application to the removal of methylene blue. Inorg. Chem. Commun. 2021, 133, 108986. [Google Scholar] [CrossRef]
- Samadder, R.; Akter, N.; Roy, A.C.; Uddin, M.M.; Hossen, M.J.; Azam, M.S. Magnetic nanocomposite based on polyacrylic acid and carboxylated cellulose nanocrystal for the removal of cationic dye. RSC Adv. 2020, 10, 11945–11956. [Google Scholar] [CrossRef]
- Sankararamakrishnan, N.; Singh, N.; Srivastava, I. Hierarchical nano Fe(0)@FeS doped cellulose nanofibres derived from agrowaste-potential bionanocomposite for treatment of organic dyes. Int. J. Biol. Macromol. 2020, 151, 713–722. [Google Scholar] [CrossRef]
- Yu, Z.; Hu, C.; Dichiara, A.B.; Jiang, W.; Gu, J. Cellulose nanofibril/carbon nanomaterial hybrid aerogels for adsorption removal of cationic and anionic organic dyes. Nanomaterials 2020, 10, 169. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Elgarhy, G.S.; El-Subruiti, G.M.; Omer, A.M. Carboxymethyl cellulose/carboxylated graphene oxide composite microbeads for efficient adsorption of cationic methylene blue dye. Int. J. Biol. Macromol. 2020, 154, 307–318. [Google Scholar] [CrossRef]
- Sayğılı, G.A.; Güzel, F. Chemical Modification of a Cellulose-Based Material to Improve Its Adsorption Capacity for Anionic Dyes. J. Dispers. Sci. Technol. 2017, 38, 381–392. [Google Scholar] [CrossRef]
- Salimpour, S.A.; Malek, R.M.A.; Mazaheri, F. Dye adsorption of cotton fabric grafted with PPI dendrimers: Isotherm and kinetic studies. J. Environ. Manag. 2015, 163, 53–61. [Google Scholar] [CrossRef]
- Obradith, C.; Jency, D.R.; Andrés, M. Adsorption of Common Laboratory Dyes Using Natural Fibers from Luffa cylindrica. J. Chem. Educ. 2018, 95, 2233–2237. [Google Scholar] [CrossRef]
- Grabi, H.; Derridj, F.; Lemlikchi, W.; Guénin, E. Studies of the potential of a native natural biosorbent for the elimination of an anionic textile dye Cibacron Blue in aqueous solution. Sci. Rep. 2021, 11, 9705. [Google Scholar] [CrossRef]
- Rigueto, C.V.T.; Piccin, J.S.; Dettmer, A.; Rosseto, M.; Dotto, G.L.; de Oliveira Schmitz, A.P.; Perondi, D.; de Freitas, T.S.M.; Loss, R.A.; Geraldi, C.A.Q. Water hyacinth (Eichhornia crassipes) roots, an amazon natural waste, as an alternative biosorbent to uptake a reactive textile dye from aqueous solutions. Ecol. Eng. 2020, 150, 105817. [Google Scholar] [CrossRef]
- Boumaza, S.; Yenounne, A.; Hachi, W.; Kaouah, F.; Bouhamidi, Y.; Trari, M. Application of Typha angustifolia (L.) Dead Leaves Waste as Biomaterial for the Removal of Cationic Dye from Aqueous Solution. Int. J. Environ. Res. 2018, 12, 561–573. [Google Scholar] [CrossRef]
- Jabli, M.; Gamha, E.; Sebeia, N.; Hamdaoui, M. Almond shell waste (Prunus dulcis): Functionalization with [dimethy-diallyl-ammonium-chloride-diallylamin-co-polymer] and chitosan polymer and its investigation in dye adsorption. J. Mol. Liq. 2017, 240, 35–44. [Google Scholar] [CrossRef]
- Khodabandehloo, A.; Shayesteh, H.; Rahbar-Kelishami, A. Methylene blue removal using Salix babylonica (Weeping willow) leaves powder as a low-cost biosorbent in batch mode: Kinetic, equilibrium, and thermodynamic studies. J. Mol. Liq. 2017, 244, 540–548. [Google Scholar] [CrossRef]
- Abdelkarim, S.; Mohammed, H.; Nouredine, B. Sorption of Methylene Blue Dye from Aqueous Solution Using an Agricultural Waste. Trends Green Chem. 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Ammar, C.; Alminderej, F.M.; EL-Ghoul, Y.; Jabli, M.; Shafiquzzaman, M. Preparation and Characterization of a New Polymeric Multi-Layered Material Based K-Carrageenan and Alginate for Efficient Bio-Sorption of Methylene Blue Dye. Polymers 2021, 13, 411. [Google Scholar] [CrossRef]
