Composite Polymers from Leather Waste to Produce Smart Fertilizers
Abstract
:1. Introduction
2. Characteristics of Hide and Leather Wastes
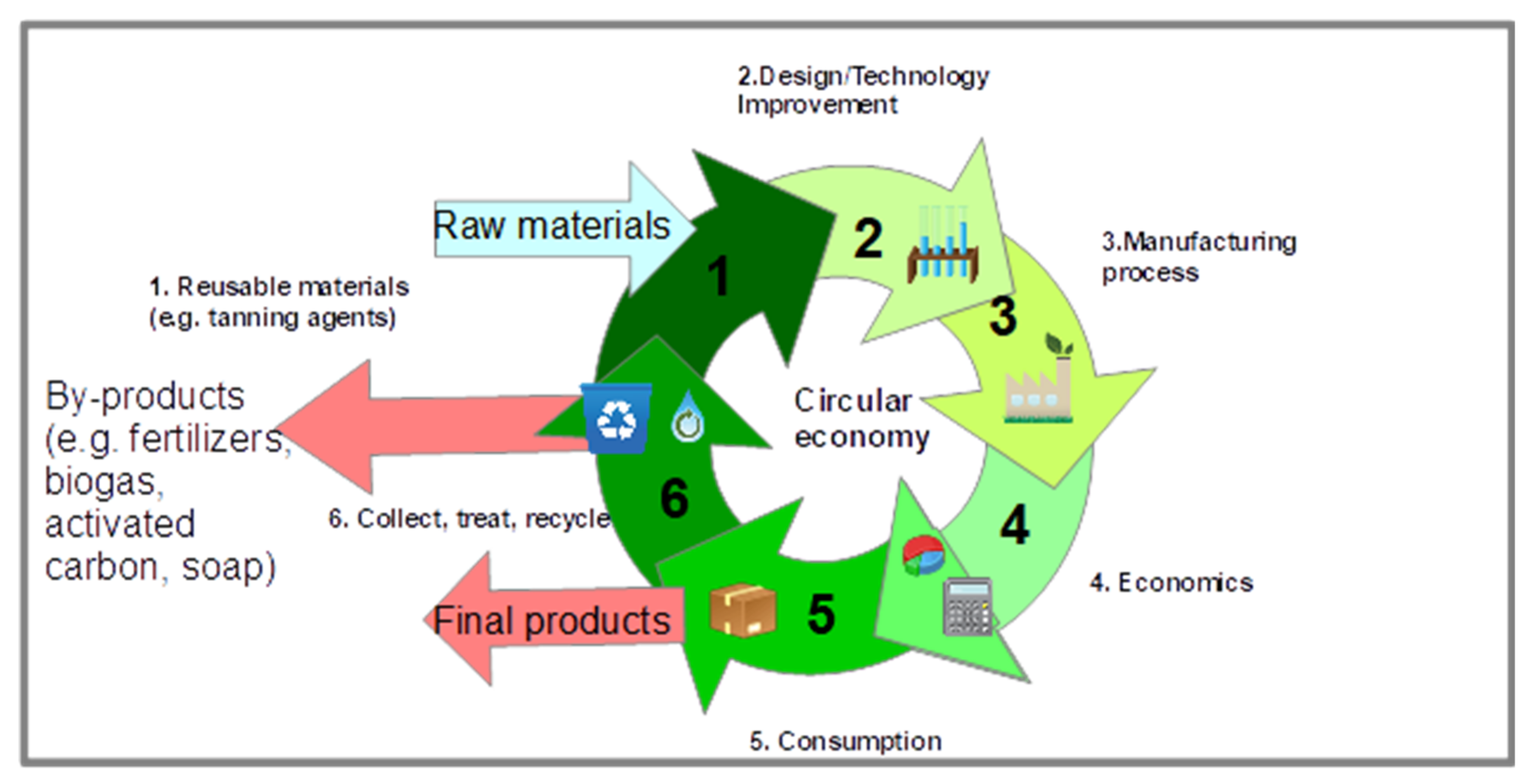
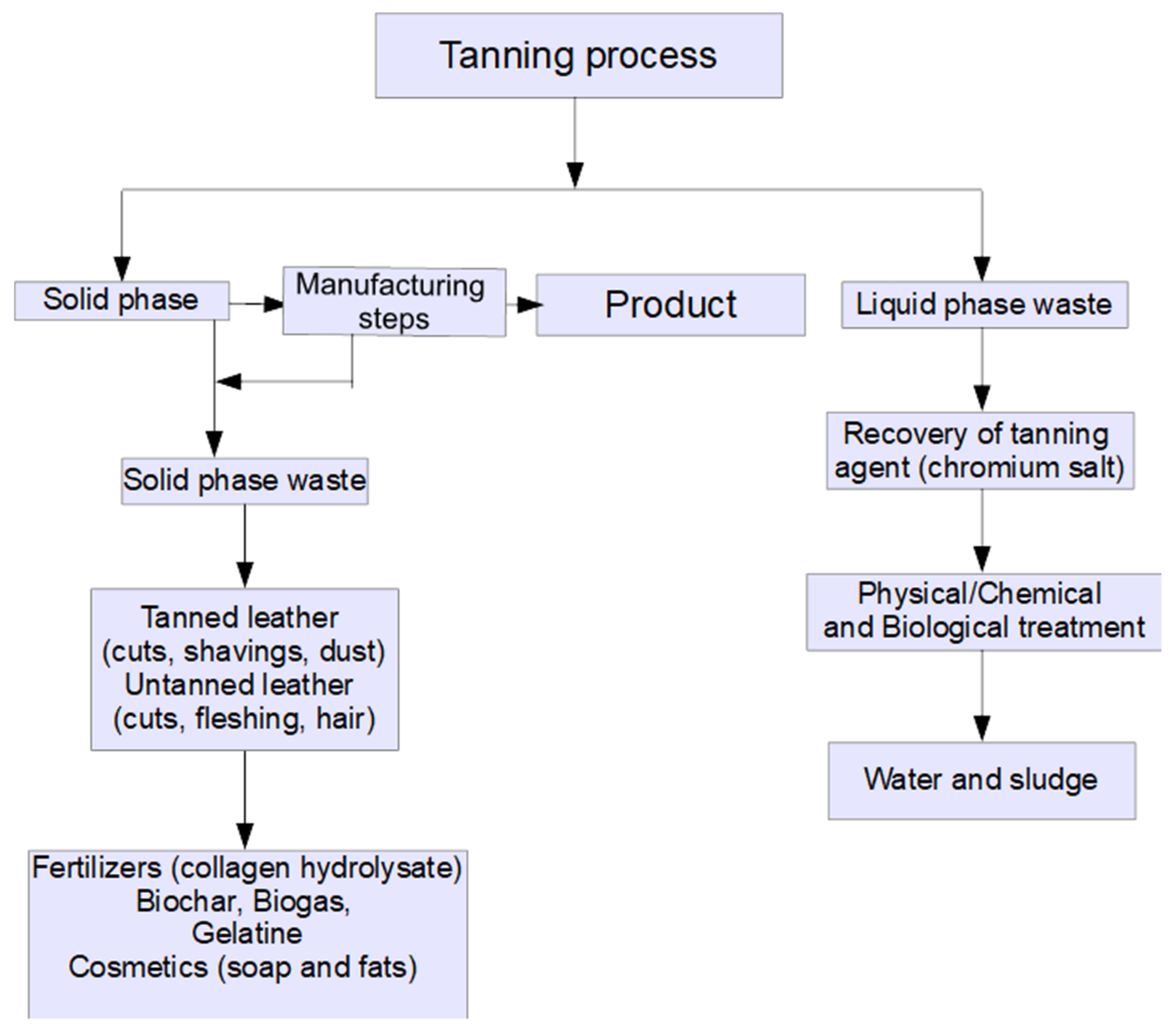
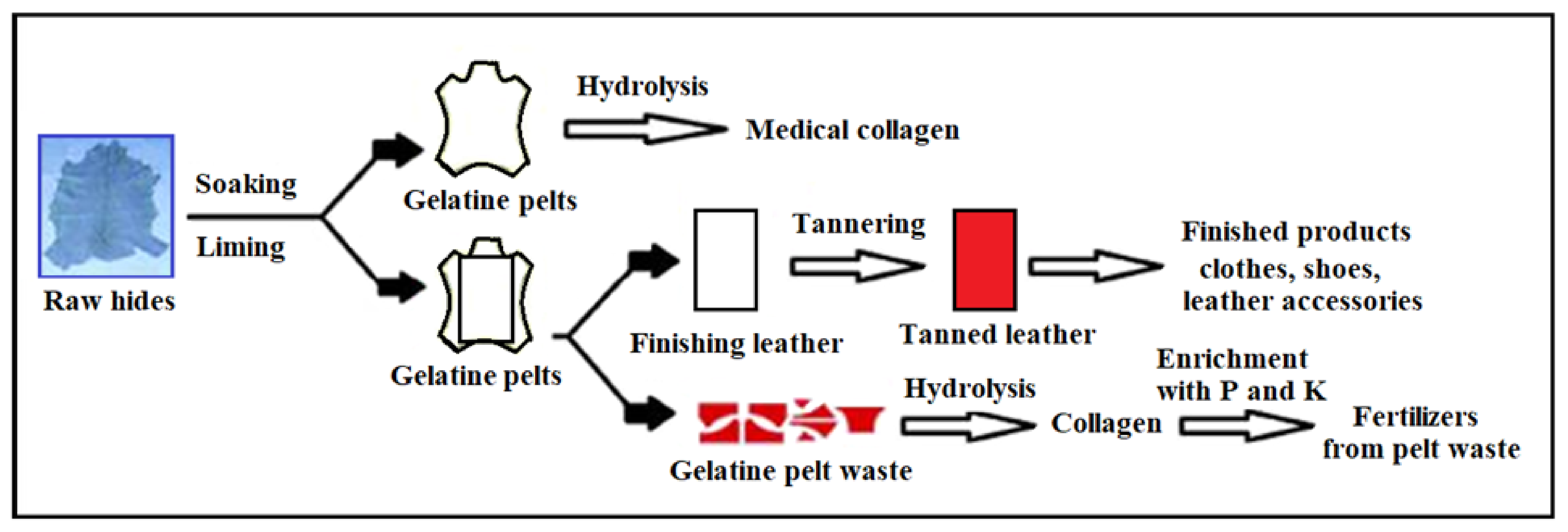
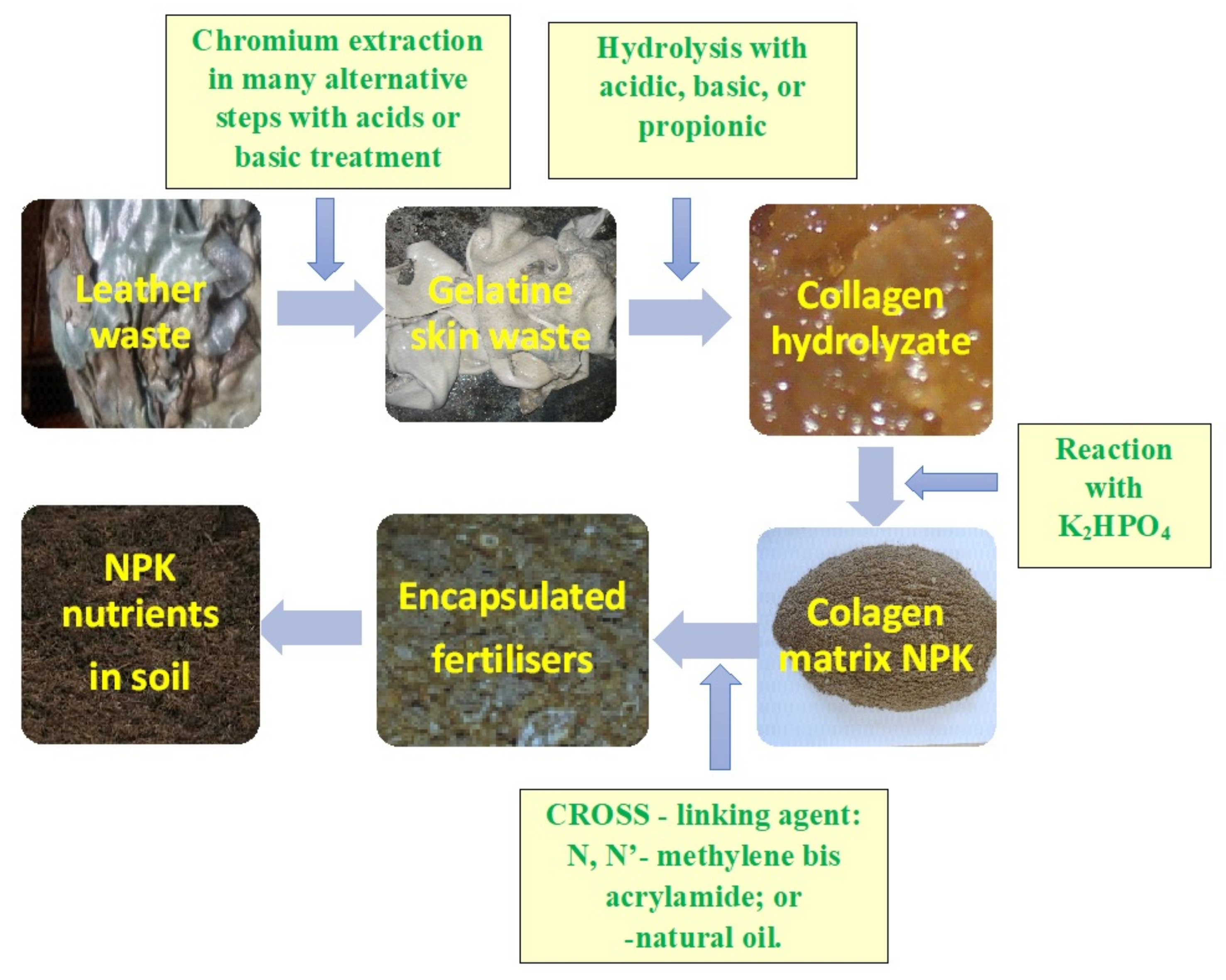
3. Hide and Leather Waste Processing
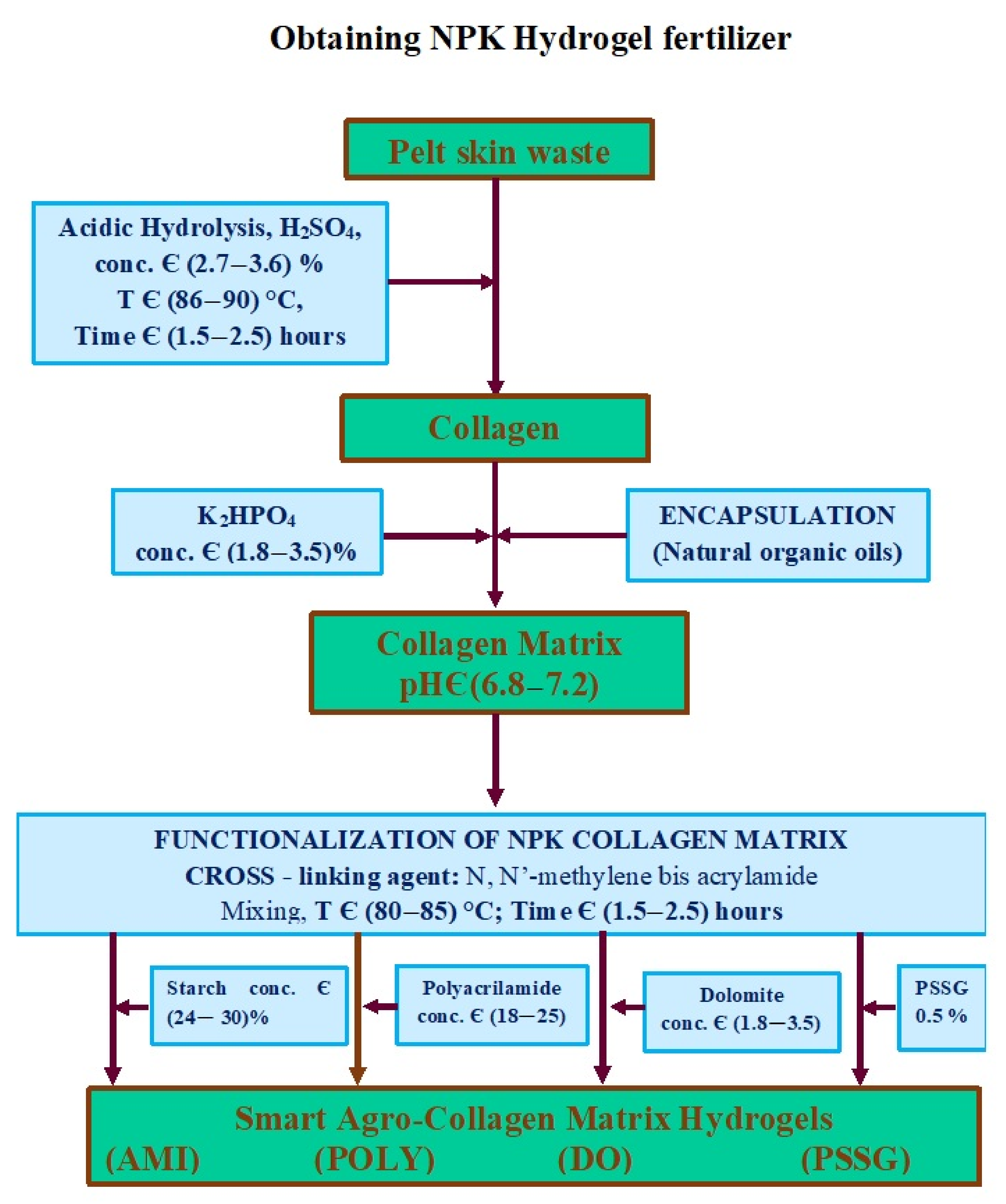
4. Preparation of Polymer Based Smart Fertilizer
5. Biodegradation of Polymers Extracted from Hide and Leather Waste
- (1)
- swelling: the ionic functional groups like hydroxyl, carboxyl, and amino can form the hydrogen bounds with water and more easily swell resulting in a