The Synthesis of Associative Copolymers with Both Amphoteric and Hydrophobic Groups and the Effect of the Degree of Association on the Instability of Emulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PASB Copolymer Synthesis Steps
2.3. FTIR, TGA, and DTG Measurement
2.4. PASB Average Molecular Weight
2.5. Viscosity Measurement of PASB
2.6. Preparation of Aqueous Phases and Emulsion
2.7. Optical Microscopic Observation
2.8. Multiple Light-Scattering Measurements
3. Results and Discussion
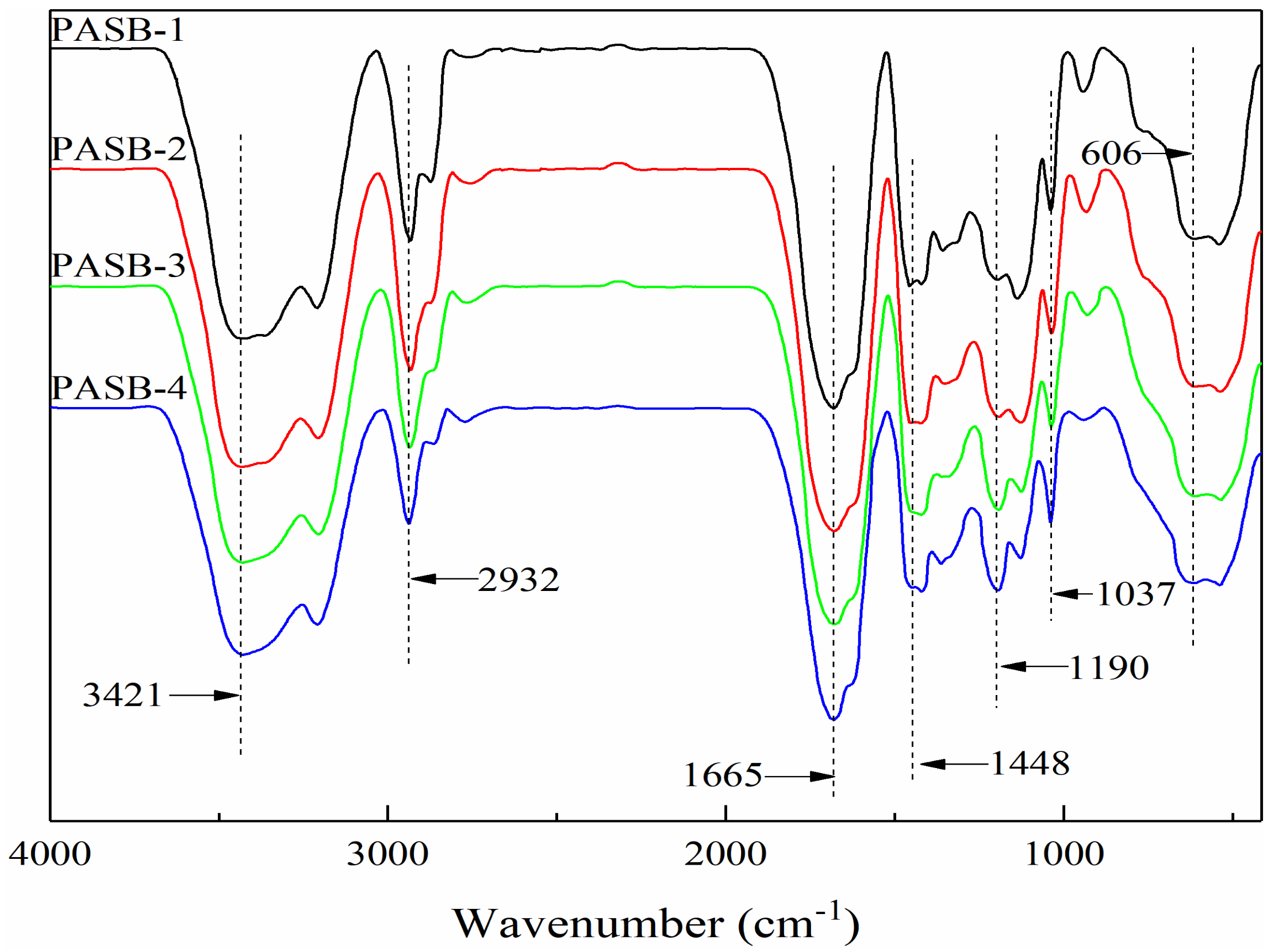
3.1. FTIR of Copolymer PASB
3.2. Thermal Properties of Copolymer PASB
3.3. Molecular Weight of Copolymer PASB
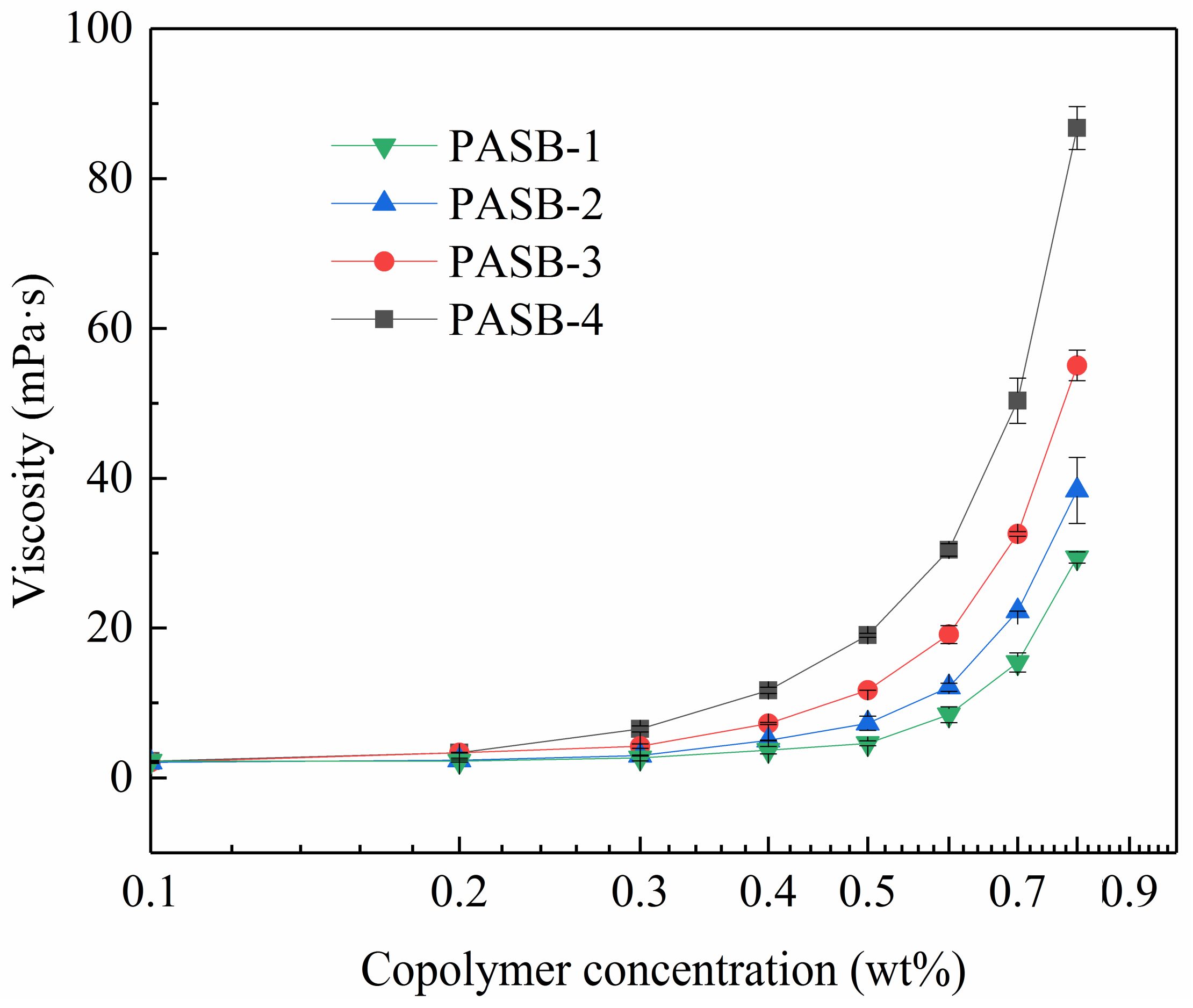
3.4. Influence of PASB Copolymer Concentration on Solution Viscosity
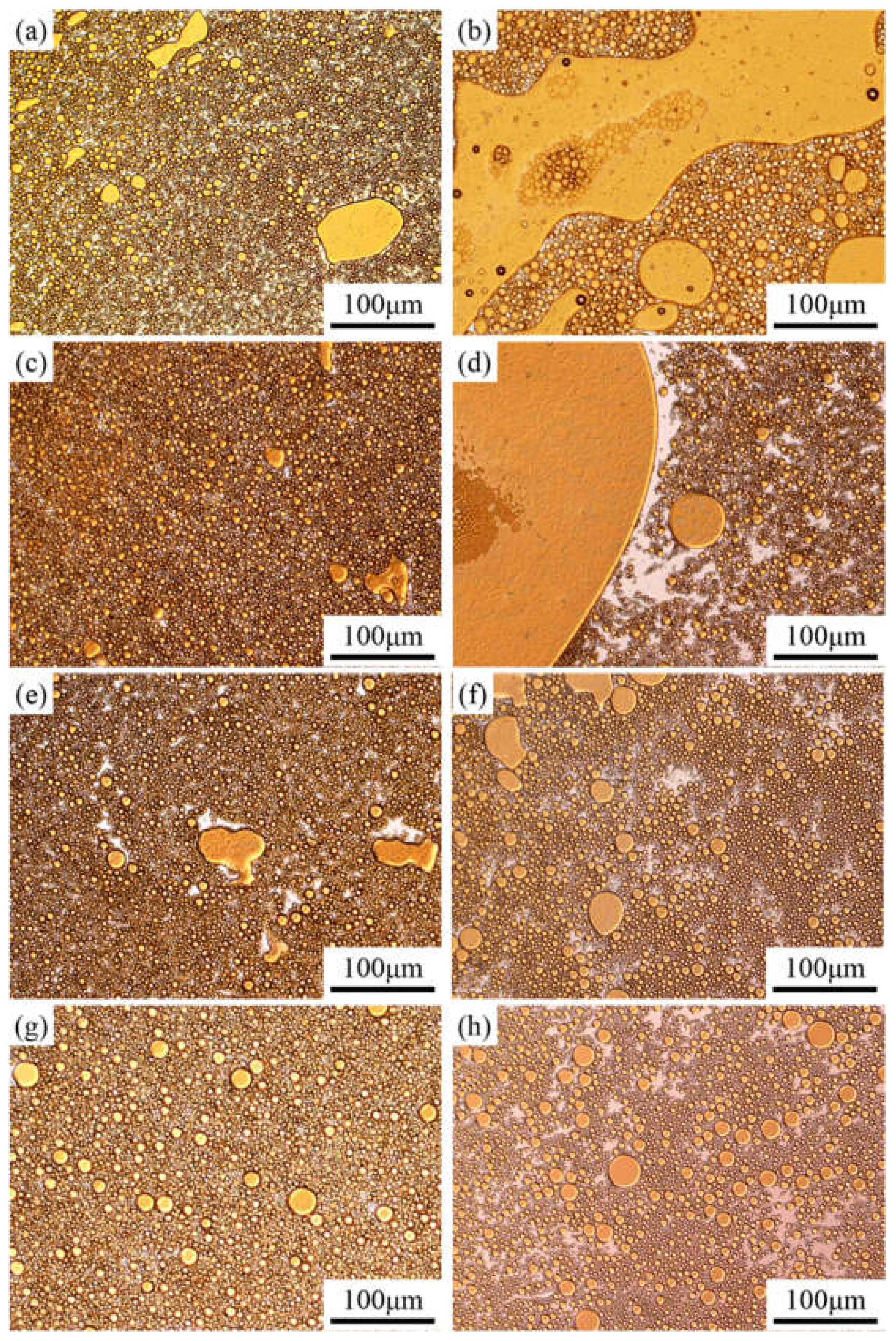
3.5. Emulsion Microscopic Morphology
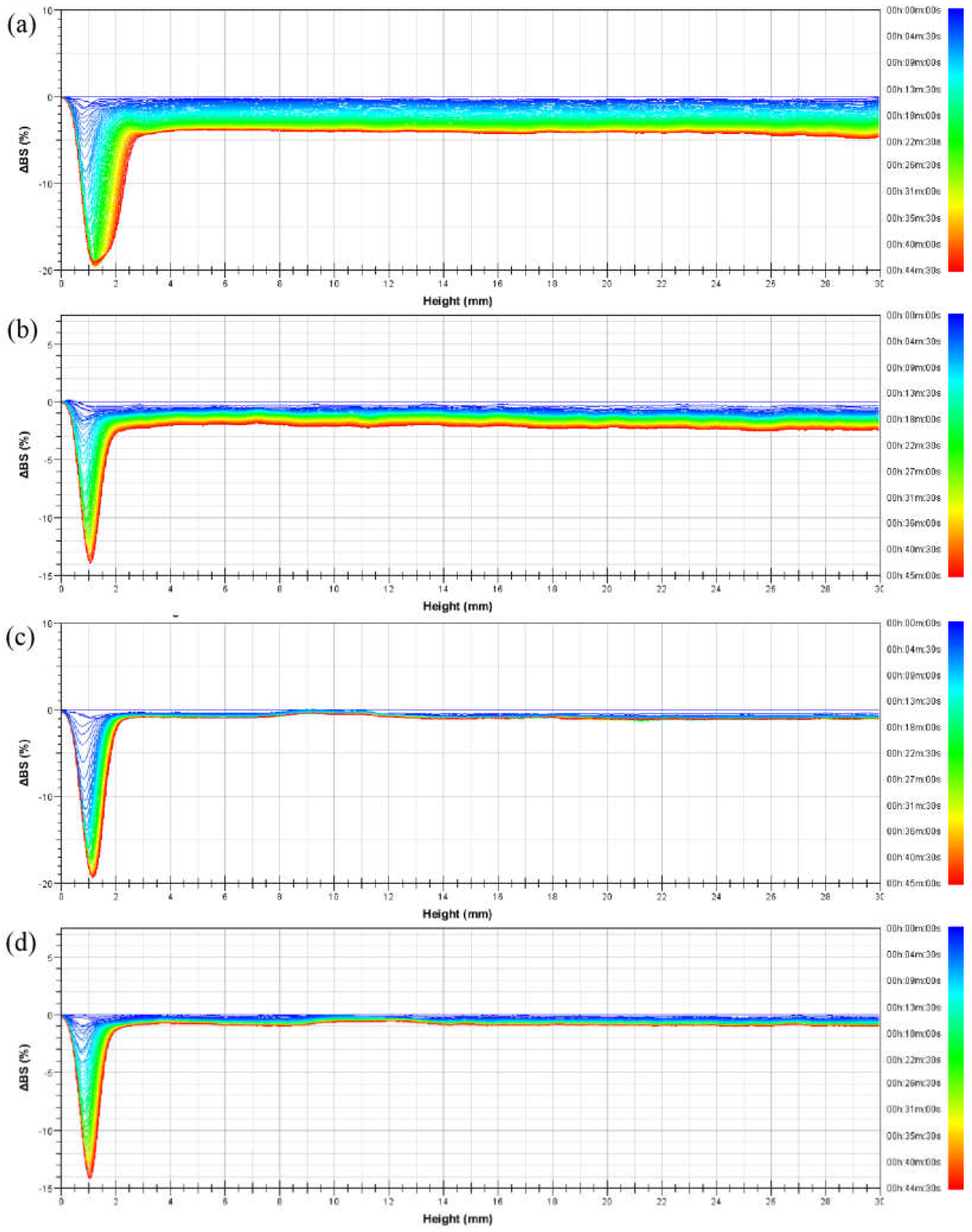