- Nascimento, G.E.; Campos, N.F.; Silva, J.J.; Barbosa, C.M.B.M.; Duarte, M.M.M.B. Adsorption of anionic dyes from an aqueous solution by banana peel and green coconut mesocarp. Desalination Water Treat. 2016, 57, 14093–14108. [Google Scholar] [CrossRef]
- N’diaye, A.D.; Ali, Y.A.E.H.; Abdallahi, O.E.M.; Bollahi, M.A.; Stitou, M.; Kankou, M.; Fahmi, D. Sorption of Malachite Green from Aqueous Solution using Typha australis Leaves as a Low Cost Sorbent. J. Environ. Treat. Tech. 2020, 8, 1023–1028. [Google Scholar]
- Salazar-Rabago, J.J.; Leyva-Ramos, R.; Rivera-Utrilla, J.; Ocampo-Perez, R.; Cerino-Cordova, F.J. Biosorption mechanism of Methylene Blue from aqueous solution onto white pine (Pinus durangensis) sawdust: Effect of operating conditions. Sustain. Environ. Res. 2016, 27, 32–40. [Google Scholar] [CrossRef]
- Rahmat, N.A.; Ali, A.A.; Salmiati, H.N.; Muhamad, M.S.; Kristanti, R.A.; Hadibarata, T. Removal of Remazol Brilliant Blue R from Aqueous Solution by Adsorption Using Pineapple Leaf Powder and Lime Peel Powder. Water Air Soil Pollut. 2016, 227, 105. [Google Scholar] [CrossRef]
- Lazim, Z.M.; Mazuin, E.; Hadibarata, T.; Yusop, Z. The removal of methylene blue and Remazol Brilliant Blue R dyes by using orange peel and spent tea leaves. J. Teknol. 2015, 74, 129–135. [Google Scholar] [CrossRef][Green Version]
- Mafra, M.R.; Igarashi-Mafra, L.; Zuim, D.R.; Vasques, E.C.; Ferreira, M.A. Adsorption of Remazol Brilliant Blue on an orange peel adsorbent. Braz. J. Chem. Eng. 2013, 30, 657–665. [Google Scholar] [CrossRef]
- Pelosi, B.T.; Lima, L.K.S.; Vieira, M.G.A. Removal of the synthetic dye Remazol Brilliant Blue R from textile industry wastewaters by biosorption on the macrophyte Salvinia natans. Braz. J. Chem. Eng. 2014, 31, 1035–1045. [Google Scholar] [CrossRef]
- Jawad, A.H.; Razuan, R.; Appaturi, J.N.; Wilson, L.D. Adsorption and mechanism study for methylene blue dye removal with carbonized watermelon (Citrullus lanatus) rind prepared via one-step liquid phase H2SO4 activation. Surf. Interfaces 2019, 16, 76–84. [Google Scholar] [CrossRef]
- Lakshmipathy, R.; Reddy, N.A.; Sarada, N.C. Optimization of brilliant green biosorption by native and acid-activated watermelon rind as low-cost adsorbent. Desalination Water Treat. 2015, 54, 235–244. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Ahmad, N.; Bello, O.S. Removal of remazol brilliant blue reactive dye from aqueous solutions using watermelon rinds as adsorbent. J. Dispers. Sci. Technol. 2015, 36, 845–858. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Ahmad, N.; Bello, O.S. Statistical optimization of adsorption process for removal of synthetic dye using watermelon rinds. Model. Earth Syst. Environ. 2017, 3, 25. [Google Scholar] [CrossRef]
- Masoudian, N.; Rajabi, M.; Ghaedi, M. Titanium oxide nanoparticles loaded onto activated carbon prepared from bio-waste watermelon rind for the efficient ultrasonic-assisted adsorption of congo red and phenol red dyes from wastewaters. Polyhedron 2019, 173, 114105. [Google Scholar] [CrossRef]
- Chigbundu, E.C.; Adebowale, K.O. Equilibrium and fractal-like kinetic studies of the sorption of acid and basic dyes onto watermelon shell (Citrullus vulgaris). Cellulose 2017, 24, 4701–4714. [Google Scholar] [CrossRef]
- Silva, F.; Nascimento, L.; Brito, M.; da Silva, K.; Paschoal, W.; Fujiyama, R. Biosorption of Methylene Blue Dye Using Natural Biosorbents Made from Weeds. Materials 2019, 12, 2486. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Lata, S.; Balasubramanian, P. Biosorption characteristics of methylene blue and malachite green from simulated wastewater onto Carica papaya wood biosorbent. Surf. Interfaces 2018, 10, 197–215. [Google Scholar] [CrossRef]
