Chemical Modification of Glycoproteins’ Carbohydrate Moiety as a General Strategy for the Synthesis of Efficient Biocatalysts by Biomimetic Mineralization: The Case of Glucose Oxidase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Periodate Oxidation
2.3. Biomimetic Mineralization
2.4. Determination of Protein Concentration
2.5. Activity Measurements
2.6. Thermal Stability Measurements
2.7. Powder X-ray Diffraction (PXRD)
2.8. Scanning Electron Microscopy (SEM)
2.9. Zeta Potential Measurements
3. Results and Discussion
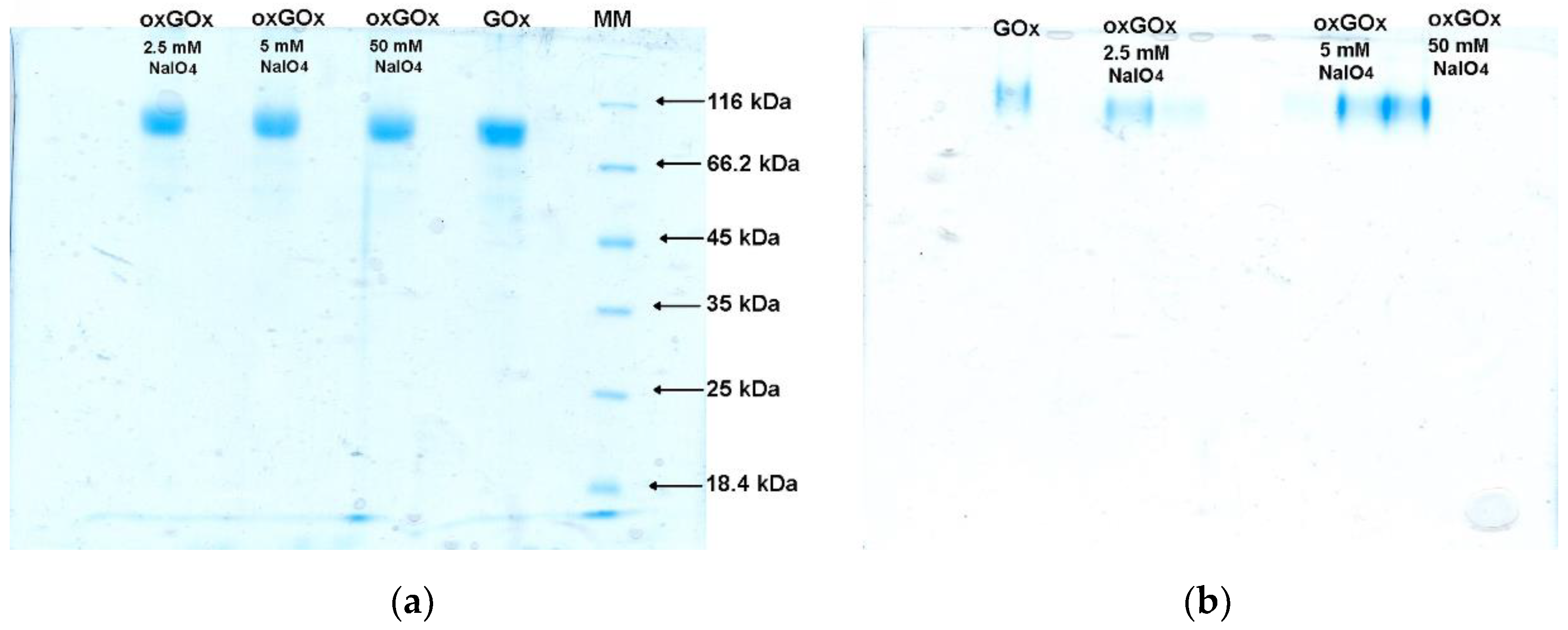
3.1. Periodate Oxidation
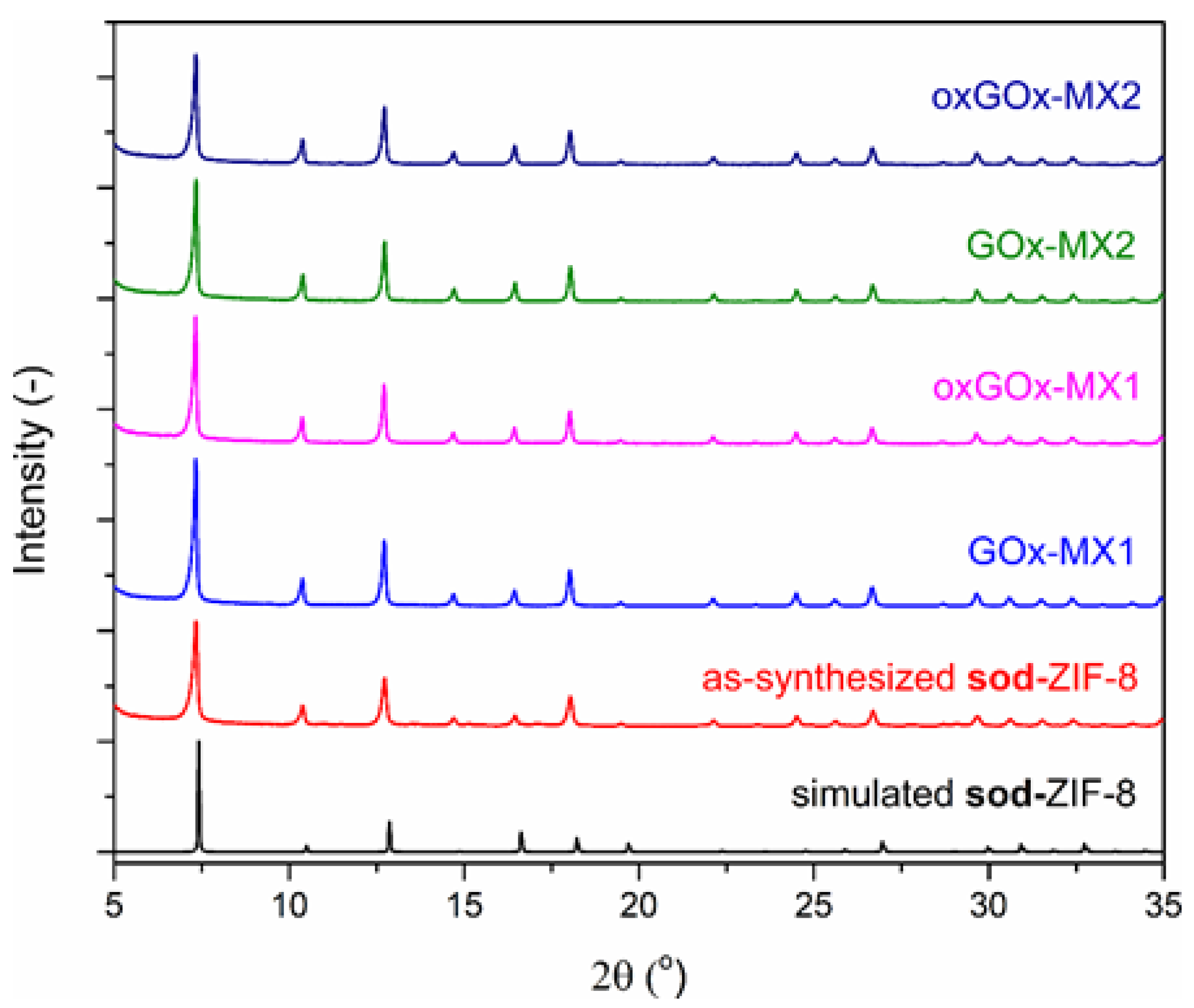
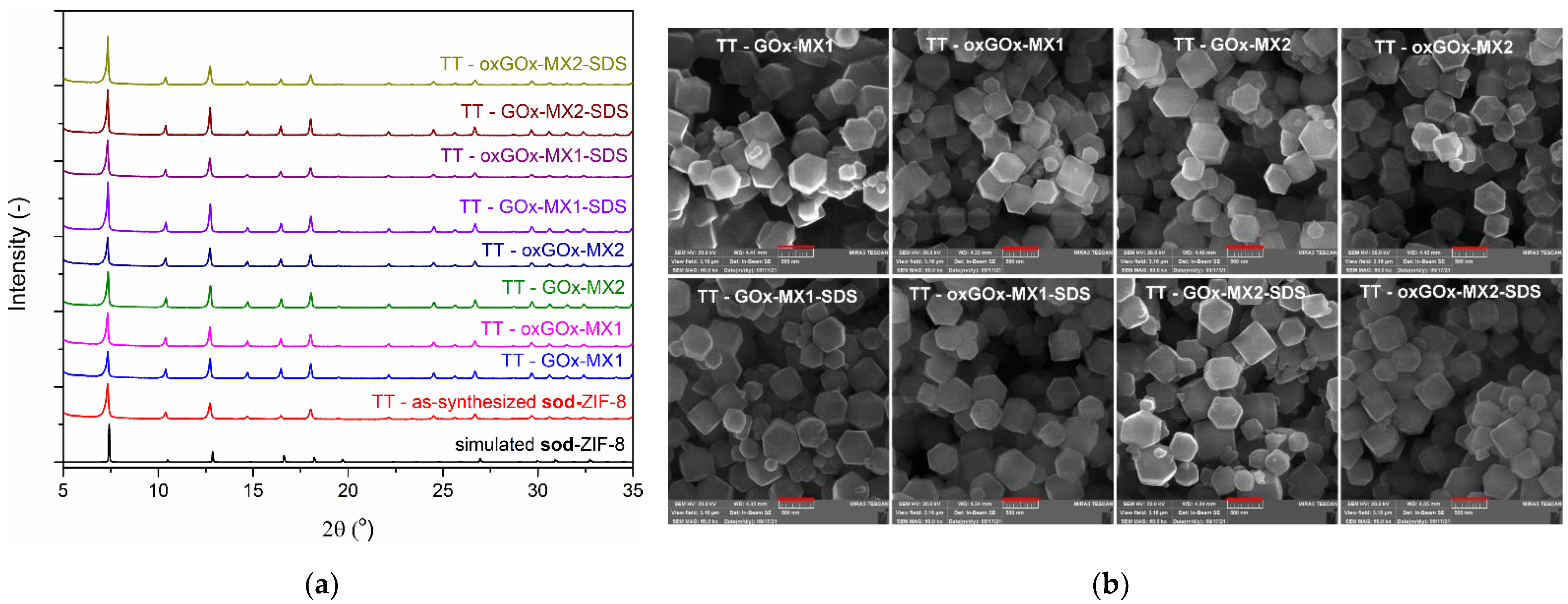
3.2. Biomimetic Mineralization Experiments
3.3. Kinetic Immobilization Performance Parameters
3.4. Thermal Stability Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Industrial Enzymes Market Growth & Trends|Size, and Share|MarketsandMarkets. Available online: https://www.marketsandmarkets.com/Market-Reports/industrial-enzymes-market-237327836.html (accessed on 8 October 2021).
