Defining Swelling Kinetics in Block Copolymer Thin Films: The Critical Role of Temperature and Vapour Pressure Ramp
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Experimental In Situ Set-Up: Monitoring Substrate Temperature and Solvent Pressure during Solvo-Thermal Vapour Annealing (STVA)
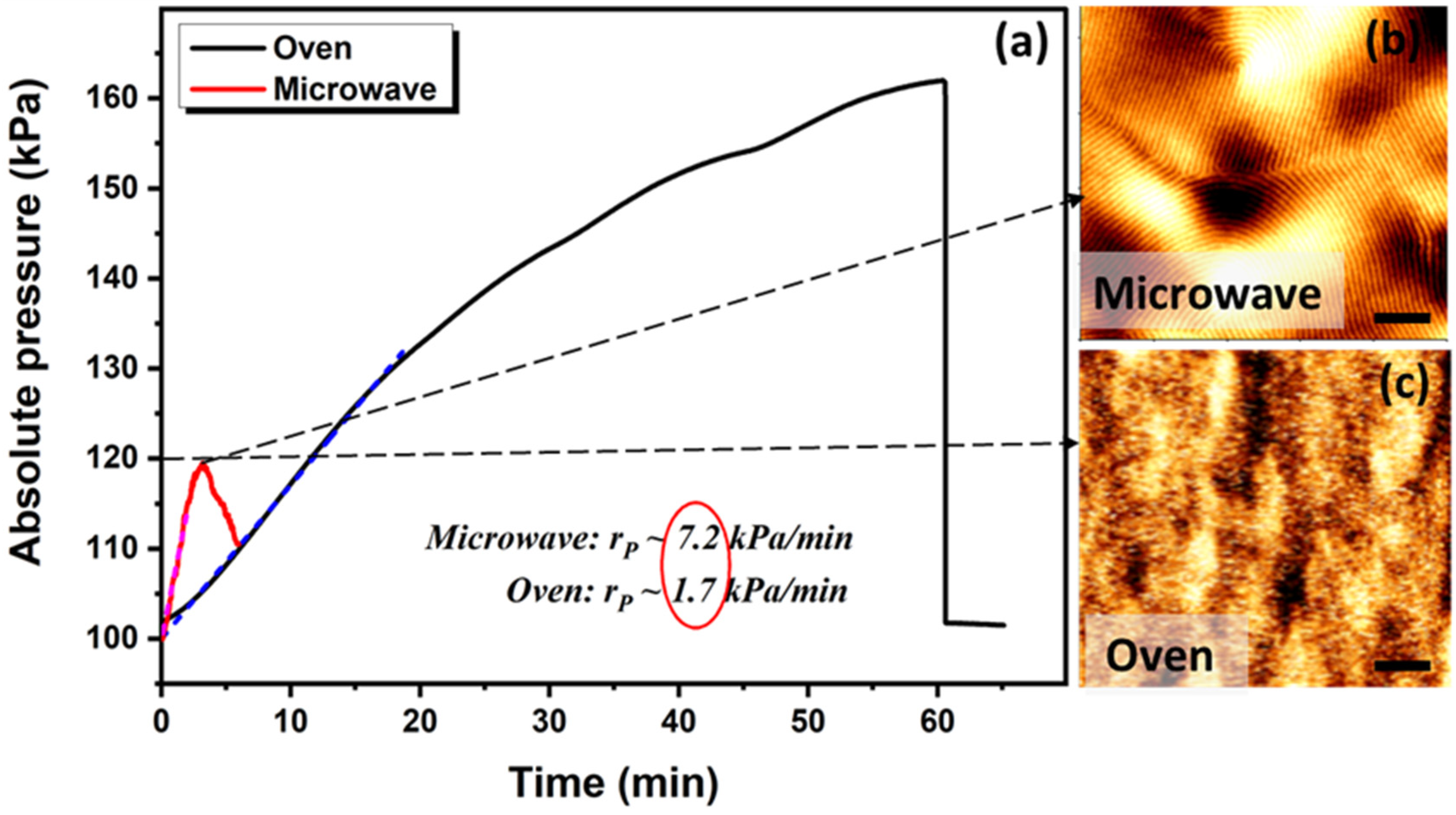
2.3. Solvo-Microwave Annealing
2.4. Film Characterisation
3. Results
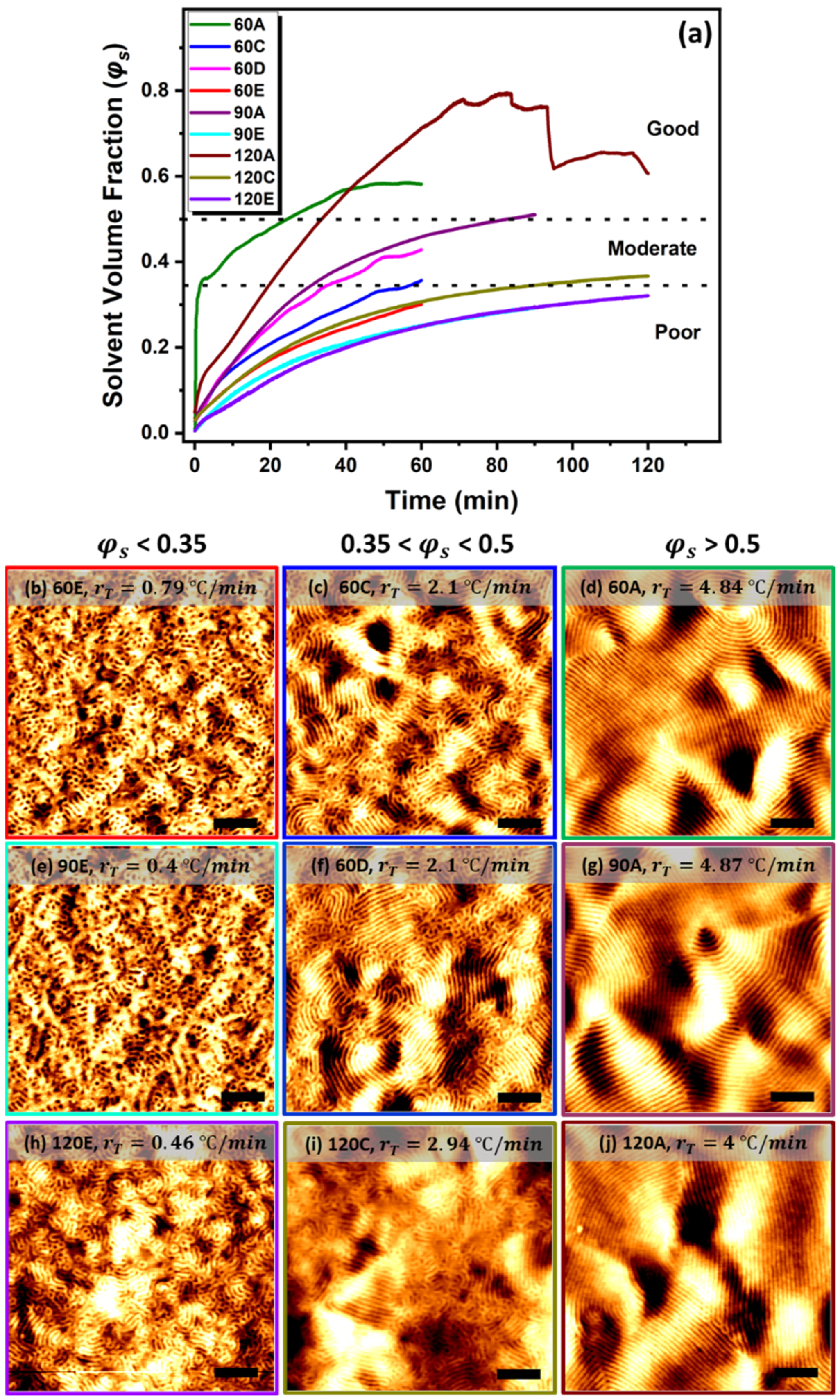
3.1. Ramp Effect on Phase Separation
3.2. Swelling Kinetics
3.2.1. Extraction of Polymer Volume Fraction
3.2.2. Solvent Volume Fraction on Phase Separation
3.3. Diffusion Kinetics
3.3.1. Mass Uptake and Case II Diffusion
3.3.2. Second Order Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keen, I.; Cheng, H.-H.; Yu, A.; Jack, K.S.; Younkin, T.R.; Leeson, M.J.; Whittaker, A.K.; Blakey, I. Behavior of Lamellar Forming Block Copolymers under Nanoconfinement: Implications for Topography Directed Self-Assembly of Sub-10 nm Structures. Macromolecules 2014, 47, 276–283. [Google Scholar] [CrossRef]
- Keen, I.; Yu, A.; Cheng, H.-H.; Jack, K.S.; Nicholson, T.M.; Whittaker, A.K.; Blakey, I. Control of the Orientation of Symmetric Poly(styrene)-block-poly(d,l-lactide) Block Copolymers Using Statistical Copolymers of Dissimilar Composition. Langmuir 2012, 28, 15876–15888. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wan, L.; Li, Z.; Suh, H.; Ren, J.; Ocola, L.E.; Hu, W.; Ji, S.; Nealey, P.F. Effect of Stereochemistry on Directed Self-Assembly of Poly(styrene-b-lactide) Films on Chemical Patterns. ACS Macro Lett. 2016, 5, 396–401. [Google Scholar] [CrossRef]
- Cheng, X.; Böker, A.; Tsarkova, L. Temperature-Controlled Solvent Vapor Annealing of Thin Block Copolymer Films. Polymers 2019, 11, 1312. [Google Scholar] [CrossRef] [Green Version]
- Ogieglo, W.; Stenbock-Fermor, A.; Juraschek, T.M.; Bogdanova, Y.; Benes, N.; Tsarkova, L.A. Synergic Swelling of Interactive Network Support and Block Copolymer Films during Solvent Vapor Annealing. Langmuir 2018, 34, 9950–9960. [Google Scholar] [CrossRef] [Green Version]
- Raybin, J.G.; Sibener, S.J. In Situ Visualization of Solvent Swelling Dynamics in Block Copolymer Films with Atomic Force Microscopy. Macromolecules 2019, 52, 5985–5994. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, L.; Steinhart, M. Swelling-Induced Morphology Reconstruction in Block Copolymer Nanorods: Kinetics and Impact of Surface Tension During Solvent Evaporation. ACS Nano 2011, 5, 1928–1938. [Google Scholar] [CrossRef] [PubMed]
