Application of a Conducting Poly-Methionine/Gold Nanoparticles-Modified Sensor for the Electrochemical Detection of Paroxetine
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Apparatus
2.2. Preparation of Solutions
2.3. Electrode Modification
2.4. Experimental Measurements of PRX
3. Results and Discussion
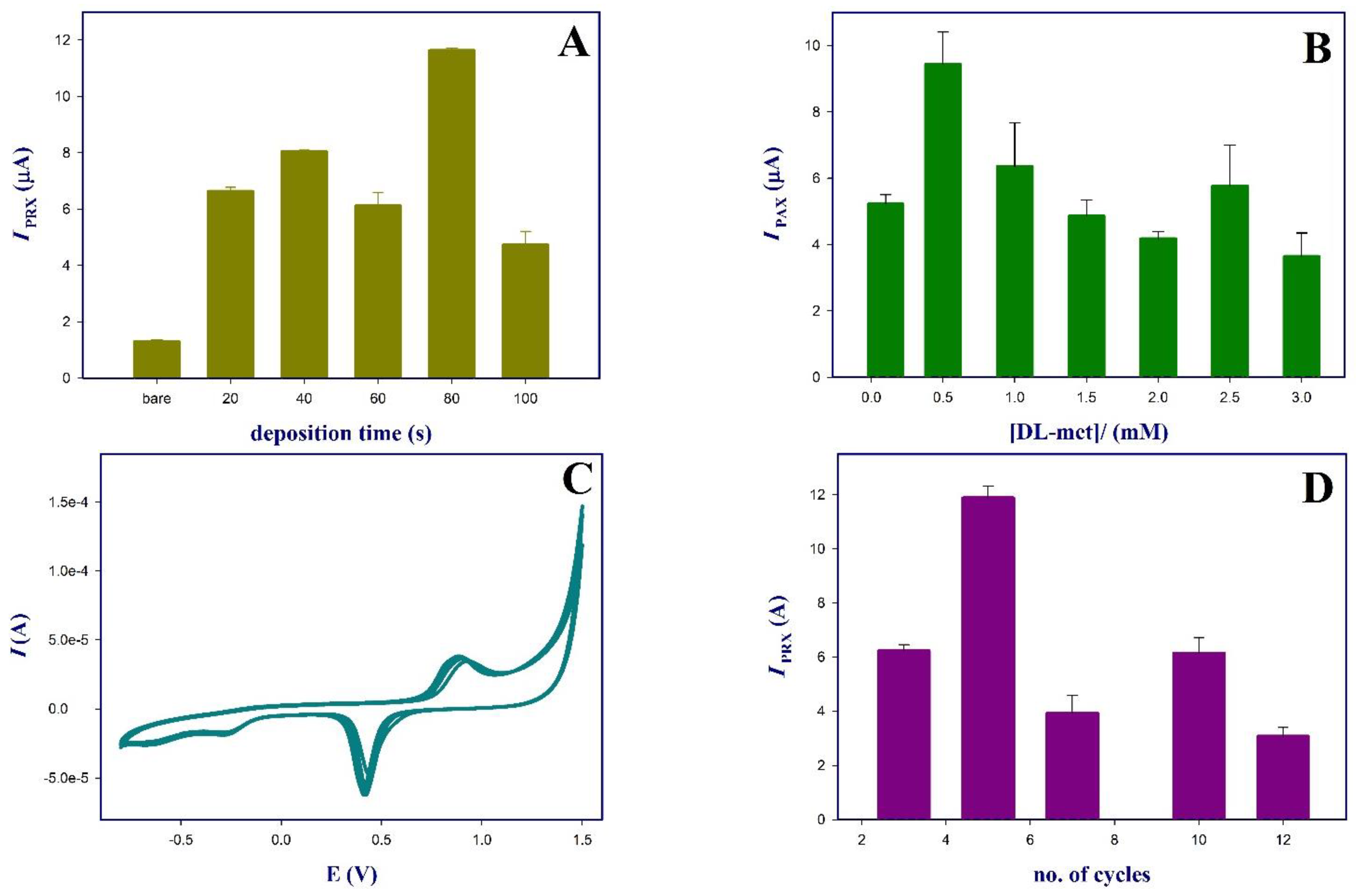
3.1. Optimization of the Sensor’s Fabrication Conditions
3.1.1. Effect of Deposition Time of AuNPs
3.1.2. Monomer (dl-met) Concentrations
3.1.3. Electropolymerization of dl-met
3.2. Effect of pH
3.3. Effect of DPV Operational Parameters
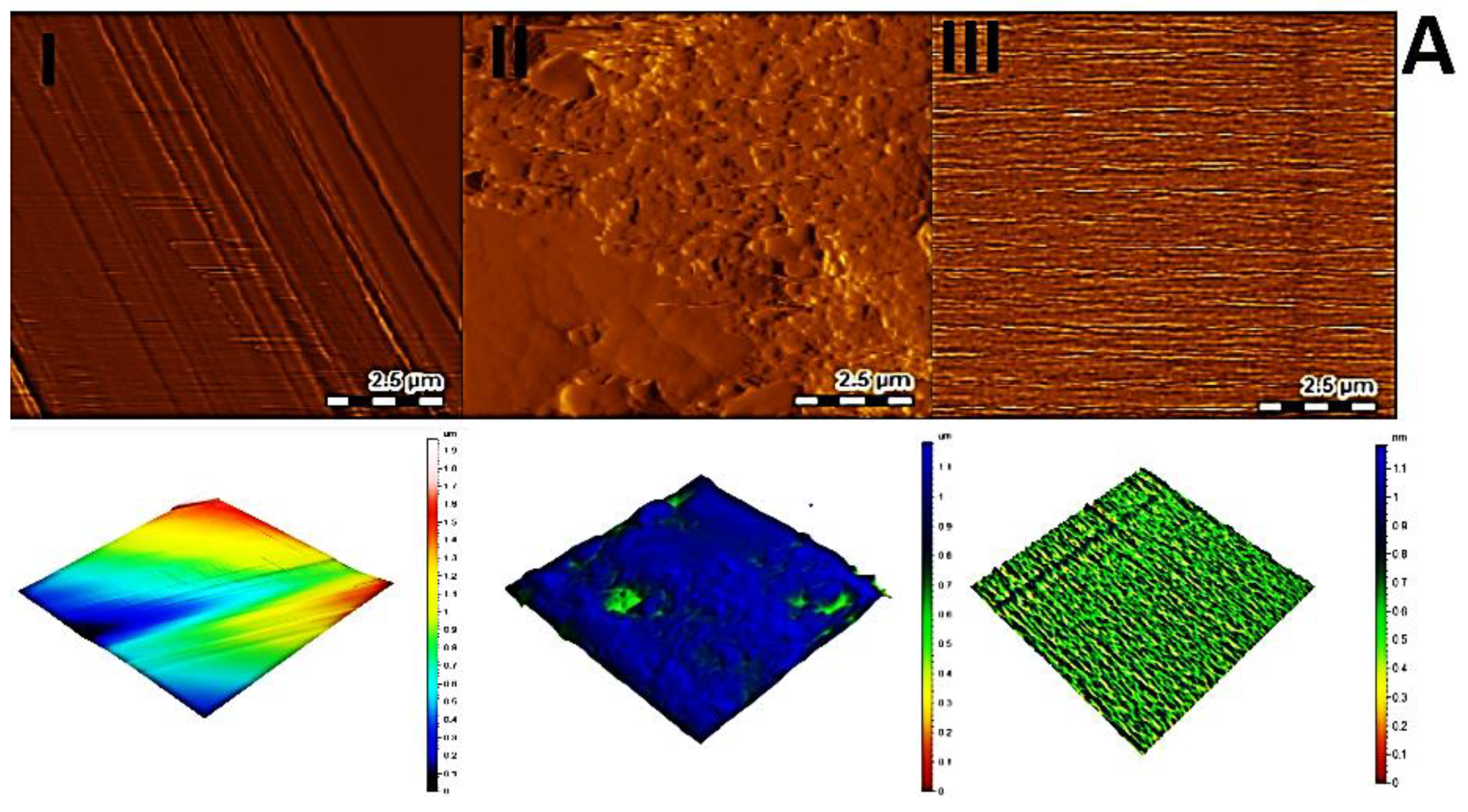
3.4. Surface Topography of the Sensor
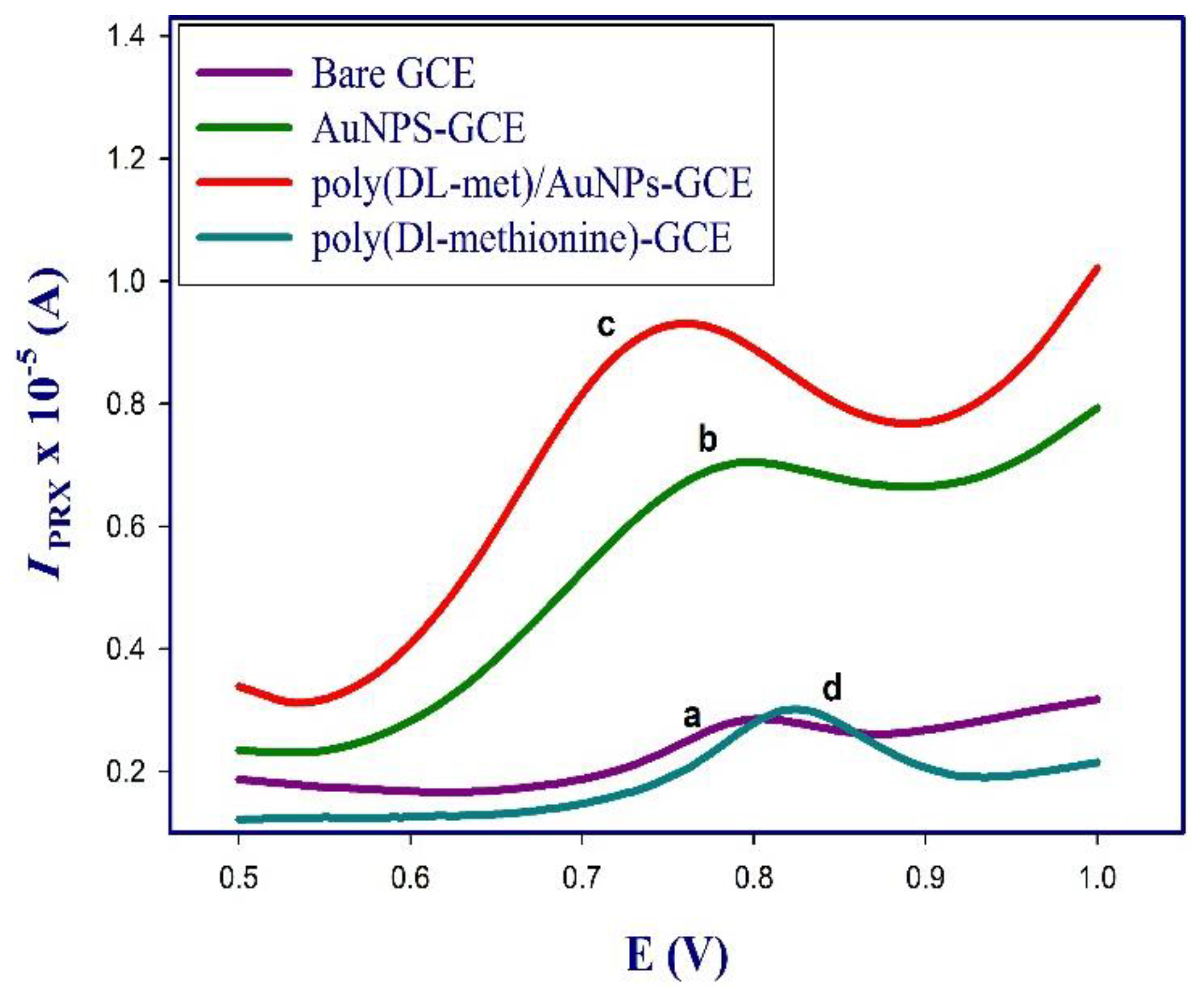
3.5. Electrochemical Characterization of the Modified Sensor
3.6. Effect of Possible Interferents on the Response (Selectivity)
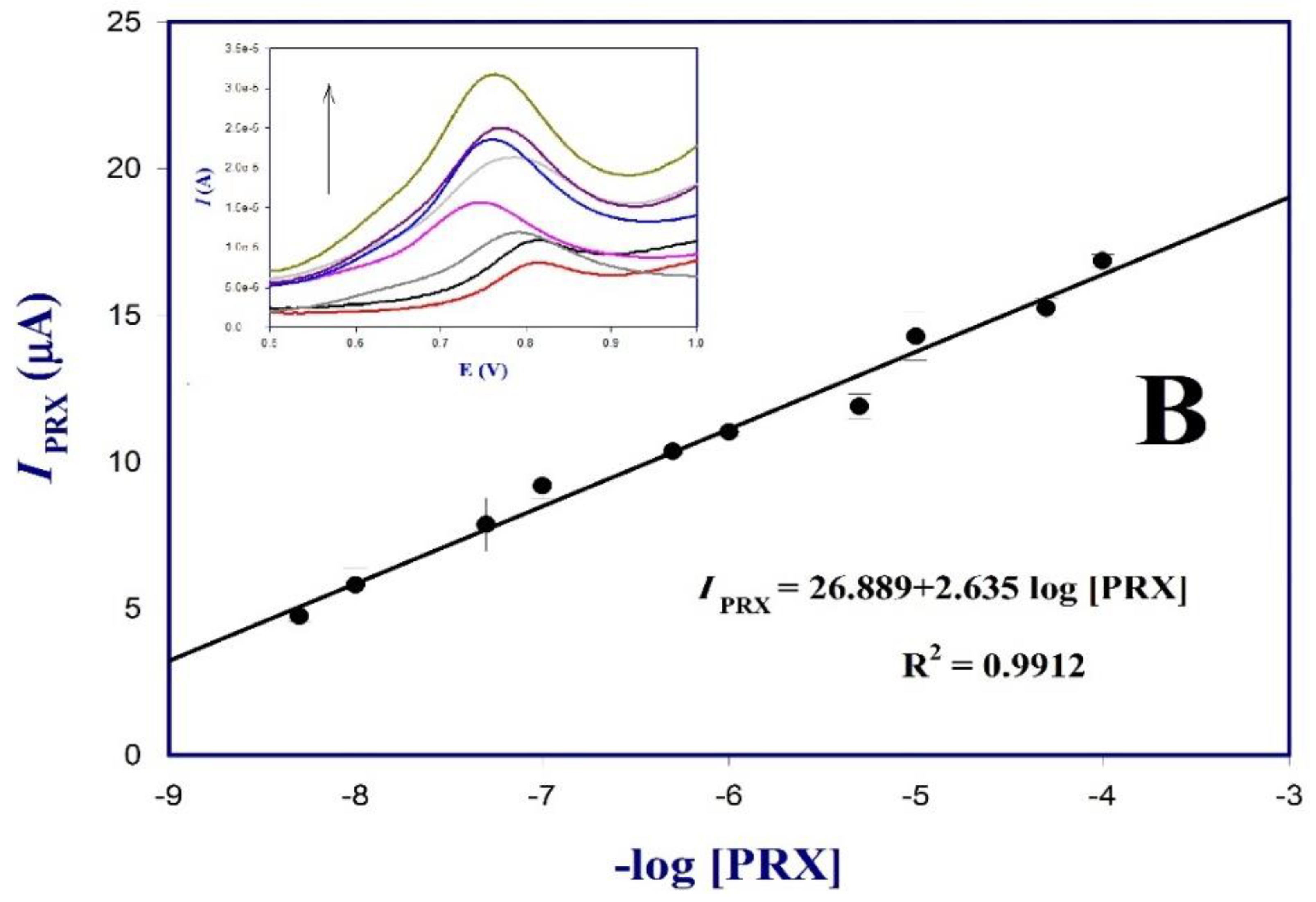
3.7. Validation of the Poly (dl-Met)/AuNPs-GCE Sensor Response
3.8. Applications of the Designed Sensor for the Detection of Paroxetine in Pharmaceutical Formulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piech, R.; Rumin, M.; Paczosa-Bator, B. High sensitive voltammetric determination of paroxetine on glassy carbon electrode modified with Nafion/MWCNTs. Int. J. Electrochem. Sci. 2014, 9, 7528–7539. [Google Scholar]
- Ajayi, R.F.; Nxusani, E.; Douman, S.F.; Jonnas, A.; Baker, P.G.L.; Iwuoha, E.I. An amperometric cytochrome P450-2D6 biosensor system for the detection of the selective serotonin reuptake inhibitors (SSRIs) paroxetine and fluvoxamine. J. Nano Res. 2016, 44, 208–228. [Google Scholar] [CrossRef]