- Honorato, A.C.; Machado, J.M.; Celante, G.; Borges, W.G.P.; Dragunski, D.C.; Caetano, J. Biosorption of methylene blue using agroindustrial residues. Rev. Bras. Eng. Agric. Amb. 2015, 19, 705–710. [Google Scholar] [CrossRef]
- Alfredo, A.P.C.; Gonçalves, G.C.; Lobo, V.S.; Montanher, S.F. Adsorção de azul de metileno em casca de batata utilizando sistemas em batelada e coluna de leito fixo. Rev. Virtual Quim. 2015, 7, 1909–1920. [Google Scholar] [CrossRef]
- Miraboutalebi, S.M.; Nikouzad, S.K.; Peydayesh, M.; Allahgholi, N.; Vafajoo, L.; McKay, G. Methylene blue adsorption via maize silk powder: Kinetic, equilibrium, thermodynamic studies and residual error analysis. Process. Saf. Environ. Prot. 2017, 106, 191–202. [Google Scholar] [CrossRef]
- Tang, Y.F.; Hu, T.; Zeng, Y.D.; Zhou, Q.; Peng, Y.Z. Effective adsorption of cationic dyes by lignin sulfonate polymer based on simple emulsion polymerization: Isotherm and kinetic studies. RSC Adv. 2015, 5, 3757–3766. [Google Scholar] [CrossRef]
- Tang, Y.; Zeng, Y.; Hu, T.; Zhou, Q.; Peng, Y. Preparation of lignin sulfonate-based mesoporous materials for adsorbing malachite green from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 2900–2910. [Google Scholar] [CrossRef]
- Afshariani, F.; Roosta, A. Experimental study and mathematical modeling of biosorption of methylene blue from aqueous solution in a packed bed of microalgae Scenedesmus. J. Clean. Prod. 2019, 225, 133–142. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Rahmanian, O.; Fazlzadeh, M.; Heidari, M. Enhancement of methylene blue adsorption onto activated carbon prepared from Date Press Cake by low frequency ultrasound. J. Mol. Liq. 2018, 264, 591–599. [Google Scholar] [CrossRef]
- Islam, M.A.; Sabar, S.; Benhouria, A.; Khanday, W.A.; Asif, M.; Hameed, B.H. Nanoporous activated carbon prepared from karanj (Pongamia pinnata) fruit hulls for methylene blue adsorption. J. Taiwan Inst. Chem. Eng. 2017, 74, 96–104. [Google Scholar] [CrossRef]
- Islam, M.A.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous activated carbon prepared from NaOH activation of rattan (Lacosperma secundiflorum) hydrochar for methylene blue removal. Ecotoxicol. Environ. Saf. 2017, 138, 279–285. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, H.M. Removal of methylene blue and phenol onto prepared activated carbon from Fox nutshell by chemical activation in batch and fixed-bed column. J. Clean. Prod. 2016, 137, 1246–1259. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Abdallah, R.; Yaseen, Z.M. Zwitterion composite chitosan-epichlorohydrin/zeolite for adsorption of methylene blue and reactive red 120 dyes. Int. J. Biol. Macromol. 2020, 163, 756–765. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, X.; Lou, T. Preparation of millimeter-sized chitosan/carboxymethyl cellulose hollow capsule and its dye adsorption properties. Carbohydr. Polym. 2020, 244, 116481. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zheng, H.; Xiong, Z.; Zhao, R.; Liu, Y.; Zhao, C.; Zheng, C. Novel anionic polyacrylamide-modify-chitosan magnetic composite nanoparticles with excellent adsorption capacity for cationic dyes and pH-independent adsorption capability for metal ions. Chem. Eng. J. 2020, 392, 123706. [Google Scholar] [CrossRef]
- Jawad, A.H.; Mubarak, N.S.A.; Abdulhameed, A.S. Hybrid Crosslinked Chitosan-Epichlorohydrin/TiO2 Nanocomposite for Reactive Red 120 Dye Adsorption: Kinetic, Isotherm, Thermodynamic, and Mechanism Study. J. Polym. Environ. 2020, 28, 624–637. [Google Scholar] [CrossRef]