3.6. Changes in the Backscattered Light Intensity of the Emulsions
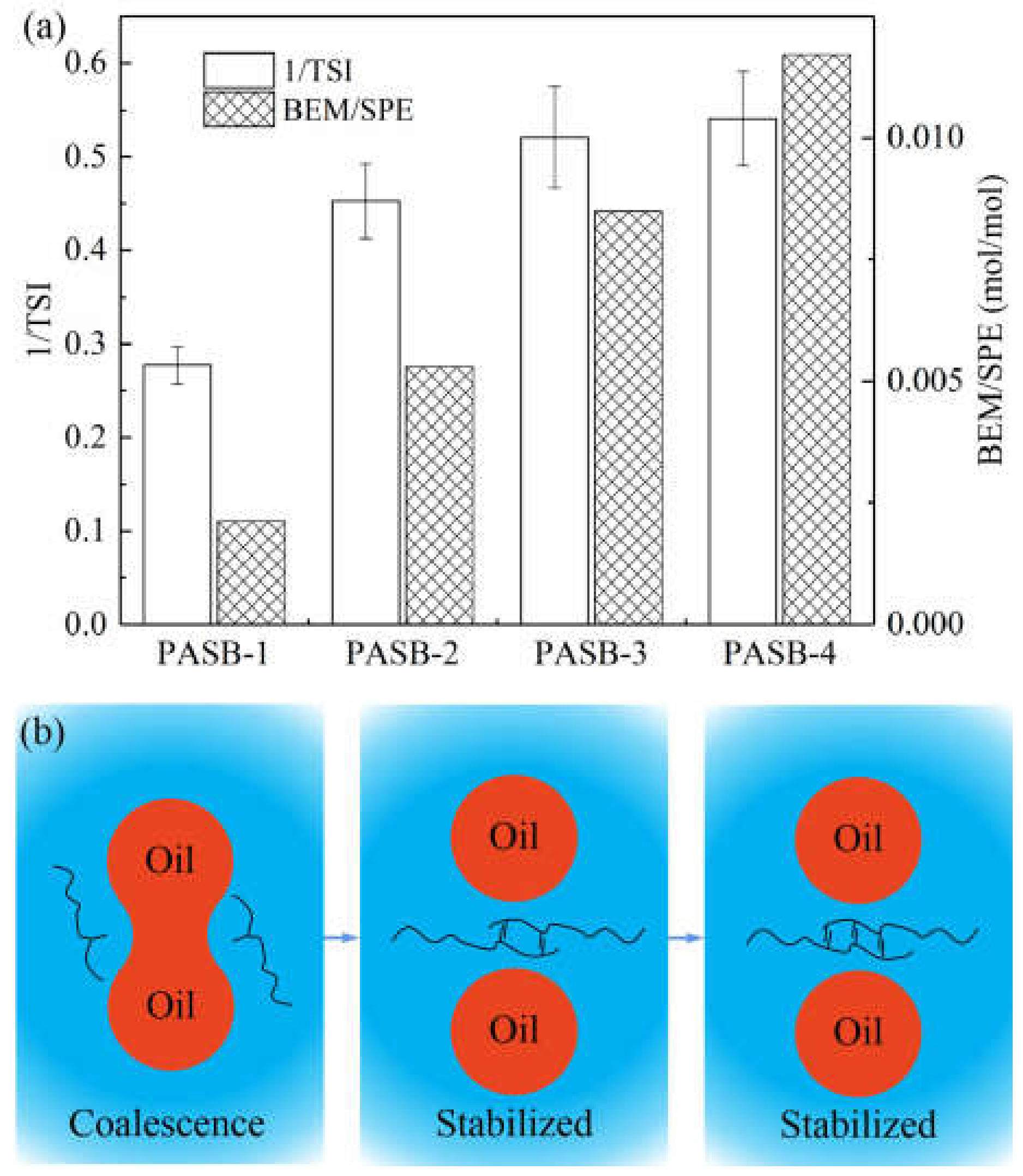
3.7. The Relationship between TSI and Reactive Monomers Ratio
4. Conclusions
- (1)
- The soap-free emulsion polymerization method was used to prepare a variety of water-soluble copolymers with both ultra-long hydrophobic chains and amphoteric groups. Through the action of a mixed initiator composed of KPS/NaHSO3/AIBA, different proportions of AM, SPE, and BEM were copolymerized to obtain four PASB copolymers.
- (2)
- The main instability processes of the emulsions prepared from PASB in 45 min were flocculation and coalescence. The phase-separation phenomenon was not significant in PASB-1 to PASB-4 emulsions. The type of all emulsions during the standing for 45 min was always O/W.
- (3)
- The behenyl functional group in the molecular chain of the synthesized PASB copolymer plays a leading role in the intermolecular association. Increasing the ratio of BEM/SPE significantly increased the degree of hydrophobic association of PASB polymers, which can cause the emulsion to reduce the occurrence of flocculation and coalescence during the standing process, but will not continue to significantly improve the stability of the emulsion, although the viscosity of the aqueous solution in the emulsion was controlled to be always equal.
