Structure Optimization of Cellulose Nanofibers/Poly(Lactic Acid) Composites by the Sizing of AKD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanocomposites
2.2.1. AKD Modification of Cellulose Nanofibers (AKD-CNF)
2.2.2. AKD-CNF/PLA Composites Preparation
2.3. Characterization
3. Results
3.1. Surface Modificaton of Cellulose Nanofibers
3.2. XRD Characterization
3.3. FTIR Characterization
3.4. TGA Analysis
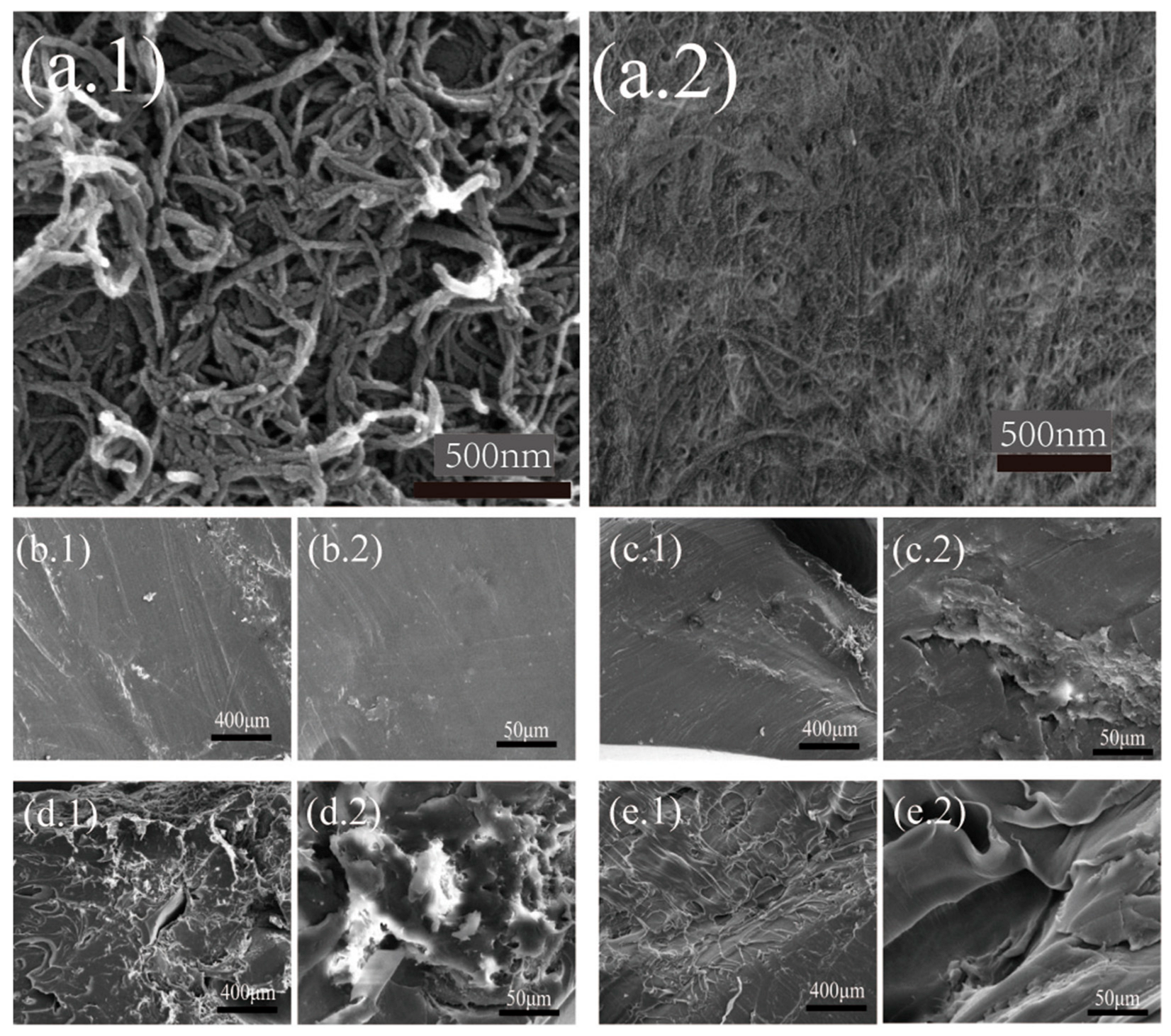
3.5. Morphology of the AKD-CNF/PLA Nanocomposites
3.6. Mechanical Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Subbaraman, R.; Tripkovic, D.; Chang, K.C.; Strmcnik, D.; Paulikas, A.P.; Hirunsit, P.; Chan, M.; Greeley, J.; Stamenkovic, V.; Narkovic, N.M. Trends in activity for the water electrolyser reactions on 3dM(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nat. Mater. 2012, 11, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. Engl. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic acid technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [Green Version]
- Abe, S.; Takagi, M. Simultaneous saccharification and fermentation of cellulose to lactic acid. Biotechnol. Bioeng. 1990, 37, 93–96. [Google Scholar] [CrossRef]
- Adsul, M.G.; Varma, A.J.; Gokhale, D.V. Lactic acid production from waste sugarcane bagasse derived cellulose. Green Chem. 2007, 9, 58–62. [Google Scholar] [CrossRef]
- Venkatesh, K.V. Simultaneous saccharification and fermentation of cellulose to lactic acid. Bioresour. Technol. 1997, 62, 91–98. [Google Scholar] [CrossRef]
- Johnson, C.M.; Sugiharto, A.B.; Roke, S. Surface and bulk structure of poly-(lactic acid) films studied by vibrational sum frequency generation spectroscopy. Chem. Phys. Lett. 2007, 449, 191–195. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(lactic acid). In Bio-Based Plastics: Materials and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013; Chapter 8; pp. 171–239. [Google Scholar]
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable antimicrobial food packaging: Trends and perspectives. Foods 2020, 9, 1438. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.K.; Koh, J.J.; Thitsartarn, W.; Zhang, X.; Kong, J.; Lin, T.T.; Li, Z.; He, C. Bend, twist, and turn: First bendable and malleable toughened PLA green composites. Adv. Funct. Mater. 2020, 30, 2001565. [Google Scholar] [CrossRef]
