Design of Promising Green Cation-Exchange-Membranes-Based Sulfonated PVA and Doped with Nano Sulfated Zirconia for Direct Borohydride Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. Synthesis of Nano Sulfated Zirconia (SO4ZrO2)
2.1.2. Preparation of SPVA/IC/SO4ZrO2 Membranes
2.2. Characterization
3. Results and Discussion
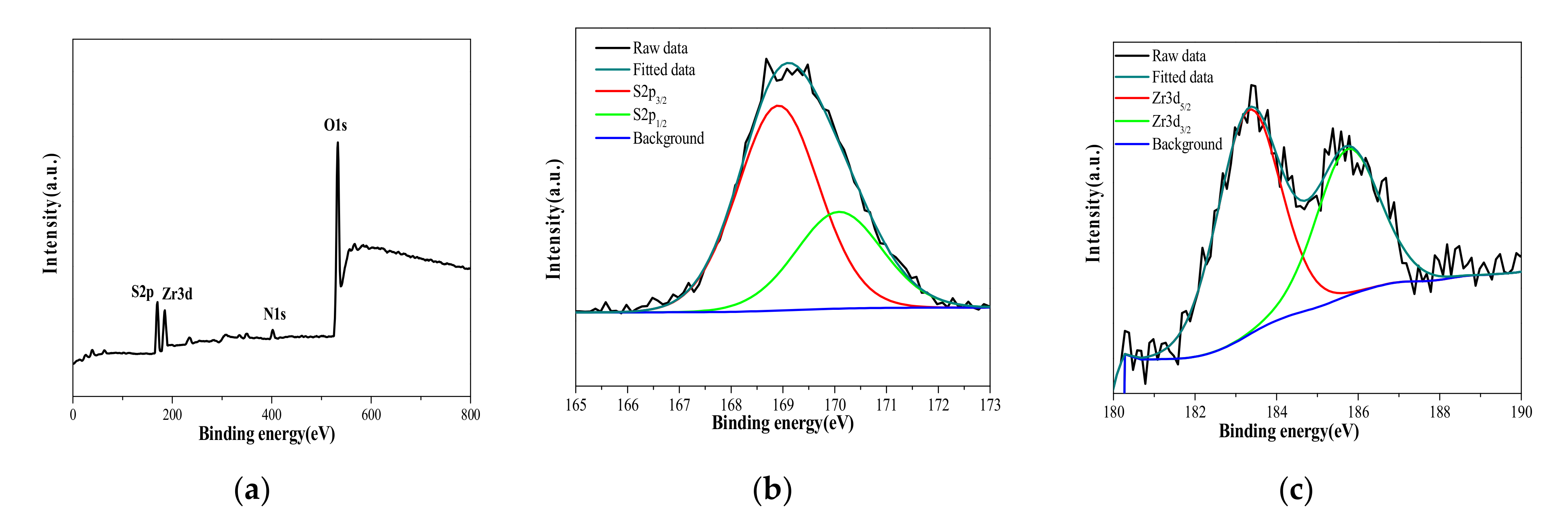
3.1. Characterization of SO4ZrO2 and Nanocomposite Membranes
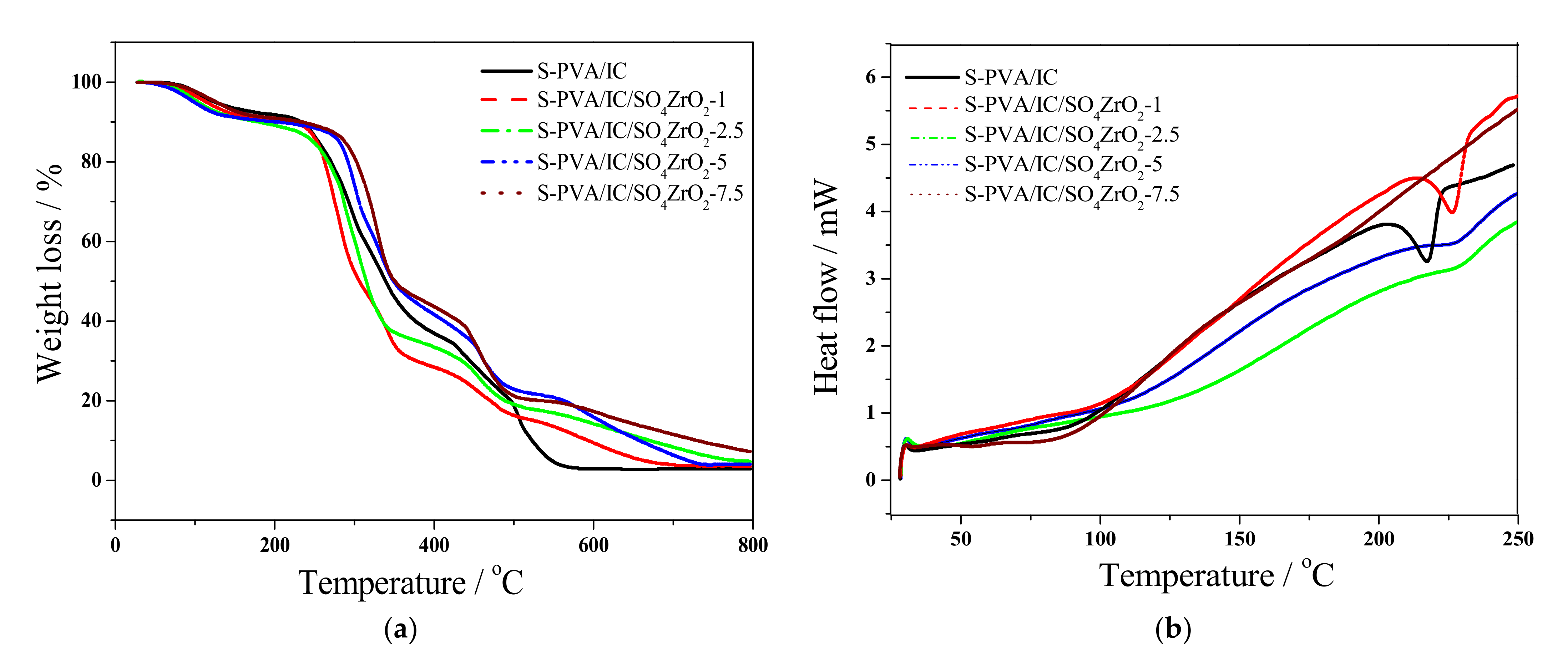
3.2. Thermal and Mechanical Analysis
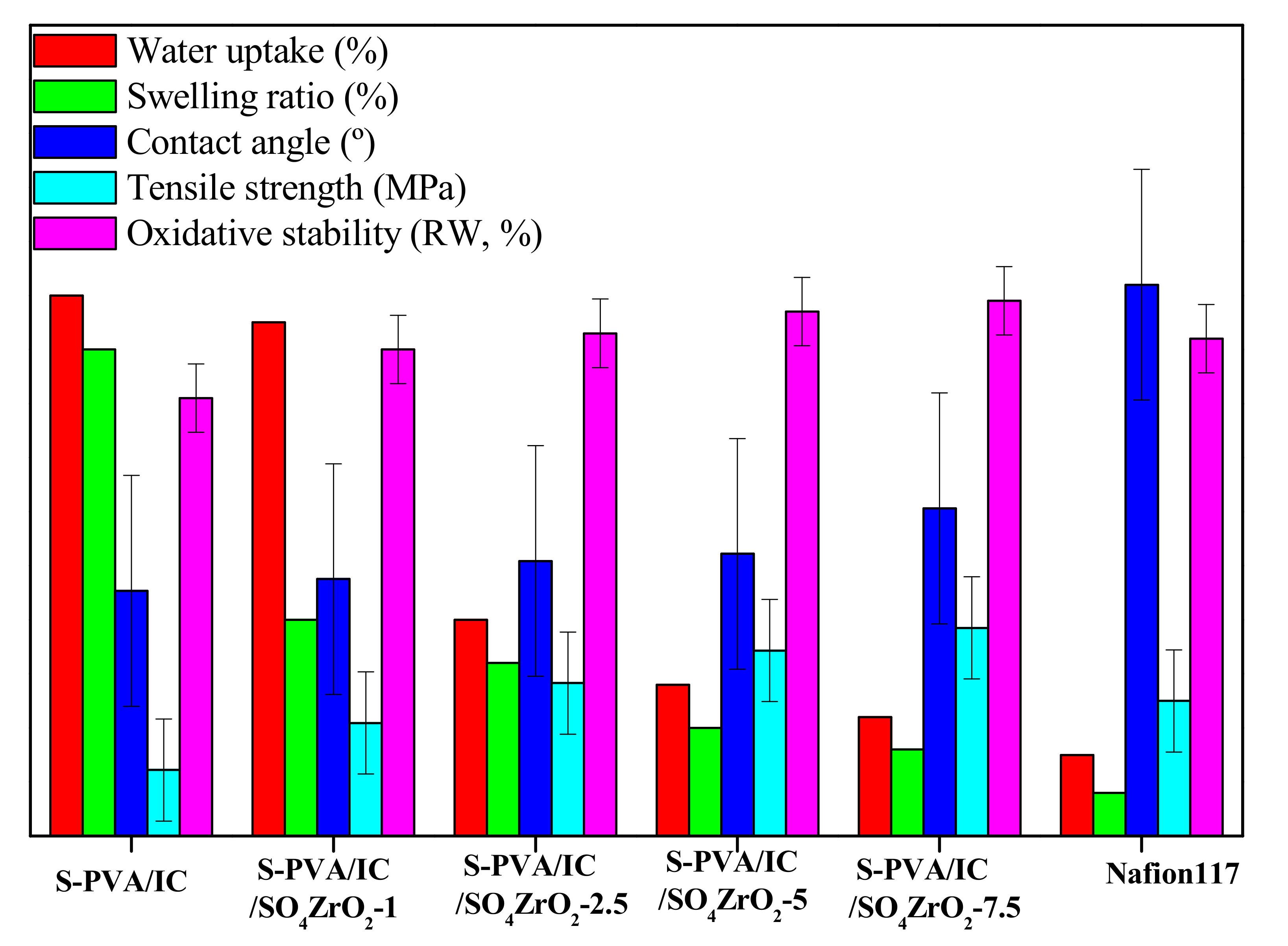
3.3. Oxidative Stability
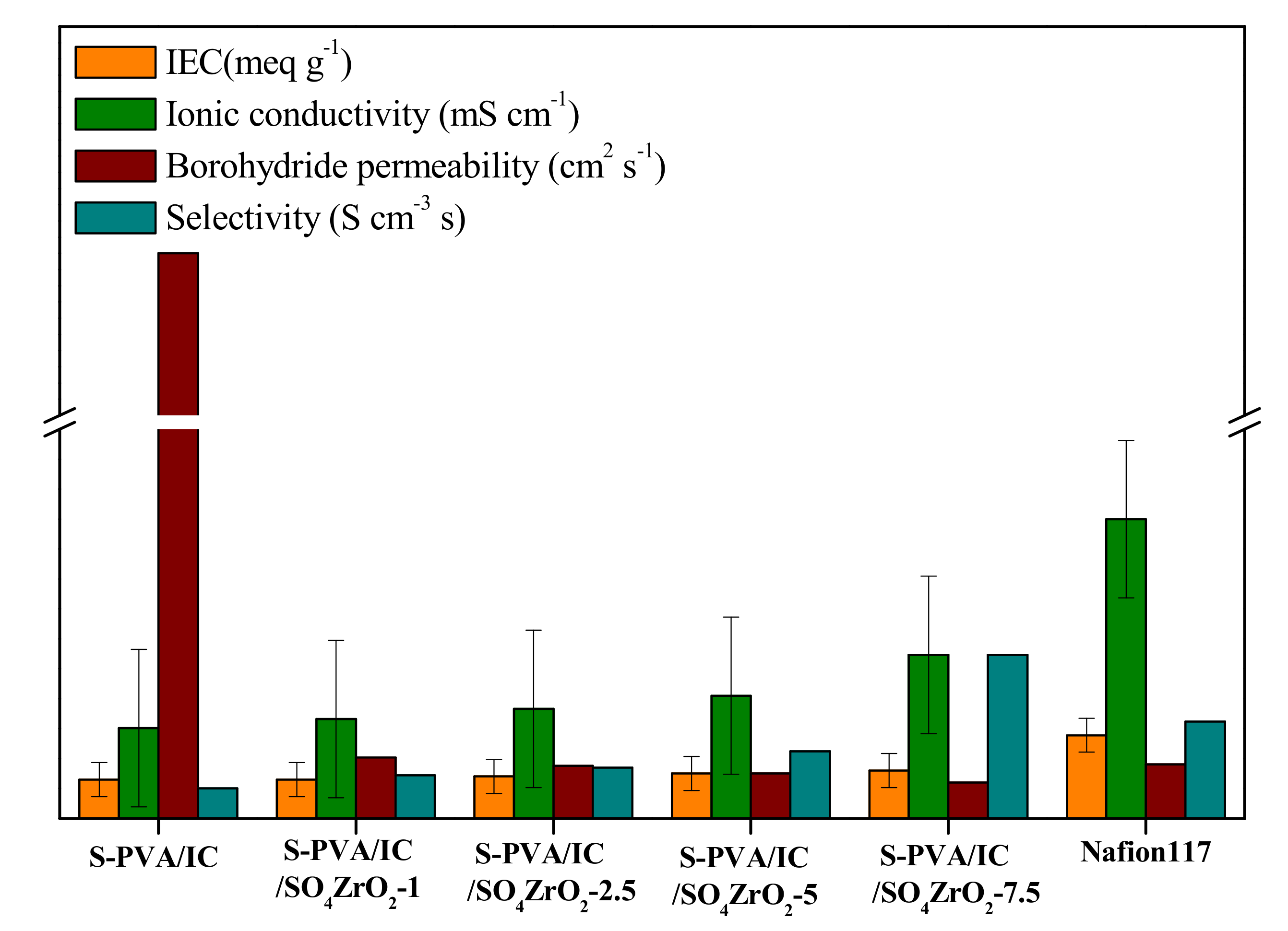
3.4. Ionic Conductivity, IEC, and Borohydride Crossover
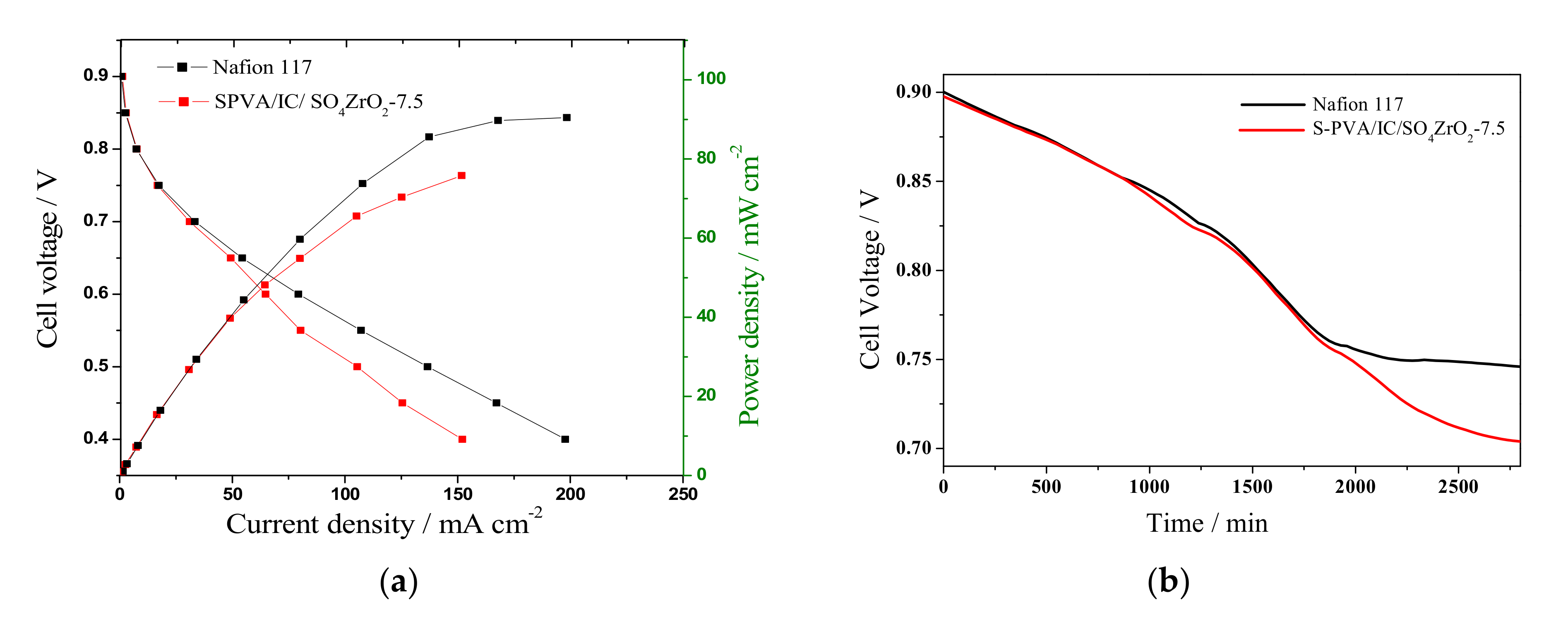
3.5. Fuel Cell Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, G.; Ling, Y.; Jiang, H.; Toghan, A. Barium Titanate as a Highly Stable Oxygen Permeable Membrane Reactor for Hydrogen Production from Thermal Water Splitting. ACS Sustain. Chem. Eng. 2021, 9, 11147–11154. [Google Scholar]
- Liu, B.; Hu, B.; Du, J.; Cheng, D.; Zang, H.Y.; Ge, X.; Su, Z. Precise Molecular Level Modification of Nafion with Bismuth Oxide Clusters for High performance Proton Exchange Membranes. Angew. Chem. Int. Ed. 2021, 133, 6141–6150. [Google Scholar] [CrossRef]
- Faisal, F.; Toghan, A.; Khalakhan, I.; Vorokhta, M.; Matolin, V.; Libuda, J. Characterization of thin CeO2 films electrochemically deposited on HOPG. Appl. Surf. Sci. 2015, 350, 142–148. [Google Scholar] [CrossRef]
- Braesch, G.; Wang, Z.; Sankarasubramanian, S.; Oshchepkov, A.G.; Bonnefont, A.; Savinova, E.R.; Ramani, V.; Chatenet, M. A high performance direct borohydride fuel cell using bipolar interfaces and noble metal-free Ni-based anodes. J. Mater. Chem. A 2020, 8, 20543–20552. [Google Scholar] [CrossRef]
- Liang, M.; Liu, Y.; Xiao, B.; Yang, S.; Wang, Z.; Han, H. An analytical model for the transverse permeability of gas diffusion layer with electrical double layer effects in proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2018, 43, 17880–17888. [Google Scholar] [CrossRef]