porous network. This behaviour is specific only for hydrogels that have more functional ionic groups. The swollen porous structure increases the pore size, allowing the release of incorporated fertilizers such as urea, phosphates, etc.
- (2)
- biodeterioration: polymer fragmentation into lower molecular mass species in abiotic reactions (oxidation, photodegradation, hydrolysis).
- (3)
- biofragmentation: the polymer is fragmentated in biotic reactions, i.e., hydrolysis of macromolecules in oligomer, dimer or monomer, the mediators being microorganisms.
- (4)
- assimilation: the monomer can be absorbed by the microorganisms and degraded for example by deamination or decarboxylation, resulting ammonia or nitrate, acids and alcohols etc.
- (5)
- mineralization: is the process of degradation of organic compounds in aerobic and anaerobic conditions to mineral compounds (nitrate, carbon dioxide, hydrogen, methane).
- (1)
- stagnant zone: 0 ÷ 6 days, the biodegradation process is initiated and the process rate is small.
- (2)
- acceleration zone: 2 ÷ 50 days, the biodegradability is linearly increasing.
- (3)
- slowing zone: 14 ÷ 56 days, the biodegradability rate is decreasing.
- (4)
- stationary zone: 41 ÷ 75 days, biodegradation reaches its maximum degree and the biodegradability rate goes to zero.
