Osteoinductive Moldable and Curable Bone Substitutes Based on Collagen, BMP-2 and Highly Porous Polylactide Granules, or a Mix of HAP/β-TCP
Abstract
:1. Introduction
2. Materials and Methods
2.1. Components’ Fabrication
2.1.1. Highly Porous Polylactide Granules’ Fabrication
2.1.2. Collagen-Fibronectin Hydrogel Fabrication
2.1.3. BMP-2 Impregnation
2.1.4. Fabrication of Composite Materials
2.2. Investigation of Physical and Mechanical Properties
2.3. In Vitro Stvitroudies
2.3.1. Cell Viability
2.3.2. Study of Cell Adhesion
2.4. In Vivo Studies
2.4.1. Orthotopic Osteogenesis Model: Implantation into a Rat Critical-SIZE Calvarial Defect
2.4.2. Ectopic Osteogenesis Model: Subcutaneous Implantation in Rats
2.4.3. Euthanasia, Histological Examination, and Morphometry
2.5. Statistical Analysis
3. Results
3.1. Mechanical Properties
3.1.1. Collagen Hydrogels
3.1.2. Hydrogel Filled with a Mixture of HAP/β-TCP
3.1.3. Hydrogel Filled with Highly Porous PLA Granules
3.1.4. Comparison of Fillers’ Mechanical Properties
3.2. In Vitro Research
3.2.1. Collagen and Collagen-Fibronectin Gels
3.2.2. The Mixture of HAP Granules with β-TCP
3.2.3. Highly Porous PLA Granules
3.3. In Vivo Study
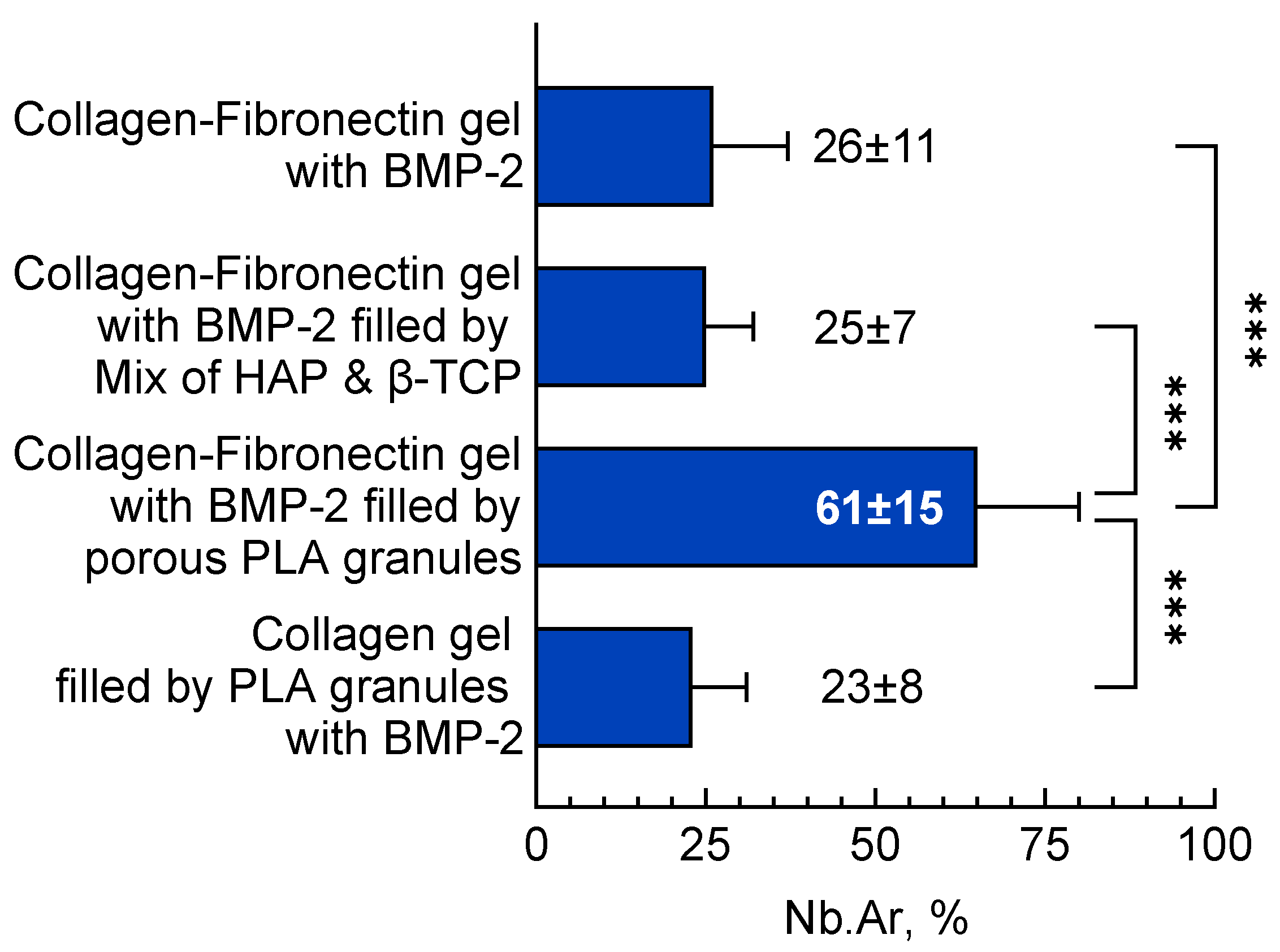
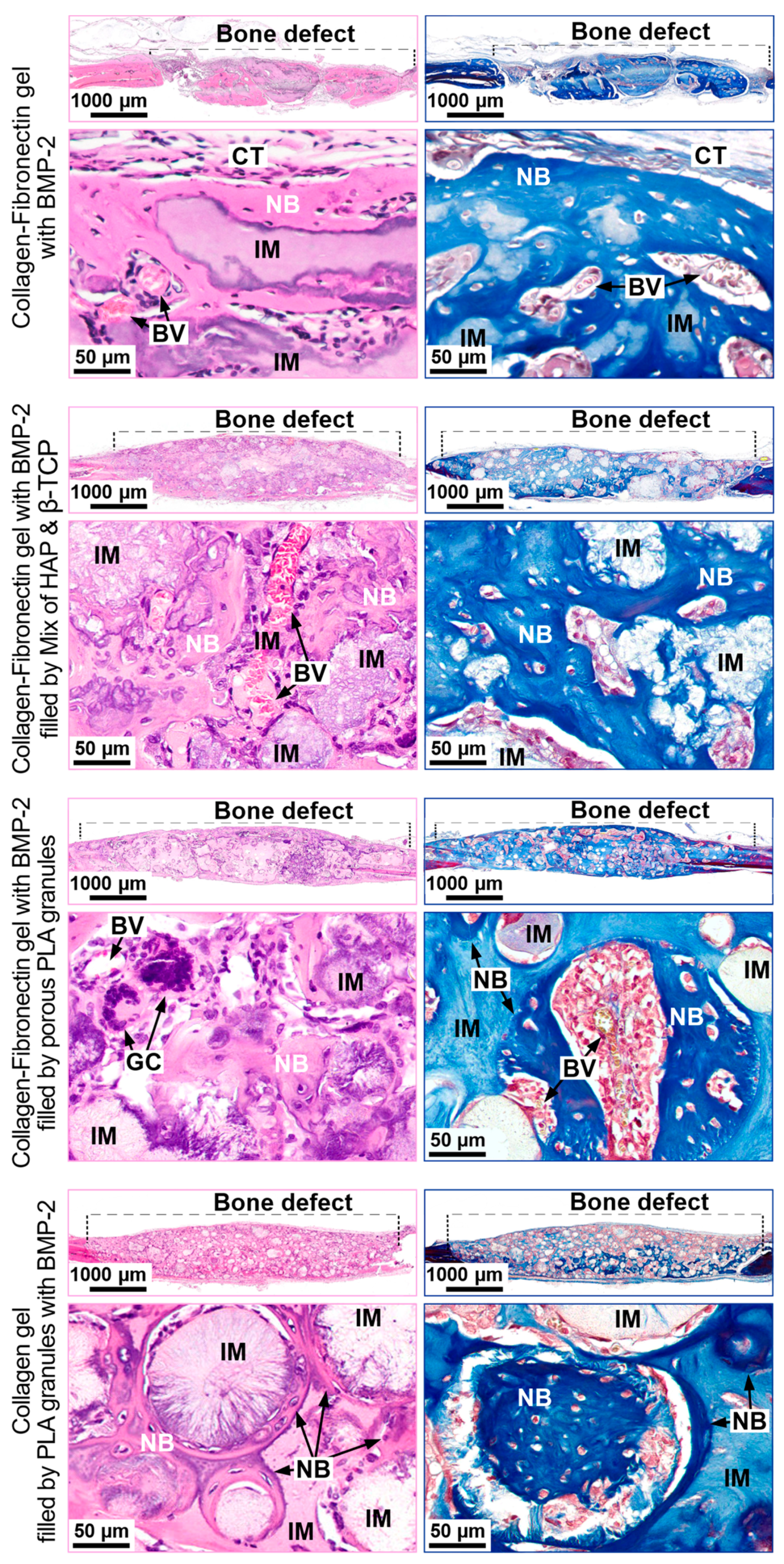
3.3.1. Orthotopic Osteogenesis Model
Collagen Fibronectin Gel with BMP-2
Collagen-Fibronectin Gel with BMP-2 Filled with HAP/β-TCP Granules
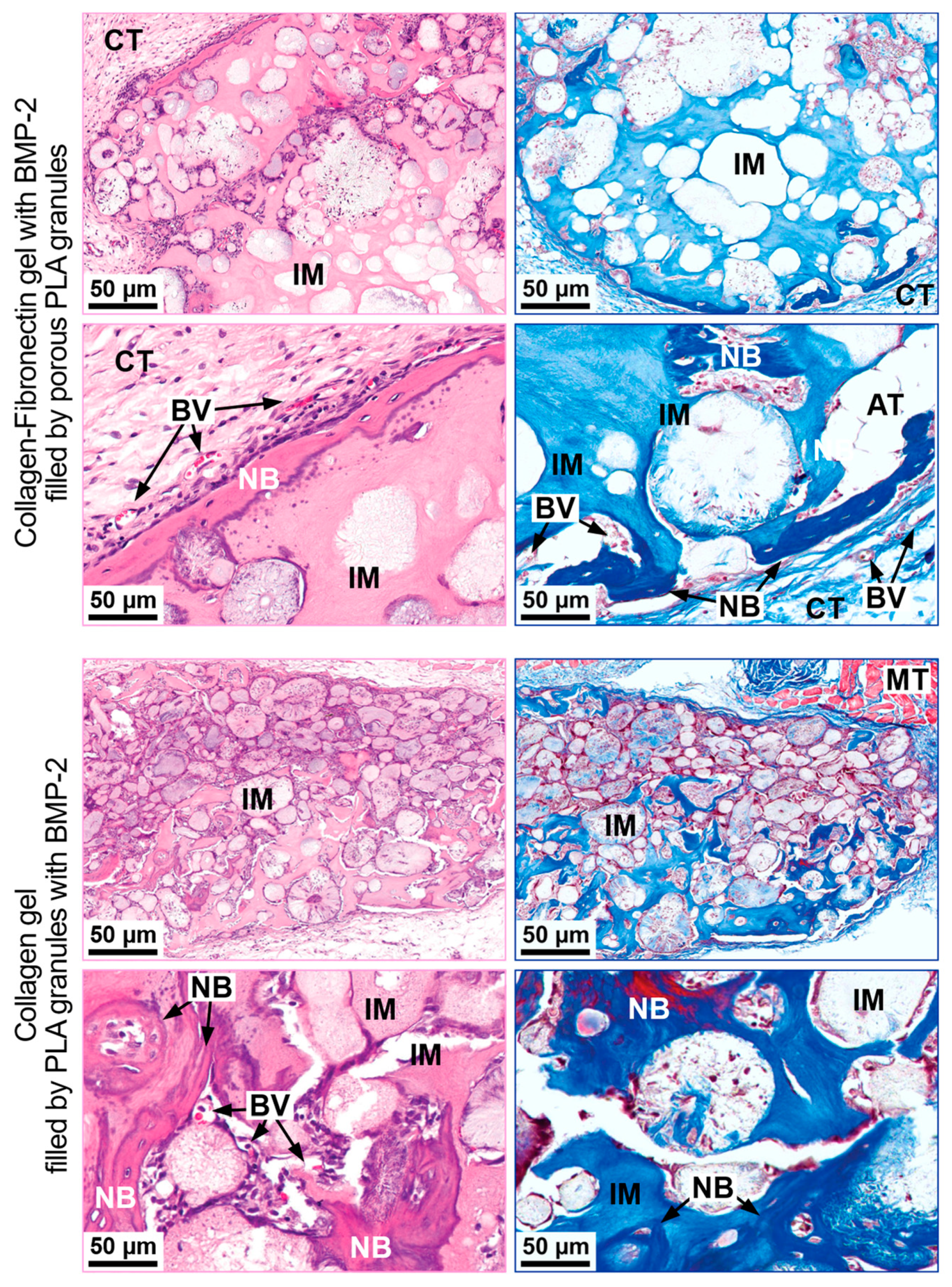
Collagen-Fibronectin Gel with BMP-2 Filled with PLA Granules
Collagen Gel with PLA Granules Impregnated with BMP-2
3.3.2. Ectopic Osteogenesis Model
Collagen-Fibronectin Gel with BMP-2 Filled with PLA Granules