- Liang, M.; Fu, C.; Xiao, B.; Luo, L.; Wang, Z. A fractal study for the effective electrolyte diffusion through charged porous media. Int. J. Heat Mass Transf. 2019, 137, 365–371. [Google Scholar] [CrossRef]
- Gouda, M.H.; Tamer, T.M.; Mohy Eldin, M.S. A highly selective novel green cation exchange membrane doped with ceramic nanotubes material for direct methanol fuel cells. Energies 2021, 14, 5664. [Google Scholar] [CrossRef]
- Gouda, M.H.; Elessawy, N.A.; Toghan, A. Development of effectively costed and performant novel cation exchange ceramic nanocomposite membrane based sulfonated PVA for direct borohydride fuel cells. J. Ind. Eng. Chem. 2021, 100, 212–219. [Google Scholar] [CrossRef]
- Gouda, M.; Tamer, T.; Konsowa, A.; Farag, H.; Eldin, M.M. Organic-Inorganic Novel Green Cation Exchange Membranes for Direct Methanol Fuel Cells. Energies 2021, 14, 4686. [Google Scholar] [CrossRef]
- Sanli, A.E.; Gordesel, M.; Yilmaz, E.S.; Ozden, S.K.; Gunlu, G.; Uysal, B.Z. Performance improvement in direct borohydride/peroxide fuel cells. Int. J. Hydrog. Energy 2017, 42, 8119–8129. [Google Scholar] [CrossRef]
- Demirci, U.B.; Akdim, O.; Andrieux, J.; Hannauer, J.; Chamoun, R.; Miele, P. Sodium Borohydride Hydrolysis as Hydrogen Generator: Issues, State of the Art and Applicability Upstream from a Fuel Cell. Fuel Cells 2010, 10, 335–350. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Choudhury, N.A.; Sahai, Y. A comprehensive review of direct borohydride fuel cells. Renew. Sustain. Energy Rev. 2010, 14, 183–199. [Google Scholar] [CrossRef]
- Ong, B.C.; Kamarudin, S.K.; Basri, S. Direct liquid fuel cells: A review. Int. J. Hydrog. Energy 2017, 42, 10142–10157. [Google Scholar] [CrossRef]
- Merino-Jiménez, I.; León, C.P.; Shah, A.A.; Walsh, F.C. Developments in direct borohydride fuel cells and remaining challenges. J. Power Source 2012, 219, 339–357. [Google Scholar] [CrossRef]
- Sljukić, B.; Morais, A.L.; Santos, D.M.F.; Sequeira, C.A.C. Anion-or cation-exchange membranes for NaBH4/H2O2 fuel cells? Membranes 2012, 2, 478–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, D.; Sequeira, C. Effect of Membrane Separators on the Performance of Direct Borohydride Fuel Cells. J. Electrochem. Soc. 2011, 159, B126–B132. [Google Scholar] [CrossRef]
