Adsorption of Paraquat by Poly(Vinyl Alcohol)-Cyclodextrin Nanosponges
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
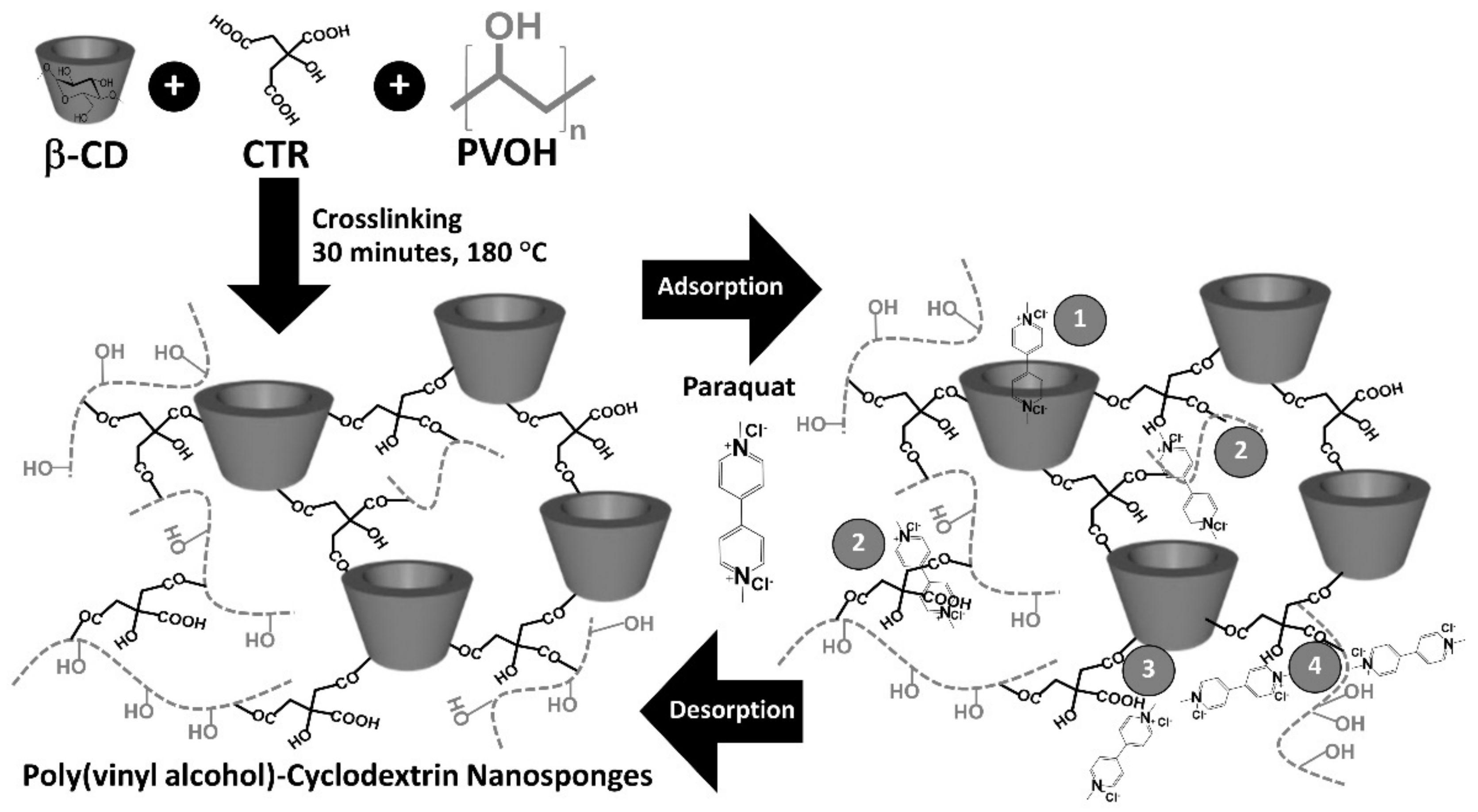
2.2. Nanosponges Preparation
2.3. Nanosponges Characterization
2.4. Adsorption Study
2.4.1. Preliminary Adsorption Study
2.4.2. Kinetics Study
2.4.3. Isotherm Study
2.4.4. Reusability Study
3. Results and Discussion
3.1. Preparation and Characterization of Nanosponges
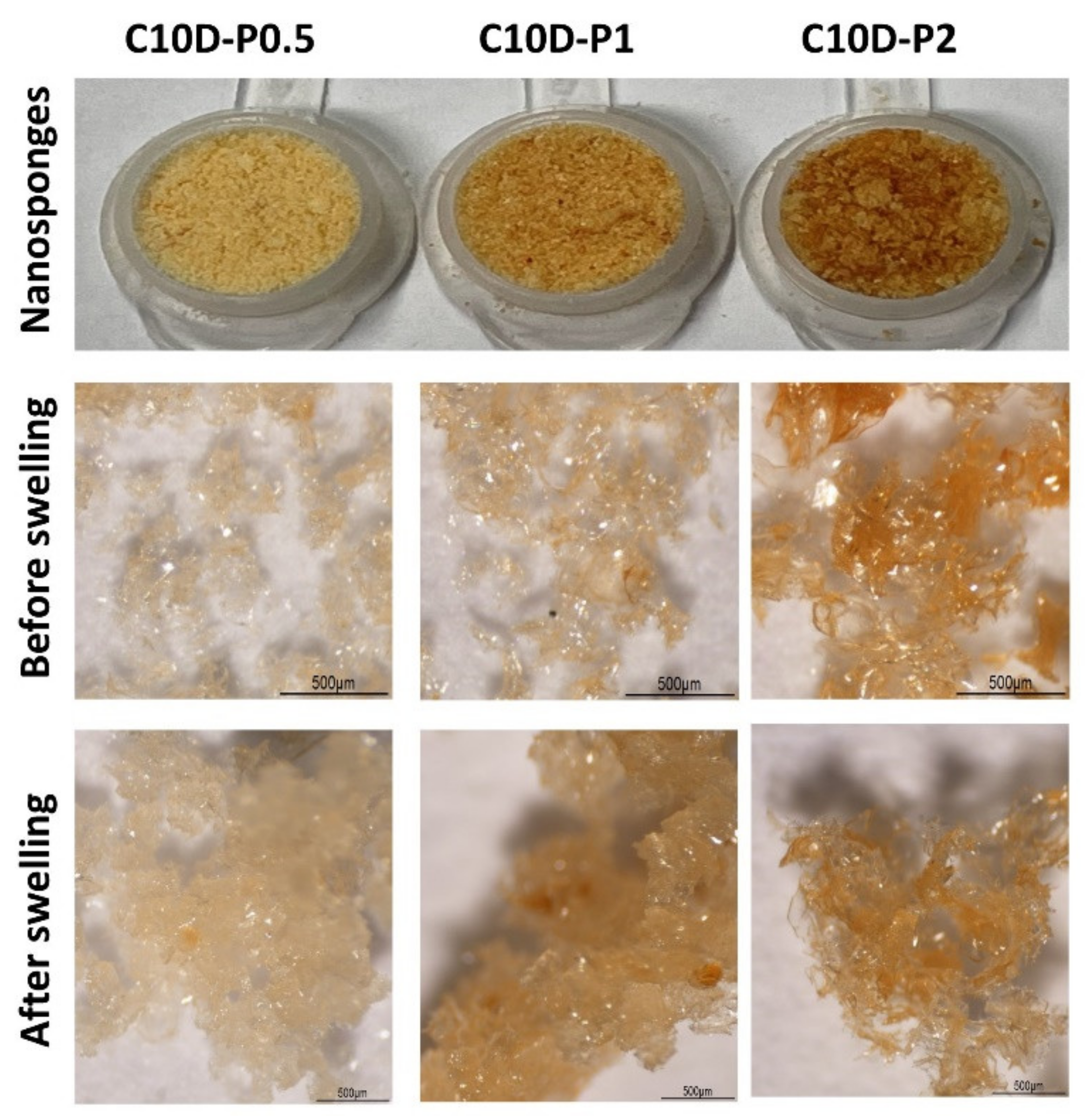
3.1.1. Physicochemical Properties of Nanosponges
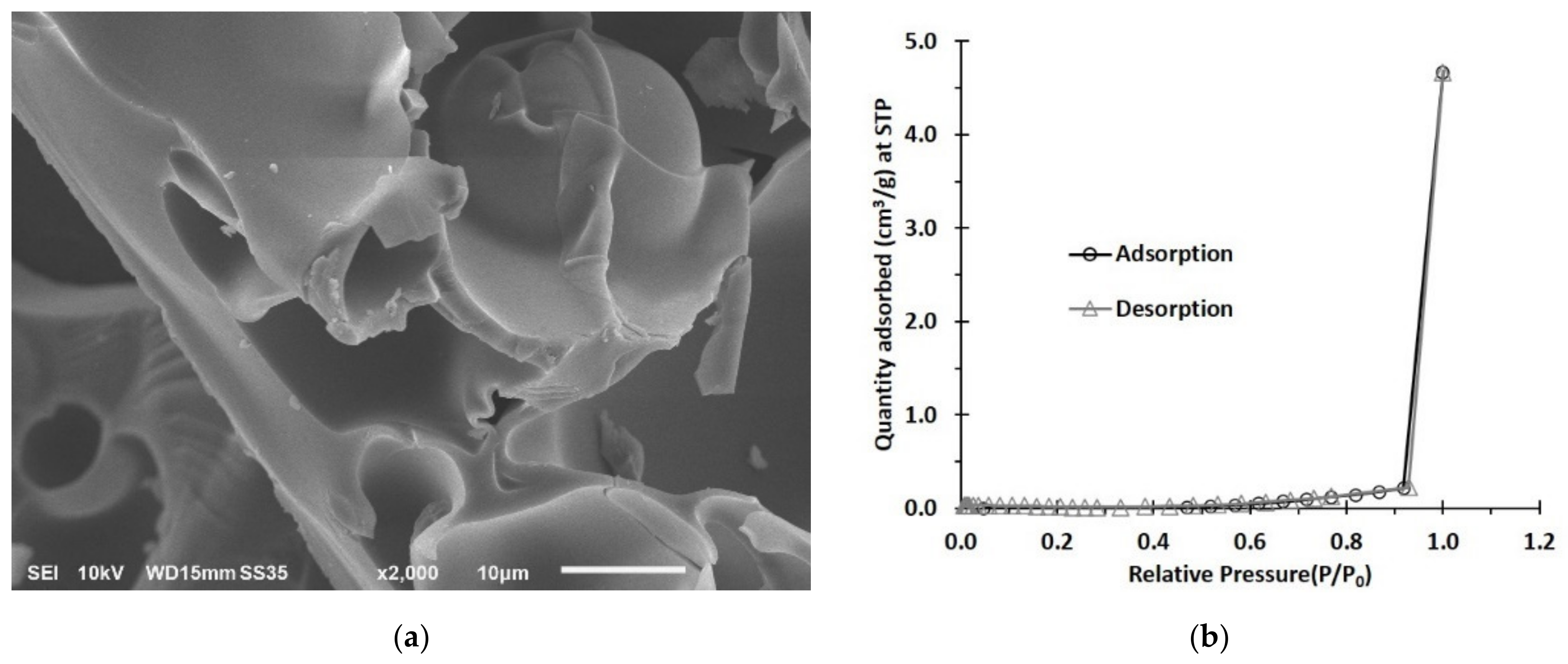
3.1.2. SEM and BET Investigation
3.1.3. TGA Analysis
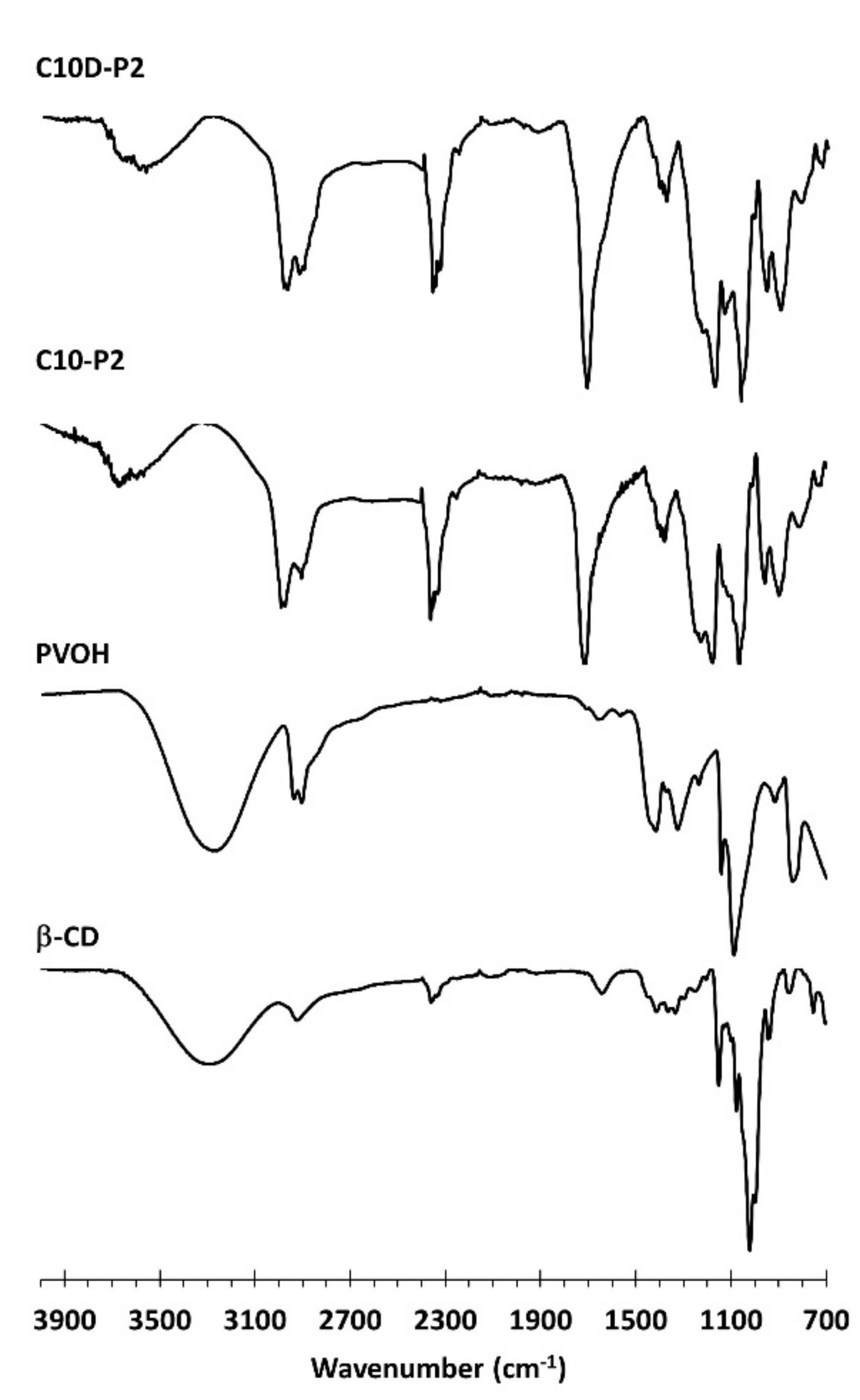
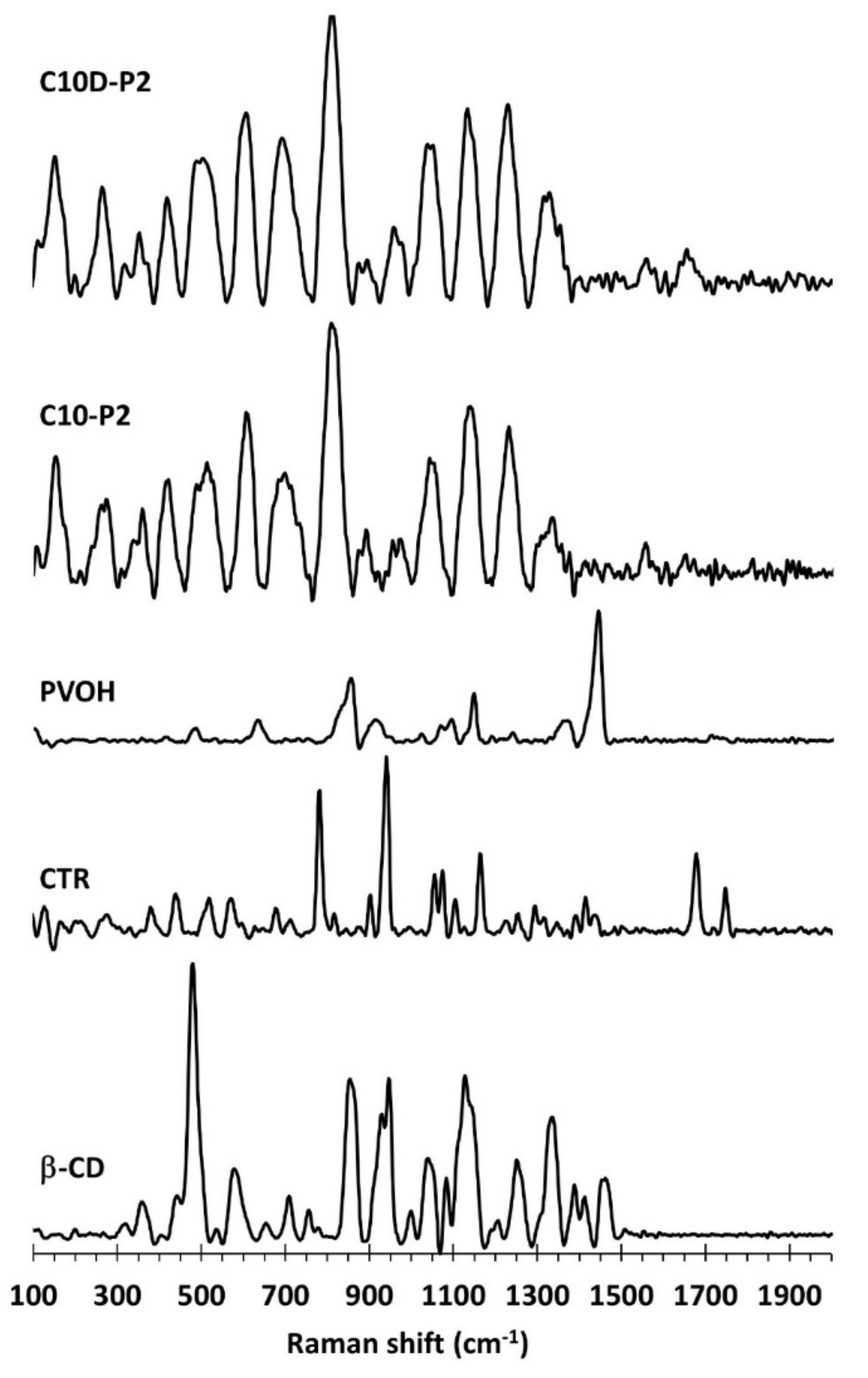
3.1.4. ATR-FTIR and Raman Exploration
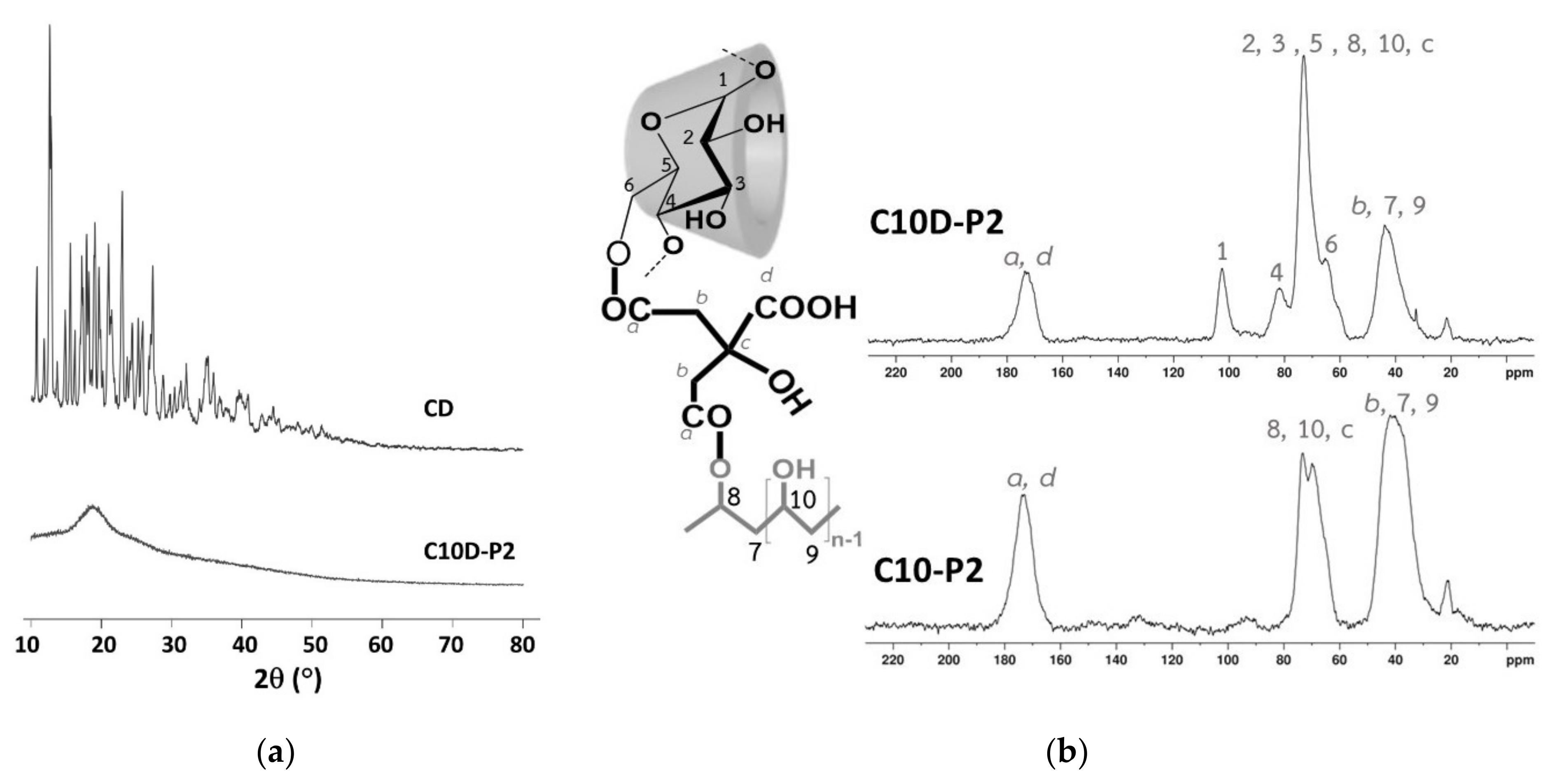
3.1.5. XRD and NMR Characterization
3.2. Adsorption Study
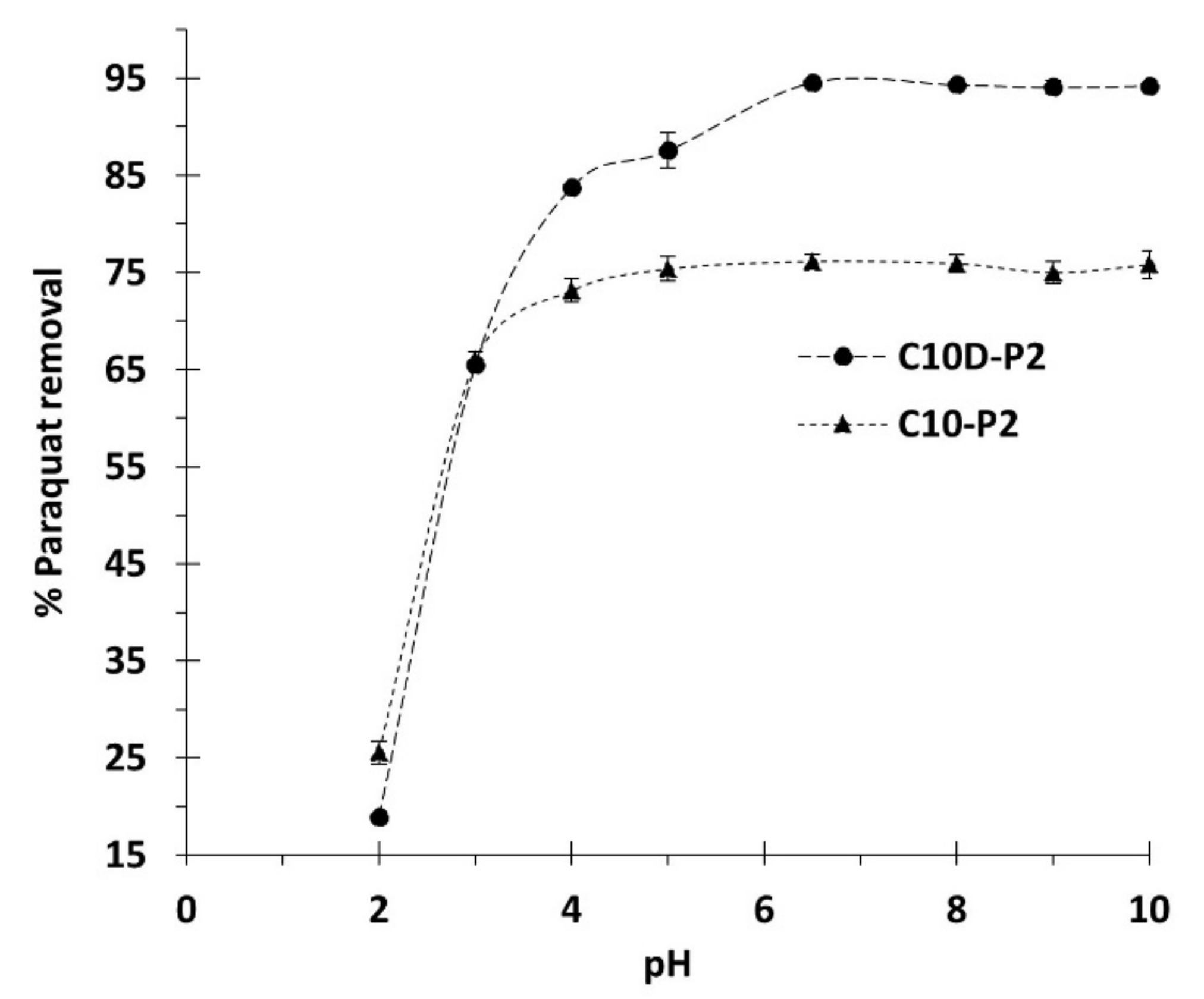
3.2.1. Preliminary Adsorption Study
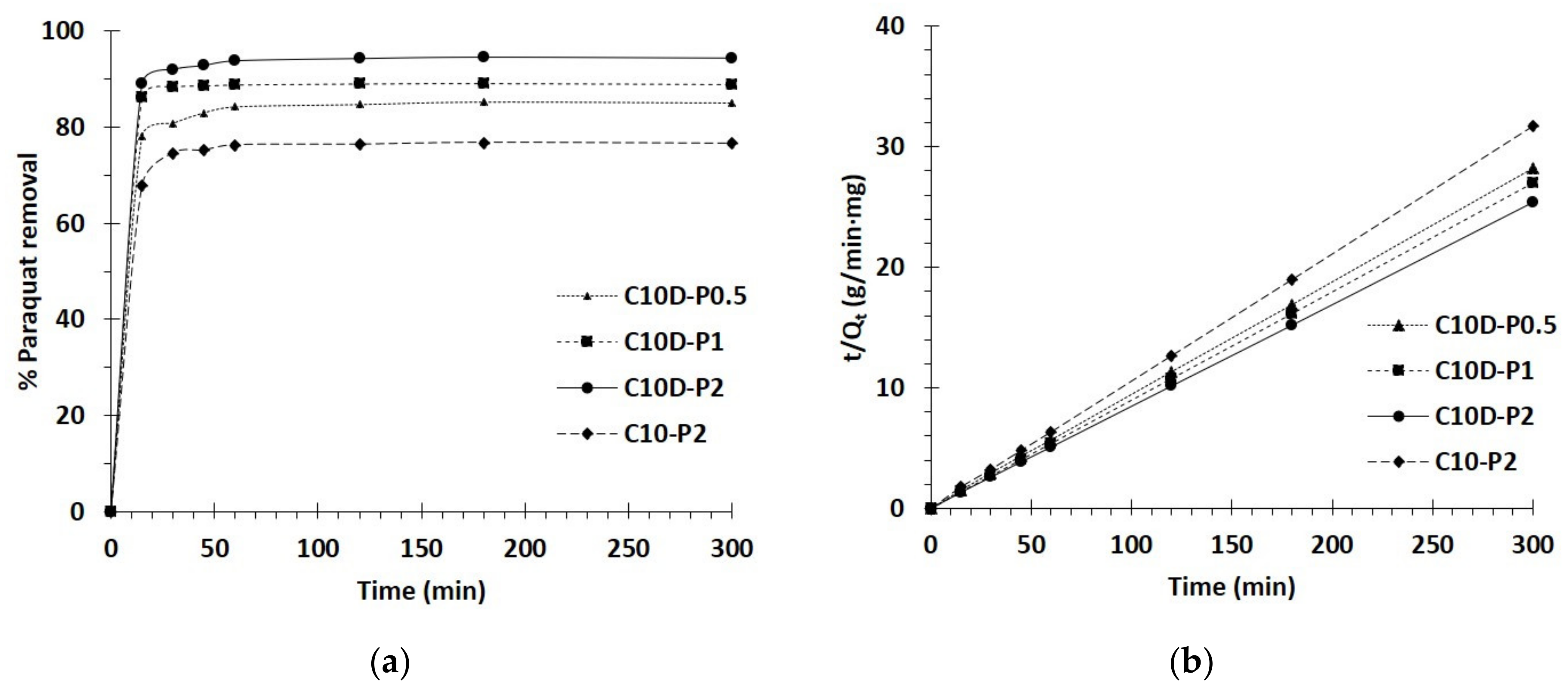
3.2.2. Kinetics Study
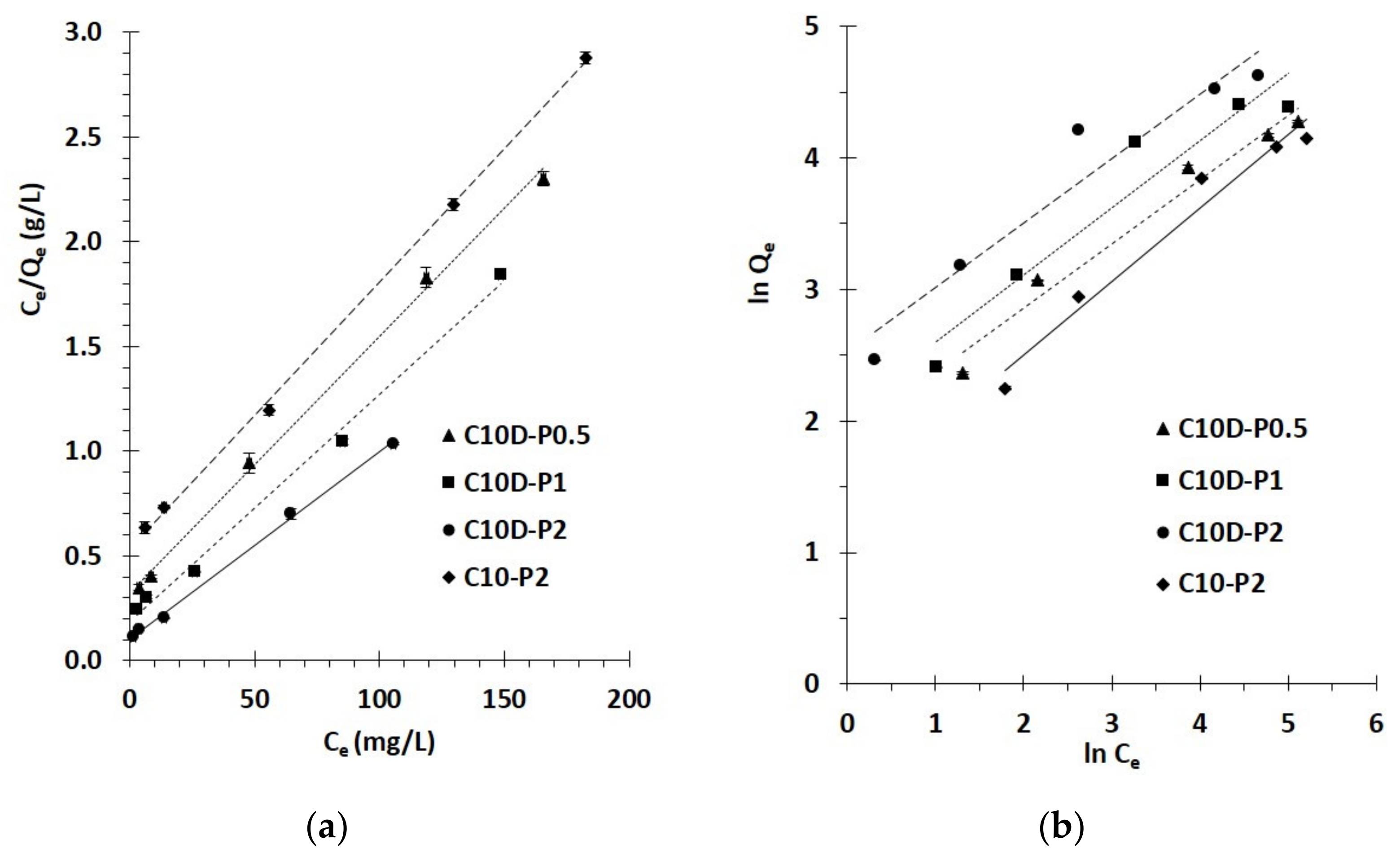
3.2.3. Isotherm Study
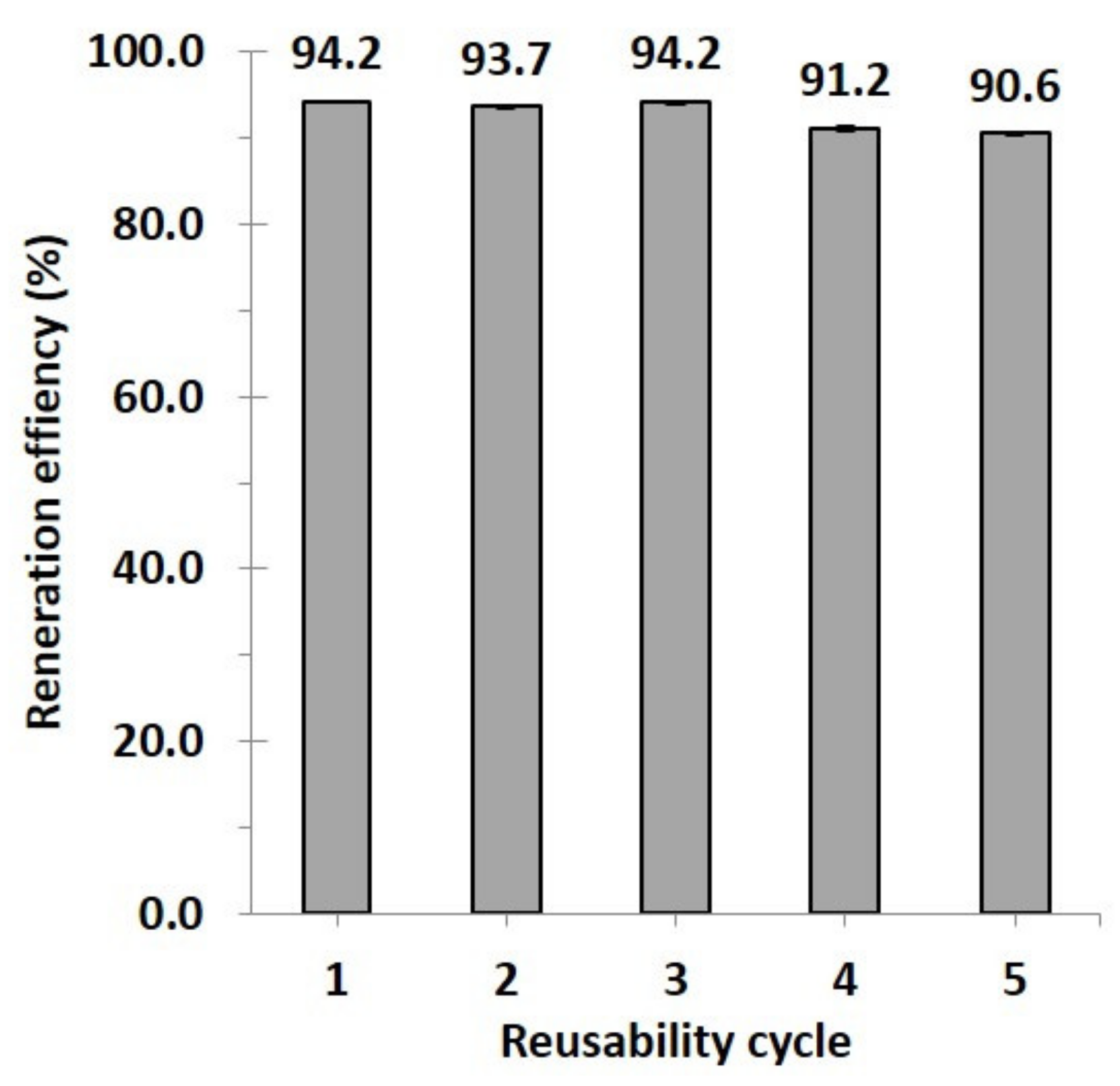
3.2.4. Reusability Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Núñez, O.; Kim, J.-B.; Moyano, E.; Galceran, M.T.; Terabe, S. Analysis of the Herbicides Paraquat, Diquat and Difenzoquat in Drinking Water by Micellar Electrokinetic Chromatography Using Sweeping and Cation Selective Exhaustive Injection. J. Chromatogr. A 2002, 961, 65–75. [Google Scholar] [CrossRef]
- Recena, M.C.P.; Caldas, E.D.; Pires, D.X.; Pontes, E.R.J.C. Pesticides Exposure in Culturama, Brazil—Knowledge, Attitudes, and Practices. Environ. Res. 2006, 102, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhan, H.; Bhatt, P.; Chen, S. Paraquat Degradation From Contaminated Environments: Current Achievements and Perspectives. Front. Microbiol. 2019, 10, 1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennis, P.G.; Kukulies, T.; Forstner, C.; Orton, T.G.; Pattison, A.B. The Effects of Glyphosate, Glufosinate, Paraquat and Paraquat-Diquat on Soil Microbial Activity and Bacterial, Archaeal and Nematode Diversity. Sci. Rep. 2018, 8, 2119. [Google Scholar] [CrossRef] [Green Version]
- Elenga, N.; Merlin, C.; Le Guern, R.; Kom-Tchameni, R.; Ducrot, Y.-M.; Pradier, M.; Ntab, B.; Dinh-Van, K.-A.; Sobesky, M.; Mathieu, D.; et al. Clinical Features and Prognosis of Paraquat Poisoning in French Guiana: A Review of 62 Cases. Medicine 2018, 97, e9621. [Google Scholar] [CrossRef]
- Shadnia, S.; Ebadollahi-Natanzi, A.; Ahmadzadeh, S.; Karami-Mohajeri, S.; Pourshojaei, Y.; Rahimi, H.R. Delayed Death Following Paraquat Poisoning: Three Case Reports and a Literature Review. Toxicol. Res. 2018, 7, 745–753. [Google Scholar] [CrossRef] [Green Version]
- Jafari, F.; Moradi, S.; Nowroozi, A.; Sadrjavadi, K.; Hosseinzadeh, L.; Shahlaei, M. Exploring the Binding Mechanism of Paraquat to DNA by a Combination of Spectroscopic, Cellular Uptake, Molecular Docking and Molecular Dynamics Simulation Methods. New J. Chem. 2017, 41, 14188–14198. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, X.; Lu, G.; Zhou, J.; Kang, J.; Zhang, J. Identify the Early Predictor of Mortality in Patients with Acute Paraquat Poisoning. BioMed Res. Int. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Somu, B.; Halkur Shankar, S.; Baitha, U.; Biswas, A. Paraquat Poisoning. QJM An. Int. J. Med. 2020, 113, 752. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-W.; Kim, D.-S. Paraquat: Toxicology and Impacts of Its Ban on Human Health and Agriculture. Weed Sci. 2020, 68, 208–213. [Google Scholar] [CrossRef]
- Predictors of Mortality in Paraquat Poisoning: A Two-Year Retrospective Analysis From A Tertiary Care Teaching Hospital in South India. IJFMT 2021, 15, 4435–4443. [CrossRef]
- Burns, I.G.; Hayes, M.H.B.; Stacey, M. Studies of the Adsorption of Paraquat on Soluble Humic Fractions by Gel Filtration and Ultrafiltration Techniques. Pestic. Sci. 1973, 4, 629–641. [Google Scholar] [CrossRef]