Collagen Gel with PLA Granules Impregnated with BMP-2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bone Grafts and Substitutes Market by Product and Application: Global Opportunity Analysis and Industry Forecast, 2021–2028. Available online: www.alliedmarketresearch.com/press-release/bone-graft-substitutes-market.html (accessed on 4 October 2021).
- Schmidt, A.H. Autologous Bone Graft: Is It Still the Gold Standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone Grafts: Which Is the Ideal Biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef]
- Drosos, G.I. Use of Demineralized Bone Matrix in the Extremities. WJO 2015, 6, 269. [Google Scholar] [CrossRef]
- Sallent, I.; Capella-Monsonís, H.; Procter, P.; Bozo, I.Y.; Deev, R.V.; Zubov, D.; Vasyliev, R.; Perale, G.; Pertici, G.; Baker, J.; et al. The Few Who Made It: Commercially and Clinically Successful Innovative Bone Grafts. Front. Bioeng. Biotechnol. 2020, 8, 952. [Google Scholar] [CrossRef]
- Drugwatch, Infuse Bone Graft—Alternatives, Controversy & Complications. 2021, pp. 1–11. Available online: www.drugwatch.com/infuse (accessed on 4 October 2021).
- Yousefi, A.-M. A Review of Calcium Phosphate Cements and Acrylic Bone Cements as Injectable Materials for Bone Repair and Implant Fixation. J. Appl. Biomater. Funct. Mater. 2019, 17, 228080001987259. [Google Scholar] [CrossRef] [PubMed]
- Vasilyev, A.V.; Kuznetsova, V.S.; Bukharova, T.B.; Grigoriev, T.E.; Zagoskin, Y.D.; Nedorubova, I.A.; Babichenko, I.I.; Chvalun, S.N.; Goldstein, D.V.; Kulakov, A.A. Influence of the Degree of Deacetylation of Chitosan and BMP-2 Concentration on Biocompatibility and Osteogenic Properties of BMP-2/PLA Granule-Loaded Chitosan/β-Glycerophosphate Hydrogels. Molecules 2021, 26, 261. [Google Scholar] [CrossRef]