6. Release Mechanism of Nutrients
6.1. Hydrogel Modelling—Kinetic Modelling of Nutrients Release
6.2. Hydrogel Modelling—Swelling Process
7. Future Perspectives of the Production of Smart Fertilizers from Leather Waste
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Helaly, P.M.; Abo-Elela, S.I. Protection of surface water from eutrophication via controlled release of phosphate fertilizer. J. Control. Release 1990, 12, 39–44. [Google Scholar] [CrossRef]
- Huang, J.; Xu, C.C.; Ridoutt, B.; Wang, X.C.; Ren, P.A. Nitrogen and phosphorus losses and eutrophication potential associated with fertilizer application to cropland in China. J. Clean. Prod. 2017, 159, 171–179. [Google Scholar] [CrossRef]
- Ashitha, A.; Rakhimol, K.R.; Jyothis, M. Fate of the conventional fertilizers in environment. In Controlled Release Fertilizers for Sustainable Agriculture, 1st ed.; Lewu, F.B., Volova, T., Thomas, S., Rakhimol, K.R., Eds.; Academic Press: London, UK, 2020; pp. 25–39. [Google Scholar]
- Li, P.; Kong, D.; Zhang, H.; Xu, L.; Li, C.; Wu, M.; Jiao, J.; Li, D.; Xu, L.; Li, H.; et al. Different regulation of soil structure and resource chemistry under animal-and plant-derived organic fertilizers changed soil bacterial communities. Appl. Soil Ecol. 2021, 165, 104020. [Google Scholar] [CrossRef]
- Naghdi, A.A.; Piri, S.; Khaligi, A.; Morad, P.J. Enhancing the qualitative and quantitative traits of potato by biological, organic, and chemical fertilizers. Saudi Soc. Agric. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Hui, K.; Tang, J.; Cui, Y.; Xi, B.; Tan, W. Accumulation of phthalates under high versus low nitrogen addition in a soil-plant system with sludge organic fertilizers instead of chemical fertilizers. Environ. Pollut. 2021, 291, 118193. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Li, Z.; Wang, H.; Liang, H. An overview of phthalate acid ester pollution in China over the last decade: Environmental occurrence and human exposure. Sci. Total Environ. 2018, 645, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.; Santanen, A.; Mäkelä, P. Recycling sludge on cropland as fertilizer–Advantages and risks. Resour. Conserv. Recycl. 2020, 155, 104647. [Google Scholar] [CrossRef]
- Bigalke, M.; Ulrich, A.; Rehmus, A.; Keller, A. Accumulation of cadmium and uranium in arable soils in Switzerland. Environ. Pollut. 2017, 221, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.J.; Singh, R.; Mishra, U.K.; Shahi, S.K. Role of Bio-Fertilizer in Organic Agriculture: A Review. Res. J. Recent Sci. 2013, 2, 39–41. [Google Scholar]
- Negi, Y.K.; Sajwan, P.; Uniyal, S.; Mishra, A.C. Enhancement in yield and nutritive qualities of strawberry fruits by the application of organic manures and biofertilizers. Sci. Hortic. 2021, 283, 110038. [Google Scholar] [CrossRef]
- Lubkowski, K.; Grzmil, B. Controlled release fertilizers. Polish, J. Chem. Technol. 2007, 9, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahma, N. Controlled release fertilizer: A review on developments, applications and potential in agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef] [PubMed]
- León, O.; Soto, D.; Antúnez, A.; Fernández, R.; González, J.; Piña, C.; Muñoz-Bonilla, A.; Fernandez-García, M. Hydrogels based on oxidized starches from different botanical sources for release of fertilizers. Int. J. Biol. Macromol. 2019, 136, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Rashedul Islam, M.; Tanveer, S.; Chen, C.C. Modeling swelling behavior of hydrogels in aqueous organic solvent. Chem. Eng. Sci. 2021, 242, 116744. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Chen, G.; Yi, S.H.; Wang, Y.; Liu, Q.; Wang, R. Multifunctional porous hydrogel with nutrient controlled-release and excellent biodegradation. J. Environ. Chem. Eng. 2021, 9, 106146. [Google Scholar] [CrossRef]
- Kazanskii, K.S.; Dubrovskii, S.A. Chemistry and physics of “agricultural” hydrogels. Adv. Polym. Sci. 1992, 104, 97–133. [Google Scholar]
- Azeem, B.; Ku Shaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar] [PubMed]
- Qiao, D.; Liu, H.; Yu, L.; Bao, X.; Simon, G.P.; Petinakis, E.; Chen, L. Preparation and characterization of slow-release fertilizer encapsulated by starch-based superabsorbent polymer. Carbohydr. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Essawy, H.A.; Ghazy, M.B.; El-Hai, F.A.; Mohamed, M.F. Superabsorbent hydrogels via graft polymerization of acrylic acid from chitosan-cellulose hybrid and their potential in controlled release of soil nutrients. Int. J. Biol. Macromol. 2016, 89, 144–151. [Google Scholar] [CrossRef]
- Hu, Z.; Yi, S.; Hu, W.; Tang, Y.; Wang, R. Synthesis and urea-loading of a novel biosuperabsorbent polymer based on leather waste. J. Soc. Leather Technol. Chem. 2015, 99, 51–57. [Google Scholar]
- Senna, A.M.; Botaro, V.R. Biodegradable hydrogel derived from cellulose acetate and EDTA as a reduction substrate of leaching NPK compound fertilizer and water retention in soil. J. Control. Release 2017, 260, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, Y.; Huang, S.; Zhao, Y. Synthesis composite hydrogels from inorganic-organic hybrids based on leftover rice for environment-friendly controlled-release urea fertilizers. Sci. Total Environ. 2018, 615, 422–430. [Google Scholar] [CrossRef]
- Langmaier, F.; Kolozmik, K.; Sukop, S.; Mladek, M. Products of enzymatic decomposition of chrome- tanned leather waste. J. Soc. Leather Technol. Chem. 1999, 83, 187–195. [Google Scholar]
- Ockerman, H.W.; Basu, L. BY-PRODUCTS Hides and Skins in Encyclopedia of Meat Sciences, 2nd ed.; Michael Dikeman, M., Devine, C., Eds.; Academic Press: London, UK, 2014; pp. 112–124. [Google Scholar]
- Mascianà, P. World Statistical Compendium for Raw Hides and Skins, Leather and Leather footwear 1999–2015. Available online: fao.org (accessed on 1 October 2021).
- Sundar, V.J.; Gnanamani, A.; Muralidharan, C.; Chandrababu, N.K.; Mandal, A.B. Recovery and utilization of proteinous wastes of leather making: A review. Rev. Environ. Sci. Biotechnol. 2011, 10, 151–163. [Google Scholar] [CrossRef]
- Cabrera-Codony, A.; Ruiz, B.; Gil, R.R.; Popartan, L.A.; Santos-Clotas, E.; Martín, M.; Fuente, E. From biocollagenic waste to efficient biogas purification: Applying circular economy in the leather industry. Environ. Technol. Innov. 2021, 21, 101229. [Google Scholar] [CrossRef]
- Kanagaraj, J.; Velappan, K.C.; Chandra Babu, N.K.; Sadulla, S. Solid wastes generation in the leather industry and its utilization for cleaner environment: A review. J. Sci. Ind. Res. 2006, 65, 541–548. [Google Scholar] [CrossRef]
- Brugnoli, F.; Král, I. Life Cycle Assessment, Carbon Footprint. In Proceedings of the Leather Processing, Eighteenth Session of the Leather and Leather Products Industry Panel, Eighteenth Session of the Leather and Leather Products Industry, Shanghai, China, 1–5 September 2012; Available online: open.unido.org (accessed on 29 September 2021).
