Use of Poly(vinyl alcohol)-Malic Acid (CLHPMA) Hydrogels and Chitosan Coated Calcium Alginate (CCCA) Microparticles as Potential Sorbent Phases for the Extraction and Quantitative Determination of Pesticides from Aqueous Solutions
Abstract
:1. Introduction
- (1)
- Polyelectrolyte complex (PEC), composed of CT and CaALG. The polyelectrolyte complexes result from the interaction of macromolecules that have complementary ionizable electrostatic groups. The use of natural polyelectrolytes to prepare polyelectrolyte complexes is due to their advantages in terms of their low cost and compatibility, and their minimal consumption of organic solvents. They are also safe and stable and have been approved for use in humans [24]. In this way, chitosan-calcium alginate complexes (CCAC), and chitosan-coated calcium alginate microparticles (CCCA) were synthesized.
- (2)
2. Theoretical Section
2.1. ALG-CT Chains
2.2. PVA-MA Substructures
2.3. ALG-CT and PVA-MA Particles
2.4. Molecular Dynamics Simulations (MDS)
3. Experimental
3.1. Reagents
3.2. Synthesis and Characterization of Materials
3.2.1. Synthesis of Hydrogels Based on Poly(vinyl alcohol) (PVA) Crosslinked with Malic Acid (MA) (CLPHMA)
3.2.2. Synthesis of Microparticles Based on Polyelectrolyte Complexes (PEC), Composed of CT and CaALG: Calcium Alginate-Chitosan Complexes (CCAC), and Chitosan-Coated Calcium Alginate Microparticles (CCCA)
3.2.3. Swelling Studies
3.2.4. Characterization of Synthesized Materials by Infrared Spectroscopy and Thermo-Gravimetric Analysis
3.2.5. Analysis of Synthesized Materials by Scanning Electron Microscopy (SEM)
3.3. Capture and Desorption Assays
3.4. Chromatographic Method
4. Results and Discussion
4.1. Molecular Dynamics Simulation Results
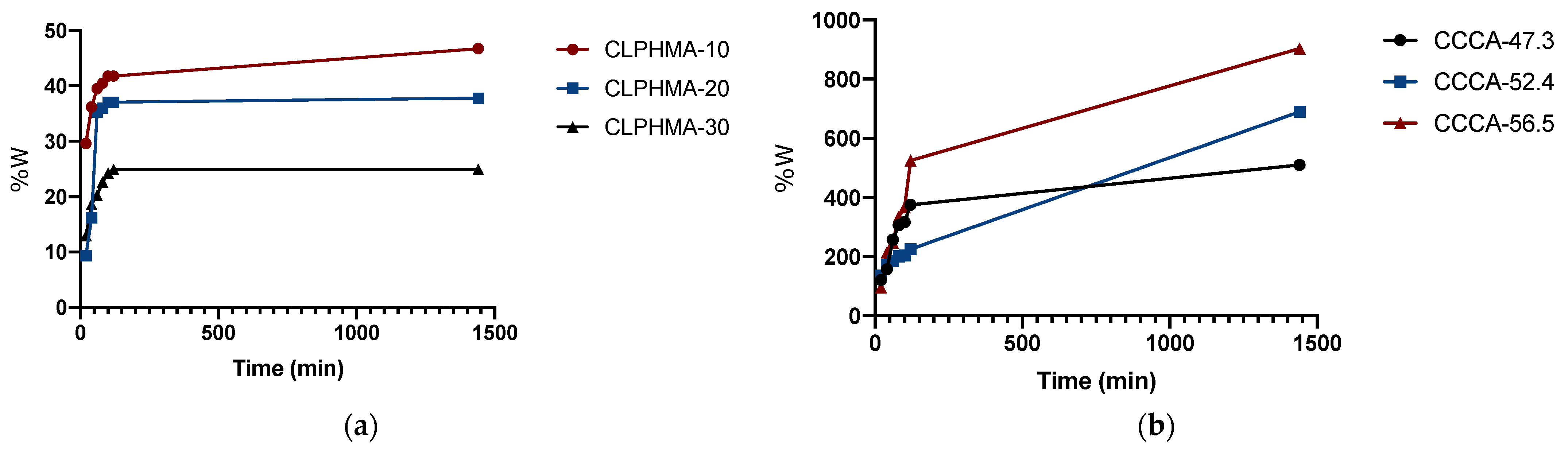
4.2. Swelling Study of CLPHMA and CCCA
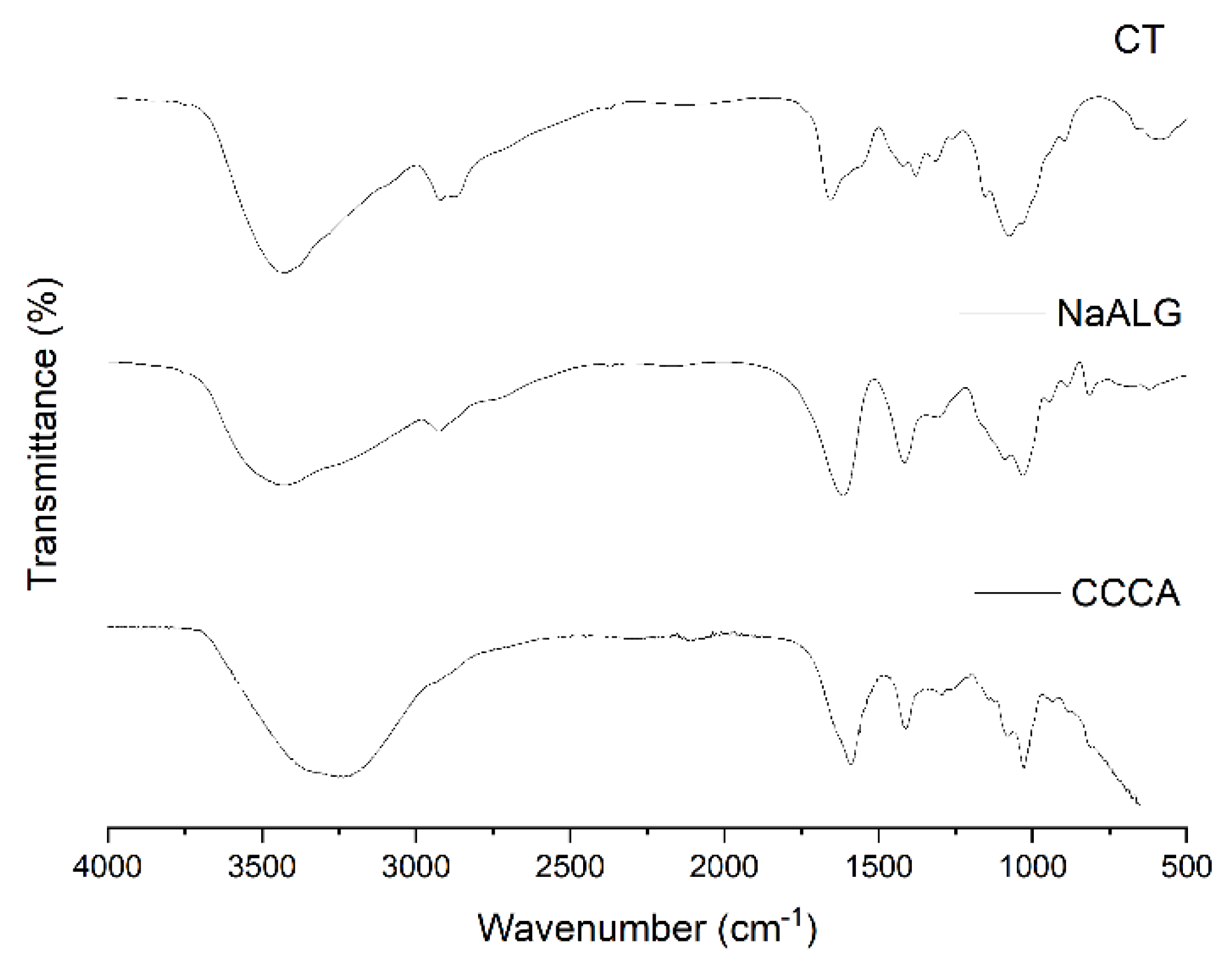
4.3. CT, NaALG and CCCA ATR-FTIR and TGA Analysis
4.4. CCCA Microparticles SEM Analysis
4.5. Capture and Desorption Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dar, M.A.; Kaushik, G.; Villarreal-Chiu, J.F. Pollution status and bioremediation of chlorpyrifos in environmental matrices by the application of bacterial communities: A review. J. Environ. Manag. 2019, 239, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Fatunsin, O.T.; Oyeyiola, A.O.; Moshood, M.O.; Akanbi, L.M.; Fadahunsi, D.E. Dietary risk assessment of organophosphate and carbamate pesticide residues in commonly eaten food crops. Sci. Afr. 2020, 8, e00442. [Google Scholar] [CrossRef]