- Talati, A.; Pantazatos, S.P.; Hirsch, J.; Schneier, F. A pilot study of gray matter volume changes associated with paroxetine treatment and response in social anxiety disorder. Psychiatry Res.-Neuroimaging 2015, 231, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Viviani, R.; Abler, B.; Seeringer, A.; Stingl, J.C. Effect of paroxetine and bupropion on human resting brain perfusion: An arterial spin labeling study. Neuroimage 2012, 61, 773–779. [Google Scholar] [CrossRef]
- Fani, N.; Ashraf, A.; Afzal, N.; Jawed, F.; Kitayama, N.; Reed, L.; Bremner, J.D. Increased neural response to trauma scripts in posttraumatic stress disorder following paroxetine treatment: A pilot study. Neurosci. Lett. 2011, 491, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Wichitnithad, W.; Nantaphol, S.; Vicheantawatchai, P.; Kiatkumjorn, T.; Wangkangwan, W.; Rojsitthisak, P. Development and validation of liquid chromatography-tandem mass spectrometry method for simple analysis of sumatriptan and its application in bioequivalence study. Pharmaceuticals 2020, 13, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanamadala, G.; Pradesh, A. Method for the simultaneous determination of paroxetine. Int. J. Pharm. 2014, 4, 448–457. [Google Scholar]
- Vergi-Athanasiou, N.; Atta-Politou, J.; Koupparis, M.; Spyropoulos, J. Development and validation of an HPLC method, with fluorescence detection, for simultaneous determination of paroxetine and its metabolites in plasma. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 1641–1655. [Google Scholar] [CrossRef]