- Vasilyev, A.V.; Kuznetsova, V.S.; Bukharova, T.B.; Zagoskin, Y.D.; Leonov, G.E.; Grigoriev, T.E.; Chvalun, S.N.; Goldshtein, D.V.; Kulakov, A.A. Chitosan Hydrogels Biocompatibility Improvement with the Perspective of Use as a Base for Osteoplastic Materials in Dentistry. Stomatologiya 2019, 98, 12. [Google Scholar] [CrossRef]
- Shekhter, A.B. Connective Tissue as an Integral System: Role of Cell-Cell and Cell-Matrix Interactions. Connect. Tissue Res. 1986, 15, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Vasilyev, A.V.; Bukharova, T.B.; Kuznetsova, V.S.; Zagoskin, Y.D.; Minaeva, S.A.; Grigoriev, T.E.; Antonov, E.N.; Osidak, E.O.; Galitsyna, E.V.; Babichenko, I.I.; et al. Comparison of Impregnated Bone Morphogenetic Protein-2 Release Kinetics from Biopolymer Scaffolds. Inorg. Mater. Appl. Res. 2019, 10, 1093–1100. [Google Scholar] [CrossRef]
- Suliman, S.; Xing, Z.; Wu, X.; Xue, Y.; Pedersen, T.O.; Sun, Y.; Døskeland, A.P.; Nickel, J.; Waag, T.; Lygre, H.; et al. Release and Bioactivity of Bone Morphogenetic Protein-2 Are Affected by Scaffold Binding Techniques In Vitro and In Vivo. J. Control. Release 2015, 197, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.B.; Keong, T.K.; Cheng, C.H.; Saim, A.B.; Idrus, R.B.H. Tricalcium Phosphate/Hydroxyapatite (TCP-HA) Bone Scaffold as Potential Candidate for the Formation of Tissue Engineered Bone. Indian J. Med. Res. 2013, 137, 1093–1101. [Google Scholar]
- Osidak, E.O.; Osidak, M.S.; Sivogrivov, D.E.; Portnaya, T.S.; Grunina, T.M.; Soboleva, L.A.; Lunin, V.G.; Karyagina, A.S.; Domogatskii, S.P. Regulation of the Binding of the BMP-2 Growth Factor with Collagen by Blood Plasma Fibronectin. Appl. Biochem. Microbiol. 2014, 50, 226–231. [Google Scholar] [CrossRef]