- Jing, Y.; Krauss, M.; Zschieschang, S.; Miltner, A.; Butkovskyi, A.; Eggen, T.; Kastner, M.; Nowak, K.M. Superabsorbent polymer as a supplement substrate of constructed wetland to retain pesticides from agricultural runoff. Water Res. 2021, 207, 117776. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, X.; Liu, T.; Fan, H.; Liu, H.; Li, S.; Wang, D.; Ding, L. Dynamic microwave-assisted extraction combined with liquid phase microextraction based on the solidification of a floating drop for the analysis of organochlorine pesticides in grains followed by GC. Food Sci. Hum. Wellness 2021, 10, 375–382. [Google Scholar] [CrossRef]
- Cequier, E.; Sakhi, A.K.; Haug, L.S.; Thomsen, C. Exposure to organophosphorous pesticides in Norwegian mothers and their children: Diurnal variability in concentrations of their biomarkers and associations with food consumption. Sci. Total Environ. 2017, 590–591, 655–662. [Google Scholar] [CrossRef]
- Dervilly-Pinel, G.; Guérin, T.; Minvielle, B.; Travel, A.; Normand, J.; Bourin, M.; Royer, E.; Dubreil, E.; Mompelat, S.; Hommet, S.; et al. Micropollutants and chemical residues in organic and conventional meat. Food Chem. 2017, 232, 218–228. [Google Scholar] [CrossRef]
- Mahmoud, A.F.A.; Ikenaka, Y.; Yohannes, Y.B.; Darwish, W.S.; Eldaly, E.A.; Morshdy, A.E.M.A.; Nakayama, S.M.M.; Mizukawa, H.; Ishizuka, M. Distribution and health risk assessment of organochlorine pesticides (OCPs) residue in edible cattle tissues from northeastern part of Egypt: High accumulation level of OCPs in tongue. Chemosphere 2016, 144, 1365–1371. [Google Scholar] [CrossRef]
- Sookhtanlou, M.; Allahyari, M.S.; Surujlal, J. Health risk of potato farmers exposed to overuse of chemical pesticides in Iran. Saf. Health 2021. [Google Scholar] [CrossRef]
- Fernández-Peralbo, M.A.; Luque de Castro, M.D. Preparation of urine samples prior to targeted or untargeted metabolomics mass-spectrometry analysis. TrAC-Trends Anal. Chem. 2012, 41, 75–85. [Google Scholar] [CrossRef]
- Wang, Q.; Zuo, Z.; Cheung, C.K.C.; Leung, S.S.Y. Updates on thermosensitive hydrogel for nasal, ocular and cutaneous delivery. Int. J. Pharm. 2019, 559, 86–101. [Google Scholar] [CrossRef]
- Coukouma, A.E.; Asher, S.A. Increased volume responsiveness of macroporous hydrogels. Sens. Actuators B Chem. 2018, 255, 2900–2903. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Farsangi, Z.J.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Wu, T.; Huang, J.; Jiang, Y.; Hu, Y.; Ye, X.; Liu, D.; Chen, J. Formation of hydrogels based on chitosan/alginate for the delivery of lysozyme and their antibacterial activity. Food Chem. 2018, 240, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, S.; Feng, W. PVA hydrogel properties for biomedical application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1228–1233. [Google Scholar] [CrossRef]
- Meng, X.; Tian, F.; Yang, J.; He, C.N.; Xing, N.; Li, F. Chitosan and alginate polyelectrolyte complex membranes and their properties for wound dressing application. J. Mater. Sci. Mater. Med. 2010, 21, 1751–1759. [Google Scholar] [CrossRef]