- Pandey, R.P.; Shukla, G.; Manohar, M.; Shahi, V.K. Graphene oxide based nanohybrid proton exchange membranes for fuel cell applications: An overview. Adv. Colloid Interface Sci. 2017, 240, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Zhang, H.; Luo, X.D.; Chen, N. A comparative study of Nafion and sulfonated poly(ether ether ketone) membrane performance for iron-chromium redox flow battery. Ionics 2019, 25, 4219–4229. [Google Scholar] [CrossRef]
- Ye, Y.-S.; Rick, J.; Hwang, B.-J. Water Soluble Polymers as Proton Exchange Membranes for Fuel Cells. Polymers 2012, 4, 913–963. [Google Scholar] [CrossRef] [Green Version]
- Pourzare, K.; Farhadi, S.; Mansourpanah, Y. Advanced nanocomposite membranes for fuel cell applications: A comprehensive review. Biofuel Res. J. 2016, 3, 496–513. [Google Scholar] [CrossRef] [Green Version]
- Akay, R.G.; Ata, K.C.; Kadıoğlu, T.; Çelik, C. Evaluation of SPEEK/PBI blend membranes for possible direct borohydride fuel cell (DBFC) application. Int. J. Hydrog. Energy 2018, 43, 18702–18711. [Google Scholar] [CrossRef]
- Ata, K.C.; Kadıoğlu, T.; Türkmen, A.C.; Çelik, C.; Akay, R.G. Investigation of the effects of SPEEK and its clay composite membranes on the performance of Direct Borohydride Fuel Cell. Int. J. Hydrog. Energy 2020, 45, 5430–5437. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, Y.; Wang, Y.; Chai, W.; Yang, M. Measurement and modeling of the effect of composition rati-os on the properties of poly(vinyl alcohol)/poly(vinyl pyrrolidone) membranes. Mater. Des. 2016, 103, 249–258. [Google Scholar] [CrossRef]
- Maarouf, S.; Tazi, B.; Guenoun, F. Preparation and characterization of new composite membranes containing polyvinylpyrrolidone, polyvinyl alcohol, sulfosuccinic acid, silicotungstic acid and silica for direct methanol fuel cell applications. J. Mater. Environ. Sci. 2017, 8, 2870–2876. [Google Scholar]