- (4)
- The stability of the emulsions, which were prepared from isoviscosity aqueous solutions controlled by the concentration of the associative copolymers, was increased with the degree of association of copolymers.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ea, S.; Fw, O. Acrylamide and Polyacrylamide: A Review of Production, Use, Environmental Fate and Neurotoxicity. Rev. Environ. Health 1991, 9, 215–228. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, Biochemistry, and Safety of Acrylamide. A Review. J. Agric. Food Chem. 2003, 51, 4504–4526. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.S.; Sultan, A.S.; Al-Mubaiyedh, U.A.; Hussein, I.A. Review on Polymer Flooding: Rheology, Adsorption, Stability, and Field Applications of Various Polymer Systems. Polym. Rev. 2015, 55, 491–530. [Google Scholar] [CrossRef]
- Zhang, G.; Seright, R.S.S. Effect of Concentration on HPAM Retention in Porous Media. SPE J. 2014, 19, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Corredor, L.M.; Aliabadian, E.; Husein, M.; Chen, Z.; Maini, B.; Sundararaj, U. Heavy Oil Recovery by Surface Modified Silica Nanoparticle/HPAM Nanofluids. Fuel 2019, 252, 622–634. [Google Scholar] [CrossRef]
- Rezaei, A.; Abdi-Khangah, M.; Mohebbi, A.; Tatar, A.; Mohammadi, A.H. Using Surface Modified Clay Nanoparticles to Improve Rheological Behavior of Hydrolized Polyacrylamid (HPAM) Solution for Enhanced Oil Recovery with Polymer Flooding. J. Mol. Liq. 2016, 222, 1148–1156. [Google Scholar] [CrossRef]
- Bashir Abdullahi, M.; Rajaei, K.; Junin, R.; Bayat, A.E. Appraising the Impact of Metal-Oxide Nanoparticles on Rheological Properties of HPAM in Different Electrolyte Solutions for Enhanced Oil Recovery. J. Pet. Sci. Eng. 2019, 172, 1057–1068. [Google Scholar] [CrossRef]
- Jiang, G.; Peng, S.; Li, X.; Yang, L.; Soares, J.B.P.; Li, G. Preparation of Amphoteric Starch-Based Flocculants by Reactive Extrusion for Removing Useless Solids from Water-Based Drilling Fluids. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 343–350. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, L.; Wang, Y.; Dai, H.; Wang, D.; Cai, H. Influences of Partially Hydrolyzed Polyacrylamide (HPAM) Residue on the Flocculation Behavior of Oily Wastewater Produced from Polymer Flooding. Sep. Purif. Technol. 2008, 62, 199–204. [Google Scholar] [CrossRef]
- Mohd Azmi, L.H.; Williams, D.R.; Ladewig, B.P. Polymer-Assisted Modification of Metal-Organic Framework MIL-96 (Al): Influence of HPAM Concentration on Particle Size, Crystal Morphology and Removal of Harmful Environmental Pollutant PFOA. Chemosphere 2021, 262, 128072. [Google Scholar] [CrossRef]
- Liravi, M.; Pakzad, H.; Moosavi, A.; Nouri-Borujerdi, A. A Comprehensive Review on Recent Advances in Superhydrophobic Surfaces and Their Applications for Drag Reduction. Prog. Org. Coat. 2020, 140, 105537. [Google Scholar] [CrossRef]
- Rashidi, M.; Blokhus, A.M.; Skauge, A. Viscosity Study of Salt Tolerant Polymers. J. Appl. Polym. Sci. 2010, 117, 1551–1557. [Google Scholar] [CrossRef] [Green Version]
- Spildo, K.; Sæ, E.I.Ø. Effect of Charge Distribution on the Viscosity and Viscoelastic Properties of Partially Hydrolyzed Polyacrylamide. Energy Fuels 2015, 29, 5609–5617. [Google Scholar] [CrossRef]
- Ding, M.; Han, Y.; Liu, Y.; Wang, Y.; Zhao, P.; Yuan, Y. Oil Recovery Performance of a Modified HAPAM with Lower Hydrophobicity, Higher Molecular Weight: A Comparative Study with Conventional HAPAM, HPAM. J. Ind. Eng. Chem. 2019, 72, 298–309. [Google Scholar] [CrossRef]