- Sinturel, C.; Grosso, D.; Boudot, M.; Amenitsch, H.; Hillmyer, M.A.; Pineau, A.; Vayer, M. Structural Transitions in Asymmetric Poly(styrene)-block-Poly(lactide) Thin Films Induced by Solvent Vapor Exposure. ACS Appl. Mater. Interfaces 2014, 6, 12146–12152. [Google Scholar] [CrossRef]
- Dinachali, S.S.; Bai, W.; Tu, K.-H.; Choi, H.K.; Zhang, J.; Kreider, M.E.; Cheng, L.-C.; Ross, C.A. Thermo-Solvent Annealing of Polystyrene-Polydimethylsiloxane Block Copolymer Thin Films. ACS Macro Lett. 2015, 4, 500–504. [Google Scholar] [CrossRef]
- Gotrik, K.W.; Ross, C.A. Solvothermal Annealing of Block Copolymer Thin Films. Nano Lett. 2013, 13, 5117–5122. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.M.; Maher, M.J.; Janes, D.W.; Ellison, C.J.; Willson, C.G. Block Copolymer Lithography. Macromolecules 2014, 47, 2–12. [Google Scholar] [CrossRef]
- Kim, B.H.; Kim, J.Y.; Kim, S.O. Directed self-assembly of block copolymers for universal nanopatterning. Soft Matter 2013, 9, 2780–2786. [Google Scholar] [CrossRef]
- Lohmüller, T.; Aydin, D.; Schwieder, M.; Morhard, C.; Louban, I.; Pacholski, C.; Spatz, J.P. Nanopatterning by block copolymer micelle nanolithography and bioinspired applications. Biointerphases 2011, 6, MR1–MR12. [Google Scholar] [CrossRef] [PubMed]
- Kathrein, C.C.; Bai, W.; Currivan-Incorvia, J.A.; Liontos, G.; Ntetsikas, K.; Avgeropoulos, A.; Böker, A.; Tsarkova, L.; Ross, C.A. Combining Graphoepitaxy and Electric Fields toward Uniaxial Alignment of Solvent-Annealed Polystyrene–b–Poly(dimethylsiloxane) Block Copolymers. Chem. Mater. 2015, 27, 6890–6898. [Google Scholar] [CrossRef]
- Frascaroli, J.; Brivio, S.; Ferrarese Lupi, F.; Seguini, G.; Boarino, L.; Perego, M.; Spiga, S. Resistive Switching in High-Density Nanodevices Fabricated by Block Copolymer Self-Assembly. ACS Nano 2015, 9, 2518–2529. [Google Scholar] [CrossRef]
- You, B.K.; Park, W.I.; Kim, J.M.; Park, K.-I.; Seo, H.K.; Lee, J.Y.; Jung, Y.S.; Lee, K.J. Reliable Control of Filament Formation in Resistive Memories by Self-Assembled Nanoinsulators Derived from a Block Copolymer. ACS Nano 2014, 8, 9492–9502. [Google Scholar] [CrossRef] [PubMed]
- Mokarian-Tabari, P.; Senthamaraikannan, R.; Glynn, C.; Collins, T.W.; Cummins, C.; Nugent, D.; O’Dwyer, C.; Morris, M.A. Large Block Copolymer Self-Assembly for Fabrication of Subwavelength Nanostructures for Applications in Optics. Nano Lett. 2017, 17, 2973–2978. [Google Scholar] [CrossRef]