- Pintauro, P.N. Perspectives on Membranes and Separators for Electrochemical Energy Conversion and Storage Devices. Polym. Rev. 2015, 55, 201–207. [Google Scholar] [CrossRef]
- Choudhury, N.; Ma, J.; Sahai, Y. High performance and eco-friendly chitosan hydrogel membrane electrolytes for direct borohydride fuel cells. J. Power Source 2012, 210, 358–365. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Zhang, Y.; Zhu, Y. Preparation and characterization of graphene oxide reinforced PVA film with boric acid as crosslinker. J. Appl. Polym. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Ye, Y.S.; Cheng, M.Y.; Xie, X.L.; Rick, J.; Huang, Y.J.; Chang, F.C.; Hwang, B.J. Alkali doped polyvinyl alcohol/graphene electrolyte for direct methanol alkaline fuel cells. J. Power Source 2013, 239, 424–432. [Google Scholar] [CrossRef]
- Gouda, M.H.; Elessawy, N.A.; Santos, D.M.F. Synthesis and Characterization of Novel Green Hybrid Nano-composites for Application as Proton Exchange Membranes in Direct Borohydride Fuel Cells. Energies 2020, 13, 1180. [Google Scholar] [CrossRef] [Green Version]
- Gouda, M.H.; Gouveia, W.; El Essawy, N.A.; Šljukić, B.; Nassr, A.B.A.A.; Santos, D.M.F. Simple design of PVA-based blend doped with SO4(PO4)-functionalised TiO2 as an effective membrane for direct borohydride fuel cells. Int. J. Hydrogen Energy 2020, 45, 15226–15238. [Google Scholar] [CrossRef]
- Gouda, M.; Gouveia, W.; Afonso, M.; Šljukić, B.; El Essawy, N.; Santos, D. Novel Ternary Polymer Blend Membranes Doped with SO4/PO4-TiO2 for Low Temperature Fuel Cells. In Proceedings of the 5th World Congress on Mechanical, Chemical, and Material Engineering (MCM’19), Paper No. ICCPE 106. Lisbon, Portugal, 15–17 August 2019. [Google Scholar] [CrossRef]
- Gouda, M.; Gouveia, W.; Afonso, M.; Šljukić, B.; El Essawy, N.; Nassr, A.; Santos, D. Poly (vinyl alcohol)-based crosslinked ternary polymer blend doped with sulfonated graphene oxide as a sustainable composite membrane for direct borohydride fuel cells. J. Power Source 2019, 432, 92–101. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Farag, H.A.; Tamer, T.M.; Konsowa, A.H.; Gouda, M.H. Development of novel iota carrageenan-g-polyvinyl alcohol polyelectrolyte membranes for direct methanol fuel cell application. Polym. Bull. 2020, 77, 4895–4916. [Google Scholar] [CrossRef]