- Agrawal, N. Determination of paroxetine in pharmaceutical preparations using HPLC with electrochemical detection. Open Anal. Chem. J. 2013, 7, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Reddy, L.S.S.; Rajkumar, T.; Evangiline, D.J.S. Method development and validation of visible spectroscopic method for the estimation of paroxetine hydrochloride in pure and tablet dosage form. Chem. Sci. Trans. 2017, 6, 679–684. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, K.; Xu, H.; Yan, B.; Gao, F.; Shi, Y.; Du, Y. Engineered photoelectrochemical platform for the ultrasensitive detection of caffeic acid based on flower-like MoS2 and PANI nanotubes nanohybrid. Sens. Actuators B Chem. 2018, 276, 322–330. [Google Scholar] [CrossRef]
- Švancara, I.; Vytřas, K.; Kalcher, K.; Walcarius, A.; Wang, J. Carbon paste electrodes in facts, numbers, and notes: A review on the occasion of the 50-years jubilee of carbon paste in electrochemistry and electroanalysis. Electroanalysis 2009, 21, 7–28. [Google Scholar] [CrossRef]
- Oghli, A.H.; Soleymanpour, A. Polyoxometalate/reduced graphene oxide modified pencil graphite sensor for the electrochemical trace determination of paroxetine in biological and pharmaceutical media. Mater. Sci. Eng. C 2020, 108, 110407. [Google Scholar] [CrossRef]
- Brycht, M.; Skrzypek, S.; Karadas-Bakirhan, N.; Smarzewska, S.; Bozal-Palabiyik, B.; Ozkan, S.A.; Uslu, B. Voltammetric behavior and determination of antidepressant drug paroxetine at carbon-based electrodes. Ionics 2015, 21, 2345–2354. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Lin, Y.; Liu, M.; Fang, G.; Wang, S. Coral-like Au1Pt3 alloy nanoparticles with multiple surface defects modified by poly(L-methionine) membrane for the selective detection of dopamine in biological samples. J. Alloys Compd. 2020, 815, 152643. [Google Scholar] [CrossRef]