- Paradossi, G.; Cavalieri, F.; Chiessi, E.; Spagnoli, C.; Cowman, M.K. Poly(vinyl alcohol) as versatile biomaterial for potential biomedical applications. J. Mater. Sci. Mater. Med. 2003, 14, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Barjas, G.; Gallardo, F.; Nesic, A.; Taboada, E.; Marican, A.; Mirabal-Gallardo, Y.; Avila-Salas, F.; Delgado, N.; de Armas-Ricard, M.; Valdes, O. Utilization of industrial by-product fungal biomass from Aspergillus niger and Fusarium culmorum to obtain biosorbents for removal of pesticide and metal ions from aqueous solutions. J. Environ. Chem. Eng. 2020, 8, 104355. [Google Scholar] [CrossRef]
- El Harmoudi, H.; El Gaini, L.; Daoudi, E.; Rhazi, M.; Boughaleb, Y.; El Mhammedi, M.A.; Migalzka-Zalas, A.; Bakasse, M. Removal of 2,4-D from aqueous solutions by adsorption processes using two biopolymers: Chitin and chitosan and their optical properties. Opt. Mater. 2014, 36, 1471–1477. [Google Scholar] [CrossRef]
- Etcheberry, M.; Cappa, V.; Trelles, J.; Zanini, G. Montmorillonite-alginate beads: Natural mineral and biopolymers based sorbent of paraquat herbicides. J. Environ. Chem. Eng. 2017, 5, 5868–5875. [Google Scholar] [CrossRef]
- Lu, L.C.; Wang, C.I.; Sye, W.F. Applications of chitosan beads and porous crab shell powder for the removal of 17 organochlorine pesticides (OCPs) in water solution. Carbohydr. Polym. 2011, 83, 1984–1989. [Google Scholar] [CrossRef]
- Avila-Salas, F.; Marican, A.; Villaseñor, J.; Arenas-Salinas, M.; Argandoña, Y.; Caballero, J.; Durán-Lara, E.F. In-Silico Design, Synthesis and Evaluation of a Nanostructured Hydrogel as a Dimethoate Removal Agent. Nanomaterials 2018, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Hui, B.; Zhang, Y.; Ye, L. Preparation of PVA hydrogel beads and adsorption mechanism for advanced phosphate removal. Chem. Eng. J. 2014, 235, 207–214. [Google Scholar] [CrossRef]
- Valdés, O.; Ávila-Salas, F.; Marican, A.; Fuentealba, N.; Villaseñor, J.; Arenas-Salinas, M.; Argandoña, Y.; Durán-Lara, E.F. Methamidophos removal from aqueous solutions using a super adsorbent based on crosslinked poly(vinyl alcohol) hydrogel. J. Appl. Polym. Sci. 2018, 135, 45964. [Google Scholar] [CrossRef]
- Sabitha, P.; Vijaya Ratna, J.; Ravindra Reddy, K. Design and evaluation of controlled release chitosan-calcium alginate microcapsules of anti tubercular drugs for oral use. Int. J. Chemtech Res. 2010, 2, 88–98. [Google Scholar]
- Maestro, S. Schrödinger Release 2021-1; LLC: New York, NY, USA, 2020. [Google Scholar]
- Shelley, J.C.; Cholleti, A.; Frye, L.L.; Greenwood, J.R.; Timlin, M.R.; Uchimaya, M. Epik: A software program for pKa prediction and protonation state generation for drug-like molecules. J. Comput. Aided. Mol. Des. 2007, 21, 681–691. [Google Scholar] [CrossRef]