- Humbert, H.; Gallard, H.; Suty, H.; Croué, J.-P. Natural Organic Matter (NOM) and Pesticides Removal Using a Combination of Ion Exchange Resin and Powdered Activated Carbon (PAC). Water Res. 2008, 42, 1635–1643. [Google Scholar] [CrossRef]
- Jindakaraked, M.; Khan, E.; Kajitvichyanukul, P. Biodegradation of Paraquat by Pseudomonas Putida and Bacillus Subtilis Immobilized on Ceramic with Supplemented Wastewater Sludge. Environ. Pollut. 2021, 286, 117307. [Google Scholar] [CrossRef]
- Thongpitak, J.; Pumas, P.; Pumas, C. Paraquat Degradation by Biological Manganese Oxide (BioMnOx) Catalyst Generated From Living Microalga Pediastrum Duplex AARL G060. Front. Microbiol. 2020, 11, 575361. [Google Scholar] [CrossRef]
- Ghavi, A.; Bagherian, G.; Rezaei-Vahidian, H. Degradation of Paraquat Herbicide Using Hybrid AOP Process: Statistical Optimization, Kinetic Study, and Estimation of Electrical Energy Consumption. Environ. Sci. Eur. 2021, 33, 117. [Google Scholar] [CrossRef]
- Lenzi, G.G.; Freitas, P.; Fidelis, M.Z.; Ribeiro, M.A.; Brackmann, R.; Colpini, L.M.S.; Tusset, A.M. Paraquat Degradation by Photocatalysis: Experimental Desing and Optimization. J. Environ. Sci. Health Part B 2021, 56, 523–531. [Google Scholar] [CrossRef]
- Pourzad, A.; Sobhi, H.R.; Behbahani, M.; Esrafili, A.; Kalantary, R.R.; Kermani, M. Efficient Visible Light-Induced Photocatalytic Removal of Paraquat Using N-Doped TiO2@SiO2@Fe3O4 Nanocomposite. J. Mol. Liq. 2020, 299, 112167. [Google Scholar] [CrossRef]
- Tariq, S.R.; Chotana, G.A.; Rashid, A. Photocatalytic Degradation of Paraquat Dichloride in the Presence of ZnO.WO3 Composite. Int. J. Environ. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- M’Bra, I.C.; Atheba, G.P.; Robert, D.; Drogui, P.; Trokourey, A. Photocatalytic Degradation of Paraquat Herbicide Using a Fixed Bed Reactor Containing TiO2 Nanoparticles Coated onto β-SiC Alveolar Foams. AJAC 2019, 10, 171–184. [Google Scholar] [CrossRef] [Green Version]
- Phuinthiang, P.; Kajitvichyanukul, P. Degradation of Paraquat from Contaminated Water Using Green TiO2 Nanoparticles Synthesized from Coffea Arabica, L. in Photocatalytic Process. Water Sci. Technol. 2019, 79, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Marien, C.B.D.; Le Pivert, M.; Azaïs, A.; M’Bra, I.C.; Drogui, P.; Dirany, A.; Robert, D. Kinetics and Mechanism of Paraquat’s Degradation: UV-C Photolysis vs UV-C Photocatalysis with TiO2/SiC Foams. J. Hazard. Mater. 2019, 370, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Mehmandost, N.; García-Valverde, M.T.; Laura Soriano, M.; Goudarzi, N.; Lucena, R.; Chamjangali, M.A.; Cardenas, S. Heracleum Persicum Based Biosorbent for the Removal of Paraquat and Diquat from Waters. J. Environ. Chem. Eng. 2020, 8, 104481. [Google Scholar] [CrossRef]
- Togue Kamga, F. Modeling Adsorption Mechanism of Paraquat onto Ayous (Triplochiton Scleroxylon) Wood Sawdust. Appl. Water Sci. 2019, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Ha, N.T.H.; Toan, N.C.; Kajitvichyanukul, P. Enhanced Paraquat Removal from Contaminated Water Using Cell-Immobilized Biochar. Clean Techn. Environ. Policy 2021. [Google Scholar] [CrossRef]
- Dehgani, Z.; Sedghi asl, M.; Ghaedi, M.; Sabzehmeidani, M.M.; Adhami, E. Removal of Paraquat from Aqueous Solutions by a Bentonite Modified Zero-Valent Iron Adsorbent. New J. Chem. 2020, 44, 13368–13376. [Google Scholar] [CrossRef]
- Rasaie, A.; Sabzehmeidani, M.M.; Ghaedi, M.; Ghane-Jahromi, M.; Sedaratian-Jahromi, A. Removal of Herbicide Paraquat from Aqueous Solutions by Bentonite Modified with Mesoporous Silica. Mater. Chem. Phys. 2021, 262, 124296. [Google Scholar] [CrossRef]
- Brenes-Guillén, L.; Fuentes-Schweizer, P.; García-Piñeres, A.; Uribe-Lorío, L. Tolerance and Sorption of Bromacil and Paraquat by Thermophilic Cyanobacteria Leptolyngbya 7M from Costa Rican Thermal Springs. J. Contam. Hydrol. 2019, 226, 103539. [Google Scholar] [CrossRef]
- Mueanpun, N.; Srisuk, N.; Chaiammart, N.; Panomsuwan, G. Nanoporous Activated Carbons Derived from Water Ferns as an Adsorbent for Removal of Paraquat from Contaminated Water. Materialia 2021, 15, 100986. [Google Scholar] [CrossRef]