- Al-Jabari, M.; Sawalha, H.; Pugazhendhi, A.; Rene, E.R. Cleaner production and resource recovery opportunities in leather tanneries: Technological applications and perspectives. Bioresour. Technol. Rep. 2021, in press. [Google Scholar] [CrossRef]
- Sreeram, K.J.; Saravanabhavan, S.; Raghava Rao, J.; Unni Nair, B. Use of chromium-collagen wastes for the removal of tannins from wastewater. Ind. Eng. Chem. Res. 2004, 43, 5310–5317. [Google Scholar] [CrossRef]
- Dixit, S.; Yadav, A.; Dwivedi, P.D.; Das, M. Toxic hazards of leather industry and technologies to combat threat: A review. J. Clean. Prod. 2015, 87, 39–49. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Mikula, K.; Witek-Krowiak, A.; Izydorczyk, G.; Kuligowski, K.; Bandrow, P.; Kułazynski, M. Progress in sustainable technologies of leather wastes valorization as solutions for the circular economy. J. Clean. Prod. 2021, 313, 127902. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-based fertilizers: A practical approach towards circular economy. Bioresour. Technol. 2020, 295, 122223. [Google Scholar] [CrossRef]
- Kiliç, E.; Puig, R.; Zengin, G.; Zengin, C.A.; Fullana-i-Palmer, P. Corporate carbon footprint for country Climate Change mitigation: A case study of a tannery in Turkey. Sci. Total Environ. 2018, 635, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Vidaurre-Arbizu, M.; Perez-Bou, S.; Zuazua-Ros, A.; Martín-Gomez, C. From the leather industry to building sector: Exploration of potential applications of discarded solid wastes. J. Clean. Prod. 2021, 291, 125960. [Google Scholar] [CrossRef]
- Hu, J.; Xiao, Z.; Zhou, R.; Deng, W.; Wang, M.; Ma, S. Ecological utilization of leather tannery waste with circular economy model. J. Clean. Prod. 2011, 19, 221–228. [Google Scholar] [CrossRef]
- Yılmaz, O.; Cem Kantarli, I.; Yuksel, M.; Saglam, M.; Yanik, J. Conversion of leather wastes to useful products. Resour. Conserv. Recycl. 2007, 49, 436–448. [Google Scholar] [CrossRef]
- Gil, R.R.; Girón, R.P.; Lozano, M.S.; Ruiz, B.; Fuente, E. Pyrolysis of biocollagenic wastes of vegetable tanning. Optimization and kinetic study. J. Anal. Appl. Pyrolysis 2012, 98, 129–136. [Google Scholar] [CrossRef]
- Amdouni, S.; Hassen Trabelsi, A.B.; Mabrouk Elasmi, A.; Chagtmi, R.; Haddad, K.; Jamaaoui, F.; Khedhira, H.; Cherif, C. Tannery fleshing wastes conversion into high value-added biofuels and biochars using pyrolysis process. Fuel 2021, 294, 120423. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Joo, H.S.; Yang, Y.H. Biowaste-to-bioenergy using biological methods—A mini-review. Energy Convers. Manag. 2018, 177, 640–660. [Google Scholar] [CrossRef]
- Simioni, T.; Borges Agustini, C.; Dettmer, A.; Gutterres, M. Nutrient balance for anaerobic co-digestion of tannery wastes: Energy efficiency, waste treatment and cost-saving. Bioresour. Technol. 2020, 308, 123255. [Google Scholar] [CrossRef] [PubMed]
- Pecha, J.; Barinova, M.; Kolomaznik, K.; Nguyen, T.N.; Dao, A.T.; Le, V.T. Technological-economic optimization of enzymatic hydrolysis used for the processing of chrome-tanned leather waste. Process. Saf. Environ. Prot. 2021, 152, 220–229. [Google Scholar] [CrossRef]
- Cem Kantarli, I.; Jale, Y. Activated carbon from leather shaving wastes and its application in removal of toxic materials. J. Hazard. Mater. 2010, 179, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Yue, Q.; Gao, B.; Li, Q.; Wang, Y.; Ngo, H.H.; Guo, W. Porous structure and adsorptive properties of hide waste activated carbons prepared via potassium silicate activation. J. Anal. Appl. Pyrolysis 2014, 109, 311–314. [Google Scholar] [CrossRef]
- Sanek, L.; Pecha, J.; Kolomaznik, K.; Barinova, M. Biodiesel production from tannery fleshings: Feedstock pretreatment and process modeling. Fuel 2015, 148, 16–24. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Thanikaivelan, P.; Krishnaraj, K.; Chandrasekaran, B. Transforming chromium containing collagen wastes into flexible composite sheets using cellulose derivatives: Structural, thermal and mechanical investigations. Polym. Compos. 2011, 32, 1009–1017. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Narayanan, N.T.; Mohana Reddy, A.L.; Gupta, B.K.; Chandrasekaran, B.; Talapatra, S.; Ajayan, P.M.; Thanikaivelan, P. Transforming collagen wastes into doped nanocarbons for sustainable energy applications. Green Chem. 2012, 14, 1689–1695. [Google Scholar] [CrossRef]
- Alibardi, L.; Cossu, R. Pre-treatment of tannery sludge for sustainable landfilling. Waste Manag. 2016, 52, 202–211. [Google Scholar] [CrossRef]
- Rai, D.; Eary, L.E.; Zachara, J.M. Environmental chemistry of chromium. Sci. Total Environ. 1989, 86, 15–23. [Google Scholar] [CrossRef]
- Tôrres Filho, A.; Lange, L.C.; Caldeira Bandeira de Melo, G.; Praes, G.E. Pyrolysis of chromium rich tanning industrial wastes and utilization of carbonized wastes in metallurgical process. Waste Manag. 2016, 48, 448–456. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, J.; Zhang, Y.; Zhou, J.; Shi, B. Conversion of tannery solid waste to an adsorbent for high-efficiency dye removal from tannery wastewater: A road to circular utilization. Chemosphere 2021, 263, 127987. [Google Scholar] [CrossRef]
- Sathish, M.; Madhan, B.; Rao, J.R. Leather solid waste: An eco-benign raw material for leather chemical preparation—A circular economy example. Waste Manag. 2019, 87, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Pati, A.; Chaudhary, R.; Subramani, S. A review on management of chrome-tanned leather shavings: A holistic paradigm to combat the environmental issues. Environ. Sci. Pollut. Res. 2014, 21, 11266–11282. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, P.; Horan, N.J. Optimising the biogas production from leather fleshing waste by co-digestion with MSW. Bioresour. Technol. 2009, 100, 4117–4120. [Google Scholar] [CrossRef] [PubMed]
- Priebe, G.P.S.; Kipper, E.; Gusmao, A.L.; Marcilio, N.R.; Gutterres, M. Anaerobic digestion of chrome-tanned leather waste for biogas production. J. Clean. Prod. 2016, 129, 410–416. [Google Scholar] [CrossRef]
- Agustini, C.; da Costa, M.; Gutterres, M. Biogas production from tannery solid wastes—Scale-up and cost saving analysis. J. Clean. Prod. 2018, 187, 158–164. [Google Scholar] [CrossRef]