- Bogard, F.; Bach, T.; Abbes, B.; Bliard, C.; Maalouf, C.; Bogard, V.; Beaumont, F.; Polidori, G. A comparative review of Nettle and Ramie fiber and their use in biocomposites, particularly with a PLA matrix. J. Nat. Fibers 2021, 1–25. [Google Scholar] [CrossRef]
- Vinod, A.; Sanjay, M.R.; Suchart, S.; Jyotishhumar, P. Renewable and sustainable biobased materials: An assessment on biofibers, biofilms, biopolymers and biocomposites. J. Clean. Prod. 2020, 258, 120978. [Google Scholar] [CrossRef]
- Qian, S.; Sheng, K. PLA toughened by bamboo cellulose nanowhiskers: Role of silane compatibilization on the PLA bionanocomposite properties. Compos. Sci. Technol. 2017, 148, 59–69. [Google Scholar] [CrossRef]
- Gazzotti, S.; Farina, H.; Lesma, G.; Rampazzo, R.; Piergiovanni, L.; Ortenzi, M.A.; Silvani, A. Polylactide/cellulose nanocrystals: The in situ polymerization approach to improved nanocomposites. Eur. Polym. J. 2017, 94, 173–184. [Google Scholar] [CrossRef]
- Trifol, J.; Plackett, D.; Sillard, C.; Szabo, P.; Bras, J.; Daugaard, A.E. Hybrid poly(lactic acid)/nanocellulose/nanoclay composites with synergistically enhanced barrier properties and improved thermomechanical resistance. Polym. Int. 2016, 65, 988–995. [Google Scholar] [CrossRef] [Green Version]
- Oksman, K.; Aitomaki, Y.; Mathew, A.P.; Siqueira, G.; Zhou, Q.; Butylina, S.; Tanpichai, S.; Zhou, X.; Hooshmand, S. Review of the recent developments in cellulose nanocomposite processing. Compos. Part A Appl. Sci. Manuf. 2016, 83, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Hydroxypropyl cellulose films filled with halloysite nanotubes/wax hybrid microspheres. Ind. Eng. Chem. Res. 2021, 60, 1656–1665. [Google Scholar] [CrossRef]
- Prochon, M.; Dzeikala, O. Biopolymer composites as an alternative to materials for the production of ecological packaging. Polymers 2021, 13, 592. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Xu, X.; Wang, S. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef] [Green Version]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Effects of halloysite content on the thermo-mechanical performances of composite bioplastics. Appl. Clay Sci. 2020, 185, 105416. [Google Scholar] [CrossRef] [Green Version]