- Tongur, T.; Ayranci, E. Adsorption and Electrosorption of Paraquat, Diquat and Difenzoquat from Aqueous Solutions onto Activated Carbon Cloth as Monitored by in-Situ Uv–Visible Spectroscopy. J. Environ. Chem. Eng. 2021, 9, 105566. [Google Scholar] [CrossRef]
- Mohammadzadeh Kakhki, R.; Karimian, A.; Saadati Rad, M. Highly Efficient Removal of Paraquat Pesticide from Aqueous Solutions Using a Novel Nano Kaolin Modified with Sulfuric Acid via Host–Guest Interactions. J. Incl. Phenom. Macrocycl. Chem. 2020, 96, 307–313. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Zhang, Y.-F.; Sun, X.-W.; Li, Y.-J.; Zhang, Y.-M.; Wei, T.-B.; Yao, H.; Lin, Q. Linear Tri-Pillar[5]Arene-Based Acceptor for Efficiently Separate Paraquat from Water through Collaboration Effect. Mater. Sci. Eng. C 2021, 118, 111358. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Wang, Z.-H.; Yao, X.-Q.; Zhang, Y.-M.; Wei, T.-B.; Yao, H.; Lin, Q. Novel Tripodal-Pillar[5]Arene-Based Chemical Sensor for Efficient Detection and Removal Paraquat by Synergistic Effect. Sens. Actuators B Chem. 2021, 327, 128885. [Google Scholar] [CrossRef]
- Shetty, D.; Boutros, S.; Skorjanc, T.; Garai, B.; Asfari, Z.; Raya, J.; Trabolsi, A. Fast and Efficient Removal of Paraquat in Water by Porous Polycalix[n]Arenes (n = 4, 6, and 8). J. Mater. Chem. A 2020, 8, 13942–13945. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Mansour Lakouraj, M.; Hasantabar, V. Synthesis and Characterization of Amphiphilic Star Copolymer of Polyaniline and Polyacrylic Acid Based on Calix[4]Resorcinarene as an Efficient Adsorbent for Removal of Paraquat Herbicide from Water. Mater. Today Commun. 2020, 25, 101523. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, S.; Nisa, K.; Kumar Sharma, D. Efficient System for Encapsulation and Removal of Paraquat and Diquat from Aqueous Solution: 4-Sulfonatocalix[n]Arenes and Its Magnetite Modified Nanomaterials. J. Environ. Chem. Eng. 2019, 7, 103130. [Google Scholar] [CrossRef]
- Dehghani, Z.; Sedghi-Asl, M.; Ghaedi, M.; Sabzehmeidani, M.M.; Adhami, E. Ultrasound-Assisted Adsorption of Paraquat Herbicide from Aqueous Solution by Graphene Oxide/Mesoporous Silica. J. Environ. Chem. Eng. 2021, 9, 105043. [Google Scholar] [CrossRef]
- Li, H.; Qi, H.; Yin, M.; Chen, Y.; Deng, Q.; Wang, S. Carbon Tubes from Biomass with Prominent Adsorption Performance for Paraquat. Chemosphere 2021, 262, 127797. [Google Scholar] [CrossRef]
- Li, H.; Miao, Q.; Chen, Y.; Yin, M.; Qi, H.; Yang, M.; Deng, Q.; Wang, S. Modified Carbon Spheres as Universal Materials for Adsorption of Cationic Harmful Substances (Paraquat and Dyes) in Water. Microporous Mesoporous Mater. 2020, 297, 110040. [Google Scholar] [CrossRef]
- Jodeh, S.; Hanbali, G.; Tighadouini, S.; Radi, S.; Hamed, O.; Jodeh, D. Removal and Extraction Efficiency of Quaternary Ammonium Herbicides Paraquat (PQ) from Aqueous Solution by Ketoenol–Pyrazole Receptor Functionalized Silica Hybrid Adsorbent (SiNPz). BMC Chem. 2019, 13, 86. [Google Scholar] [CrossRef]
- Brigante, M.; Schulz, P.C. Adsorption of Paraquat on Mesoporous Silica Modified with Titania: Effects of PH, Ionic Strength and Temperature. J. Colloid. Interface Sci. 2011, 363, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.; Soares, S.; Trindade, T.; Daniel-da-Silva, A. Magnetic Hybrid Nanosorbents for the Uptake of Paraquat from Water. Nanomaterials 2017, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Zhang, J.; He, Q.; Chen, X.; Jin, M. Fabrication of Benzenesulfonic Acid Groups Modified Magnetic Microspheres as an MSPE Adsorbent for Fast Determination of Paraquat and Diquat in Human Urine Combined with UPLC-HRMS. J. Chromatogr. B 2020, 1136, 121880. [Google Scholar] [CrossRef] [PubMed]
- Ilari, R.; Etcheverry, M.; Waiman, C.V.; Zanini, G.P. A Simple Cation Exchange Model to Assess the Competitive Adsorption between the Herbicide Paraquat and the Biocide Benzalkonium Chloride on Montmorillonite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125797. [Google Scholar] [CrossRef]