- Chen, Y.; Long, W.; Xu, H. Efficient removal of Acid Red 18 from aqueous solution by in-situ polymerization of polypyrrole-chitosan composites. J. Mol. Liq. 2019, 287, 110888. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Mastuli, M.S. Mesoporous Crosslinked Chitosan-Activated Charcoal Composite for the Removal of Thionine Cationic Dye: Comprehensive Adsorption and Mechanism Study. J. Polym. Environ. 2020, 28, 1095–1105. [Google Scholar] [CrossRef]
- Mohammad, A.T.; Abdulhameed, A.S.; Jawad, A.H. Box-Behnken design to optimize the synthesis of new crosslinked chitosan-glyoxal/TiO2 nanocomposite: Methyl orange adsorption and mechanism studies. Int. J. Biol. Macromol. 2019, 129, 98–109. [Google Scholar] [CrossRef]
- Li, Z.; Sellaoui, L.; Dotto, G.L.; Lamine, A.B.; Bonilla-Petriciolet, A.; Hanafy, H.; Erto, A. Interpretation of the adsorption mechanism of Reactive Black 5 and Ponceau 4R dyes on chitosan/polyamide nanofibers via advanced statistical physics model. J. Mol. Liq. 2019, 285, 165–170. [Google Scholar] [CrossRef]
- Zeng, M.; Wu, W.; Fang, J.; Li, S.; Zhou, Z. Fabrication of chitosan/alginate porous sponges as adsorbents for the removal of acid dyes from aqueous solution. J. Mater. Sci. 2019, 54, 9995–10008. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; Lou, T. Preparation of fibrous chitosan/sodium alginate composite foams for the adsorption of cationic and anionic dyes. J. Hazard. Mater. 2020, 403, 124054. [Google Scholar] [CrossRef]
- Wang, Q.; Ju, J.; Tan, Y.; Hao, L.; Ma, Y.; Wu, Y.; Zhang, H.; Xia, Y.; Sui, K. Controlled synthesis of sodium alginate electrospun nanofiber membranes for multi-occasion adsorption and separation of methylene blue. Carbohydr. Polym. 2019, 205, 125–134. [Google Scholar] [CrossRef]
- Jiao, C.; Li, T.; Wang, J.; Wang, H.; Zhang, X.; Han, X.; Zhaofang, D.; Yali, S.; Yuyue, C. Efficient removal of dyes from aqueous solution by a porous sodium alginate/gelatin/graphene oxide triple-network composite aerogel. J. Polym. Environ. 2020, 28, 1492–1502. [Google Scholar] [CrossRef]
- Ma, M.; Liu, Z.; Hui, L.; Shang, Z.; Yuan, S.; Dai, L.; Liu, P.; Liu, X.; Ni, Y. Lignin-containing cellulose nanocrystals/sodium alginate beads as highly effective adsorbents for cationic organic dyes. Int. J. Biol. Macromol. 2019, 139, 640–646. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.; Drumm, F.C.; Grassi, P.; Schadeck Netto, M.; Allasia, D.; Dotto, G.L. Paddle cactus (Tacinga palmadora) as potential low-cost adsorbent to treat textile effluents containing crystal violet. Chem. Eng. Commun. 2020, 207, 1368–1379. [Google Scholar] [CrossRef]
- Pang, X.; Sellaoui, L.; Franco, D.; Dotto, G.L.; Georgin, J.; Bajahzar, A.; Belmabrouk, H.; Lamine, A.B.; Bonilla-Petriciolet, A.; Li, Z. Adsorption of crystal violet on biomasses from pecan nutshell, para chestnut husk, araucaria bark and palm cactus: Experimental study and theoretical modeling via monolayer and double layer statistical physics models. Chem. Eng. J. 2019, 378, 122101. [Google Scholar] [CrossRef]
- Louati, I.; Fersi, M.; Hadrich, B. Prickly pear cactus cladodes powder of Opuntia ficus indica as a cost effective biosorbent for dyes removal from aqueous solutions. 3 Biotech 2018, 8, 478. [Google Scholar] [CrossRef]
- El Maguana, Y.; Elhadiri, N.; Bouchdoug, M.; Benchanaa, M.; Jaouad, A. Activated carbon from prickly pear seed cake: Optimization of preparation conditions using experimental design and its application in dye removal. Int. J. Chem. Eng. 2019, 2019, 8621951. [Google Scholar] [CrossRef]
- Pelaez-Cid, A.A.; Herrera-González, A.M.; Salazar-Villanueva, M.; Bautista-Hernandez, A. Elimination of textile dyes using activated carbons prepared from vegetable residues and their characterization. J. Environ. Manag. 2016, 181, 269–278. [Google Scholar] [CrossRef]