- Kannan, A.; Sivanesan, A.; Kalaivani, G.; Manivel, A.; Sevvel, R. A highly selective and simultaneous determination of ascorbic acid, uric acid and nitrite based on a novel poly-N-acetyl-l-methionine (poly-NALM) thin film. RSC Adv. 2016, 6, 96898–96907. [Google Scholar] [CrossRef]
- Akbari Hasanjani, H.R.; Zarei, K. An electrochemical sensor for attomolar determination of mercury(II) using DNA/poly-L-methionine-gold nanoparticles/pencil graphite electrode. Biosens. Bioelectron. 2019, 128, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Akkapinyo, C.; Subannajui, K.; Poo-Arporn, Y.; Poo-Arporn, R.P. Disposable electrochemical sensor for food colorants detection by reduced graphene oxide and methionine film modified screen printed carbon electrode. Molecules 2021, 26, 2312. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Wang, M.; Kumara Swamy, B.E.; Praveen, M.; Zhao, W. Poly (alanine)/NaOH/ MoS2/MWCNTs modified carbon paste electrode for simultaneous detection of dopamine, ascorbic acid, serotonin and guanine. Colloids Surf. B Biointerfaces 2020, 196, 111299. [Google Scholar] [CrossRef]
- Charithra, M.M.; Manjunatha, J.G. Electroanalytical determination of acetaminophen using polymerized carbon nanocomposite based sensor. Chem. Data Collect. 2021, 33, 100718. [Google Scholar] [CrossRef]
- Bonyadi, S.; Ghanbari, K. Electrochemical synthesis of Poly(melamine)-Poly (aspartic acid) copolymer for highly sensitive and selective determination of dopamine. Mater. Chem. Phys. 2021, 267, 124683. [Google Scholar] [CrossRef]