- Akinoglu, G.E.; Mir, S.H.; Gatensby, R.; Rydzek, G.; Mokarian-Tabari, P. Block Copolymer Derived Vertically Coupled Plasmonic Arrays for Surface-Enhanced Raman Spectroscopy. ACS Appl. Mater. Interfaces 2020, 12, 23410–23416. [Google Scholar] [CrossRef]
- Park, S.; Lee, D.H.; Xu, J.; Kim, B.; Hong, S.W.; Jeong, U.; Xu, T.; Russell, T.P. Macroscopic 10-Terabit–per–Square-Inch Arrays from Block Copolymers with Lateral Order. Science 2009, 323, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.A.; Hillmyer, M.A. Nanoporous Membranes Derived from Block Copolymers: From Drug Delivery to Water Filtration. ACS Nano 2010, 4, 3548–3553. [Google Scholar] [CrossRef]
- Rose, F.; Bosworth, J.K.; Dobisz, E.A.; Ruiz, R. Three-dimensional mesoporous structures fabricated by independent stacking of self-assembled films on suspended membranes. Nanotechnology 2010, 22, 035603. [Google Scholar] [CrossRef]
- Kim, E.; Park, S.; Han, Y.-S.; Kim, T.-H. Effect of solvent selectivity on supramolecular assemblies of block copolymer by solvent-vapor annealing. Polymer 2018, 150, 214–222. [Google Scholar] [CrossRef]
- Kim, Y.C.; Shin, T.J.; Hur, S.-M.; Kwon, S.J.; Kim, S.Y. Shear-solvo defect annihilation of diblock copolymer thin films over a large area. Sci. Adv. 2019, 5, eaaw3974. [Google Scholar] [CrossRef] [Green Version]
- Jung, F.A.; Berezkin, A.V.; Tejsner, T.B.; Posselt, D.; Smilgies, D.-M.; Papadakis, C.M. Solvent Vapor Annealing of a Diblock Copolymer Thin Film with a Nonselective and a Selective Solvent: Importance of Pathway for the Morphological Changes. Macromol. Rapid Commun. 2020, 41, 2000150. [Google Scholar] [CrossRef]
- Bai, W.; Hannon, A.F.; Gotrik, K.W.; Choi, H.K.; Aissou, K.; Liontos, G.; Ntetsikas, K.; Alexander-Katz, A.; Avgeropoulos, A.; Ross, C.A. Thin Film Morphologies of Bulk-Gyroid Polystyrene-block-polydimethylsiloxane under Solvent Vapor Annealing. Macromolecules 2014, 47, 6000–6008. [Google Scholar] [CrossRef]
- Di, Z.; Posselt, D.; Smilgies, D.-M.; Li, R.; Rauscher, M.; Potemkin, I.I.; Papadakis, C.M. Stepwise Swelling of a Thin Film of Lamellae-Forming Poly(styrene-b-butadiene) in Cyclohexane Vapor. Macromolecules 2012, 45, 5185–5195. [Google Scholar] [CrossRef]
- Mokarian-Tabari, P.; Cummins, C.; Rasappa, S.; Simao, C.; Sotomayor Torres, C.M.; Holmes, J.D.; Morris, M.A. Study of the Kinetics and Mechanism of Rapid Self-Assembly in Block Copolymer Thin Films during Solvo-Microwave Annealing. Langmuir 2014, 30, 10728–10739. [Google Scholar] [CrossRef]