- Kim, H.R.; Jang, J.W.; Park, J.W. Carboxymethyl chitosan-modified magnetic-cored dendrimer as an amphoteric adsorbent. J. Hazard. Mater. 2016, 317, 608–616. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Joneidi-Yekta, H.; Mohseni, M. Adsorption of anionic and cationic dyes from aqueous solution using gelatin-based magnetic nanocomposite beads comprising carboxylic acid functionalized carbon nanotube. Chem. Eng. J. 2017, 308, 1133–1144. [Google Scholar] [CrossRef]
- Yang, D.; Qiu, L.; Yang, Y. Efficient adsorption of methyl orange using a modified chitosan magnetic composite adsorbent. J. Chem. Eng. Data 2016, 61, 3933–3940. [Google Scholar] [CrossRef]
- Sahraei, R.; Sekhavat-Pour, Z.; Ghaemy, M. Novel magnetic bio-sorbent hydrogel beads based on modified gum tragacanth/graphene oxide: Removal of heavy metals and dyes from water. J. Clean. Prod. 2017, 142, 2973–2984. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Mosallanezhad, A. Facile and green rout to prepare magnetic and chitosan-crosslinked κ-carrageenan bionanocomposites for removal of methylene blue. J. Water Process. Eng. 2016, 10, 143–155. [Google Scholar] [CrossRef]
- Soares, S.F.; Simões, T.R.; Trindade, T.; Daniel-da-Silva, A.L. Highly efficient removal of dye from water using magnetic carrageenan/silica hybrid nano-adsorbents. Water Air Soil Pollut. 2017, 228, 87. [Google Scholar] [CrossRef]
- Gulnaz, O.; Sahmurova, A.; Kama, S. Removal of reactive red 198 from aqueous solution by Potamogeton crispus. Chem. Eng. J. 2011, 174, 579–585. [Google Scholar] [CrossRef]
- Demarchi, C.A.; Debrassi, A.; Buzzi, F.C.; Nedelko, N.; Ślawska-Waniewska, A.; Dłużewski, P.; Magro, J.D.; Scapinello, J.; Rodrigues, C.A. Adsorption of the dye Remazol Red 198 (RR198) by O-carboxymethylchitosan-N-lauryl/γ-Fe2O3 magnetic nanoparticles. Arab. J. Chem. 2019, 12, 3444–3453. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mostafapour, F.K.; Mahvi, A.H. Decolorization of reactive red 198 by adsorption onto ZnCl2 activated pistachio hull wastes. Int. J. Environ. Health Eng. 2014, 3, 38–45. [Google Scholar] [CrossRef]
- Malakootian, M.; Mansoorian, H.J.; Hosseini, A.; Khanjani, N. Evaluating the efficacy of alumina/carbon nanotube hybrid adsorbents in removing Azo Reactive Red 198 and Blue 19 dyes from aqueous solutions. Process. Saf. Environ. Prot. 2015, 96, 125–137. [Google Scholar] [CrossRef]
- Tayebi, H.; Dalirandeh, Z.; Rad, A.S.; Mirabi, A. Synthesis of polyaniline/Fe3O4 magnetic nanoparticles for removal of reactive red 198 from textile waste water: Kinetic, isotherm, and thermodynamic studies. Desalination Water Treat. 2016, 57, 22551–22563. [Google Scholar] [CrossRef]
- Elkady, M.F.; Ibrahim, A.M.; El-Latif, M.M.A. Assessment of the adsorption kinetics, equilibrium and thermodynamic for the potential removal of reactive red dye using eggshell biocomposite beads. Desalination 2011, 278, 412–423. [Google Scholar] [CrossRef]
- Alimohammadi, Z.; Younesi, H.; Bahramifar, N. Batch and column adsorption of reactive red 198 from textile industry effluent by microporous activated carbon developed from walnut shells. Waste Biomass Valorization 2016, 7, 1255–1270. [Google Scholar] [CrossRef]
- Toprak, F.; Armagan, B.; Cakici, A. Systematic approach for the optimal process conditions of Reactive Red 198 adsorption by pistachio nut shell using Taguchi method. Desalination Water Treat. 2012, 48, 96–105. [Google Scholar] [CrossRef]
