Development of Oil and Gas Stimulation Fluids Based on Polymers and Recycled Produced Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Simulated Produced Water
2.3. Viscosity Measurement
3. Results and Discussion
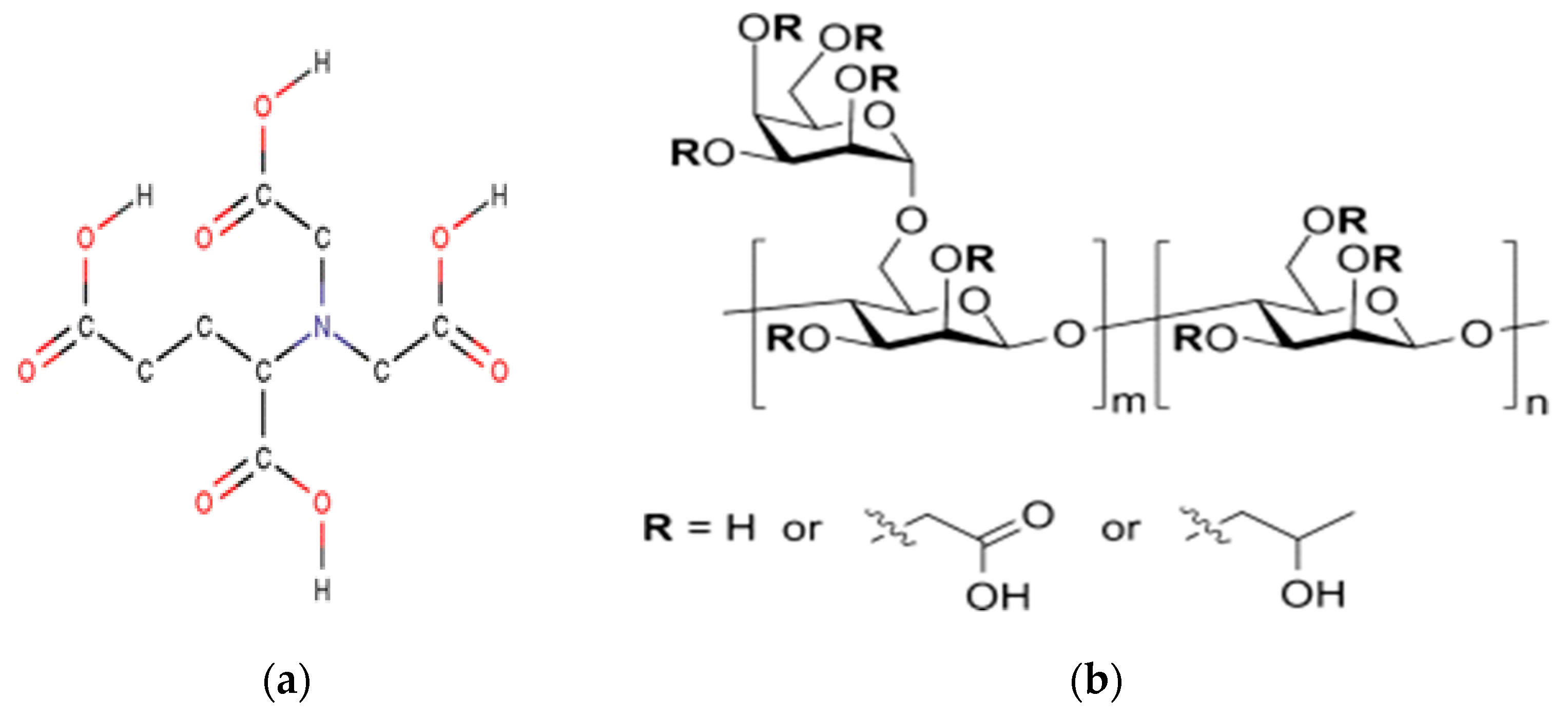
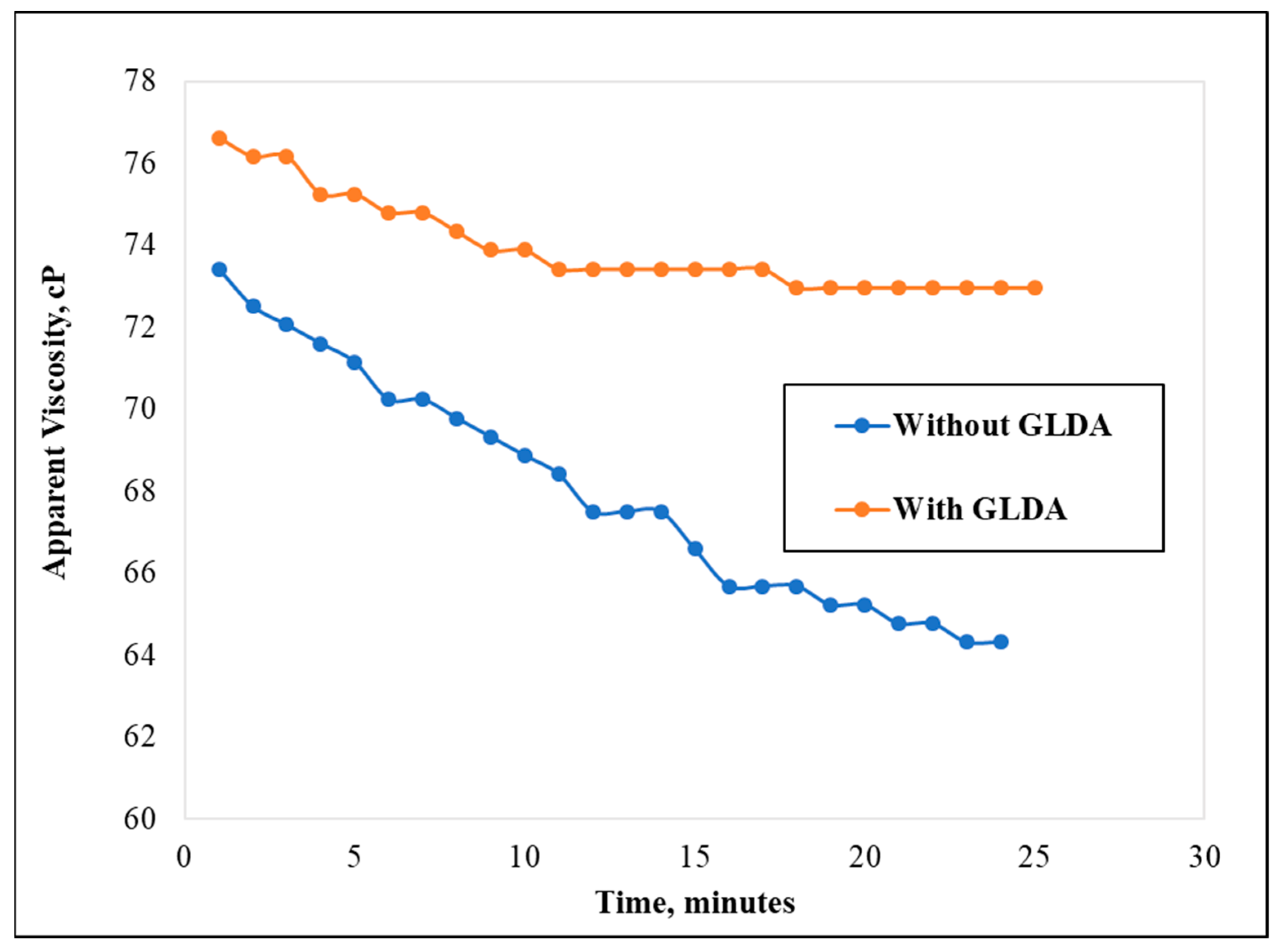
3.1. Effect of Chelating Agent’s Presence
3.2. Effect of GLDA on Water Softening
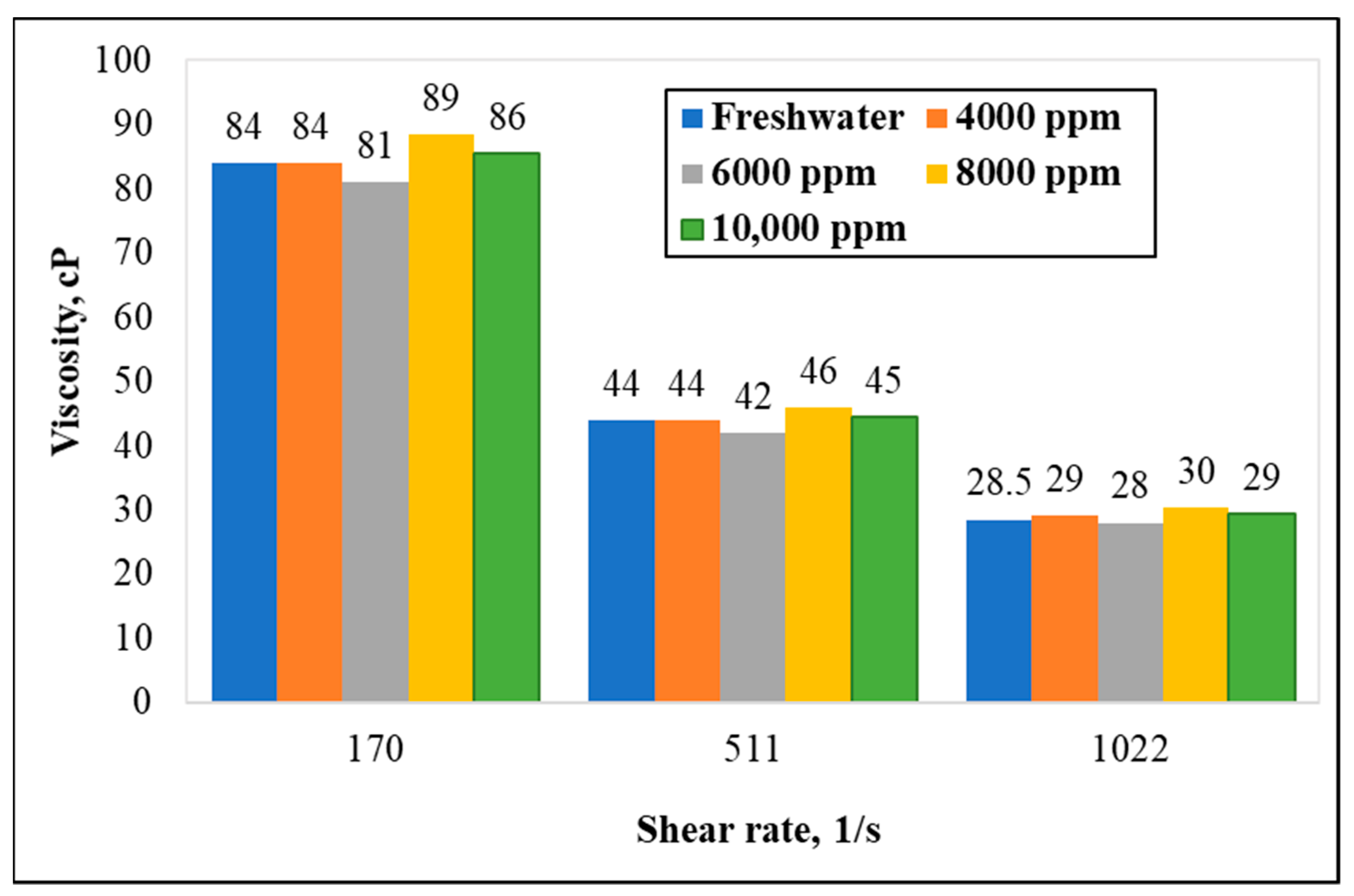