- Case, D.A.; Aktulga, H.M.; Belfon, K.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E., III; Cruzeiro, V.W.D.; Darden, T.A.; Duke, R.E.; et al. Amber 2021. University of California, San Francisco. Available online: https://ambermd.org/index.php (accessed on 10 September 2021).
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, W.L.; Tirado-Rives, J. The OPLS Potential Functions for Proteins. Energy Minimizations for Crystals of Cyclic Peptides and Crambin. J. Am. Chem. Soc. 1988, 110, 1657–1666. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Giorgino, T. Computing diffusion coefficients in macromolecular simulations: The Diffusion Coefficient Tool for VMD. J. Open Source Softw. 2019, 4, 1698. [Google Scholar] [CrossRef] [Green Version]
- Schanuel, F.S.; Santos, K.S.R.; Monte-Alto-Costa, A.; de Oliveira, M.G. Combined Nitric Oxide-releasing Poly(vinyl alcohol) Film/F127 Hydrogel for Accelerating Wound Healing. Colloids Surf. B Biointerfaces 2015, 130, 182–191. [Google Scholar] [CrossRef]
- Guastaferro, M.; Reverchon, E.; Baldino, L. Polysaccaride-Based Aerogel Production for Biomedical Applications: A Comparative Review. Materials 2021, 14, 1631. [Google Scholar] [CrossRef]
- Gonzalez-Rodrıguez, M.L.; Holgado, M.A.; Sanchez-Lafuente, C.; Rabasco, A.M.; Fini, A. Alginate/chitosan particulate systems for sodium diclofenac release. Int. J. Pharm. Sci. Res. 2002, 232, 225–234. [Google Scholar] [CrossRef]
- Xu, Y.; Zhan, C.; Fan, L.; Wang, L.; Zheng, H. Preparation of dual crosslinked alginate–chitosan blend gel beads and in vitro controlled release in oral site-specific drug delivery system. Int. J. Pharm. 2007, 336, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Caetano, L.; Almeida, A.; Gonçalves, L. Effect of Experimental Parameters on Alginate/Chitosan Microparticles for BCG Encapsulation. Mar. Drugs 2016, 14, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaari, N.; Kamarudin, S.K. Sodium alginate/alumina composite biomembrane preparation and performance in DMFC application. Polym. Test. 2020, 81, 106183. [Google Scholar] [CrossRef]
- Balau, L.; Lisa, G.; Popa, M.; Tura, V.; Melnig, V. Physico-chemical properties of Chitosan films. Open Chem. 2004, 2, 638–647. [Google Scholar] [CrossRef]
- Sankalia, M.G.; Mashru, R.C.; Sankalia, J.M.; Sotariya, V.B. Reversed chitosan—Alginate polyelectrolyte complex for stability improvement of alpha-amylase: Optimization and physicochemical characterization. Eur. J. Pharm. Biopharm. 2007, 65, 215–232. [Google Scholar] [CrossRef]
- Valdés, O.; Alexandrova, L.; Zaldivar, D.; Katime, I. A comparative study of two polyelectrolyte complexes. J. Appl. Polym. Sci. 2012, 123, 2283–2290. [Google Scholar] [CrossRef]