- Haffad, H.; Zbair, M.; Anfar, Z.; Ahsaine, H.A.; Bouhlal, H.; Khallok, H. Removal of reactive red-198 dye using chitosan as an adsorbent: Optimization by Central composite design coupled with response surface methodology. Toxin Rev. 2019, 40, 225–237. [Google Scholar] [CrossRef]
- EL-Ghoul, Y.; Ammar, C.; Alminderej, F.M.; Shafiquzzaman, M. Design and Evaluation of a New Natural Multi-Layered Biopolymeric Adsorbent System-Based Chitosan/Cellulosic Nonwoven Material for the Biosorption of Industrial Textile Effluents. Polymers 2021, 13, 322. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Peng, H.; Wang, Z.; Wu, J.; Liu, Z. Dye Adsorption from Aqueous Solution by Cellulose/chitosan Composite: Equilibrium, Kinetics, and Thermodynamics. Fibers Polym. 2018, 19, 340–349. [Google Scholar] [CrossRef]
- Azari, A.; Noorisepehr, M.; Dehganifard, E.; Karimyan, K.; Hashemi, S.Y.; Kalhori, E.M.; Norouzi, R.; Agarwal, S.; Gupta, V.K. Experimental Design, Modeling and Mechanism of Cationic Dyes Biosorption on to Magnetic Chitosan-lutaraldehyde Composite. Int. J. Biol. Macromol. 2019, 131, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U.; Siddique, T.A.; Talebian, S.; Lee, J.J.L.; Salleh, A.; Ang, B.C.; Afifi, A.M. Effect of Deacetylation on Property of Electrospun Chitosan/PVA Nanofibrous Membrane and Removal of Methyl Orange, Fe(III) and Cr(VI) ions. Carbohydr. Polym. 2017, 177, 32–39. [Google Scholar] [CrossRef]
- Anitha, T.; Kumar, S.P.; Kumar, S.K. Synthesis of Nano-sized Nhitosan Blended Polyvinyl Alcohol for the Removal of Eosin Yellow Dye from Aqueous Solution. J. Water Process. Eng. 2016, 13, 127–136. [Google Scholar] [CrossRef]
- Muinde, V.M.; Onyari, J.M.; Wamalwa, B.; Wabomba, J.N. Adsorption of Malachite Green Dye from Aqueous Solutions using Mesoporous Chitosan–zinc oxide Composite Material. Environ. Chem. Ecotoxicol. 2020, 2, 115–125. [Google Scholar] [CrossRef]
- Insulation Coatings Market by Type (Acrylic, Polyurethane, Epoxy, Mullite, YSZ), End-Use Industry (Industrial, Building, and Construction, Aerospace, Automotive, Marine), and Region (North America, South America, Europe, APAC, MEA)—Global Forecast to 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/insulation-coatings-market-26484290.html (accessed on 1 December 2021).
- Che, K.; Lyu, P.; Wan, F.; Ma, M. Investigations on Aging Behavior and Mechanism of Polyurea Coating in Marine Atmosphere. Materials 2019, 12, 3636. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wang, X.; Ji, C.; Liu, Q.; Gao, Z.; Zhang, K.; Zhao, C. Experimental and numerical simulation study on polyurea-coated fuel tank subjected to combined action of blast shock waves and fragments. Thin-Walled Struct. 2021, 169, 108436. [Google Scholar] [CrossRef]
- Ma, L.; Dong, X.; Chen, M.; Zhu, L.; Wang, C.; Yang, F.; Dong, Y. Fabrication and Water Treatment Application of Carbon Nanotubes (CNTs)-Based Composite Membranes: A Review. Membranes 2017, 7, 16. [Google Scholar] [CrossRef]
- García-Picazo, F.J.; Pérez-Sicairos, S.; Fimbres-Weihs, G.A.; Lin, S.W.; Salazar-Gastélum, M.I.; Trujillo-Navarrete, B. Preparation of Thin-Film Composite Nanofiltration Membranes Doped with N- and Cl-Functionalized Graphene Oxide for Water Desalination. Polymers 2021, 13, 1637. [Google Scholar] [CrossRef] [PubMed]
- Agboola, O.; Fayomi, O.S.I.; Ayodeji, A.; Ayeni, A.O.; Alagbe, E.E.; Sanni, S.E.; Okoro, E.E.; Moropeng, L.; Sadiku, R.; Kupolati, K.W.; et al. A Review on Polymer Nanocomposites and Their Effective Applications in Membranes and Adsorbents for Water Treatment and Gas Separation. Membranes 2021, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Amari, A.; Alzahrani, F.M.; Katubi, K.M.; Alsaiari, N.S.; Tahoon, M.A.; Rebah, F.B. Clay-Polymer Nanocomposites: Preparations and Utilization for Pollutants Removal. Materials 2021, 14, 1365. [Google Scholar] [CrossRef]