3.3. Effect of Chelating Agent Concentration
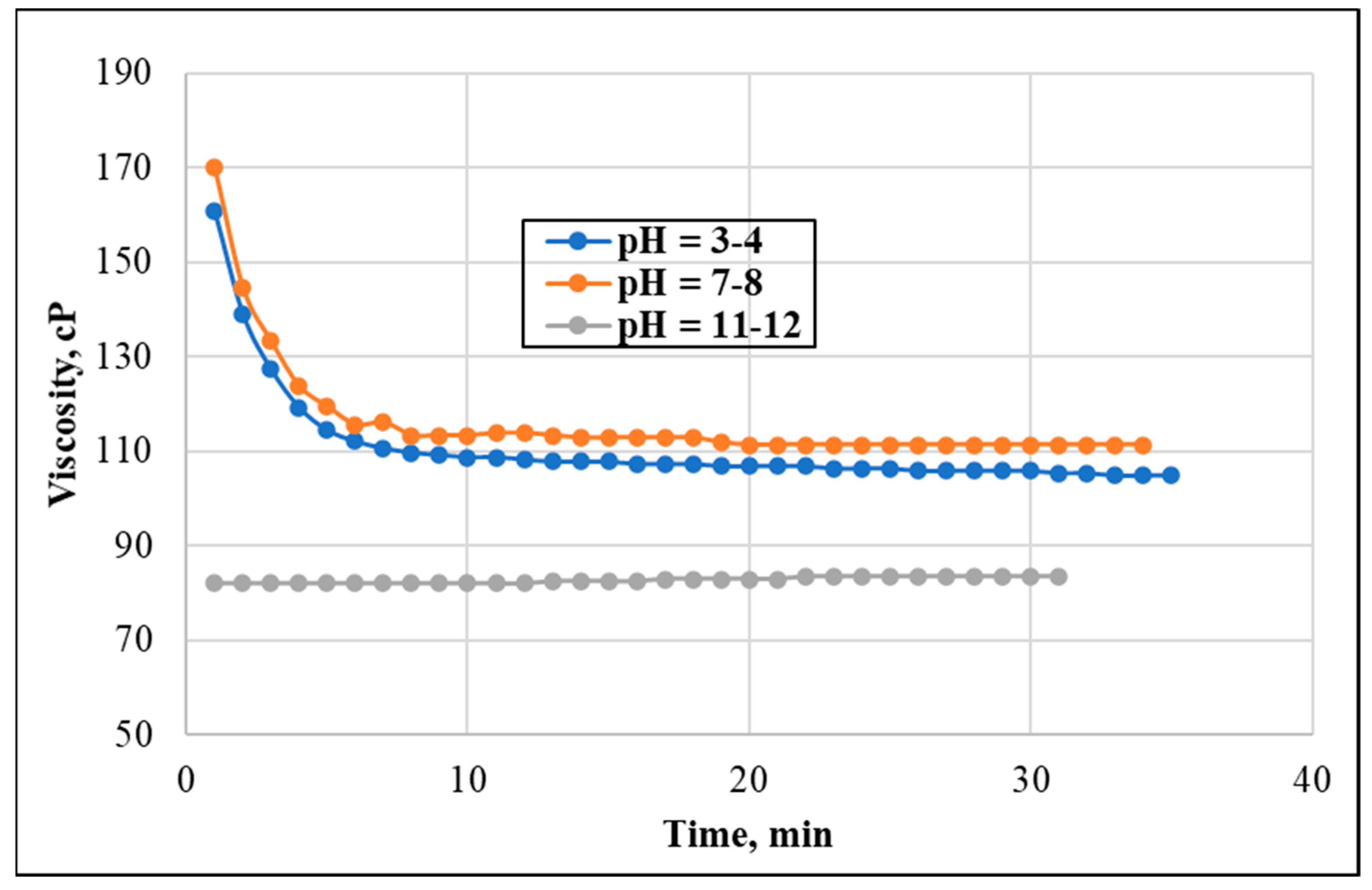
3.4. Effect of pH
3.5. Effect of CMHPG Concentration
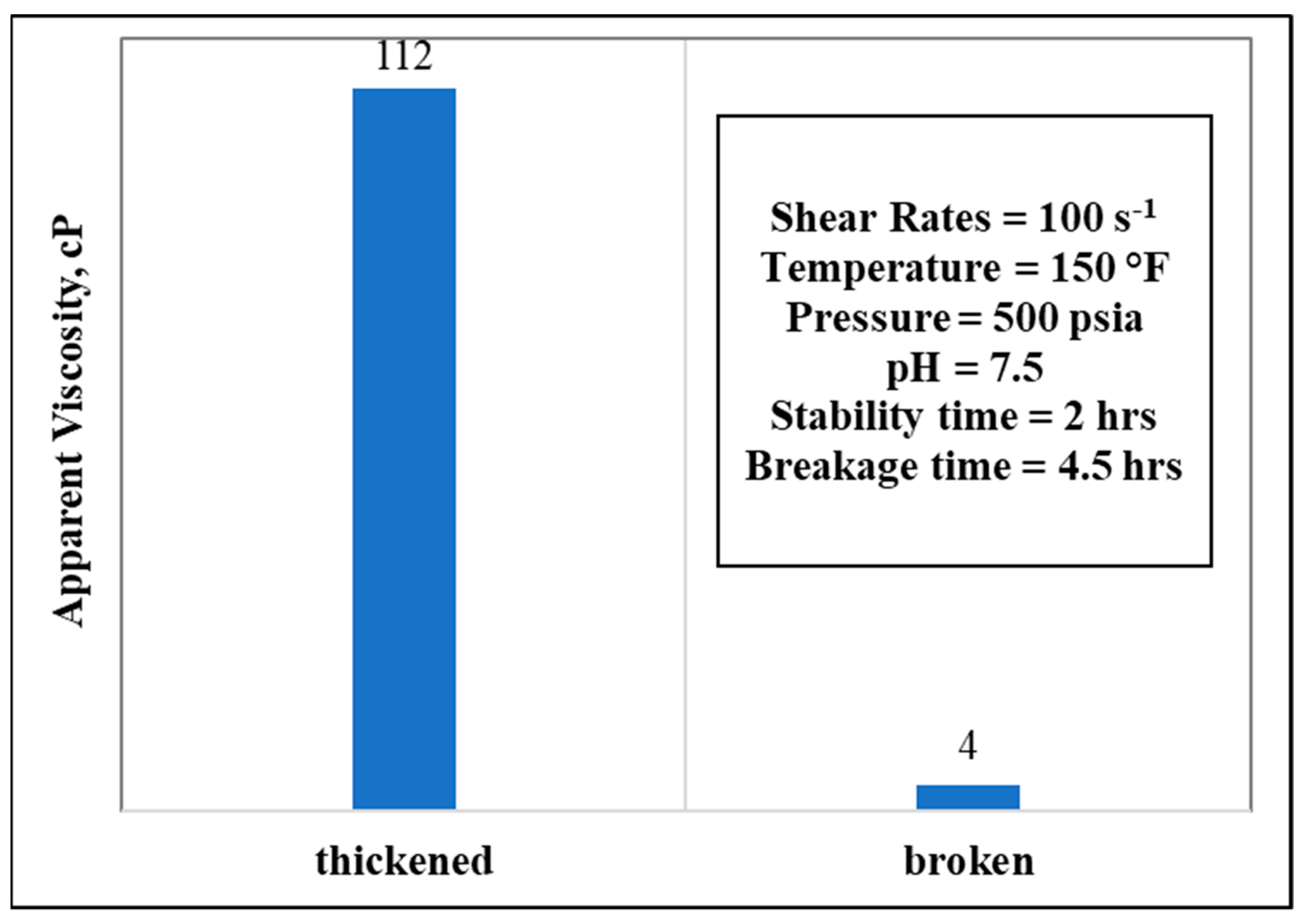