- Wang, M.; Orr, A.A.; He, S.; Dalaijamts, C.; Chiu, W.A.; Tamamis, P.; Phillips, T.D. Montmorillonites Can Tightly Bind Glyphosate and Paraquat Reducing Toxin Exposures and Toxicity. ACS Omega 2019, 4, 17702–17713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-F.; Tu, C.-W.; Lee, R.-H.; Yang, C.-H.; Hung, W.-C.; Andrew Lin, K.-Y. Study of Various Diameter and Functionality of TEMPO-Oxidized Cellulose Nanofibers on Paraquat Adsorptions. Polym. Degrad. Stab. 2019, 161, 206–212. [Google Scholar] [CrossRef]
- Asgari, S.; Sun, L.; Lin, J.; Weng, Z.; Wu, G.; Zhang, Y.; Lin, M. Nanofibrillar Cellulose/Au@Ag Nanoparticle Nanocomposite as a SERS Substrate for Detection of Paraquat and Thiram in Lettuce. Microchim. Acta 2020, 187, 390. [Google Scholar] [CrossRef]
- Junthip, J. Water-Insoluble Cyclodextrin Polymer Crosslinked with Citric Acid for Paraquat Removal from Water. J. Macromol. Sci. Part. A 2019, 56, 555–563. [Google Scholar] [CrossRef]
- Köse, K.; Tüysüz, M.; Aksüt, D.; Uzun, L. Modification of Cyclodextrin and Use in Environmental Applications. Environ. Sci. Pollut Res. 2021. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Crini, G. Environmental Applications of Water-Insoluble β-Cyclodextrin–Epichlorohydrin Polymers. Prog. Polym. Sci. 2013, 38, 344–368. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Winterton, P.; Fourmentin, S.; Wilson, L.D.; Fenyvesi, É.; Crini, G. Water-Insoluble β-Cyclodextrin–Epichlorohydrin Polymers for Removal of Pollutants from Aqueous Solutions by Sorption Processes Using Batch Studies: A Review of Inclusion Mechanisms. Prog. Polym. Sci. 2018, 78, 1–23. [Google Scholar] [CrossRef]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Rubin Pedrazzo, A.; Cecone, C.; Cavalli, R.; Trotta, F. History of Cyclodextrin Nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Tian, Y.; Liu, J. Cyclodextrin-Based Adsorbents for the Removal of Pollutants from Wastewater: A Review. Environ. Sci. Pollut. Res. 2021, 28, 1317–1340. [Google Scholar] [CrossRef]
- Cova, T.F.; Murtinho, D.; Aguado, R.; Pais, A.A.C.C.; Valente, A.J.M. Cyclodextrin Polymers and Cyclodextrin-Containing Polysaccharides for Water Remediation. Polysaccharides 2021, 2, 2. [Google Scholar] [CrossRef]
- Zhao, B.; Jiang, L.; Jia, Q. Advances in Cyclodextrin Polymers Adsorbents for Separation and Enrichment: Classification, Mechanism and Applications. Chin. Chem. Lett. 2021, S1001841721004319. [Google Scholar] [CrossRef]
- Rizzi, V.; Gubitosa, J.; Signorile, R.; Fini, P.; Cecone, C.; Matencio, A.; Trotta, F.; Cosma, P. Cyclodextrin Nanosponges as Adsorbent Material to Remove Hazardous Pollutants from Water: The Case of Ciprofloxacin. Chem. Eng. J. 2021, 411, 128514. [Google Scholar] [CrossRef]
- Rubin Pedrazzo; Smarra; Caldera; Musso; Dhakar; Cecone; Hamedi; Corsi; Trotta Eco-Friendly β-Cyclodextrin and Linecaps Polymers for the Removal of Heavy Metals. Polymers 2019, 11, 1658. [CrossRef] [Green Version]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Guernelli, S.; Noto, R.; Riela, S. Synthesis and Characterization of Halloysite–Cyclodextrin Nanosponges for Enhanced Dyes Adsorption. ACS Sustain. Chem. Eng. 2017, 5, 3346–3352. [Google Scholar] [CrossRef] [Green Version]
- Varan, C.; Anceschi, A.; Sevli, S.; Bruni, N.; Giraudo, L.; Bilgiç, E.; Korkusuz, P.; İskit, A.B.; Trotta, F.; Bilensoy, E. Preparation and Characterization of Cyclodextrin Nanosponges for Organic Toxic Molecule Removal. Int. J. Pharm. 2020, 585, 119485. [Google Scholar] [CrossRef]
- Liao, X.; Wang, B.; Zhang, Q. Synthesis of Glycopolymer Nanosponges with Enhanced Adsorption Performances for Boron Removal and Water Treatment. J. Mater. Chem. A 2018, 6, 21193–21206. [Google Scholar] [CrossRef]