- Akbari, A.; Majumder, M.; Tehrani, A. Polylactic Acid (PLA) carbon nanotube nanocomposites. In Handbook of Polymer Nanocomposites. Processing, Performance and Application, 1st ed.; Kar, K.K., Pandey, J.K., Rana, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Volume B: Carbon Nanotube Based Polymer Composite, pp. 283–297. [Google Scholar]
- Lizundia, E.; Fortunati, E.; Dominici, F.; Vilas, J.L.; Leon, L.M.; Armentano, I.; Rorre, L.; Kenny, J.M. PLLA-grafted cellulose nanocrystals: Role of the CNC content and grafting on the PLA bionanocomposite film properties. Carbohydr. Polym. 2016, 142, 105–113. [Google Scholar] [CrossRef]
- Basilissi, L.; Silvestro, G.D.; Farina, H.; Ortenzi, M.A. Synthesis and characterization of PLA nanocomposites containing nanosilica modified with different organosilanes II: Effect of the organosilanes on the properties of nanocomposites: Thermal characterization. J. Appl. Polym. Sci. 2013, 128, 3057–3063. [Google Scholar] [CrossRef]
- Gazzotti, S.; Rampazzo, R.; Hakkarainen, M.; Bussini, D.; Ortenzi, M.A.; Farina, H.; Lesma, G.; Silvani, A. Cellulose nanofibrils as reinforcing agents for PLA-based nanocomposites: An in situ approach. Compos. Sci. Technol. 2019, 171, 94–102. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Zhou, Q.; Iannoni, A.; Saino, E.; Visai, L.; Berglund, L.A.; Kenny, J.M. Multifunctional bionanocomposite films of poly(lactic acid), cellulose nanocrystals and silver nanoparticles. Carbohyd. Polym. 2012, 87, 1596–1605. [Google Scholar] [CrossRef]
- Khosravi, A.; Fereidoon, A.; Khorasani, M.M.; Naderi, G.; Ganjali, M.R.; Zarrintaj, P.; Saeb, M.R.; Gutierrez, T.J. Soft and hard sections from cellulose-reinforced poly(lactic acid)-based food packaging films: A critical review. Food Packag. Shelf Life 2020, 23, 100429. [Google Scholar] [CrossRef]
- Dhar, P.; Tarafder, D.; Kumar, A.; Katiyar, V. Thermally recyclable polylactic acid/cellulose nanocrystal films through reactive extrusion process. Polymer 2016, 87, 268–282. [Google Scholar] [CrossRef]
- Li, L.; Neivandt, D.J. The mechanism of Alkyl Ketene Dimer (AKD) sizing on cellulose model films studied by sum frequency generation vibrational spectroscopy. Cellulose 2019, 26, 3415–3435. [Google Scholar] [CrossRef]
- Kumar, S.; Chauhan, V.S.; Chakrabarti, S.K. Separation and analysis techniques for bound and unbound Alkyl Ketene Dimer (AKD) in paper: A review. Arab. J. Chem. 2016, 9, S1636–S1642. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.C. Paper Chemistry; Cahpaman and Hall: New York, NY, USA, 1991; pp. 192–214. [Google Scholar]