- Gouda, M.H.; Konsowa, A.H.; Farag, H.A.; Elessawy, N.A.; Tamer, T.M.; Mohy Eldin, M.S. Novel nanocomposite membranes based on cross-linked eco-friendly polymers doped with sulfated titania nano-tubes for direct methanol fuel cell application. Nanomater. Nanotechnol. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Selvasekarapandian, S.; Premalatha, M.; Monisha, S.; Boopathi, G.; Aristatil, G.; Arun, A.; Madeswaran, S. Proton-conducting I-Carrageenan-based biopolymer electrolyte for fuel cell application. Ionics 2016, 23, 2775–2780. [Google Scholar] [CrossRef]
- Sedesheva, Y.S.; Ivanov, V.S.; Wozniak, A.I.; Yegorov, A.S. Proton-Exchange Membranes Based on Sulfonated Polymers. Orient. J. Chem. 2016, 32, 2283–2296. [Google Scholar] [CrossRef]
- Awang, N.; Ismail, A.; Jaafar, J.; Matsuura, T.; Junoh, H.; Othman, M.H.D.; Rahman, M. Functionalization of polymeric materials as a high performance membrane for direct methanol fuel cell: A review. React. Funct. Polym. 2015, 86, 248–258. [Google Scholar] [CrossRef]
- Sacca, A.; Gatto, I.; Carbone, A.; Pedicini, R.; Passalacqua, E. ZrO2–Nafion composite membranes for polymer electrolyte fuel cells (PEFCs) at intermediate temperature. J. Power Source 2006, 163, 47–51. [Google Scholar] [CrossRef]
- D’Epifanio, A.; Navarra, M.A.; Weise, F.C.; Mecheri, B.; Farrington, J.; Licoccia, S.; Greenbaum, S. Composite Nafion/Sulfated Zirconia Membranes: Effect of the Filler Surface Properties on Proton Transport Characteristics. Chem. Mater. 2009, 22, 813–821. [Google Scholar] [CrossRef] [Green Version]
- Giffin, G.A.; Piga, M.; Lavina, S.; Navarra, M.A.; D’Epifanio, A.; Scrosati, B.; Di Noto, V. Characterization of sulfated-zirconia/Nafion® composite membranes for proton exchange membrane fuel cells. J. Power Source 2012, 198, 66–75. [Google Scholar] [CrossRef]