- Krishnan, R.G.; Saraswathyamma, B. Electro-generated poly (cysteine) film as a sensor platform towards the simultaneous electroanalysis of hydrazine and hydroxylamine. Mater. Chem. Phys. 2021, 271, 124880. [Google Scholar] [CrossRef]
- Venkataprasad, G.; Reddy, T.M.; Shaikshavali, P.; Gopal, P. A novel electrochemical sensor based on multi-walled carbon nanotubes/poly (L-Methionine) for the investigation of 5-Nitroindazole: A voltammetric study. Anal. Chem. Lett. 2018, 8, 457–474. [Google Scholar] [CrossRef]
- Ojani, R.; Raoof, J.B.; Maleki, A.A.; Safshekan, S. Simultaneous and sensitive detection of dopamine and uric acid using a poly(L-methionine)/gold nanoparticle-modified glassy carbon electrode. Cuihua Xuebao/Chinese J. Catal. 2014, 35, 423–429. [Google Scholar] [CrossRef]
- Golkarieh, A.-M.; Nasirizadeh, N.; Jahanmardi, R. Fabrication of an electrochemical sensor with Au nanorods-graphene oxide hybrid nanocomposites for in situ measurement of cloxacillin. Mater. Sci. Eng. C 2020, 118, 111337. [Google Scholar] [CrossRef]
- Dehghani, M.; Nasirizadeh, N.; Yazdanshenas, M.E. Determination of cefixime using a novel electrochemical sensor produced with gold nanowires/graphene oxide/electropolymerized molecular imprinted polymer. Mater. Sci. Eng. C 2019, 96, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, S.; Sridevi, V. In Situ electrodeposited gold nanoparticles on polyaniline-modified electrode surface for the detection of dopamine in presence of ascorbic acid and uric acid. Electrocatalysis 2021, 12, 415–435. [Google Scholar] [CrossRef]