- Salazar, S.; Yutronic, N.; Jara, P. Magnetic β-Cyclodextrin Nanosponges for Potential Application in the Removal of the Neonicotinoid Dinotefuran from Wastewater. IJMS 2020, 21, 4079. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Singh, P.; Singhal, A. Cyclodextrin-Based Nanostructured Materials for Sustainable Water Remediation Applications. Environ. Sci. Pollut Res. 2020, 27, 32432–32448. [Google Scholar] [CrossRef]
- Huang, W.; Hu, Y.; Li, Y.; Zhou, Y.; Niu, D.; Lei, Z.; Zhang, Z. Citric Acid-Crosslinked β-Cyclodextrin for Simultaneous Removal of Bisphenol A, Methylene Blue and Copper: The Roles of Cavity and Surface Functional Groups. J. Taiwan Inst. Chem. Eng. 2018, 82, 189–197. [Google Scholar] [CrossRef]
- Moulahcene, L.; Skiba, M.; Senhadji, O.; Milon, N.; Benamor, M.; Lahiani-Skiba, M. Inclusion and Removal of Pharmaceutical Residues from Aqueous Solution Using Water-Insoluble Cyclodextrin Polymers. Chem. Eng. Res. Des. 2015, 97, 145–158. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, L.; Zhu, C.-S.; Shen, X.; Zhang, X.; Sha, B. Comparative Study of Polymer Containing β-Cyclodextrin and –COOH for Adsorption toward Aniline, 1-Naphthylamine and Methylene Blue. J. Hazard. Mater. 2009, 171, 241–246. [Google Scholar] [CrossRef]
- Li, W.; Liu, H.; Li, L.; Liu, K.; Liu, J.; Tang, T.; Jiang, W. Green Synthesis of Citric Acid-Crosslinked β-Cyclodextrin for Highly Efficient Removal of Uranium(VI) from Aqueous Solution. J. Radioanal. Nucl. Chem. 2019, 322, 2033–2042. [Google Scholar] [CrossRef]
- Pirouzmand, M.; Sani, P.S.; Ghasemi, Z.; Azizi, S. Citric Acid-Crosslinked β-Cyclodextrin Supported Zinc Peroxide as a Biocompatible H2O2 Scavenger. J. Biol. Inorg. Chem. 2020, 25, 411–417. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.X.; Wang, P.L.; Zhang, Y.; Cheng, B.W.; Sun, Q.M.; Li, F. Synthesis of β-Cyclodextrin Immobilized Starch and Its Application for the Removal of Dyestuff from Waste-Water. J. Polym. Environ. 2019, 27, 929–941. [Google Scholar] [CrossRef]
- Zhao, J.; Zou, Z.; Ren, R.; Sui, X.; Mao, Z.; Xu, H.; Zhong, Y.; Zhang, L.; Wang, B. Chitosan Adsorbent Reinforced with Citric Acid Modified β-Cyclodextrin for Highly Efficient Removal of Dyes from Reactive Dyeing Effluents. Eur. Polym. J. 2018, 108, 212–218. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.; Wang, J.; Lu, J.; Zhou, Y. Polydopamine Modified Cyclodextrin Polymer as Efficient Adsorbent for Removing Cationic Dyes and Cu2+. J. Hazard. Mater. 2020, 389, 121897. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Y.; Zhao, B.; Jia, Q. Supramolecular Adsorbents in Extraction and Separation Techniques–A Review. Anal. Chim. Acta 2020, 1122, 97–113. [Google Scholar] [CrossRef]
- Trotta, F. Cyclodextrin Nanosponges and their Applications. In Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine; Bilensoy, E., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 323–342. ISBN 978-0-470-92681-9. [Google Scholar]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-Based Nanosponges as Drug Carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099. [Google Scholar] [CrossRef]
- Kardooni, R.; Kiasat, A.R.; Eskandari Sabzi, N. Hyper-Cross-Linked β-Cyclodextrin Nanosponge: A Three-Dimensional, Porous and Biodegradable Catalyst in the One-Pot Synthesis of Kojic Acid-Based Heterocyclic Compounds. Res. Chem. Intermed. 2020, 46, 1857–1868. [Google Scholar] [CrossRef]
- Haimhoffer, Á.; Rusznyák, Á.; Réti-Nagy, K.; Vasvári, G.; Váradi, J.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Fenyvesi, F. Cyclodextrins in Drug Delivery Systems and Their Effects on Biological Barriers. Sci. Pharm. 2019, 87, 33. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Porcino, M.; Qiu, J.; Constantin, D.; Martineau-Corcos, C.; Gref, R. Doxorubicin-Loaded Metal-Organic Frameworks Nanoparticles with Engineered Cyclodextrin Coatings: Insights on Drug Location by Solid State NMR Spectroscopy. Nanomaterials 2021, 11, 945. [Google Scholar] [CrossRef]