- Zawadzki, J.; Kaczmarek, H. Thermal treatment of chitosan in various conditions. Carbohydr. Polym. 2010, 80, 394–400. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; Álvarez, C.; O’Donnell, C.P. Ultrasound applications for the extraction, identification and delivery of food proteins and bioactive peptides. Trends Food Sci. Technol. 2015, 46, 60–67. [Google Scholar] [CrossRef]

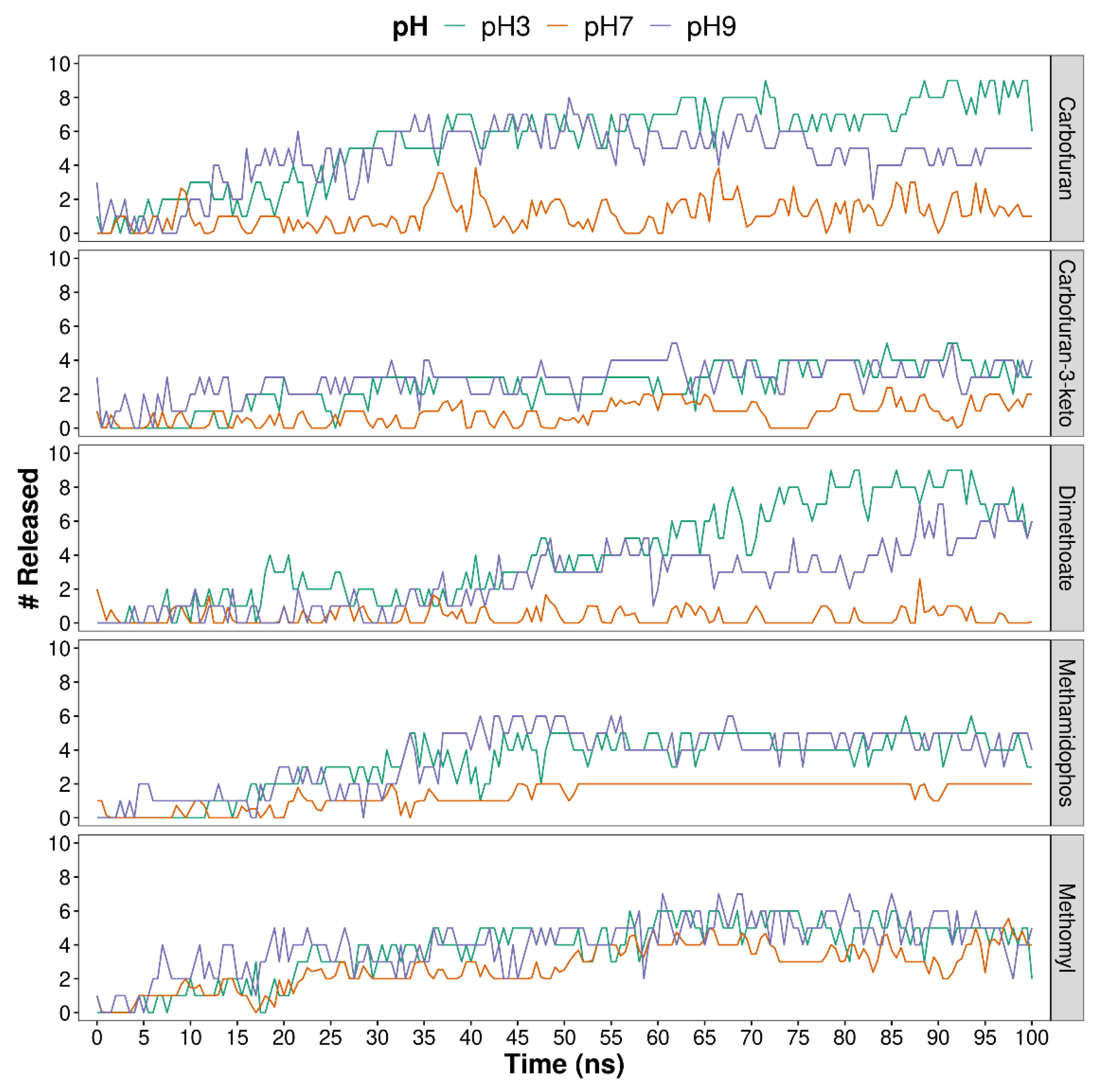

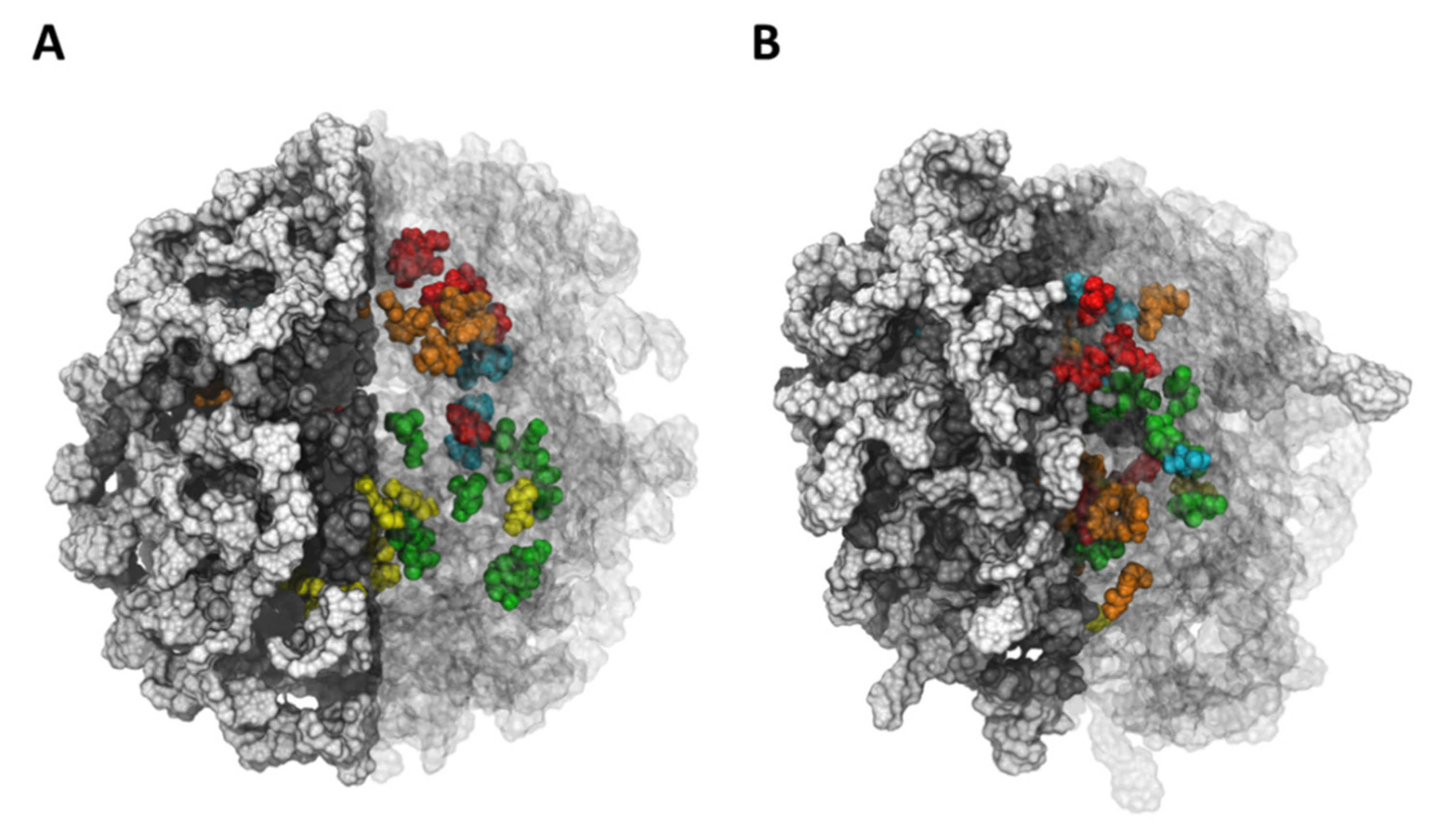
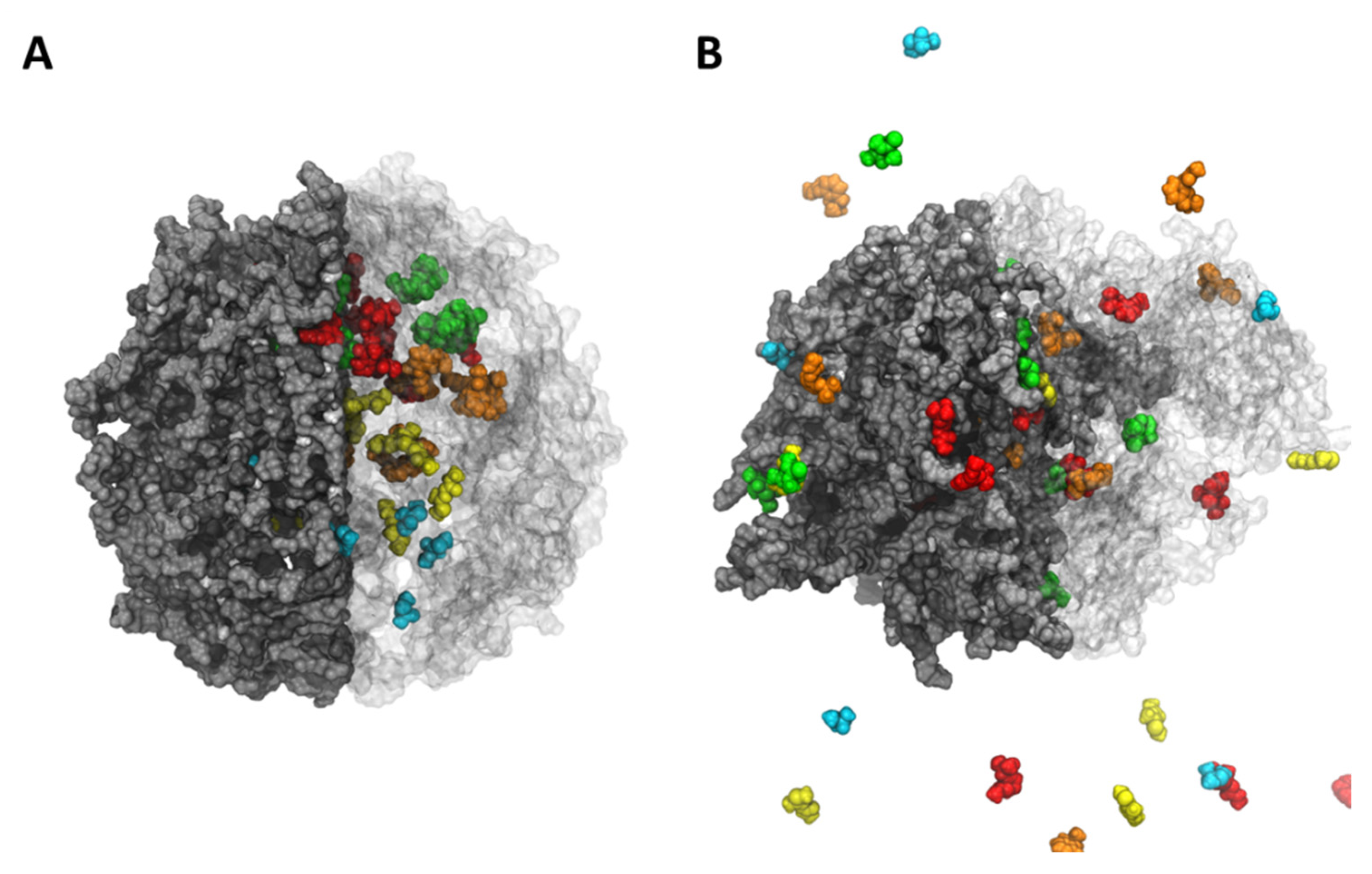


| System | Compound | # Release | D.C. (Å/ns) | # Release per Hydrogel | D.C. (Å/ns) per Hydrogel |
|---|---|---|---|---|---|
| ALG-CT at pH 9 | CFN | 4.53 | 96.6 | 3.5 | 59.2 |
| KCF | 2.87 | 19.1 | |||
| DMT | 2.60 | 40.4 | |||
| MTD R | 3.58 | 86.6 | |||
| MTD S | 66.5 | ||||
| MTL | 4.07 | 46.3 | |||
| ALG-CT at pH 7 | CFN | 1.1 | 1.5 | 1.3 | 7.8 |
| KCF | 0.8 | 8.8 | |||
| DMT | 0.4 | 0.5 | |||
| MTD R | 1.4 | 6.6 | |||
| MTD S | 4.6 | ||||
| MTL | 2.7 | 24.9 | |||
| ALG-CT at pH 3 | CFN | 5.5 | 40.3 | 3.8 | 32.9 |
| KCF | 2.5 | 28.2 | |||
| DMT | 4.1 | 22.5 | |||
| MTD R | 3.3 | 2.2 | |||
| MTD S | 41.3 | ||||
| MTL | 3.7 | 63.2 |
| Hydrogel | Compound | # Release | D.C. (Å/ns) | # Release per Hydrogel | D.C (Å/ns) per Hydrogel |
|---|---|---|---|---|---|
| PVA-MA with 10% crosslinker | CFN | 5.6 | 74.3 | 6.4 | 102.4 |
| KCF | 6.3 | 66.5 | |||
| DMT | 6.0 | 120.6 | |||
| MTD R | 7.1 | 146.7 | |||
| MTD S | 64.7 | ||||
| MTL | 6.9 | 141.5 | |||
| PVA-MA with 20% crosslinker | CFN | 5.1 | 57.2 | 6.1 | 78.8 |
| KCF | 6.0 | 47.5 | |||
| DMT | 5.4 | 55.1 | |||
| MTD R | 6.6 | 60.1 | |||
| MTD S | 80.6 | ||||
| MTL | 7.6 | 172.4 | |||
| PVA-MA with 30% crosslinker | CFN | 5.4 | 76.3 | 5.7 | 85.4 |
| KCF | 4.7 | 41.2 | |||
| DMT | 4.7 | 72.6 | |||
| MTD R | 7.8 | 108.4 | |||
| MTD S | 95.8 | ||||
| MTL | 6.1 | 118.0 |
| Exp | Capture Time (min) | Desorption Time (min) | CLPHMA Composition (%) | pH of Desorption Solution (-) | mg/L of Compound Released per mg of CLPHMA | ||||
|---|---|---|---|---|---|---|---|---|---|
| CFN | KCF | DMT | MTD | MTL | |||||
| 1 | 120 (1) | 60 (−1) | 30 (1) | 3 (−1) | 0.0005 | ND | ND | ND | 0.0004 |
| 2 | 10 (−1) | 120 (1) | 10 (−1) | 9 (1) | 0.0009 | ND | ND | ND | ND |
| 3 | 120 (1) | 60 (−1) | 30 (1) | 3 (−1) | 0.0010 | 0.0014 | ND | 0.0024 | ND |
| 4 | 120 (1) | 60 (−1) | 30 (1) | 3 (−1) | 0.0009 | ND | 0.0015 | ND | 0.0004 |
| 5 | 120 (1) | 120 (1) | 30 (1) | 9 (1) | 0.0005 | ND | ND | ND | ND |
| 6 | 10 (−1) | 60 (−1) | 10 (−1) | 3 (−1) | 0.0005 | ND | ND | 0.0014 | 0.0004 |
| 7 | 120 (1) | 120 (1) | 30 (1) | 9 (1) | 0.0007 | 0.0009 | ND | ND | ND |
| 8 | 10 (−1) | 120 (1) | 10 (−1) | 9 (1) | 0.0010 | ND | ND | ND | ND |
| 9 | 10 (−1) | 120 (1) | 10 (−1) | 9 (1) | 0.0006 | 0.0010 | ND | 0.0022 | 0.0007 |
| 10 | 10 (−1) | 120 (1) | 10 (−1) | 9 (1) | 0.0007 | ND | ND | ND | 0.0003 |