- Zhou, F.; Jing, W.; Liu, S.; Mao, Q.; Xu, Y.; Han, F.; Wei, Z.; Jiang, Z. Electrodeposition of gold nanoparticles on ZnO nanorods for improved performance of enzymatic glucose sensors. Mater. Sci. Semicond. Process. 2020, 105, 104708. [Google Scholar] [CrossRef]
- Menon, S.; Jesny, S.; Girish Kumar, K. A voltammetric sensor for acetaminophen based on electropolymerized-molecularly imprinted poly(o-aminophenol) modified gold electrode. Talanta 2018, 179, 668–675. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Wu, K.; Zhang, L.; Ge, S.; Yu, J. A molecularly imprinted polypyrrole for ultrasensitive voltammetric determination of glyphosate. Microchim. Acta 2017, 184, 1959–1967. [Google Scholar] [CrossRef]
- Kamal Ahmed, R.; Saad, E.M.; Fahmy, H.M.; El Nashar, R.M. Design and application of molecularly imprinted Polypyrrole/Platinum nanoparticles modified platinum sensor for the electrochemical detection of Vardenafil. Microchem. J. 2021, 171, 106771. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Atty, S.A.; Yehia, A.M.; Foster, C.W.; Banks, C.E.; Allam, N.K. Electrochemical determination of the serotonin reuptake inhibitor, dapoxetine, using cesium-gold nanoparticles. ACS Omega 2017, 2, 6628–6635. [Google Scholar] [CrossRef]
- Abdel Ghani, N.T.; Abdulla, H.; Rizk, M.S.; Dena, A.S.A.; El Nashar, R.M. Molecularly imprinted polymer/reduced graphene oxide‒based carbon‒paste sensor for highly sensitive determination of the anti‒HCV drug daclatasvir dihydrochloride. Sens. Actuators B Chem. 2019, 283, 6–17. [Google Scholar] [CrossRef]
- Satana, H.E.; Dogan-Topal, B.; Ozkan, S.A. Electrochemical characterization and rapid voltammetric determination of riluzole in pharmaceuticals and human serum. Anal. Lett. 2011, 44, 976–990. [Google Scholar] [CrossRef]