- Chassenieux, C.; Nicolai, T.; Benyahia, L. Rheology of Associative Polymer Solutions. Curr. Opin. Colloid Interface Sci. 2011, 16, 18–26. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, G.; Peng, S.; He, Y.; Wang, J. Amphoteric Polymer as an Anti-Calcium Contamination Fluid-Loss Additive in Water-Based Drilling Fluids. Energy Fuels 2016, 30, 7221–7228. [Google Scholar] [CrossRef]
- Argillier, J.-F.; Audibert, A.; Lecourtier, J.; Moan, M.; Rousseau, L. Solution and Adsorption Properties of Hydrophobically Associating Water-Soluble Polyacrylamides. Colloids Surf. A: Physicochem. Eng. Asp. 1996, 113, 247–257. [Google Scholar] [CrossRef]
- Wormuth, K. Superparamagnetic Latex via Inverse Emulsion Polymerization. J. Colloid Interface Sci. 2001, 241, 366–377. [Google Scholar] [CrossRef]
- Volpert, E.; Selb, J.; Candau, F. Influence of the Hydrophobe Structure on Composition, Microstructure, and Rheology in Associating Polyacrylamides Prepared by Micellar Copolymerization. Macromolecules 1996, 29, 1452–1463. [Google Scholar] [CrossRef]
- Fried, J.R. Polymer Science and Technology; Prentice Hall: Hoboken, NJ, USA, 2014; ISBN 978-0-13-703955-5. [Google Scholar]
- Zhou, J.; Chen, X.; Ma, J. Synthesis of Cationic Fluorinated Polyacrylate Copolymer by RAFT Emulsifier-Free Emulsion Polymerization and Its Application as Waterborne Textile Finishing Agent. Dye. Pigment. 2017, 139, 102–109. [Google Scholar] [CrossRef]
- Yamamoto, T.; Arakawa, K.; Takahashi, Y.; Sumiyoshi, M. Antimicrobial Activities of Low Molecular Weight Polymers Synthesized through Soap-Free Emulsion Polymerization. Eur. Polym. J. 2018, 109, 532–536. [Google Scholar] [CrossRef]
- Cochin, D.; Laschewsky, A.; Nallet, F. Emulsion Polymerization of Styrene Using Conventional, Polymerizable, and Polymeric Surfactants. A Comparative Study. Macromolecules 1997, 30, 2278–2287. [Google Scholar] [CrossRef]
- Unzué, M.J.; Schoonbrood, H.A.S.; Asua, J.M.; Goñi, A.M.; Sherrington, D.C.; Stähler, K.; Goebel, K.-H.; Tauer, K.; Sjöberg, M.; Holmberg, K. Reactive Surfactants in Heterophase Polymerization. VI. Synthesis and Screening of Polymerizable Surfactants (Surfmers) with Varying Reactivity in High Solids Styrene—Butyl Acrylate—Acrylic Acid Emulsion Polymerization. J. Appl. Polym. Sci. 1997, 66, 1803–1820. [Google Scholar] [CrossRef]
- Dickinson, E. 2—Hydrocolloids and emulsion stability. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2009; pp. 23–49. ISBN 978-1-84569-414-2. [Google Scholar]
- Gohtani, S.; Yoshii, H. 6—Microstructure, composition, and their relationship with emulsion stability. In Food Microstructure and Its Relationship with Quality and Stability; Devahastin, S., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2018; pp. 97–122. ISBN 978-0-08-100764-8. [Google Scholar]
- Friberg, S.E. Emulsion Stability. In Emulsions—A Fundamental and Practical Approach; Sjöblom, J., Ed.; NATO ASI Series; Springer: Dordrecht, The Netherlands, 1992; pp. 1–24. ISBN 978-94-011-2460-7. [Google Scholar]
- Wilde, P.J. Interfaces: Their Role in Foam and Emulsion Behaviour. Curr. Opin. Colloid Interface Sci. 2000, 5, 176–181. [Google Scholar] [CrossRef]
- Li, G.; Wang, K.; Lu, C. Wet-Etched Asymmetric Spherical Nanoparticles with Controllable Pit Structures and Application in Non-Aqueous Foams. Soft Matter 2021, 17, 4848–4856. [Google Scholar] [CrossRef] [PubMed]