- Mok, C.F.; Ching, Y.C.; Muhamad, F.; Abu Osman, N.A.; Hai, N.D.; Che Hassan, C.R. Adsorption of Dyes Using Poly(Vinyl Alcohol) (PVA) and PVA-Based Polymer Composite Adsorbents: A Review. J. Polym. Environ. 2020, 28, 775–793. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, L.; Zhu, C.-S.; Huang, W.-Q.; Hu, J.-L. Water-Insoluble β-Cyclodextrin Polymer Crosslinked by Citric Acid: Synthesis and Adsorption Properties toward Phenol and Methylene Blue. J. Incl. Phenom. Macrocycl. Chem. 2009, 63, 195–201. [Google Scholar] [CrossRef]
- Oughlis-Hammache, F.; Skiba, M.; Hallouard, F.; Moulahcene, L.; Kebiche-Senhadji, O.; Benamor, M.; Lahiani-Skiba, M. Synthesis and Characterization of Poly(Vinyl-Alcohol)-Poly(β-Cyclodextrin) Copolymer Membranes for Aniline Extraction. Membr. Water Treat. 2016, 7, 223–240. [Google Scholar] [CrossRef]
- Liang, H.; Zou, C. Adsorption of Naphthenic Acids from Oil Sand Process-affected Water with Water-insoluble Poly(β-cyclodextrin-citric Acid). Can. J. Chem. Eng. 2019, 97, 1894–1902. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, L.; Zhu, C.; Tian, Z.; Shen, X. Synthesis and Properties of Water-Insoluble β-Cyclodextrin Polymer Crosslinked by Citric Acid with PEG-400 as Modifier. Carbohydr. Polym. 2009, 78, 125–130. [Google Scholar] [CrossRef]
- Rubin Pedrazzo, A.; Trotta, F.; Hoti, G.; Cesano, F.; Zanetti, M. Sustainable Mechanochemical Synthesis of β-Cyclodextrin Polymers by Twin Screw Extrusion. Environ. Sci. Pollut Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, S.; Roh, H.; Oh, S.; Kim, S.; Kim, M.; Kim, D.; Park, J. Preparation and Characterization of Superabsorbent Polymers Based on Starch Aldehydes and Carboxymethyl Cellulose. Polymers 2018, 10, 605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Uppaluri, R.; Das, C. Feasibility of Poly-Vinyl Alcohol/Starch/Glycerol/Citric Acid Composite Films for Wound Dressing Applications. Int. J. Biol. Macromol. 2019, 131, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Bi, J.; Zhang, Z.; Zhu, A.; Chen, D.; Zhou, X.; Zhang, L.; Tian, W. The Effect of Citric Acid on the Structural Properties and Cytotoxicity of the Polyvinyl Alcohol/Starch Films When Molding at High Temperature. Carbohydr. Polym. 2008, 74, 763–770. [Google Scholar] [CrossRef]
- Ghorpade, V.S.; Yadav, A.V.; Dias, R.J. Citric Acid Crosslinked Cyclodextrin/Hydroxypropylmethylcellulose Hydrogel Films for Hydrophobic Drug Delivery. Int. J. Biol. Macromol. 2016, 93, 75–86. [Google Scholar] [CrossRef]
- Patil, N.V.; Netravali, A.N. Cyclodextrin-Based “Green” Wrinkle-Free Finishing of Cotton Fabrics. Ind. Eng. Chem. Res. 2019, 58, 20496–20504. [Google Scholar] [CrossRef]
- Truong, Y.B.; Choi, J.; Mardel, J.; Gao, Y.; Maisch, S.; Musameh, M.; Kyratzis, I.L. Functional Cross-Linked Electrospun Polyvinyl Alcohol Membranes and Their Potential Applications. Macromol. Mater. Eng. 2017, 302, 1700024. [Google Scholar] [CrossRef] [Green Version]
- Gieroba, B.; Kalisz, G.; Sroka-Bartnicka, A.; Płazińska, A.; Płaziński, W.; Starek, M.; Dąbrowska, M. Molecular Structure of Cefuroxime Axetil Complexes with α-, β-, γ-, and 2-Hydroxypropyl-β-Cyclodextrins: Molecular Simulations and Raman Spectroscopic and Imaging Studies. IJMS 2021, 22, 5238. [Google Scholar] [CrossRef]
- Sierpe, R.; Lang, E.; Jara, P.; Guerrero, A.R.; Chornik, B.; Kogan, M.J.; Yutronic, N. Gold Nanoparticles Interacting with β-Cyclodextrin–Phenylethylamine Inclusion Complex: A Ternary System for Photothermal Drug Release. ACS Appl. Mater. Interfaces 2015, 7, 15177–15188. [Google Scholar] [CrossRef]
- Rachmawati, H.; Edityaningrum, C.A.; Mauludin, R. Molecular Inclusion Complex of Curcumin–β-Cyclodextrin Nanoparticle to Enhance Curcumin Skin Permeability from Hydrophilic Matrix Gel. AAPS PharmSciTech 2013, 14, 1303–1312. [Google Scholar] [CrossRef] [Green Version]
- Ceborska, M.; Zimnicka, M.; Kowalska, A.A.; Dąbrowa, K.; Repeć, B. Structural Diversity in the Host–Guest Complexes of the Antifolate Pemetrexed with Native Cyclodextrins: Gas Phase, Solution and Solid State Studies. Beilstein J. Org. Chem. 2017, 13, 2252–2263. [Google Scholar] [CrossRef]