- Navarra, M.; Abbati, C.; Scrosati, B. Properties and fuel cell performance of a Nafion-based, sulfated zirconia-added, composite membrane. J. Power Source 2008, 183, 109–113. [Google Scholar] [CrossRef]
- Ren, S.; Sun, G.; Li, C.; Song, S.; Xin, Q.; Yang, X. Sulfated zirconia–Nafion composite membranes for higher temperature direct methanol fuel cells. J. Power Source 2006, 157, 724–726. [Google Scholar] [CrossRef]
- Tominaka, S.; Momma, T.; Scrosati, B.; Osaka, T. Sulfated zirconia as a proton conductor for fuel cells: Stability to hydrolysis and influence on catalysts. J. Power Source 2010, 195, 4065–4071. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhang, H.; Hu, J.; Yi, B. Preparation and characterization of sulfated zirconia (SO42−/ZrO2)/Nafion composite membranes for PEMFC operation at high temperature/low humidity. J. Membr. Sci. 2006, 280, 148–155. [Google Scholar] [CrossRef]
- Deshmukh, K.; Ahamed, M.B.; Sadasivuni, K.K.; Ponnamma, D.; Deshmukh, R.R.; Pasha, S.K.K.; AlMaadeed, M.A.-A.; Chidambaram, K. Graphene oxide reinforced polyvinyl alcohol/polyethylene glycol blend composites as high-performance dielectric material. J. Polym. Res. 2016, 23, 1–13. [Google Scholar] [CrossRef]
- Li, A.; Xiao, L.; Jiang, Z.; Tian, X.; Luo, L.; Liu, W.; Xu, Z.L.; Yang, H.; Jiang, Z.J. Sulfonic acid functionalized graphene oxide paper sandwiched in sulfonatedpoly (ether ether ketone): A proton exchange membrane with high performance for semi-passive direct methanol fuel cells. Int. J. Hydrog. Energy 2017, 42, 16731–16740. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, S.; Du, Y.; Yuan, L.; Wang, S.; Yang, J.; Deng, F.; Xiao, F. Solvent-Free Preparation of Nanosized Sulfated Zirconia with Brønsted Acidic Sites from a Simple Calcination. J. Phys. Chem. B 2005, 109, 2567–2572. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.H.; Elessawy, N.A.; Toghan, A. Novel crosslinked sulfonated PVA/PEO doped with phosphated titanium oxide nanotubes as effective green cation exchange membrane for direct borohydride fuel cells. Polymers 2021, 13, 2050. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.H.; Konsowa, A.H.; Farag, H.A.; Elessawy, N.A.; Tamer, T.M.; Eldin, M.S.M. Development novel eco-friendly proton exchange membranes doped with nano sulfated zirconia for direct methanol fuel cells. J. Polym. Res. 2021, 28, 1–10. [Google Scholar] [CrossRef]