- Angardi, V.; Ettehadi, A.; Yücel, Ö. Critical Review of Emulsion Stability and Characterization Techniques in Oil Processing. J. Energy Resour. Technol. 2021, 144, 040801. [Google Scholar] [CrossRef]
- Goodarzi, F.; Zendehboudi, S. A Comprehensive Review on Emulsions and Emulsion Stability in Chemical and Energy Industries. Can. J. Chem. Eng. 2019, 97, 281–309. [Google Scholar] [CrossRef] [Green Version]
- Mcclements, D.J. Critical Review of Techniques and Methodologies for Characterization of Emulsion Stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef]
- Mengual, O.; Meunier, G.; Cayré, I.; Puech, K.; Snabre, P. TURBISCAN MA 2000: Multiple Light Scattering Measurement for Concentrated Emulsion and Suspension Instability Analysis. Talanta 1999, 50, 445–456. [Google Scholar] [CrossRef]
- Wang, K.; Li, G.; Zhang, B. Opposite Results of Emulsion Stability Evaluated by the TSI and the Phase Separation Proportion. Colloids Surf. A: Physicochem. Eng. Asp. 2018, 558, 402–409. [Google Scholar] [CrossRef]
- Jiménez-Regalado, E.J.; Cadenas-Pliego, G.; Pérez-Álvarez, M.; Hernández-Valdez, Y. Study of Three Different Families of Water-Soluble Copolymers: Synthesis, Characterization and Viscoelastic Behavior of Semidilute Solutions of Polymers Prepared by Solution Polymerization. Polymer 2004, 45, 1993–2000. [Google Scholar] [CrossRef]


| Items | Value |
|---|---|
| K+ (mg/L) | 18.7 |
| Na+ (mg/L) | 3907.3 |
| Ca2+ (mg/L) | 41.4 |
| Mg2+ (mg/L) | 118.4 |
| Cl− (mg/L) | 4230.7 |
| SO42− (mg/L) | 138.0 |
| HCO3− (mg/L) | 3675.2 |
| Total salinity (mg/L) | 12129.7 |
| Sample | AM (mol%) | SPE (mol%) | BEM (mol%) | KPS (mmol) | NaHSO3 (mmol) | AIBA (mmol) | Yield (%) |
|---|---|---|---|---|---|---|---|
| PASB-1 | 94.8 | 5 | 0.2 | 0.022 | 0.045 | 0.133 | 93.06 |
| PASB-2 | 94.5 | 5 | 0.5 | 0.022 | 0.045 | 0.133 | 92.73 |
| PASB-3 | 94.2 | 5 | 0.8 | 0.022 | 0.045 | 0.133 | 89.20 |
| PASB-4 | 93.9 | 5 | 1.1 | 0.022 | 0.045 | 0.133 | 87.27 |
| Sample | Mw. App (g × mol−1) | A2 (10−5 mol × mL × g−2) |
|---|---|---|
| PASB-1 | (1.134 ± 0.093) × 106 | 6.458 ± 0.967 |
| PASB-2 | (9.703 ± 0.517) × 105 | 5.681 ± 0.881 |
| PASB-3 | (8.073 ± 0.355) × 105 | 3.861 ± 1.019 |
| PASB-4 | (5.981 ± 0.191) × 105 | 4.269 ± 0.949 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, G.; Chen, Y.; Wang, K.; Yang, E. The Synthesis of Associative Copolymers with Both Amphoteric and Hydrophobic Groups and the Effect of the Degree of Association on the Instability of Emulsions. Polymers 2021, 13, 4041. https://doi.org/10.3390/polym13224041
Zhang X, Li G, Chen Y, Wang K, Yang E. The Synthesis of Associative Copolymers with Both Amphoteric and Hydrophobic Groups and the Effect of the Degree of Association on the Instability of Emulsions. Polymers. 2021; 13(22):4041. https://doi.org/10.3390/polym13224041
Chicago/Turabian StyleZhang, Xiaotong, Gen Li, Yuhao Chen, Keliang Wang, and Erlong Yang. 2021. "The Synthesis of Associative Copolymers with Both Amphoteric and Hydrophobic Groups and the Effect of the Degree of Association on the Instability of Emulsions" Polymers 13, no. 22: 4041. https://doi.org/10.3390/polym13224041
APA StyleZhang, X., Li, G., Chen, Y., Wang, K., & Yang, E. (2021). The Synthesis of Associative Copolymers with Both Amphoteric and Hydrophobic Groups and the Effect of the Degree of Association on the Instability of Emulsions. Polymers, 13(22), 4041. https://doi.org/10.3390/polym13224041