- Elbagerma, M.A.; Edwards, H.G.M.; Munshi, T.; Scowen, I.J. Identification of a New Cocrystal of Citric Acid and Paracetamol of Pharmaceutical Relevance. Cryst. Eng. Comm. 2011, 13, 1877–1884. [Google Scholar] [CrossRef] [Green Version]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference Database of Raman Spectra of Biological Molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Zampieri, M.; Lazaro, S.R.; Paskocimas, C.A.; Ferreira, A.G.; Longo, E.; Varela, J.A. Structural Analysis of Ti And Pb Citrate Using NMR and FT-Raman Signals and Quantum Mechanics Simulations. J. Sol-Gel Sci. Technol. 2006, 37, 9–17. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, X.; Li, Y.; Chen, J.; Lin, J.; Wang, J.; Lei, J.; Chen, R. Quantitative Determination of Citric Acid in Seminal Plasma by Using Raman Spectroscopy. Appl. Spectrosc. 2013, 67, 757–760. [Google Scholar] [CrossRef]
- Liao, G.-M.; Yang, C.-C.; Hu, C.-C.; Pai, Y.-L.; Lue, S.J. Novel Quaternized Polyvinyl Alcohol/Quaternized Chitosan Nano-Composite as an Effective Hydroxide-Conducting Electrolyte. J. Membr. Sci. 2015, 485, 17–29. [Google Scholar] [CrossRef]
- Salazar, J.D.R. Study of Structural, Thermic, μ-Raman and Optic Transformation of PVA/TiO2 Polymeric Membranes. Scientia Technica 2018, 23, 543–552. [Google Scholar] [CrossRef]
- Casanova, E.; Pelé-Meziani, C.; Guilminot, É.; Mevellec, J.-Y.; Riquier-Bouclet, C.; Vinçotte, A.; Lemoine, G. The Use of Vibrational Spectroscopy Techniques as a Tool for the Discrimination and Identification of the Natural and Synthetic Organic Compounds Used in Conservation. Anal. Methods 2016, 8, 8514–8527. [Google Scholar] [CrossRef]
- Munnier, E.; Al Assaad, A.; David, S.; Mahut, F.; Vayer, M.; Van Gheluwe, L.; Yvergnaux, F.; Sinturel, C.; Soucé, M.; Chourpa, I.; et al. Homogeneous Distribution of Fatty Ester-based Active Cosmetic Ingredients in Hydrophilic Thin Films by Means of Nanodispersion. Int. J. Cosmet Sci. 2020, 42, 512–519. [Google Scholar] [CrossRef]
- Musuc, A.M.; Anuta, V.; Atkinson, I.; Popa, V.T.; Sarbu, I.; Mircioiu, C.; Abdalrb, G.A.; Mitu, M.A.; Ozon, E.A. Development and Characterization of Orally Disintegrating Tablets Containing a Captopril-Cyclodextrin Complex. Pharmaceutics 2020, 12, 744. [Google Scholar] [CrossRef]
- Ghorpade, V.S.; Yadav, A.V.; Dias, R.J. Citric Acid Crosslinked β -Cyclodextrin/Carboxymethylcellulose Hydrogel Films for Controlled Delivery of Poorly Soluble Drugs. Carbohydr. Polym. 2017, 164, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Malanga, M.; Manet, I.; Manoli, F.; Tuza, K.; Aykaç, A.; Ladavière, C.; Fenyvesi, E.; Vargas-Berenguel, A.; Gref, R.; et al. Citric Acid–γ-Cyclodextrin Crosslinked Oligomers as Carriers for Doxorubicin Delivery. Photochem. Photobiol. Sci. 2013, 12, 1841. [Google Scholar] [CrossRef] [PubMed]
- Zbair, M.; Anfar, Z.; Ait Ahsaine, H.; Khallok, H. Kinetics, Equilibrium, Statistical Surface Modeling and Cost Analysis of Paraquat Removal from Aqueous Solution Using Carbonated Jujube Seed. RSC Adv. 2019, 9, 1084–1094. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Gao, D.-W.; Pan, H.; Hao, L.; Wang, P. Equilibrium and Kinetics of Aniline Adsorption onto Crosslinked Sawdust-Cyclodextrin Polymers. RSC Adv. 2014, 4, 40071–40077. [Google Scholar] [CrossRef]
- Junthip, J. Coating of PET Textiles with Anionic Cyclodextrin Polymer for Paraquat Removal from Aqueous Solution. Fibers Polym. 2018, 19, 2335–2343. [Google Scholar] [CrossRef]
- Jiang, L.-W.; Zeng, F.-T.; Zhang, Y.; Xu, M.-Y.; Xie, Z.-W.; Wang, H.-Y.; Wu, Y.-X.; He, F.-A.; Jiang, H.-L. Preparation of a Novel Fe3O4/Graphite Oxide Nanosheet/Citric Acid-Crosslinked β-Cyclodextrin Polymer Composite to Remove Methylene Blue from Water. Adv. Powder Technol. 2021, 32, 492–503. [Google Scholar] [CrossRef]
- Zhou, K.; Li, Y.; Li, Q.; Du, Q.; Wang, D.; Sui, K.; Wang, C.; Li, H.; Xia, Y. Kinetic, Isotherm and Thermodynamic Studies for Removal of Methylene Blue Using β-Cyclodextrin/Activated Carbon Aerogels. J. Polym. Environ. 2018, 26, 3362–3370. [Google Scholar] [CrossRef]