- Mokarian-Tabari, P.; Collins, T.W.; Holmes, J.D.; Morris, M.A. Brushless and controlled microphase separation of lamellar polystyrene-b-polyethylene oxide thin films for block copolymer nanolithography. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 904–909. [Google Scholar] [CrossRef]
- Gu, X.; Gunkel, I.; Hexemer, A.; Gu, W.; Russell, T.P. An In Situ Grazing Incidence X-Ray Scattering Study of Block Copolymer Thin Films During Solvent Vapor Annealing. Adv. Mater. 2014, 26, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Yager, K.G.; Ross, C.A. In Situ Characterization of the Self-Assembly of a Polystyrene–Polydimethylsiloxane Block Copolymer during Solvent Vapor Annealing. Macromolecules 2015, 48, 8574–8584. [Google Scholar] [CrossRef]
- Peng, J.; Kim, D.H.; Knoll, W.; Xuan, Y.; Li, B.; Han, Y. Morphologies in solvent-annealed thin films of symmetric diblock copolymer. J. Chem. Phys. 2006, 125, 064702. [Google Scholar] [CrossRef]
- Knoll, A.; Magerle, R.; Krausch, G. Phase behavior in thin films of cylinder-forming ABA block copolymers: Experiments. J. Chem. Phys. 2004, 120, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Phillip, W.A.; Hillmyer, M.A.; Cussler, E.L. Cylinder Orientation Mechanism in Block Copolymer Thin Films Upon Solvent Evaporation. Macromolecules 2010, 43, 7763–7770. [Google Scholar] [CrossRef]
- Xu, X.; Man, X.; Doi, M.; Ou-Yang, Z.-c.; Andelman, D. Defect Removal by Solvent Vapor Annealing in Thin Films of Lamellar Diblock Copolymers. Macromolecules 2019, 52, 9321–9333. [Google Scholar] [CrossRef]
- Mokarian-Tabari, P.; Collins, T.W.; Holmes, J.D.; Morris, M.A. Cyclical “Flipping” of Morphology in Block Copolymer Thin Films. ACS Nano 2011, 5, 4617–4623. [Google Scholar] [CrossRef]
- Alfrey, T., Jr.; Gurnee, E.F.; Lloyd, W.G. Diffusion in glassy polymers. J. Polym. Sci. Part C Polym. Symp. 1966, 12, 249–261. [Google Scholar] [CrossRef]
- Astaluta, G.; Sarti, G.C. A class of mathematical models for sorption of swelling solvents in glassy polymers. Polym. Eng. Sci. 1978, 18, 388–395. [Google Scholar] [CrossRef]
- Gall, T.P.; Kramer, E.J. Diffusion of deuterated toluene in polystyrene. Polymer 1991, 32, 265–271. [Google Scholar] [CrossRef]
- Hui, C.Y.; Wu, K.C.; Lasky, R.C.; Kramer, E.J. Case-II diffusion in polymers. I. Transient swelling. J. Appl. Phys. 1987, 61, 5129–5136. [Google Scholar] [CrossRef]