| 11 | 120 (1) | 60 (−1) | 30 (1) | 3 (−1) | 0.0005 | ND | 0.0011 | ND | ND |
| 12 | 120 (1) | 120 (1) | 30 (1) | 9 (1) | 0.0008 | 0.0008 | ND | ND | ND |
| 13 | 10 (−1) | 60 (−1) | 10 (−1) | 3 (−1) | 0.0009 | 0.0008 | ND | ND | ND |
| 14 | 10 (−1) | 60 (−1) | 10 (−1) | 3 (−1) | ND | ND | ND | ND | ND |
| 15 | 10 (−1) | 60 (−1) | 10 (−1) | 3 (−1) | ND | ND | ND | ND | ND |
| 16 | 120 (1) | 120 (1) | 30 (1) | 9 (1) | 0.0008 | ND | ND | ND | ND |
| 17 | 10 (−1) | 90 (0) | 10 (−1) | 7.3 (0.43) | 0.0005 | ND | ND | ND | ND |
| 18 | 65 (0) | 90 (0) | 20 (0) | 7.3 (0.43) | 0.0005 | ND | 0.0014 | ND | ND |
| 19 | 120 (1) | 60 (−1) | 30 (1) | 7.3 (0.43) | 0.0021 | ND | ND | ND | 0.0005 |
| 20 | 10 (−1) | 60 (−1) | 20 (0) | 7.3 (0.43) | 0.0008 | ND | 0.0012 | ND | ND |
| 21 | 65 (0) | 120 (1) | 30 (1) | 7.3 (0.43) | 0.0006 | ND | ND | ND | ND |
| 22 | 120 (1) | 120 (1) | 10 (−1) | 7.3 (0.43) | 0.0012 | ND | ND | 0.0031 | 0.0004 |
| Exp | Capture Time (min) | Desorption Time (min) | CCCA Composition (%) | pH of Desorption Solution (-) | mg/L of Compound Released per mg of CCCA | ||||
|---|---|---|---|---|---|---|---|---|---|
| CFN | KCF | DMT | MTD | MTL | |||||
| 1 | 120 (1) | 60 (−1) | 56.5 (1) | 3 (−1) | 0.0065 | 0.0132 | 0.0088 | 0.0095 | 0.0253 |
| 2 | 10 (−1) | 120 (1) | 47.3 (−1) | 9 (1) | 0.0033 | 0.0030 | 0.0043 | 0.0141 | 0.0029 |
| 3 | 120 (1) | 60 (−1) | 56.5 (1) | 3 (−1) | 0.0031 | 0.0020 | 0.0012 | 0.0087 | 0.0023 |
| 4 | 120 (1) | 60 (−1) | 56.5 (1) | 3 (−1) | 0.0035 | 0.0025 | 0.0060 | 0.0142 | 0.0019 |
| 5 | 120 (1) | 120 (1) | 56.5 (1) | 9 (1) | 0.0034 | 0.0052 | 0.0015 | 0.0019 | 0.0072 |
| 6 | 10 (−1) | 60 (−1) | 47.3 (−1) | 3 (−1) | 0.0017 | 0.0010 | 0.0058 | 0.0051 | 0.0010 |
| 7 | 120 (1) | 120 (1) | 56.5 (1) | 9 (1) | 0.0026 | 0.0052 | 0.0019 | 0.0051 | 0.0061 |
| 8 | 10 (−1) | 120 (1) | 47.3 (−1) | 9 (1) | 0.0019 | 0.0016 | 0.0017 | 0.0073 | 0.0020 |
| 9 | 10 (−1) | 120 (1) | 47.3 (−1) | 9 (1) | 0.0010 | 0.0011 | 0.0020 | 0.0034 | ND |
| 10 | 10 (−1) | 120 (1) | 47.3 (−1) | 9 (1) | 0.0010 | 0.0008 | 0.0034 | 0.0023 | 0.0006 |
| 11 | 120 (1) | 60 (−1) | 56.5 (1) | 3 (−1) | 0.0017 | 0.0026 | 0.0035 | 0.0017 | ND |
| 12 | 120 (1) | 120 (1) | 56.5 (1) | 9 (1) | 0.0022 | 0.0011 | 0.0011 | 0.0078 | 0.0006 |
| 13 | 10 (−1) | 60 (−1) | 47.3 (−1) | 3 (−1) | 0.0026 | 0.0008 | 0.0271 | 0.0087 | ND |
| 14 | 10 (−1) | 60 (−1) | 47.3 (−1) | 3 (−1) | 0.0011 | 0.0013 | 0.0017 | ND | 0.0003 |
| 15 | 10 (−1) | 60 (−1) | 47.3 (−1) | 3 (−1) | 0.0007 | 0.0008 | ND | 0.0022 | ND |
| 16 | 120 (1) | 120 (1) | 56.5 (1) | 9 (1) | 0.0018 | 0.0023 | ND | 0.0025 | 0.0004 |
| 17 | 10 (−1) | 90 (0) | 47.3 (−1) | 7.3 (0.43) | 0.0014 | 0.0013 | ND | 0.0035 | 0.0009 |
| 18 | 65 (0) | 90 (0) | 52.4 (0.11) | 7.3 (0.43) | 0.0018 | 0.0018 | ND | ND | 0.0016 |
| 19 | 120 (1) | 60 (−1) | 56.5 (1) | 7.3 (0.43) | 0.0014 | 0.0014 | ND | 0.0032 | ND |