| Nanosponges | Composition (% w/v) | % Yield | IEC (mmol/g) | % Swelling | % Paraquat Removal | β-CD Content (mmol/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-CD | CTR | PVOH | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | |
| C2.5D-P2 | 10 | 2.5 | 2 | 52.9 | 2.0 | 2.13 | 0.02 | 199.2 | 1.2 | 66.0 | 1.9 | 0.365 | 0.003 |
| C5D-P2 | 10 | 5 | 2 | 57.5 | 1.1 | 2.93 | 0.01 | 147.2 | 1.4 | 84.2 | 0.5 | 0.351 | 0.005 |
| C10D-P2 | 10 | 10 | 2 | 60.2 | 2.1 | 3.14 | 0.03 | 96.4 | 0.5 | 94.5 | 0.1 | 0.335 | 0.003 |
| C10D-P1 | 10 | 10 | 1 | 63.4 | 1.5 | 3.20 | 0.02 | 101.6 | 0.7 | 89.0 | 0.2 | 0.328 | 0.002 |
| C10D-P0.5 | 10 | 10 | 0.5 | 68.6 | 1.3 | 3.27 | 0.03 | 104.1 | 0.8 | 85.2 | 0.5 | 0.314 | 0.004 |
| C10-P2 | 0 | 10 | 2 | 39.9 | 2.6 | 3.84 | 0.05 | 135.3 | 0.6 | 75.9 | 0.8 | - | - |
| C10-P1 | 0 | 10 | 1 | 21.3 | 2.3 | 4.07 | 0.03 | 143.6 | 0.4 | 69.2 | 1.0 | - | - |
| C10-P0.5 | 0 | 10 | 0.5 | 17.3 | 1.3 | 4.46 | 0.04 | 166.7 | 0.9 | 21.4 | 0.4 | - | - |
| Nanosponges | Qe (exp) | Pseudo First-Order | Pseudo Second-Order | Adsorption Mechanism | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | Qe (cal) | k1 | R2 | Qe (cal) | k2 | h | t1/2 | k31 | k32 | ||
| C10D-P0.5 | 10.6 | 0.7031 | 2.8 | 0.0104 | 0.9997 | 10.7 | 0.1248 | 14.2 | 0.8 | 0.2011 | 0.0163 |
| C10D-P1 | 11.1 | 0.7155 | 3.0 | 0.0111 | 0.9994 | 11.1 | 0.1670 | 20.7 | 0.5 | 0.1511 | 0.0148 |
| C10D-P2 | 11.8 | 0.7133 | 3.1 | 0.0117 | 0.9998 | 11.8 | 0.4709 | 65.8 | 0.2 | 0.0807 | 0.0131 |
| C10-P2 | 9.6 | 0.7541 | 2.1 | 0.0064 | 0.9991 | 9.5 | 0.1126 | 10.2 | 0.9 | 0.2640 | 0.0217 |
| Nano-Sponges | Qm (exp) | Langmuir Isotherm | Freundlich Isotherm | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | Qm (cal) | KL | χ2 | RL for C0 (mg/L) | R2 | Qm (cal) | Kf | 1/n | χ2 | ||||||
| 25 | 50 | 150 | 250 | 300 | |||||||||||
| C10D-P0.5 | 71.9 | 0.9975 | 82.0 | 0.04 | 1.2 | 0.51 | 0.34 | 0.15 | 0.10 | 0.08 | 0.9706 | 52.9 | 6.5 | 0.49 | 6.9 |
| C10D-P1 | 80.5 | 0.9939 | 92.6 | 0.06 | 1.6 | 0.41 | 0.26 | 0.10 | 0.07 | 0.05 | 0.9245 | 69.2 | 7.4 | 0.51 | 1.9 |
| C10D-P2 | 102.0 | 0.9979 | 112.4 | 0.09 | 1.0 | 0.32 | 0.19 | 0.07 | 0.04 | 0.04 | 0.9307 | 120.4 | 12.5 | 0.49 | 2.8 |
| C10-P2 | 63.5 | 0.9990 | 78.7 | 0.02 | 3.0 | 0.63 | 0.46 | 0.22 | 0.14 | 0.12 | 0.9665 | 40.6 | 4.0 | 0.56 | 12.9 |
| Adsorbent | Adsorption Dosage | Paraquat Concentration (mg/L) | Maximum Adsorption Capacity |
|---|---|---|---|
| PVOH-Cyclodextrin nanosponges (This work) | 0.02 g in 0.01 L | 25–250 mg/L | 112.2 mg/g |
| Cyclodextrin nanosponges [48] | 0.02 g in 0.01 L | 10–200 mg/L | 21.9 mg/g |
| Cellulose nanofiber [46] | 0.02 g in 0.2 L | - | 108 g/g |
| Montmorillonite [45] | 0.002 g in 0.1 L | - | 0.44 mol/kg |
| Magnetic adsorbent [42] | 0.0025 g in 0.005 L | 30–900 mg/L | 242.2 mg/g |
| Carbon nanotubes [38] | 0.002 g in 0.005 L | 70–250 mg/L | 218.6 mg/g |
| Graphene oxide [37] | 0.02 g in 0.025 L | 4–24 mg/L | 29.15 mg/g |
| Calixarene [34] | 0.025 g in 0.005 L | 0.5–2.0 mmol/L | 419 mg/g |
| Activated carbon [29] | 0.01 g in 0.01 L | 1.5–45 mg/L | 20 mg/g |
| Microorganisms [28] | 0.005 g in 0.015 L | 0–285.7 mg/L | 24.4 mg/g |
| Bentonite [27] | 0.04 g in 0.025 L | 4–24 mg/L | 11.75 mg/g |
| Bio-based material [23] | 0.069 g in 0.025 L | 25–85 mg/L | 20.58 mg/g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martwong, E.; Chuetor, S.; Junthip, J. Adsorption of Paraquat by Poly(Vinyl Alcohol)-Cyclodextrin Nanosponges. Polymers 2021, 13, 4110. https://doi.org/10.3390/polym13234110
Martwong E, Chuetor S, Junthip J. Adsorption of Paraquat by Poly(Vinyl Alcohol)-Cyclodextrin Nanosponges. Polymers. 2021; 13(23):4110. https://doi.org/10.3390/polym13234110
Chicago/Turabian StyleMartwong, Ekkachai, Santi Chuetor, and Jatupol Junthip. 2021. "Adsorption of Paraquat by Poly(Vinyl Alcohol)-Cyclodextrin Nanosponges" Polymers 13, no. 23: 4110. https://doi.org/10.3390/polym13234110
APA StyleMartwong, E., Chuetor, S., & Junthip, J. (2021). Adsorption of Paraquat by Poly(Vinyl Alcohol)-Cyclodextrin Nanosponges. Polymers, 13(23), 4110. https://doi.org/10.3390/polym13234110