- Garnier, G.; Wright, J.; Godbout, L.; Yu, L. Wetting mechanism of alkyl ketene dimers on cellulose films. Colloids Surf. A Physicochem. Eng. Asp. 1998, 145, 153–165. [Google Scholar] [CrossRef]


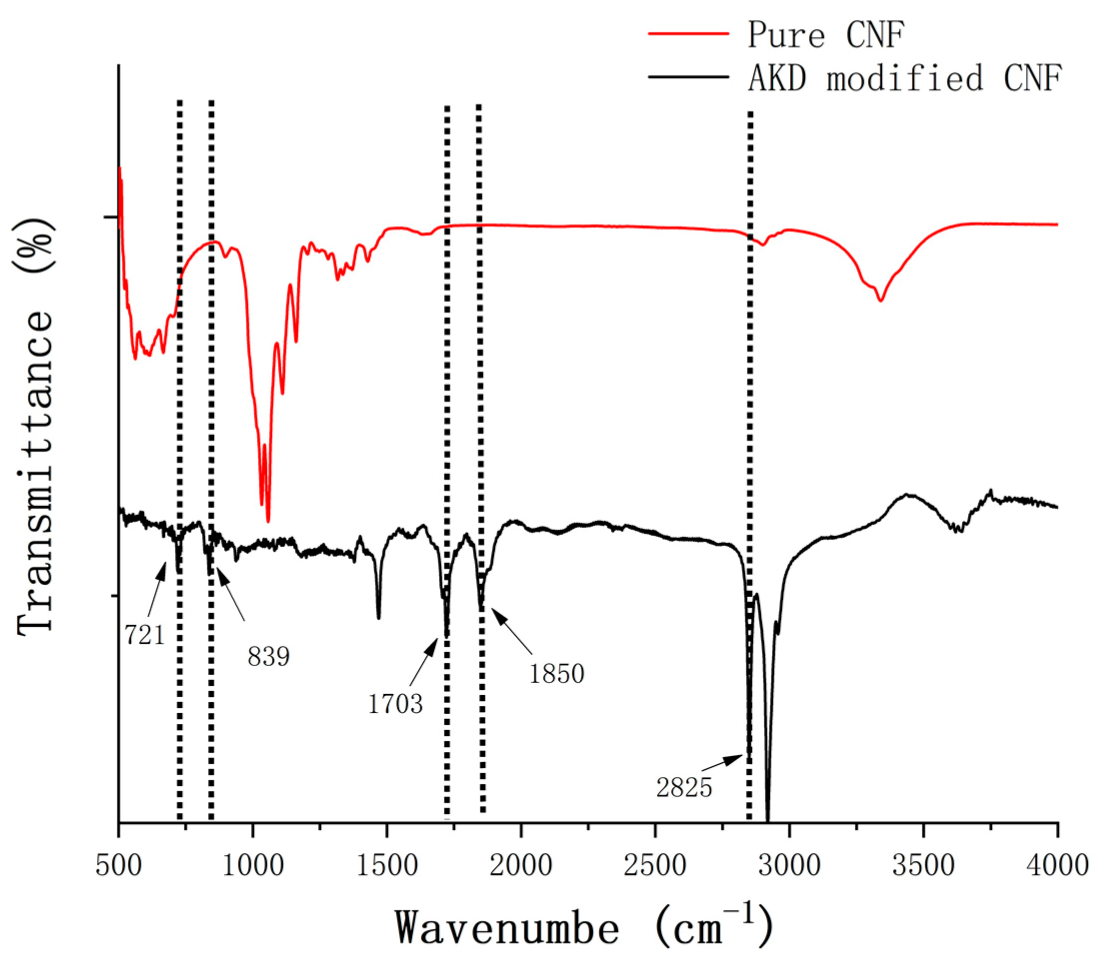


| Pure CNF | AKD Modified CNF | ||
|---|---|---|---|
| Wavenumber (cm−1) | Vibrational Band Assignment | Wavenumber (cm−1) | Vibrational Band Assignment |
| 896 | β-glucosidic bond | 721 | cis alkene sp2 C-H bend |
| 1063 | C-O stretching | 839 | In-plane bending of C-H |
| 1376 | C-H stretching | 1703 | ν C = O |
| 1645 | O-H bending | 1850 | ν C = O |
| 2920 | C-H stretching | 2825 | ν CH3 |
| 3406 | Hydrogen bonded O-H stretching | ||
| Sample | Temperature at DTG Peaks (°C) | Temperature at CNF Decomposition (°C) |
|---|---|---|
| PLA | 402.6 | N/A |
| 1 wt.% AKD-CNF/PLA | 391 | 356 |
| 2.5 wt.% AKD-CNF/PLA | 394 | 359 |
| 5 wt.% AKD-CNF/PLA | 396.3 | 361 |
| 1 wt.% CNF/PLA | 319 | 300.6 |
| 2.5 wt.% CNF/PLA | 327.6 | 318.9 |
| 5 wt.% CNF/PLA | 344 | 325 |
| Sample | PLA | 1 wt.% AKD-CNF/PLA | 2.5 wt.% AKD-CNF/PLA | 5 wt.% AKD-CNF/PLA | |
|---|---|---|---|---|---|
| Parameter | |||||
| Stress at breaking point (MPa) | 80 | 321 | 380 | 427 | |
| Ultimate elongation (%) | 2.28 | 3.37 | 2.10 | 2.08 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Cao, M.; Li, J.; Wang, C.; Li, S. Structure Optimization of Cellulose Nanofibers/Poly(Lactic Acid) Composites by the Sizing of AKD. Polymers 2021, 13, 4119. https://doi.org/10.3390/polym13234119
Li L, Cao M, Li J, Wang C, Li S. Structure Optimization of Cellulose Nanofibers/Poly(Lactic Acid) Composites by the Sizing of AKD. Polymers. 2021; 13(23):4119. https://doi.org/10.3390/polym13234119
Chicago/Turabian StyleLi, Lei, Minjian Cao, Jingdan Li, Cong Wang, and Shengjuan Li. 2021. "Structure Optimization of Cellulose Nanofibers/Poly(Lactic Acid) Composites by the Sizing of AKD" Polymers 13, no. 23: 4119. https://doi.org/10.3390/polym13234119
APA StyleLi, L., Cao, M., Li, J., Wang, C., & Li, S. (2021). Structure Optimization of Cellulose Nanofibers/Poly(Lactic Acid) Composites by the Sizing of AKD. Polymers, 13(23), 4119. https://doi.org/10.3390/polym13234119