- Agustini, C.B.; Spier, F.; da Costa, M.; Gutterres, M. Biogas production for anaerobic co-digestion of tannery solid wastes under presence and absence of the tanning agent. Resour. Conserv. Recycl. 2018, 130, 51–59. [Google Scholar] [CrossRef]
- Diamantis, V.; Eftaxias, A.; Stamatelatou, K.; Noutsopoulos, C.; Vlachokostas, C.; Aivasidis, A. Bioenergy in the era of circular economy: Anaerobic digestion technological solutions to produce biogas from lipid-rich wastes. Renew. Energy 2021, 168, 438–447. [Google Scholar] [CrossRef]
- Puhazhselvan, P.; Aparna, R.; Ayyadurai, N.; Gowthaman, M.K.; Saravanan, P.; Kamini, N.R. Enzyme based cleaner process for enhanced recovery of lipids from tannery fleshing waste. J. Clean. Prod. 2017, 144, 187–191. [Google Scholar] [CrossRef]
- Stefan, D.S.; Manea-Saghin, A.M.; Meghea, A.; Stefan, M. Biodegradation of composite fertilizers in aerobic aqueous and composting conditions. In Proceedings of the 19th International Multidisciplinary Scientific GeoConference SGEM, Albena, Bulgaria, 30 June–6 July 2019; Volume 19, pp. 49–56. [Google Scholar]
- Ockerman, H.W.; Hansen, E.I. Animal By-Product Processing, 1st ed.; VCH: Wienheim, Germany, 1988; pp. 89–131. [Google Scholar]
- Tahiri, S.; Albizane, A.; Messaoudi, A.; Azzi, M.; Bennazha, J.; Younssi, S.A.; Bouhria, M. Thermal behaviour of chrome shavings and of sludges recovered after digestion of tanned solid wastes with calcium hydroxide. Waste Manag. 2007, 27, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, L.F.; Taylor, M.M.; DiMaio, G.L.; Brown, E.M.; Marmer, W.N.; Carrió, R.; Celma, P.J.; Cot, J. Processing of leather waste: Pilot scale studies on chrome shavings. Isolation of potentially valuable protein products and chromium. Waste Manag. 1998, 18, 211–218. [Google Scholar] [CrossRef]
- Famielec, S. Chromium Concentrate Recovery from Solid Tannery Waste in a Thermal Process. Materials 2020, 13, 1533. [Google Scholar] [CrossRef] [PubMed]
- El Boushy, A.R.; van der Poel, A.F.B.; Koene, J.I.A.; Dieleman, S.H. Tanning waste by-product from cattle hides, its suitability as a feedstuff. Bioresour. Technol. 1991, 35, 321–323. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Jin, Y.; Chen, M.; Luo, J.; Zhu, X.; Zhang, Y. Study on the removal of chromium(III) from leather waste by a two-step method. J. Ind. Eng. Chem. 2019, 79, 172–180. [Google Scholar] [CrossRef]
- Teles, F.R.R.; Cabral, J.M.S.; Santos, J.A.L. Enzymatic degreasing of a solid waste from the leather industry by lipases. Biotechnol. Lett. 2001, 23, 1159–1163. [Google Scholar] [CrossRef]
- Stefan, D.S.; Dima, R.; Pantazi, M.; Ferdes, M.; Meghea, A. Identifying Microorganisms Able to Perform Biodegradation of Leather Industry Waste. Mol. Cryst. Liq. Cryst. 2012, 556, 301–308. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walters, B.D.; Stegemann, J.P. Strategies for directing the structure and function of three-dimensional collagen biomaterials across length scales. Acta Biomater. 2014, 10, 1488–1501. [Google Scholar] [CrossRef] [Green Version]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, E.; Lowman, A.; Marcolongo, M. Synthesis and Characterization of an Injectable Hydrogel with Tunable Mechanical Properties for Soft Tissue Repair. Biomacromolecules 2006, 7, 3223–3228. [Google Scholar] [CrossRef]
- Rutz, A.L.; Shah, R.N. Protein Based Hydrogels in Polymeric Hydrogels as Smart Biomaterials, 1st ed.; Kalia, S., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 73–104. [Google Scholar]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Horue, M.; Berti, I.R.; Cacicedo, M.L.; Castro, G.R. Microbial production and recovery of hybrid biopolymers from wastes for industrial applications—A review. Bioresour. Technol. 2021, 340, 125671. [Google Scholar] [CrossRef]
- Sionkowska, A. Collagen blended with natural polymers: Recent advances and trends. Prog. Polym. Sci. 2021, 122, 101452. [Google Scholar] [CrossRef]
- Ucar, B. Natural biomaterials in brain repair: A focus on collagen. Neurochem. Int. 2021, 146, 105033. [Google Scholar] [CrossRef]
- Stefan, D.S.; Zainescu, G.; Manea-Saghin, A.M.; Triantaphyllidou, I.E.; Tzoumani, I.; Tatoulis, T.I.; Syriopoulos, G.T.; Meghea, A. Collagen-Based Hydrogels Composites from Hide Waste to Produce Smart Fertilizers. Materials 2020, 13, 4396. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, X.; Wang, Y.; Hu, Z.; Wang, R. Characterization of slow-release collagen-g-poly(acrylic acid-co-2-acrylamido-2-methyl-1-propane sulfonic acid)–iron(III) superabsorbent polymer containing fertilizer. J. Appl. Polym. Sci. 2019, 136, 47178. [Google Scholar] [CrossRef]
- Teramoto, N.; Hayashi, A.; Yamanaka, K.; Sakiyama, A.; Nakano, A.; Shibata, M. Preparation and Mechanical Properties of Photo-Crosslinked Fish Gelatin/Imogolite Nanofiber Composite Hydrogel. Materials 2012, 5, 2573–2585. [Google Scholar] [CrossRef] [Green Version]
- Liguori, A.; Uranga, J.; Panzavolta, S.; Guerrero, P.; de la Caba, K.; Focarete, M.L. Electrospinning of Fish Gelatin Solution Containing Citric Acid: An Environmentally Friendly Approach to Prepare Crosslinked Gelatin Fibers. Materials 2019, 12, 2808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzoumani, I.; Lainioti, G.C.; Aletras, A.J.; Zainescu, G.; Stefan, S.; Meghea, A.; Kallitsis, J.K. Modification of Collagen Derivatives with Water-Soluble Polymers for the Development of Cross-Linked Hydrogels for Controlled Release. Materials 2019, 12, 4067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masilamani, D.; Madhan, B.; Shanmugam, G.; Palanivel, S.; Narayan, B. Extraction of collagen from raw trimming wastes of tannery: A waste to wealth approach. J. Clean. Prod. 2016, 113, 338–344. [Google Scholar] [CrossRef]
- Gousterova, A.; Nustorova, M.; Christov, P.; Nedkov, P.; Neshev, G.; Vasileva-Tonkova, E. Development of a biotechnological procedure for treatment of animal wastes to obtain inexpensive biofertilizer. World J. Microbiol. Biotechnol. 2008, 24, 2647–2652. [Google Scholar] [CrossRef]
- Constantinescu, R.R.; (National Research & Development Institute for Textiles and Leather, Bucharest, Romania); Ignat, M.; (National Research & Development Institute for Textiles and Leather, Bucharest, Romania). Personal communication, 2013.