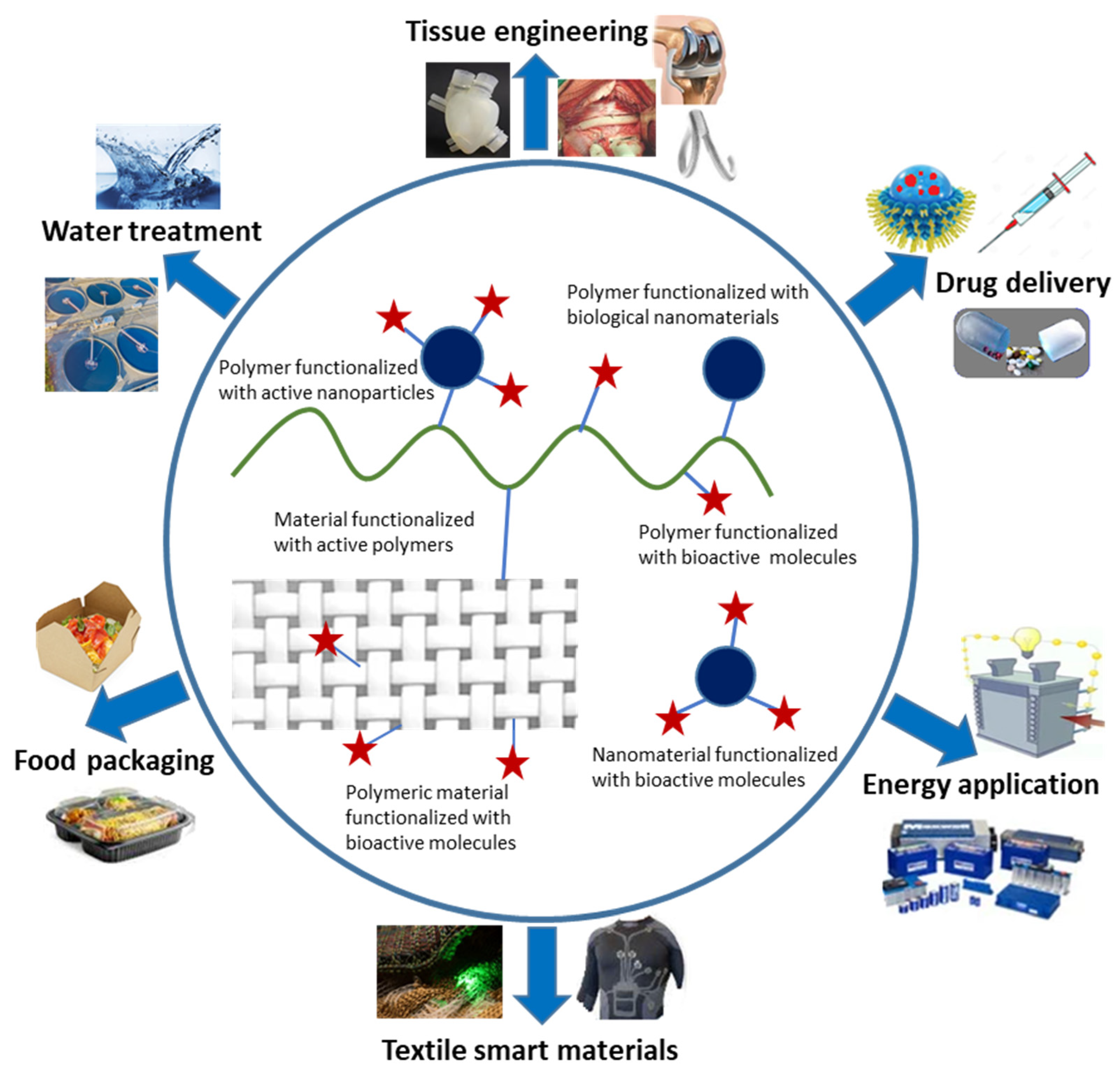
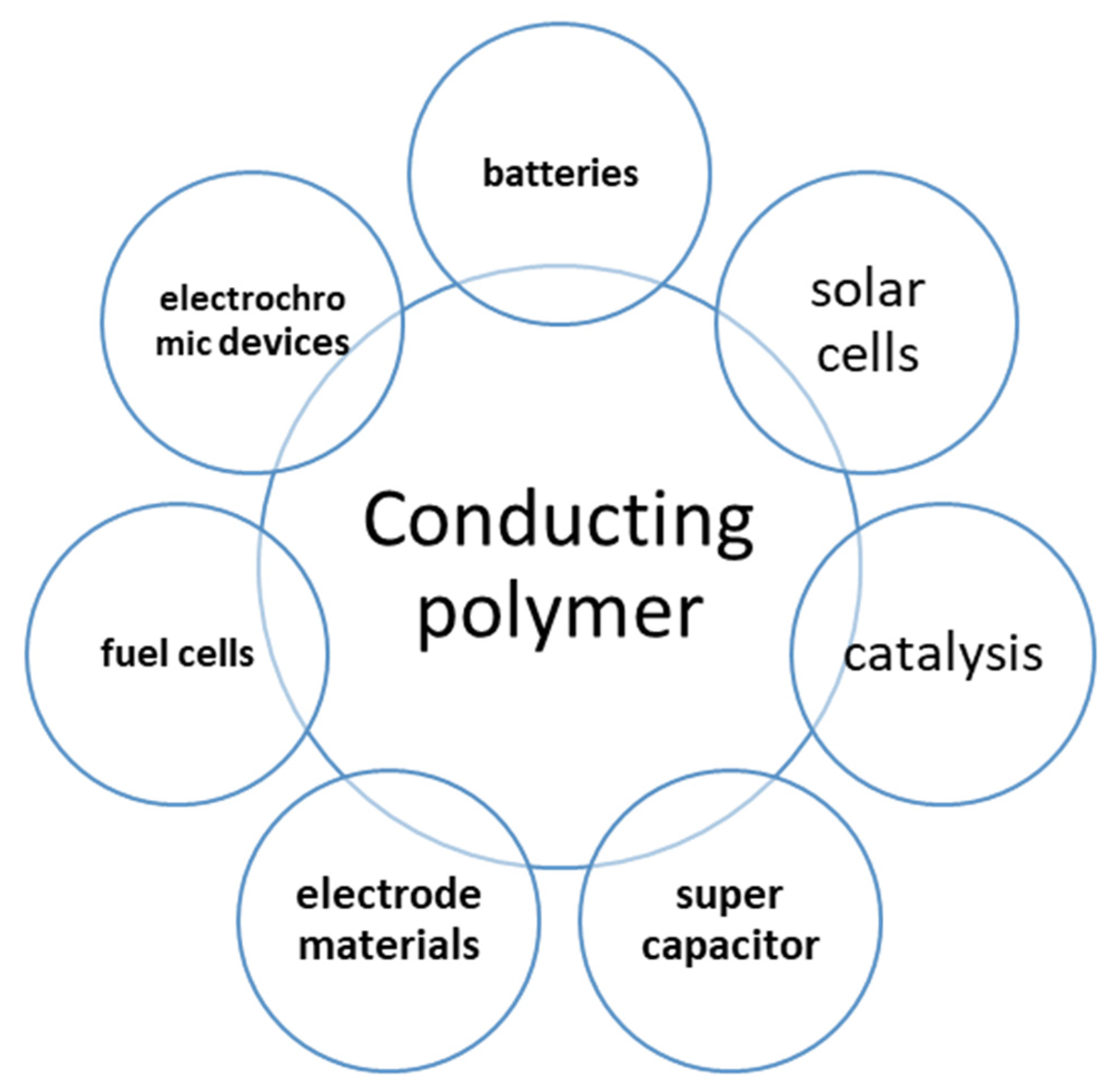

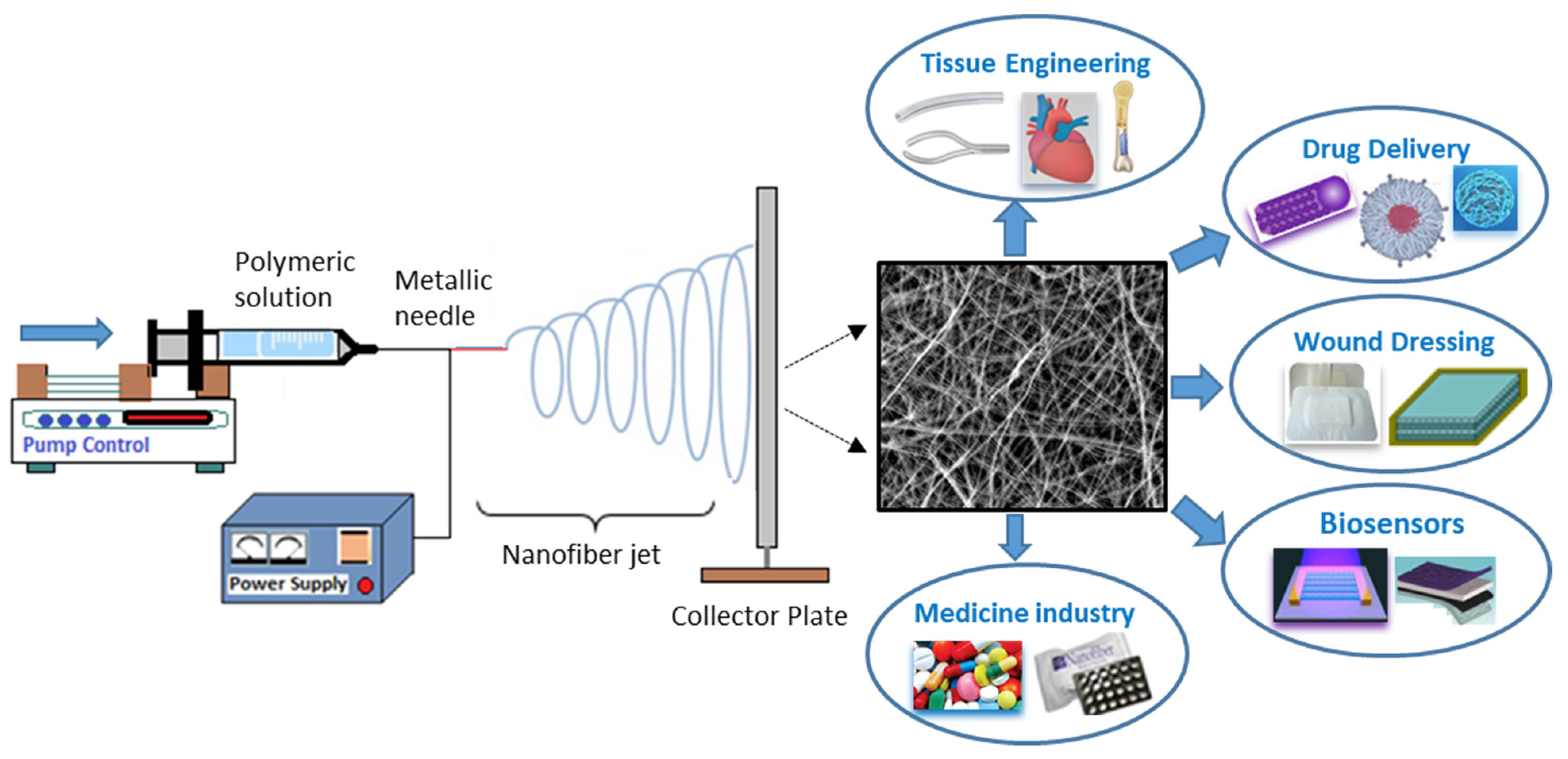
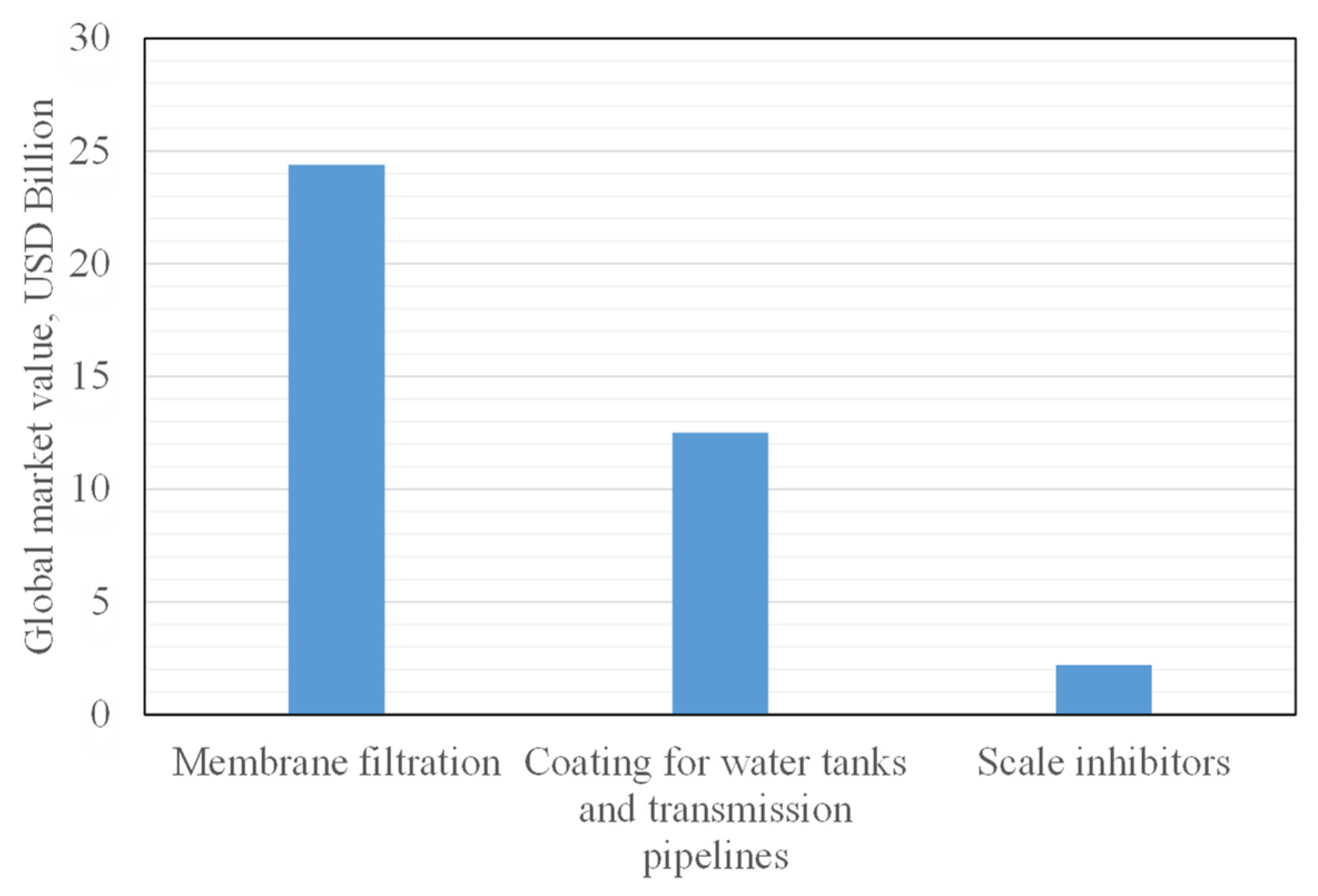
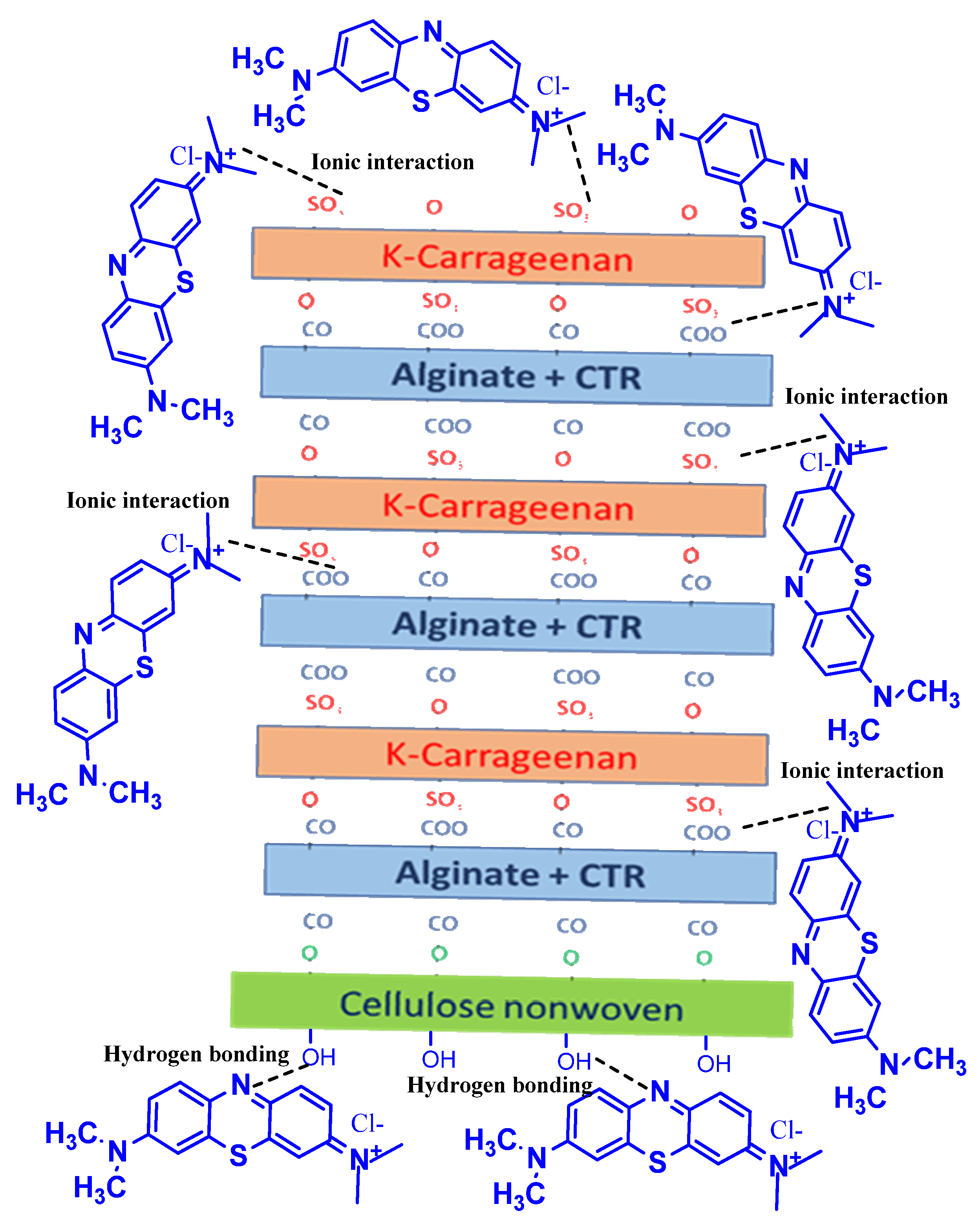
| Adsorbent | Adsorbate | qt (mg·g−1) | Adsorption Efficiency (%) | References |
|---|---|---|---|---|
| Luffa Cylindrica fibers | Malachite green/MB | 63/52 | 96.72/94.40 | [322] |
| Bean peel | Cibaron Blue | 5.72 | 95.31 | [323] |
| Eichhornia crassipes roots | 4B red reactive | 43.28 | 95 | [324] |
| Dead Typha angustifolia (L.) leaves | MB | 106.75 | 89.83 | [325] |
| Almond shell | MB | 84.9 | [326] | |
| Salix babylonica leaves | MB | 60.97 | [327] | |
| Prickly (peel) bark of cactus fruit | MB | 222 | [328] | |
| k-carrageenan/alginate/cellulose PEM | MB | 522.4 | 98.6 | [329] |
| Coconut mesocarp | Remazol golden yellow | 6.8 | 94.9 | [330] |
| Reactive gray BF-2R | 4.8 | 100 | ||
| Reactive turquoise Q-G125 | 4.1 | 96.6 | ||
| Banana peels | Remazol golden yellow | 2.5 | 70.2 | [330] |
| Reactive gray BF-2R | 1.2 | 75.4 | ||
| Reactive turquoise Q-G125 | 1.6 | 100 | ||
| Typha australis Leaves | Malachite green | 85.21 | - | [331] |
| white pine (Pinus durangensis) sawdust | MB | 87 | 86 | [332] |
| pineapple (Ananas comosus) leaf | Remazol Brilliant Blue R | 9.66 | 96.20 | [333] |
| Lime (Citrus aurantifolia) peel | Remazol Brilliant Blue R | 9.58 | 95.89 | [333] |
| Coconut bunch | Remazol Brilliant Blue R | 9.48 | 94.76 | [333] |
| Coconut fiber | Remazol Brilliant Blue R | 9.55 | 95.48 | [333] |
| Chili seeds | Remazol Brilliant Blue R | 9.40 | 93.97 | [333] |
| Guava leaves | Remazol Brilliant Blue R | 9.41 | 94.09 | [333] |
| Coconut residue | Remazol Brilliant Blue R | 9.38 | 93.85 | [333] |
| Jackfruit peels | Remazol Brilliant Blue R | 9.23 | 92.26 | [333] |
| Orange peel | Remazol Brilliant Blue R | - | 11.4 | [334,335] |
| Spent tea leaves | Remazol Brilliant Blue R | 9.7 | - | [334] |
| Salvinia natans | Remazol Brilliant Blue R | 61.9 | - | [336] |
| Durian peel | Remazol Brilliant Blue R | - | 14.9 | [336] |
| watermelon rind | MB | 200 | 99 | [337] |
| Brilliant green | 188.6 | 98 | [338] | |
| Remazol Brilliant Blue R | 333.33 | 92–97 | [339,340] | |
| Congo red CR | 17 | 100 | [341] | |
| Orange G | 27 | 85 | [342] | |
| Cyanthilium cinereum L. H. Rob weeds | MB | 76.336 | 95 | [343] |
| Paspalum maritimum (PMT) | MB | 56.179 | 97 | [343] |
| Carica papaya wood | MB | 32.25 | [344] | |
| pupunha palm | MB | 78.989 | [345] | |
| Potato shell | MB | 48.7 | [346] | |
| Scenedesmus | MB | 61.69 | [346] | |
| Maize silk powder | MB | 132.1 | [347] | |
| Lignin sulfonate polymer | Malachite green | 27.4 | 60 | [348] |
| Lignin sulfonate-g-poly(acrylic acid-r-acrylamide) | Malachite green | 97 | 97 | [349] |
| Microalgae Scenedesmus | MB | 87.69 | - | [350] |
| activated carbon prepared from Date Press Cake | MB | 613.8 | 83.3 | [351] |
| Karanj fruit hulls | MB | 239.4 | 94.4 | [352] |
| Rattan (Lacosperma secundiflorum) | MB | 359 | 96 | [353] |
| Fox nutshell | MB | 968.7 | 99.96 | [354] |
| chitosan-epichlorohydrin/zeolite composite | MB | 44.2 | 90 | [355] |
| Reactive red 120 | 45.25 | 88 | ||
| chitosan/carboxymethyl cellulose capsules | MB | 64.6 | 4.4 | [356] |
| Methyl orange | 334.8 | 37.5 | ||
| Acid blue-113 | 526.8 | 59 | ||
| Polyacrylamide-chitosan magnetic nanoparticles | MB | 1044.06 | 76.1 | [357] |
| chitosan-epichlorohydrin/TiO2 nanocomposite | Reactive red 120 | 46.3 | 99.3 | [358] |
| polypyrrole-chitosan composites | Acid red 18 | 285.71 | 98.93 | [359] |