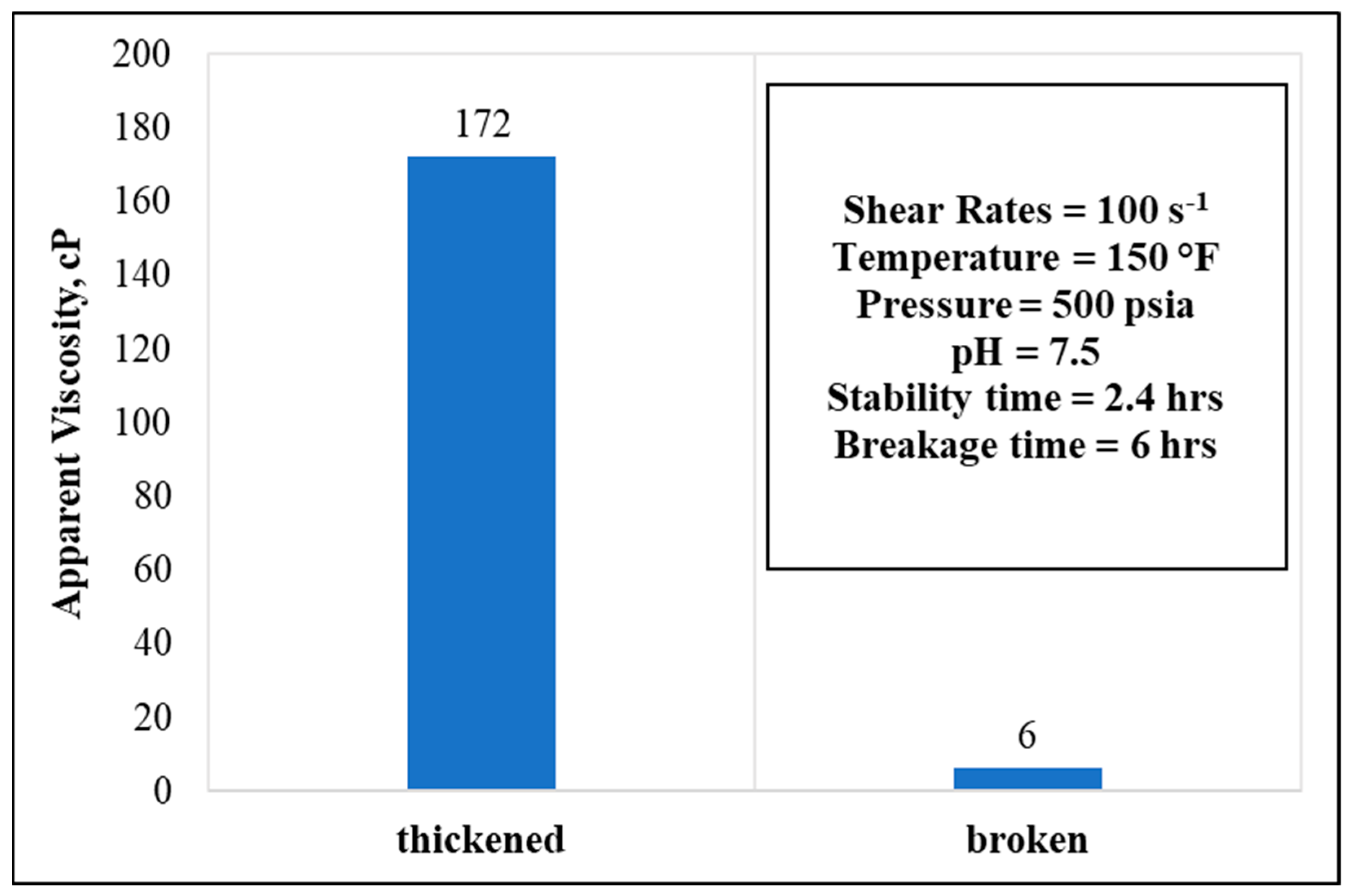
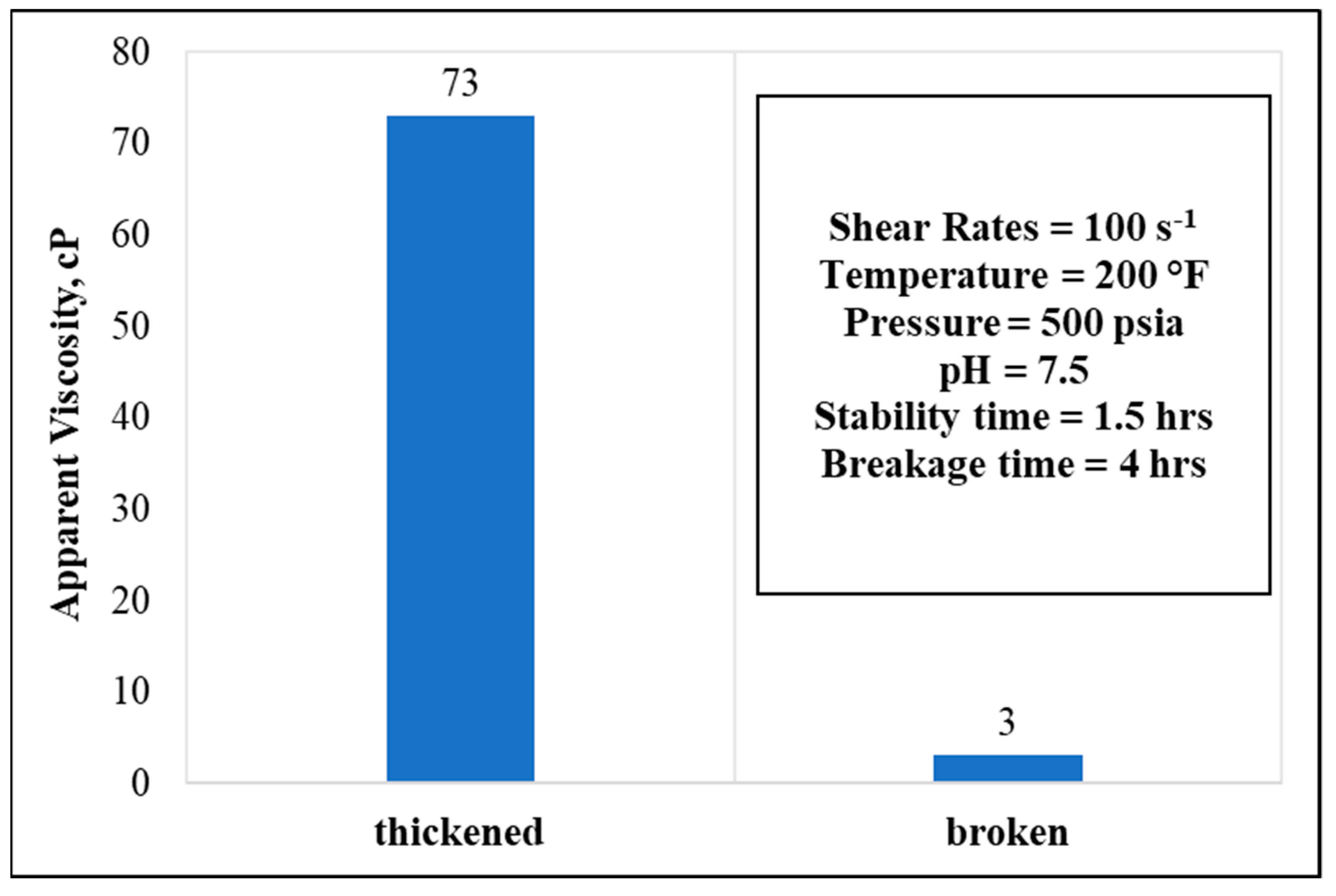
3.6. Stability and Breakage Behaviors
4. Conclusions and Recommendation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hogeboom, R.J. The Water Footprint Concept and Water’s Grand Environmental Challenges. One Earth 2020, 2, 218–222. [Google Scholar] [CrossRef]
- Reig, P.; Luo, T.; Proctor, J.N. Global Shale Gas Development: Water Availability & Business Risks. World Resources Institute. 2014. Available online: https://www.wri.org/research/global-shale-gas-development-water-availability-business-risks (accessed on 19 November 2021).