- Vasilyev, A.V.; Kuznetsova, V.S.; Bukharova, T.B.; Grigoriev, T.E.; Zagoskin, Y.D.; Galitsina, E.V.; Fatkhudinova, N.L.; Babichenko, I.I.; Chvalun, S.N.; Goldstein, D.V.; et al. Osteoinductive Potential of Highly Porous Polylactide Granules and Bio-Oss Impregnated with Low Doses of BMP-2. IOP Conf. Ser. Earth Environ. Sci. 2020, 421, 052035. [Google Scholar] [CrossRef]
- Vasilyev, A.; Volkov, A.; Bolshakova, G.; Goldstein, D. Characteristics of Neoosteogenesis in the Model of Critical Defect of Rats Parietal Bone Using Traditional and Three-Dimensional Morphometry. Genes Cells 2014, 9, 121–127. [Google Scholar]
- Parfitt, A.M. Bone Histomorphometry: Standardization of Nomenclature, Symbols and Units (Summary of Proposed System). Bone 1988, 9, 67–69. [Google Scholar] [CrossRef]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized Nomenclature, Symbols, and Units for Bone Histomorphometry: A 2012 Update of the Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Min. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [Green Version]
- Hänseler, P.; Ehrbar, M.; Kruse, A.; Fischer, E.; Schibli, R.; Ghayor, C.; Weber, F.E. Delivery of BMP-2 by Two Clinically Available Apatite Materials: In Vitro and In Vivo Comparison: Delivery of RhBMP-2 by Apatite Granules. J. Biomed. Mater. Res. 2015, 103, 628–638. [Google Scholar] [CrossRef]
- Huh, J.-B.; Yang, J.-J.; Choi, K.-H.; Bae, J.; Lee, J.-Y.; Kim, S.-E.; Shin, S.-W. Effect of RhBMP-2 Immobilized Anorganic Bovine Bone Matrix on Bone Regeneration. IJMS 2015, 16, 16034–16052. [Google Scholar] [CrossRef] [Green Version]