| 20 | 10 (−1) | 60 (−1) | 52.4 (0.11) | 7.3 (0.43) | 0.0005 | ND | 0.0012 | 0.0040 | ND |
| 21 | 65 (0) | 120 (1) | 56.5 (1) | 7.3 (0.43) | 0.0017 | 0.0017 | ND | 0.0017 | 0.0004 |
| 22 | 120 (1) | 120 (1) | 47.3 (−1) | 7.3 (0.43) | 0.0018 | 0.0026 | 0.0016 | 0.0031 | 0.0034 |
| Analyte | Influence | ANOVA Model p-Value | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| CFN | + | - | - (NSS) | + | 0.0654 |
| Equation of the fitted model | R2 (%) | ||||
| CFN = 0.000711575 + 0.000391122 × A − 0.000462556 × B + 0.000575588 × D | 38.90 | ||||
| KCF | + | + | + | - (NSS) | 0.9774 |
| Equation of the fitted model | R2 (%) | ||||
| KCF = 0.00022443 + 0.0000213213 × A + 0.0000400718 × B + 0.0000480358 × C | 2.52 | ||||
| DMT | - (NSS) | - | + | + | 0.4353 |
| Equation of the fitted model | R2 (%) | ||||
| DMT = 0.000229189 − 0.00026971 × B + 0.000158496 × C + 0.000119232 × D | 19.03 | ||||
| MTD | + | + (NSS) | - | - | 0.0752 |
| Equation of the fitted model | R2 (%) | ||||
| MTD = 0.000420364 + 0.000906789 × A − 0.000964469 × C − 0.000382322 × D | 37.72 | ||||
| MTL | + | - | - | + (NSS) | 0.5568 |
| Equation of the fitted model | R2 (%) | ||||
| MTL = 0.000137202 + 0.000168035 × A − 0.000107823 × B − 0.000175881 × C | 15.41 | ||||
| Analyte | Influence | ANOVA Model p-Value | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| CFN | + | + | + (NSS) | - | 0.2094 |
| Equation of the fitted model | R2 (%) | ||||
| CFN = 0.00213976 + 0.00043176 × A + 0.000449696 × B − 0.000705095 × D | 27.88 | ||||
| KCF | + | + | + (NSS) | - | 0.2928 |
| Equation of the fitted model | R2 (%) | ||||
| KCF = 0.00244117 + 0.00104561 × A + 0.000665528 × B − 0.000944831 × D | 24.09 | ||||
| DMT | - | + (NSS) | - | - | 0.4593 |
| Equation of the fitted model | R2 (%) | ||||
| DMT = 0.00339492 − 0.000318947 × A − 0.000913688 × C − 0.00281292 × D | 18.28 | ||||
| MTD | + | - | - | - (NSS) | 0.9874 |
| Equation of the fitted model | R2 (%) | ||||
| MTD = 0.0050002 + 0.00050246 × A − 0.000409299 × B − 0.000141824 × C − 0.0000353008 × D | 1.85 | ||||
| MTL | + | + | + (NSS) | - | 0.5987 |
| Equation of the fitted model | R2 (%) | ||||
| MTL = 0.00262437 + 0.00182125 × A + 0.000737128 × B − 0.00146819 × D | 14.25 | ||||
| Experiment (n = 3) | Sorbent Type and Agitation | Capture Time (min) | Desorption Time (min) | Sorbent Composition (%) | pH (-) |
|---|---|---|---|---|---|
| 1 | CLPHMA, Mechanical agitation | 120 (1) | 120 (1) | 30 (1) | 9 (1) |
| 2 | 120 (1) | 60 (−1) | 10 (−1) | 3 (−1) | |
| 3 | CCCA, Mechanical agitation | 120 (1) | 120 (1) | 56.5 (1) | 3 (−1) |
| 4 | 10 (−1) | 60 (−1) | 47.3 (−1) | 3 (−1) | |
| 5 | CLPHMA, Ultrasonic agitation | 120 (1) | 60 (−1) | 10 (−1) | 9 (1) |
| 6 | 10 (−1) | 120 (1) | 10 (−1) | 3 (−1) | |