- Hui, C.Y.; Wu, K.C.; Lasky, R.C.; Kramer, E.J. Case-II diffusion in polymers. II. Steady-state front motion. J. Appl. Phys. 1987, 61, 5137–5149. [Google Scholar] [CrossRef]
- Krüger, K.-M.; Sadowski, G. Fickian and Non-Fickian Sorption Kinetics of Toluene in Glassy Polystyrene. Macromolecules 2005, 38, 8408–8417. [Google Scholar] [CrossRef]
- Kwei, T.K.; Wang, T.T. Diffusion in Glassy Polymers. In Permeability of Plastic Films and Coatings: To Gases, Vapors, and Liquids; Hopfenberg, H.B., Ed.; Springer: Boston, MA, USA, 1974; pp. 63–71. [Google Scholar] [CrossRef]
- Gall, T.P.; Lasky, R.C.; Kramer, E.J. Case II diffusion: Effect of solvent molecule size. Polymer 1990, 31, 1491–1499. [Google Scholar] [CrossRef]
- Papanu, J.S.; Soane, D.S.; Bell, A.T.; Hess, D.W. Transport models for swelling and dissolution of thin polymer films. J. Appl. Polym. Sci. 1989, 38, 859–885. [Google Scholar] [CrossRef]
- Peterlin, A. Diffusion in a network with discontinuous swelling. J. Polym. Sci. Part B Polym. Lett. 1965, 3, 1083–1087. [Google Scholar] [CrossRef]
- Peterlin, A. Diffusion in a glassy polymer with discontinuous swelling. II. Concentration distribution of diffusant as function of time. Die Makromol. Chem. 1969, 124, 136–142. [Google Scholar] [CrossRef]
- Petropoulos, J.H. Sorption—longitudinal swelling kinetic correlations in polymer film—Vapor systems. J. Membr. Sci. 1984, 17, 233–244. [Google Scholar] [CrossRef]
- Sarti, G.C. Solvent osmotic stresses and the prediction of Case II transport kinetics. Polymer 1979, 20, 827–832. [Google Scholar] [CrossRef]
- Schott, H. Kinetics of swelling of polymers and their gels. J. Pharm. Sci. 1992, 81, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Sharp, J.S.; Jones, R.A.L. Swelling-induced morphology in ultrathin supported films of poly(d,l−lactide). Phys. Rev. E 2002, 66, 011801. [Google Scholar] [CrossRef]
- Thomas, N.; Windle, A.H. Case II swelling of PMMA sheet in methanol. J. Membr. Sci. 1978, 3, 337–342. [Google Scholar] [CrossRef]
- Thomas, N.; Windle, A.H. Transport of methanol in poly(methyl methacrylate). Polymer 1978, 19, 255–265. [Google Scholar] [CrossRef]
- Thomas, N.L.; Windle, A.H. A deformation model for Case II diffusion. Polymer 1980, 21, 613–619. [Google Scholar] [CrossRef]
- Vrentas, J.S.; Vrentas, C.M. Fickian diffusion in glassy polymer-solvent systems. J. Polym. Sci. Part B Polym. Phys. 1992, 30, 1005–1011. [Google Scholar] [CrossRef]