- Majee, S.; Halder, G.; Mandal, T. Formulating nitrogen-phosphorous-potassium enriched organic manure from solid waste: A novel approach of waste valorization. Process. Saf. Environ. Prot. 2019, 132, 160–168. [Google Scholar] [CrossRef]
- Majee, S.; Halder, G.; Mandal, D.D.; Tiwari, O.N.; Mandal, T. Transforming wet blue leather and potato peel into an eco-friendly bio-organic NPK fertilizer for intensifying crop productivity and retrieving value-added recyclable chromium salts. J. Hazard. Mater. 2021, 411, 125046. [Google Scholar] [CrossRef] [PubMed]
- Lima de Oliveira, D.Q.; Gomes Carvalho, K.T.; Ribeiro Bastos, A.R.; Alves de Oliveira, L.C.; João José Granate de Sá e Melo Marques, J.J.; Severina de Melo Pereira do Nascimento, R. Use of leather industry residues as nitrogen sources for elephantgrass. Rev. Bras. Ciênc. Solo 2008, 32, 417–424. [Google Scholar]
- Lima, D.Q.; Oliveira, L.C.A.; Bastos, A.R.R.; Carvalho, G.S.; Marques, J.G.S.M.; Carvalho, J.G.; de Souza, G.A. Leather Industry Solid Waste as Nitrogen Source for Growth of Common Bean Plants. Appl. Environ. Soil Sci. 2010, 2010, 703842. [Google Scholar] [CrossRef]
- Chiroma, T.M.; Ebewele, R.O.; Hymore, F.K. Comparative Assessment of Heavy Metal Levels In Soil, Vegetables and Urban Grey Waste Water Used for Irrigation in Yola And Kano. Int. Ref. J. Eng. Sci. 2014, 3, 1–9. [Google Scholar]
- Constantinescu, R.R.; Zainescu, G.; Stefan, D.S.; Sirbu, C.; Voicu, P. Protein biofertilizer development and application on soybean cultivated degraded soil. Leather Footwear, J. 2015, 15, 169–178. [Google Scholar] [CrossRef]
- Zainescu, G.; Albu, L.; Constantinescu, R.R. Study of Collagen Hydrogel Biodegradability over Time. Rev. Chim. 2018, 69, 101. [Google Scholar] [CrossRef]
- Nogueira, F.G.E.; do Prado, N.T.; Oliveira, L.C.A.; Bastos, A.R.R.; Lopes, J.H.; Carvalho, J.G. Incorporation of mineral phosphorus and potassium on leather waste (collagen): A new NcollagenPK-fertilizer with slow liberation. J. Hazard. Mater. 2010, 176, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Progr. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Du, C.W.; Zhou, J.M.; Shaviv, A. Release Characteristics of Nutrients from Polymer-coated Compound Controlled Release Fertilizers. J. Polym. Environ. 2006, 14, 223–230. [Google Scholar] [CrossRef]
- Zainescu, G.; Constantinescu, R.R.; Sirbu, C. Smart Hydrogels with Collagen Structure Made of Pelt Waste. Rev. Chim. 2017, 68, 393. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Majeed, Z.; Ramli, N.K.; Mansor, N.; Man, Z. A comprehensive review on biodegradable polymers and their blends used in controlled release fertilizer processes. Rev. Chem. Eng. 2015, 31, 69–95. [Google Scholar] [CrossRef]
- Zuriaga-Agustí, E.; Galiana-Aleixandre, M.V.; Bes-Pia, A.; Mendoza-Roca, J.A.; Risueno-Puchades, V.; Segarra, V. Pollution reduction in an eco-friendly chrome-free tanning and evaluation of the biodegradation by composting of the tanned leather wastes. J. Clean. Prod. 2015, 87, 874–881. [Google Scholar]
- China, C.R.; Hilonga, A.; Nyandoro, S.S.; Schroepfer, M.; Kanth, S.V.; Meyer, M.; Njau, K.N. Suitability of selected vegetable tannins traditionally used in leathermaking in Tanzania. J. Clean. Prod. 2020, 251, 119687. [Google Scholar] [CrossRef]
- Carsote, C.; Sendrea, C.; Micu, M.C.; Adams, A.; Badea, E. Micro-DSC, FTIR-ATR and NMR MOUSE study of the dose-dependent effects of gamma irradiation on vegetable-tanned leather: The influence of leather thermal stability. Radiat. Phys. Chem. 2021, 189, 109712. [Google Scholar] [CrossRef]
- Pradeep, S.; Sundaramoorthy, S.; Sathish, M.; Jayakumar, G.C.; Rathinam, A.; Madhan, B.; Saravanan, P.; Rao, J.R. Chromium-free and waterless vegetable-aluminium tanning system for sustainable leather manufacture. Chem. Eng. J. Adv. 2021, 7, 100108. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, R.; Mi, Z.; Lyu, S.; Ma, J. Engineering a sustainable chrome-free leather processing based on novel lightfast wet-white tanning system towards eco-leather manufacture. J. Clean. Prod. 2021, 282, 124504. [Google Scholar] [CrossRef]
- Stefan, D.S.; Meghea, I.; Apetroaei, M.R. Study of biodegradation of leather tanning with chromium and vegetal compounds. In Proceedings of the 11th International Multidisciplinary Scientific GeoConference SGEM2011, Albena, Bulgaria, 20–25 June 2011; Volume 3, pp. 221–228. [Google Scholar]
- Dhayalan, K.; Fathima, N.N.; Gnanamani, A.; Rao, J.R.; Nair, B.U.; Ramasami, T. Biodegradability of leathers through anaerobic pathway. Waste Manag. 2001, 27, 760–767. [Google Scholar]
- Ge, J.; Yu, H.; Zhong, W.; Li, W.; Yu, T. Study on the utilization of biodegradable polyurethane material from the bark of Acacia mearnsii (I). Coating material of controlled slow-release fertilizer. J. Funct. Polym. 1998, 11, 478–482. [Google Scholar]
- Chen, L.; Xie, Z.; Zhuang, X.; Chen, X.; Jing, X. Controlled release of urea encapsulated by starch-g-poly(l-lactide). Carbohydr. Polym. 2008, 72, 342–348. [Google Scholar] [CrossRef]
- Han, X.; Chen, S.; Hu, X. Controlled-release fertilizer encapsulated by starch/polyvinyl alcohol coating. Desalination 2009, 240, 21–26. [Google Scholar] [CrossRef]
- Celli, A.; Sabaa, M.W.; Jyothi, A.N.; Kalia, S. Chitosan and Starch-Based Hydrogels Via Graft Copolymerization. In Polymeric Hydrogels as Smart Biomaterials; Springer Series on Polymer and Composite Materials; Kalia, S., Ed.; Springer: Cham, Switzerland, 2016; pp. 189–234. [Google Scholar]