| Chitosan-Activated Charcoal Composite | Thionine cationic dye | 60.9 | 92.9 | [360] |
| chitosan-glyoxal/TiO2 nanocomposite | Methyl orange | 374.8 | 75.9 | [361] |
| chitosan/polyamide nanofibers | Ponceau 4R | 482.2 | - | [362] |
| Reactive Black 5 | 352.5 | - | ||
| Chitosan/alginate composite sponge | Acid red B14 | 1486.9 | - | [363] |
| fibrous chitosan/alginate composite foam | MB | 1488.1 | - | [364] |
| Acid Black-172 | 817 | - | ||
| Sodium alginate nanofiber membranes | MB | 2230 | - | [365] |
| Sodium alginate/gelatin/graphene oxide composite aerogel | MB | 322.6 | - | [366] |
| Congo red | 196.8 | - | ||
| Lignin/cellulose nanocrystals/alginate beads | MB | 1181 | - | [367] |
| Cladodes of Tacinga palmadora | Crystal violet | 228.74 | - | [368] |
| Palm cactus | Crystal violet | 220 | - | [369] |
| O. ficus indica cladodes | Acid orange | 198.9 | - | [370] |
| Cactus pear seed cake | MB | 260 | 56.48 | [371] |
| Methyl orange | 336.12 | 100 | ||
| Fruit peels (O. ficus indica) | Indigo carmine | 294 | 76–99 | [372] |
| Solophenyl blue | 909 | 76–99 | ||
| MB | 416 | 76–99 | ||
| Crystal violet | 312 | 76–99 | ||
| Carboxymethyl chitosan-modified magnetic-cored dendrimers | MB | 20.85 | - | [373] |
| Methyl orange | 96.31 | - | ||
| Gelatin-based magnetic beads | MB | 465 | - | [374] |
| Direct Red | 380 | - | ||
| Glutaraldehyde cross-linked chitosan-coated Fe3O4 nanoparticles | Methyl orange | 758 | - | [375] |
| Magnetic hydrogel beads with gum tragacanth | Congo Red | 94 | - | [376] |
| Fe3O4–κ-carrageenan cross-linked with chitosan | MB | 123 | [377] | |
| Fe3O4@SiO2–κ-carrageenan | MB | 530 | - | [378] |
| Potamogeton crispus | RR198 | 44.2 | - | [379] |
| O-carboxymethylchitosan-N-lauryl/γ-Fe2O3 magnetic nanoparticles | RR198 | 216 | - | [380] |
| Pistachio hull wastes | RR198 | 253.67 | 95.13 | [381] |
| Al2O3/MWCNTs Carbon nanotube | RR198 | 424 | 91.54 | [382] |
| Polyaniline/Fe3O4 | RR198 | 45.45 | 92.1 | [383] |
| Eggshell biocomposite beads | RR198 | 46.9 | 92 | [384] |
| Activated Carbon (Walnut Shells) | RR198 | 79.15 | 87.17 | [385] |
| Pistachio nut shell | RR198 | 108.15 | 88 | [386] |
| Chitosan | RR198 | 310.4 | 95.11 | [387] |
| Chitosan/cellulose PEM | RR198 | 819 | 99.77 | [388] |
| Cellulose/chitosan aerogels | Congo Red | 381.7 | 95 | [389] |
| Chitosan/Zeolite composite | MB | 19.23 | 84.85 | [390] |
| Chitosan/PVA composite | Methyl orange Eosin Yellow Dye | 9.34 52.91 | 92.42 86.70 | [391] [392] |
| Chitosan/ZnO | Malachite Green Dye | - | 98.50 | [393] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
EL-Ghoul, Y.; Alminderej, F.M.; Alsubaie, F.M.; Alrasheed, R.; Almousa, N.H. Recent Advances in Functional Polymer Materials for Energy, Water, and Biomedical Applications: A Review. Polymers 2021, 13, 4327. https://doi.org/10.3390/polym13244327
EL-Ghoul Y, Alminderej FM, Alsubaie FM, Alrasheed R, Almousa NH. Recent Advances in Functional Polymer Materials for Energy, Water, and Biomedical Applications: A Review. Polymers. 2021; 13(24):4327. https://doi.org/10.3390/polym13244327
Chicago/Turabian StyleEL-Ghoul, Yassine, Fahad M. Alminderej, Fehaid M. Alsubaie, Radwan Alrasheed, and Norah H. Almousa. 2021. "Recent Advances in Functional Polymer Materials for Energy, Water, and Biomedical Applications: A Review" Polymers 13, no. 24: 4327. https://doi.org/10.3390/polym13244327
APA StyleEL-Ghoul, Y., Alminderej, F. M., Alsubaie, F. M., Alrasheed, R., & Almousa, N. H. (2021). Recent Advances in Functional Polymer Materials for Energy, Water, and Biomedical Applications: A Review. Polymers, 13(24), 4327. https://doi.org/10.3390/polym13244327