- Paciolla, M.; Arismendi-Arrieta, D.J.; Moreno, A.J. Coarsening Kinetics of Complex Macromolecular Architectures in Bad Solvent. Polymers 2020, 12, 531. [Google Scholar] [CrossRef] [Green Version]
- Selkirk, A.; Prochukhan, N.; Lundy, R.; Cummins, C.; Gatensby, R.; Kilbride, R.; Parnell, A.; Baez Vasquez, J.; Morris, M.; Mokarian-Tabari, P. Optimization and Control of Large Block Copolymer Self-Assembly via Precision Solvent Vapor Annealing. Macromolecules 2021, 54, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Lundy, R.; Flynn, S.P.; Cummins, C.; Kelleher, S.M.; Collins, M.N.; Dalton, E.; Daniels, S.; Morris, M.A.; Enright, R. Controlled solvent vapor annealing of a high χ block copolymer thin film. Phys. Chem. Chem. Phys. 2017, 19, 2805–2815. [Google Scholar] [CrossRef] [PubMed]
- Kreuzer, L.P.; Widmann, T.; Hohn, N.; Wang, K.; Bießmann, L.; Peis, L.; Moulin, J.-F.; Hildebrand, V.; Laschewsky, A.; Papadakis, C.M.; et al. Swelling and Exchange Behavior of Poly(sulfobetaine)-Based Block Copolymer Thin Films. Macromolecules 2019, 52, 3486–3498. [Google Scholar] [CrossRef]
- Stenbock-Fermor, A.; Knoll, A.W.; Böker, A.; Tsarkova, L. Enhancing Ordering Dynamics in Solvent-Annealed Block Copolymer Films by Lithographic Hard Mask Supports. Macromolecules 2014, 47, 3059–3067. [Google Scholar] [CrossRef] [Green Version]
- Crank, J. A theoretical investigation of the influence of molecular relaxation and internal stress on diffusion in polymers. J. Polym. Sci. 1953, 11, 151–168. [Google Scholar] [CrossRef]
- Thomas, N.L.; Windle, A.H. A theory of case II diffusion. Polymer 1982, 23, 529–542. [Google Scholar] [CrossRef]
- Gotrik, K.W.; Hannon, A.F.; Son, J.G.; Keller, B.; Alexander-Katz, A.; Ross, C.A. Morphology Control in Block Copolymer Films Using Mixed Solvent Vapors. ACS Nano 2012, 6, 8052–8059. [Google Scholar] [CrossRef]
- Hulkkonen, H.; Salminen, T.; Niemi, T. Automated solvent vapor annealing with nanometer scale control of film swelling for block copolymer thin films. Soft Matter 2019, 15, 7909–7917. [Google Scholar] [CrossRef] [Green Version]
- Baruth, A.; Seo, M.; Lin, C.H.; Walster, K.; Shankar, A.; Hillmyer, M.A.; Leighton, C. Optimization of Long-Range Order in Solvent Vapor Annealed Poly(styrene)-block-poly(lactide) Thin Films for Nanolithography. ACS Appl. Mater. Interfaces 2014, 6, 13770–13781. [Google Scholar] [CrossRef]