- Samad, N.A.; Bruno, V.L. The urgency of preserving water resources. EnviroNews 2013, 21, 3–6. [Google Scholar]
- Sowers, J.; Vengosh, A.; Weinthal, E. Climate change, water resources, and the politics of adaptation in the Middle East and North Africa. Clim. Chang. 2010, 104, 599–627. [Google Scholar] [CrossRef]
- Zhang, L.; Hascakir, B. A review of issues, characteristics, and management for wastewater due to hydraulic fracturing in the U.S. J. Pet. Sci. Eng. 2021, 202, 108536. [Google Scholar] [CrossRef]
- Yao, S.; Chang, C.; Hai, K.; Huang, H.; Li, H. A review of experimental studies on the proppant settling in hydraulic fractures. J. Pet. Sci. Eng. 2021, 109211. [Google Scholar] [CrossRef]
- LeBas, R.; Lord, P.; Luna, D.; Shahan, T. Development and Use of High-TDS Recycled Produced Water for Crosslinked-Gel-based Hydraulic Fracturing. Presented at the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 4–6 February 2013; pp. 125–133. [Google Scholar] [CrossRef] [Green Version]
- Whalen, T. The Challenges of Reusing Produced Water. J. Pet. Technol. 2012, 64, 18–20. [Google Scholar] [CrossRef]
- Zendehboudi, S.; Bahadori, A. Shale Oil and Gas Handbook: Theory, Technologies and Challenges; Gulf Professional Publishing: Houston, TX, USA, 2016; ISBN 9780128021002. [Google Scholar]
- Cheremisinoff, N.P.; Davletshin, A. Hydraulic Fracturing Operations: Handbook of Environmental Management Practices; John Wiley & Sons: Beverly, MA, USA, 2015; ISBN 9781119099987. [Google Scholar]
- Bonapace, J.; Giglio, M.; Moggia, J.; Krenz, A. Water Conservation: Reducing Freshwater Consumption by Using Produced Water for Base Fluid in Hydraulic Fracturing-Case Histories in Argentina. Presented at the SPE Latin America and Caribbean Petroleum Engineering Conference, Mexico City, Mexico, 16–18 April 2012; pp. 199–223. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Nasr-El-Din, H.A.; De Wolf, C.A.; LePage, J.N.; Bemelaar, J.H. Evaluation of a New Environmentally Friendly Chelating Agent for High-Temperature Applications. SPE J. 2011, 16, 559–574. [Google Scholar] [CrossRef]
- Kamal, M.S.; Mohammed, M.; Mahmoud, M.; Elkatatny, S. Development of chelating agent-based polymeric gel system for hydraulic fracturing. Energies 2018, 11, 1663. [Google Scholar] [CrossRef] [Green Version]
- Abdelgawad, K.Z.; Mahmoud, M.A.; Elkatatny, S.M. Stimulation of High Temperature Carbonate Reservoirs using Seawater and GLDA Chelating Agents: Reaction Kinetics Comparative Study. Presented at the SPE Kuwait Oil & Gas Show and Conference, Kuwait City, Kuwait, 15–18 October 2017. [Google Scholar]
- Veil, J.A.; Puder, M.G.; Elcock, D.; Redweik, R.J.J.; Punder, M.G.; Elcock, D.; Redweik Jr, R.J. A White Paper Describing Produced Water from Production of Crude Oil, Natural Gas, and Coal Bed Methane; Argonne National Laboratry: Du Page, IL, USA, 2004. [Google Scholar]
- Khatib, Z.; Verbeek, P. Water to value—Produced water management for sustainable field development of mature and green fields. JPTJ Pet. Technol. 2003, 55, 26–28. [Google Scholar] [CrossRef]