- Parnian, M.J.; Rowshanzamir, S.; Moghaddam, J.A. Investigation of physicochemical and electrochemical properties of recast Nafionnanocomposite membranes using different loading of zirconia nanoparticlesfor proton exchange membrane fuel cell applications. Mater. Sci. Energy Technol. 2018, 1, 146–154. [Google Scholar]
- Yu, X.; Qiang, L. Preparation for Graphite Materials and Study on Electrochemical Degradation of Phenol by Graphite Cathodes. Adv. Mater. Phys. Chem. 2012, 2, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Mossayebi, Z.; Parnian, M.P.; Rowshanzamir, S. Effect of the Sulfated Zirconia Nanostructure Characteristics on Physicochemical and Electrochemical Properties of SPEEK Nanocompo-site Membranes for PEM Fuel Cell Applications. Macromol. Mater. Eng. 2018, 303, 1700570. [Google Scholar] [CrossRef]
- Kowsari, E.; Zare, A.; Ansari, V. Phosphoric acid-doped ionic liquid-functionalized graphene oxide/sulfonated polyimide composites as proton exchange membrane. Int. J. Hydrog. Energy 2015, 40, 13964–13978. [Google Scholar] [CrossRef]
- Bayer, T.; Cunning, B.V.; Selyanchyn, R.; Daio, T.; Nishihara, M.; Fujikawa, S.; Sasaki, K.; Lyth, S.M. Alkaline anion exchange membranes based on KOH-treated multilayer graphene oxide. J. Membr. Sci. 2016, 508, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Pandey, R.; Shahi, V. Sulphonatedimidized graphene oxide (SIGO) based polymer electrolyte membrane for improved water retention, stability and proton conductivity. J. Power Source 2015, 299, 104–113. [Google Scholar] [CrossRef]
- Shirdast, A.; Sharif, A.; Abdollahi, M. Effect of the incorporation of sulfonated chitosan/sulfonated graphene oxide on the proton conductivity of chitosan membranes. J. Power Source 2016, 306, 541–551. [Google Scholar] [CrossRef]