- Nelson, G.; Drapes, C.S.; Grant, M.A.; Gnabasik, R.; Wong, J.; Baruth, A. High-Precision Solvent Vapor Annealing for Block Copolymer Thin Films. Micromachines 2018, 9, 271. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Olsen, B.C.; Luber, E.J.; Buriak, J.M. Nanopatterning via Solvent Vapor Annealing of Block Copolymer Thin Films. Chem. Mater. 2017, 29, 176–188. [Google Scholar] [CrossRef]
- Cummins, C.; Mokarian-Tabari, P.; Andreazza, P.; Sinturel, C.; Morris, M.A. Solvothermal Vapor Annealing of Lamellar Poly(styrene)-block-poly(d,l-lactide) Block Copolymer Thin Films for Directed Self-Assembly Application. ACS Appl. Mater. Interfaces 2016, 8, 8295–8304. [Google Scholar] [CrossRef] [PubMed]
- Sharp, J.S.; Forrest, J.A.; Jones, R.A.L. Swelling of Poly(dl-lactide) and Polylactide-co-glycolide in Humid Environments. Macromolecules 2001, 34, 8752–8760. [Google Scholar] [CrossRef]
- Robinson, I.D. Swelling of coated gelatin-silver bromide emulsions. Photogr. Sci. Eng. 1964, 8, 220–224. [Google Scholar]





| Regimes | Slow Heating | Medium Heating | Fast Heating |
|---|---|---|---|
| Annealing time | Sample ID | Sample ID | Sample ID |
| 60 min | 60E, 60F | 60C, 60D | 60A |
| 90 min | 90E | 90C | 90A |
| 120 min | 120E | 120C | 120A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neppalli, S.N.; Collins, T.W.; Gholamvand, Z.; Cummins, C.; Morris, M.A.; Mokarian-Tabari, P. Defining Swelling Kinetics in Block Copolymer Thin Films: The Critical Role of Temperature and Vapour Pressure Ramp. Polymers 2021, 13, 4238. https://doi.org/10.3390/polym13234238
Neppalli SN, Collins TW, Gholamvand Z, Cummins C, Morris MA, Mokarian-Tabari P. Defining Swelling Kinetics in Block Copolymer Thin Films: The Critical Role of Temperature and Vapour Pressure Ramp. Polymers. 2021; 13(23):4238. https://doi.org/10.3390/polym13234238
Chicago/Turabian StyleNeppalli, Sudhakara Naidu, Timothy W. Collins, Zahra Gholamvand, Cian Cummins, Michael A. Morris, and Parvaneh Mokarian-Tabari. 2021. "Defining Swelling Kinetics in Block Copolymer Thin Films: The Critical Role of Temperature and Vapour Pressure Ramp" Polymers 13, no. 23: 4238. https://doi.org/10.3390/polym13234238
APA StyleNeppalli, S. N., Collins, T. W., Gholamvand, Z., Cummins, C., Morris, M. A., & Mokarian-Tabari, P. (2021). Defining Swelling Kinetics in Block Copolymer Thin Films: The Critical Role of Temperature and Vapour Pressure Ramp. Polymers, 13(23), 4238. https://doi.org/10.3390/polym13234238