- Kao, D.W.K.; Kubota, A.; Nevins, M.; Fiorellini, J.P. The Negative Effect of Combining RhBMP-2 and Bio-Oss on Bone Formation for Maxillary Sinus Augmentation. Int. J. Periodontics Restor. Dent. 2012, 32, 61–67. [Google Scholar]
- Kubota, T.; Hasuike, A.; Ozawa, Y.; Yamamoto, T.; Tsunori, K.; Yamada, Y.; Sato, S. Regenerative Capacity of Augmented Bone in Rat Calvarial Guided Bone Augmentation Model. J. Periodontal Implant Sci. 2017, 47, 77. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira Lomelino, R.; Castro-Silva, I.I.; Linhares, A.B.R.; Alves, G.G.; de Albuquerque Santos, S.R.; Gameiro, V.S.; Rossi, A.M.; Granjeiro, J.M. The Association of Human Primary Bone Cells with Biphasic Calcium Phosphate (ΒTCP/HA 70:30) Granules Increases Bone Repair. J. Mater. Sci. Mater. Med. 2012, 23, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Durham, E.L.; Howie, R.N.; Hall, S.; Larson, N.; Oakes, B.; Houck, R.; Grey, Z.; Steed, M.; LaRue, A.C.; Muise-Helmericks, R.; et al. Optimizing Bone Wound Healing Using BMP2 with Absorbable Collagen Sponge and Talymed Nanofiber Scaffold. J. Transl. Med. 2018, 16, 321. [Google Scholar] [CrossRef] [PubMed]
- Luz, E.P.C.G.; das Chagas, B.S.; de Almeida, N.T.; de Fátima Borges, M.; Andrade, F.K.; Muniz, C.R.; Castro-Silva, I.I.; Teixeira, E.H.; Popat, K.; de Freitas Rosa, M.; et al. Resorbable Bacterial Cellulose Membranes with Strontium Release for Guided Bone Regeneration. Mater. Sci. Eng.: C 2020, 116, 111175. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Susin, C.; Lee, J.; Fiorini, T.; Bisch, F.C.; Dixon, D.R.; McPherson, J.C.; Buxton, A.N.; Wikesjö, U.M.E. Effect of RhBMP-2 Dose on Bone Formation/Maturation in a Rat Critical-Size Calvarial Defect Model. J. Clin. Periodontol. 2014, 41, 827–836. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilyev, A.V.; Kuznetsova, V.S.; Bukharova, T.B.; Osidak, E.O.; Grigoriev, T.E.; Zagoskin, Y.D.; Nedorubova, I.A.; Domogatsky, S.P.; Babichenko, I.I.; Zorina, O.A.; et al. Osteoinductive Moldable and Curable Bone Substitutes Based on Collagen, BMP-2 and Highly Porous Polylactide Granules, or a Mix of HAP/β-TCP. Polymers 2021, 13, 3974. https://doi.org/10.3390/polym13223974
Vasilyev AV, Kuznetsova VS, Bukharova TB, Osidak EO, Grigoriev TE, Zagoskin YD, Nedorubova IA, Domogatsky SP, Babichenko II, Zorina OA, et al. Osteoinductive Moldable and Curable Bone Substitutes Based on Collagen, BMP-2 and Highly Porous Polylactide Granules, or a Mix of HAP/β-TCP. Polymers. 2021; 13(22):3974. https://doi.org/10.3390/polym13223974
Chicago/Turabian StyleVasilyev, Andrey Vyacheslavovich, Valeriya Sergeevna Kuznetsova, Tatyana Borisovna Bukharova, Egor Olegovich Osidak, Timofei Evgenevich Grigoriev, Yuriy Dmitrievich Zagoskin, Irina Alekseevna Nedorubova, Sergey Petrovich Domogatsky, Igor Ivanovich Babichenko, Oksana Aleksandrovna Zorina, and et al. 2021. "Osteoinductive Moldable and Curable Bone Substitutes Based on Collagen, BMP-2 and Highly Porous Polylactide Granules, or a Mix of HAP/β-TCP" Polymers 13, no. 22: 3974. https://doi.org/10.3390/polym13223974
APA StyleVasilyev, A. V., Kuznetsova, V. S., Bukharova, T. B., Osidak, E. O., Grigoriev, T. E., Zagoskin, Y. D., Nedorubova, I. A., Domogatsky, S. P., Babichenko, I. I., Zorina, O. A., Kutsev, S. I., Chvalun, S. N., Kulakov, A. A., Losev, F. F., & Goldshtein, D. V. (2021). Osteoinductive Moldable and Curable Bone Substitutes Based on Collagen, BMP-2 and Highly Porous Polylactide Granules, or a Mix of HAP/β-TCP. Polymers, 13(22), 3974. https://doi.org/10.3390/polym13223974