- Özkan, S.A.; Uslu, B.; Aboul-Enein, H.Y. Voltammetric investigation of Tamsulosin. Talanta 2003, 61, 147–156. [Google Scholar] [CrossRef]
- Sanz, C.G.; Serrano, S.H.P.; Brett, C.M.A. Electroanalysis of cefadroxil antibiotic at carbon nanotube/gold nanoparticle modified glassy carbon electrodes. ChemElectroChem 2020, 7, 2151–2158. [Google Scholar] [CrossRef]
- Tawab, M.A.H.A.; El-Moghny, M.G.A.; El Nashar, R.M. Computational design of molecularly imprinted polymer for electrochemical sensing and stability indicating study of sofosbuvir. Microchem. J. 2020, 158, 105180. [Google Scholar] [CrossRef]
- Aparecida, D.; Araújo, G.; Santos, A.L.; Massako, R. Non-Enzymatic lactose molecularly imprinted sensor based on disposable graphite paper electrode. Anal. Chim. Acta 2020, 1143, 53–64. [Google Scholar] [CrossRef]
- Zayed, S.I.M.; Issa, Y.M. Differential pulse anodic voltammetric determination of chlorzoxazone in pharmaceutical formulation using carbon paste electrode. Acta Chim. Slov. 2020, 67, 1053–1060. [Google Scholar] [CrossRef]





| Sensor Material | Analytical Method | Linear Range (M) | LOD (M) | R2 | Reference |
|---|---|---|---|---|---|
| poly (dl-met)/AuNPs-GCE | DPV | 5 × 10−11–5 × 10−9 | 1.00 × 10−11 | 0.991 | Present work |
| 5 × 10−9–1 × 10−4 | 0.995 | ||||
| rGO/PWA/PGE | DPV | 9 × 10−8–1 × 10−6 | 9.00 × 10−10 | 0.998 | [13] |
| Au/PANSA/CYP2D6 | DPV | 0.5 × 10−8–0.5 × 10−7 | 2.00 × 10−9 | 0.990 | [2] |
| BDDE | SWAdSV | 7.0 × 10−7–3.5 × 10−6 | 6.95 × 10−9 | 0.999 | [14] |
| EPGE | SWAdSV | 1.0 × 10−8–5.0 × 10−6 | 1.03 × 10−9 | 0.999 | [14] |