- Beydaghi, H.; Javanbakht, M.; Kowsari, E. Synthesis and Characterization of Poly(vinyl alcohol)/Sulfonated Graphene Oxide Nanocomposite Membranes for Use in Proton Exchange Membrane Fuel Cells (PEMFCs). Ind. Eng. Chem. Res. 2014, 53, 16621–16632. [Google Scholar] [CrossRef]
- Qiu, X.; Dong, T.; Ueda, M.; Zhang, X.; Wang, L. Sulfonated reduced graphene oxide as a conductive layer in sulfonated poly(ether ether ketone) nanocomposite membranes. J. Membr. Sci. 2017, 524, 663–672. [Google Scholar] [CrossRef]
- Cheng, T.; Feng, M.; Huang, Y.; Liu, X. SGO/SPEN-based highly selective polymer electrolyte membranes for direct methanol fuel cells. Ionics 2017, 23, 2143–2152. [Google Scholar] [CrossRef]
- Luo, T.; Xu, H.; Li, Z.; Gao, S.; Fang, Z.; Zhang, Z.; Wang, F.; Ma, B.; Zhu, C. Novel proton conducting membranes based on copolymers containing hydroxylated poly(ether ether ketone) and sulfonated polystyrenes. J. Appl. Polym. Sci. 2017, 134, 1–8. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouda, M.H.; Elessawy, N.A.; Al-Hussain, S.A.; Toghan, A. Design of Promising Green Cation-Exchange-Membranes-Based Sulfonated PVA and Doped with Nano Sulfated Zirconia for Direct Borohydride Fuel Cells. Polymers 2021, 13, 4205. https://doi.org/10.3390/polym13234205
Gouda MH, Elessawy NA, Al-Hussain SA, Toghan A. Design of Promising Green Cation-Exchange-Membranes-Based Sulfonated PVA and Doped with Nano Sulfated Zirconia for Direct Borohydride Fuel Cells. Polymers. 2021; 13(23):4205. https://doi.org/10.3390/polym13234205
Chicago/Turabian StyleGouda, Marwa H., Noha A. Elessawy, Sami A. Al-Hussain, and Arafat Toghan. 2021. "Design of Promising Green Cation-Exchange-Membranes-Based Sulfonated PVA and Doped with Nano Sulfated Zirconia for Direct Borohydride Fuel Cells" Polymers 13, no. 23: 4205. https://doi.org/10.3390/polym13234205
APA StyleGouda, M. H., Elessawy, N. A., Al-Hussain, S. A., & Toghan, A. (2021). Design of Promising Green Cation-Exchange-Membranes-Based Sulfonated PVA and Doped with Nano Sulfated Zirconia for Direct Borohydride Fuel Cells. Polymers, 13(23), 4205. https://doi.org/10.3390/polym13234205