- Mahkam, M. Synthesis and Characterization of Polymer/Silica Composite for Colon-Specific Drug Delivery. Int. J. Polym. Mater. 2011, 60, 456–468. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gumy, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Lee, P.I. Kinetics of drug release from hydrogel matrices. J. Control. Release 1985, 2, 277–288. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Nešovic, K.; Jankovic, A.; Peric-Grujic, A.; Vukašinovic-Sekulic, M.; Radetic, T.; Živkovic, L.; Park, S.J.; Rheed, K.Y.; Miškovic-Stankovic, V. Kinetic models of swelling and thermal stability of silver/poly(vinyl alcohol)/chitosan/graphene hydrogels. J. Ind. Eng. Chem. 2019, 77, 83–96. [Google Scholar] [CrossRef]
- Pareek, A.; Maheshwari, S.; Cherlo, S.; Thavva, R.S.R.; Runkana, V. Modeling drug release through stimuli responsive polymer hydrogels. Int. J. Pharm. 2017, 532, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.B.; Li, H.; Lam, K.Y. Development of a multiphysics model to characterize the responsive behavior of urea-sensitive hydrogel as biosensor. Biosens. Bioelectron. 2017, 91, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Caccavo, D. An overview on the mathematical modeling of hydrogels’ behavior for drug delivery systems. Int. J. Pharm. 2019, 560, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Sauerwein, M.; Steeb, H. Modeling of dynamic hydrogel swelling within the pore space of a porous medium. Int. J. Eng. Sci. 2020, 155, 103353. [Google Scholar] [CrossRef]
- Chun, S.W.; Kim, J.D. Swelling and Deswelling Transition of Water-Soluble Poly(N-isopropylacrylamide) by a Method of Blob Rescaling. Korean J. Chem. Eng. 2002, 19, 803–807. [Google Scholar] [CrossRef]
- Poschlad, K.; Enders, S. Thermodynamics of aqueous solutions containing poly (N-isopropylacrylamide). J. Chem. Thermodynamics 2011, 43, 262–269. [Google Scholar] [CrossRef]
- Althans, D.; Langenbach, K.; Enders, S. Influence of different alcohols on the swelling behaviour of hydrogels. Mol. Phys. 2012, 110, 1391–1402. [Google Scholar] [CrossRef]
- Sheth, S.; Barnard, E.; Hyatt, B.; Rathinam, M.; Zustiak, S.P. Predicting Drug Release from Degradable Hydrogels Using Fluorescence Correlation Spectroscopy and Mathematical Modeling. Front. Bioeng. Biotechnol. 2019, 7, 410. [Google Scholar] [CrossRef] [Green Version]
- Sauerwein, M.; Steeb, H. A modified effective stress principle for chemical active multiphase materials with internal mass exchange. Geomech. Energy Environ. 2018, 15, 19–34. [Google Scholar] [CrossRef]
- Zhi, D.; Huang, Y.; Hu, S.; Liu, H.; Hu, Y. Molecular thermodynamic model for swelling behavior and volume phase transition of multi-responsive hydrogels. Fluid Phase Equilibria. 2011, 312, 106–115. [Google Scholar] [CrossRef]
- Bayat, M.R.; Dolatabadi, R.; Baghani, M. Transient swelling response of pH-sensitive hydrogels: A monophasic constitutive model and numerical implementation. Int. J. Pharm. 2020, 577, 119030. [Google Scholar] [CrossRef]


| Hydrogel | Water Absorption Capacity | Cr (III) Adsorption Capacity |
|---|---|---|
| Biofertilizer (collagen-nitrogen and potassium) [16] | 2208 g H2O/g | 149.3 g Cr (III)/g |
| Biofertilizer (source P nutrient) Collagen-g-poly(acrylic acid-co- | 2595 g H2O/g | Not tested |
| 2-acrylamido-2-methyl-1-propane sulfonic acid)–iron(III) [81] | ||
| Collagen-polyacrylic acid-co- | ||
| 2-acrylamido-2-methyl-1-propane sulfonic acid) [81] | 3578 g H2O/g | Not tested |
| Hydrogel | Pore Diameter | Days of Controlled Nutrient Release |
|---|---|---|
| Biofertilizer (collagen-nitrogen and potassium) [16] | 1.26–6.73 μm | More than 40 |
| Biofertilizer (source P nutrient) Collagen-g-poly(acrylic acid-co-2-acrylamido-2-methyl-1-propane sulfonic acid)–iron(III) [81] | 4–9 μm | More than 30 |
| Time, Days | Biodegradation Degree | |||||||
|---|---|---|---|---|---|---|---|---|
| CH | Ref CH | AMI | POLY | PSSG | ||||
| W | W | C | W | C | W | C | W | |
| 10 | 42 | 35 | 17 | 37 | 33 | 15 | 12 | 20 |
| 20 | 58 | 48 | 33 | 50 | 48 | 27 | 21 | 35 |
| 75 | 99 | 74 | 50 | 80 | 64 | 62 | 40 | 63 |
| Fertilizer Type | k | n | R2 |
|---|---|---|---|
| CH | 0.1370 | 0.6693 | 0.9159 |
| REF-CH | 0.0480 | 0.9514 | 0.9240 |
| AMI | 0.0880 | 0.7995 | 0.9212 |
| POLY | 0.0560 | 0.9059 | 0.9212 |
| PSSG | 0.0710 | 0.8644 | 0.9097 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefan, D.S.; Bosomoiu, M.; Constantinescu, R.R.; Ignat, M. Composite Polymers from Leather Waste to Produce Smart Fertilizers. Polymers 2021, 13, 4351. https://doi.org/10.3390/polym13244351
Stefan DS, Bosomoiu M, Constantinescu RR, Ignat M. Composite Polymers from Leather Waste to Produce Smart Fertilizers. Polymers. 2021; 13(24):4351. https://doi.org/10.3390/polym13244351
Chicago/Turabian StyleStefan, Daniela Simina, Magdalena Bosomoiu, Rodica Roxana Constantinescu, and Madalina Ignat. 2021. "Composite Polymers from Leather Waste to Produce Smart Fertilizers" Polymers 13, no. 24: 4351. https://doi.org/10.3390/polym13244351
APA StyleStefan, D. S., Bosomoiu, M., Constantinescu, R. R., & Ignat, M. (2021). Composite Polymers from Leather Waste to Produce Smart Fertilizers. Polymers, 13(24), 4351. https://doi.org/10.3390/polym13244351