| Nafion-MWCNTs/GCE | DPV | 0.1 × 10−6–2.5 × 10−6 | 0.62 × 10−7 | 0.998 | [1] |
| Sample | Taken (µg mL−1) | Found (ng mL−1) | Recovery% ± SD | RSD% |
|---|---|---|---|---|
| Paroxetine CR® 37.5 | 0.27 | 0.28 | 103.04% ± 0.05 | 0.46 |
| mg/tab | 0.99 | 0.97 | 97.57% ± 0.37 | 3.11 |
| 2.87 | 2.91 | 98.62% ± 0.04 | 2.98 | |
| Paroxetine CR® 25 | 0.27 | 0.26 | 96.29% ± 0.07 | 1.24 |
| mg/tab | 0.99 | 1.04 | 105.05% ± 0.02 | 0.21 |
| 2.87 | 2.917 | 101.16% ± 0.40 | 2.98 | |
| Paroxetine CR® 12.5 | 0.27 | 0.27 | 103.40% ± 0.58 | 5.41 |
| mg/tab | 0.99 | 0.98 | 99.10% ± 0.21 | 1.80 |
| 2.87 | 2.94 | 102.50% ± 0.41 | 3.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Mhyawi, S.R.; Ahmed, R.K.; El Nashar, R.M. Application of a Conducting Poly-Methionine/Gold Nanoparticles-Modified Sensor for the Electrochemical Detection of Paroxetine. Polymers 2021, 13, 3981. https://doi.org/10.3390/polym13223981
Al-Mhyawi SR, Ahmed RK, El Nashar RM. Application of a Conducting Poly-Methionine/Gold Nanoparticles-Modified Sensor for the Electrochemical Detection of Paroxetine. Polymers. 2021; 13(22):3981. https://doi.org/10.3390/polym13223981
Chicago/Turabian StyleAl-Mhyawi, Saedah R., Riham K. Ahmed, and Rasha M. El Nashar. 2021. "Application of a Conducting Poly-Methionine/Gold Nanoparticles-Modified Sensor for the Electrochemical Detection of Paroxetine" Polymers 13, no. 22: 3981. https://doi.org/10.3390/polym13223981
APA StyleAl-Mhyawi, S. R., Ahmed, R. K., & El Nashar, R. M. (2021). Application of a Conducting Poly-Methionine/Gold Nanoparticles-Modified Sensor for the Electrochemical Detection of Paroxetine. Polymers, 13(22), 3981. https://doi.org/10.3390/polym13223981