- Dickhout, J.M.; Moreno, J.; Biesheuvel, P.M.; Boels, L.; Lammertink, R.G.H.; de Vos, W.M. Produced water treatment by membranes: A review from a colloidal perspective. J. Colloid Interface Sci. 2017, 487, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Al-Kaabi, M.A.; Ashfaq, M.Y.; Da’na, D.A. Produced water characteristics, treatment and reuse: A review. J. Water Process Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- Kalam, S.; Kamal, M.S.; Patil, S.; Hussain, S.M.S. Impact of Spacer Nature and Counter Ions on Rheological Behavior of Novel Polymer-Cationic Gemini Surfactant Systems at High Temperature. Polymers 2020, 12, 1027. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.; Aljawad, M.S.; Mahmoud, M.; Kamal, M.S.; Patil, S.; Bataweel, M. Chelating Agents Usage in Optimization of Fracturing Fluid Rheology Prepared from Seawater. Polymers 2021, 13, 2111. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Clark, P.E. Crosslinking of Guar and Guar Derivatives. SPE J. 2007, 12, 316–321. [Google Scholar] [CrossRef]





| Parameter | Concentration (mg/L) |
|---|---|
| Major Parameters | |
| TDS (Total dissolved solids) | 100–400,000 |
| TSS (Total suspended solids) | 1.2–1000 |
| COD (Chemical oxygen demand) | 1220–2600 |
| TOC (Total organic content) | 0–1500 |
| Total organic acids | 0.001–10,000 |
| Chemicals Additives | |
| Glycol | 7.7–2000 |
| Corrosion inhibitor | 0.3–10 |
| Scale inhibitor | 0.2–30 |
| BTEX | |
| Benzene | 0.032–778.51 |
| Toluene | 0.058–5.86 |
| Ethylbenzene | 0.026–399.84 |
| Xylene | 0.01–1.29 |
| Other pollutants | |
| Saturated hydrocarbons | 17–30 |
| Total oil and grease | 2–560 |
| Phenol | 0.001–10,000 |
| Metals | |
| Na | 0–150,000 |
| Sr | 0–6250 |
| Zn | 0.01–35 |
| Li | 0.038–64 |
| Al | 0.4–410 |
| As | 0.002–11 |
| Ba | 0–850 |
| Cr | 0.002–1.1 |
| Fe | 0.1–1100 |
| Mn | 0.004–175 |
| K | 24–4300 |
| Other ions | |
| B | 5–95 |
| Ca2+ | 0–74,000 |
| SO42− | 0–15,000 |
| Mg2+ | 8–6000 |
| HCO3− | 77–3990 |
| Cl− | 0–270,000 |
| Salts | g/L |
|---|---|
| NaCl, g/L | 48.6 |
| CaCl2·2H2O, g/L | 22 |
| MgCl2·6H2O, g/L | 8.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlKhowaildi, M.; Tawabini, B.; Kamal, M.S.; Mahmoud, M.; Aljawad, M.S.; Bataweel, M. Development of Oil and Gas Stimulation Fluids Based on Polymers and Recycled Produced Water. Polymers 2021, 13, 4017. https://doi.org/10.3390/polym13224017
AlKhowaildi M, Tawabini B, Kamal MS, Mahmoud M, Aljawad MS, Bataweel M. Development of Oil and Gas Stimulation Fluids Based on Polymers and Recycled Produced Water. Polymers. 2021; 13(22):4017. https://doi.org/10.3390/polym13224017
Chicago/Turabian StyleAlKhowaildi, Mustafa, Bassam Tawabini, Muhammad Shahzad Kamal, Mohamed Mahmoud, Murtada Saleh Aljawad, and Mohammed Bataweel. 2021. "Development of Oil and Gas Stimulation Fluids Based on Polymers and Recycled Produced Water" Polymers 13, no. 22: 4017. https://doi.org/10.3390/polym13224017
APA StyleAlKhowaildi, M., Tawabini, B., Kamal, M. S., Mahmoud, M., Aljawad, M. S., & Bataweel, M. (2021). Development of Oil and Gas Stimulation Fluids Based on Polymers and Recycled Produced Water. Polymers, 13(22), 4017. https://doi.org/10.3390/polym13224017