| 7 | CCCA, Ultrasonic agitation | 120 (1) | 60 (−1) | 47.3 (−1) | 9 (1) |
| 8 | 10 (−1) | 120 (1) | 56.5 (1) | 3 (−1) |
| mg/L of Compound Released per mg of CCCA (Mean ± SD) | |||||
|---|---|---|---|---|---|
| CFN | KCF | DMT | MTD | MTL | |
| 1 | 0.0036 ± 0.0010 | 0.0035 ± 0.0005 | 0.0054 ± 0.0009 | 0.0045 ± 0.0004 | 0.0115 ± 0.0028 |
| 2 | 0.0022 ± 0.0001 | 0.0014 ± 0.0001 | 0.0089 ± 0.0003 | 0.0048 ± 0.0004 | 0.0094 ± 0.0028 |
| 3 | 0.0030 ± 0.0003 | ND | 0.0176 ± 0.0034 | 0.0040 ± 0.0004 | ND |
| 4 | 0.0024 ± 0.0001 | 0.0028 ± 0.0002 | 0.0080 ± 0.0004 | 0.0059 ± 0.0011 | 0.0094 ± 0.0008 |
| 5 | ND | 0.0020 ± 0.0001 | 0.0163 ± 0.0007 | 0.0050 ± 0.0003 | 0.0050 ± 0.0008 |
| 6 | ND | 0.0017 ± 0.0002 | ND | ND | ND |
| 7 | 0.0025 ± 0.0002 | ND | 0.0132 ± 0.0010 | 0.0057 ± 0.0004 | 0.0039 ± 0.0004 |
| 8 | 0.0018 ± 0.0001 | 0.0024 ± 0.0001 | 0.0063 ± 0.0009 | 0.0051 ± 0.0005 | 0.0084 ± 0.0004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés, C.; Valdés, O.; Bustos, D.; Abril, D.; Cabrera-Barjas, G.; Pereira, A.; Villaseñor, J.; Polo-Cuadrado, E.; Carreño, G.; Durán-Lara, E.F.; et al. Use of Poly(vinyl alcohol)-Malic Acid (CLHPMA) Hydrogels and Chitosan Coated Calcium Alginate (CCCA) Microparticles as Potential Sorbent Phases for the Extraction and Quantitative Determination of Pesticides from Aqueous Solutions. Polymers 2021, 13, 3993. https://doi.org/10.3390/polym13223993
Valdés C, Valdés O, Bustos D, Abril D, Cabrera-Barjas G, Pereira A, Villaseñor J, Polo-Cuadrado E, Carreño G, Durán-Lara EF, et al. Use of Poly(vinyl alcohol)-Malic Acid (CLHPMA) Hydrogels and Chitosan Coated Calcium Alginate (CCCA) Microparticles as Potential Sorbent Phases for the Extraction and Quantitative Determination of Pesticides from Aqueous Solutions. Polymers. 2021; 13(22):3993. https://doi.org/10.3390/polym13223993
Chicago/Turabian StyleValdés, Cristian, Oscar Valdés, Daniel Bustos, Diana Abril, Gustavo Cabrera-Barjas, Alfredo Pereira, Jorge Villaseñor, Efraín Polo-Cuadrado, Gustavo Carreño, Esteban F. Durán-Lara, and et al. 2021. "Use of Poly(vinyl alcohol)-Malic Acid (CLHPMA) Hydrogels and Chitosan Coated Calcium Alginate (CCCA) Microparticles as Potential Sorbent Phases for the Extraction and Quantitative Determination of Pesticides from Aqueous Solutions" Polymers 13, no. 22: 3993. https://doi.org/10.3390/polym13223993
APA StyleValdés, C., Valdés, O., Bustos, D., Abril, D., Cabrera-Barjas, G., Pereira, A., Villaseñor, J., Polo-Cuadrado, E., Carreño, G., Durán-Lara, E. F., & Marican, A. (2021). Use of Poly(vinyl alcohol)-Malic Acid (CLHPMA) Hydrogels and Chitosan Coated Calcium Alginate (CCCA) Microparticles as Potential Sorbent Phases for the Extraction and Quantitative Determination of Pesticides from Aqueous Solutions. Polymers, 13(22), 3993. https://doi.org/10.3390/polym13223993