- Cirujano, F.G.; Dhakshinamoorthy, A. Challenges and Opportunities for the Encapsulation of Enzymes over Porous Solids for Biodiesel Production and Cellulose Valorization into Glucose. ChemCatChem 2021. [Google Scholar] [CrossRef]
- Drout, R.J.; Robison, L.; Farha, O.K. Catalytic applications of enzymes encapsulated in metal–organic frameworks. Coord. Chem. Rev. 2019, 381, 151–160. [Google Scholar] [CrossRef]
- Liang, W.; Wied, P.; Carraro, F.; Sumby, C.J.; Nidetzky, B.; Tsung, C.K.; Falcaro, P.; Doonan, C.J. Metal–Organic Framework-Based Enzyme Biocomposites. Chem. Rev. 2021, 121, 1077–1129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tu, R.; Lu, Z.; Peng, J.; Hou, C.; Wang, Z. Hierarchical mesoporous metal–organic frameworks encapsulated enzymes: Progress and perspective. Coord. Chem. Rev. 2021, 443, 214032. [Google Scholar] [CrossRef]
- Molina, M.A.; Gascón-Pérez, V.; Sánchez-Sánchez, M.; Blanco, R.M. Sustainable One-Pot Immobilization of Enzymes in/on Metal-Organic Framework Materials. Catalysts 2021, 11, 1002. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Integration of metal organic frameworks with enzymes as multifunctional solids for cascade catalysis. Dalton Trans. 2020, 49, 11059–11072. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Modica, J.A.; Howarth, A.J.; Vargas, L.E.; Moghadam, P.Z.; Snurr, R.Q.; Mrksich, M.; Hupp, J.T.; Farha, O.K. Toward Design Rules for Enzyme Immobilization in Hierarchical Mesoporous Metal-Organic Frameworks. Chem 2016, 1, 154–169. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Chen, Q.; Wang, T.C.; Vermeulen, N.A.; Mehdi, B.L.; Dohnalkova, A.; Browning, N.D.; Shen, D.; Anderson, R.; Gómez-Gualdrón, D.A.; et al. Hierarchically Engineered Mesoporous Metal-Organic Frameworks toward Cell-free Immobilized Enzyme Systems. Chem 2018, 4, 1022–1034. [Google Scholar] [CrossRef] [Green Version]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2009, 43, 58–67. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. Biointerface Between ZIF-8 and Biomolecules and their Applications. Biointerface Res. Appl. Chem. 2021, 11, 8283–8297. [Google Scholar]
- Mann, S. Biomineralization and Biomimetic Materials Chemistry. J. Mater. Chem. 1995, 5, 935–946. [Google Scholar] [CrossRef]
- Morris, W.; Stevens, C.J.; Taylor, R.E.; Dybowski, C.; Yaghi, O.M.; Garcia-Garibay, M.A. NMR and X-ray Study Revealing the Rigidity of Zeolitic Imidazolate Frameworks. J. Phys. Chem. C 2012, 116, 13307–13312. [Google Scholar] [CrossRef]
- Cousin Saint Remi, J.; Rémy, T.; Van Hunskerken, V.; van de Perre, S.; Duerinck, T.; Maes, M.; De Vos, D.; Gobechiya, E.; Kirschhock, C.E.A.; Baron, G.V.; et al. Biobutanol Separation with the Metal–Organic Framework ZIF-8. ChemSusChem 2011, 4, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Katsenis, A.D.; Puškarić, A.; Štrukil, V.; Mottillo, C.; Julien, P.A.; Užarević, K.; Pham, M.-H.; Do, T.-O.; Kimber, S.A.J.; Lazić, P.; et al. In situ X-ray diffraction monitoring of a mechanochemical reaction reveals a unique topology metal-organic framework. Nat. Commun. 2015, 6, 6662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, W.; Ricco, R.; Maddigan, N.K.; Dickinson, R.P.; Xu, H.; Li, Q.; Sumby, C.J.; Bell, S.G.; Falcaro, P.; Doonan, C.J. Control of Structure Topology and Spatial Distribution of Biomacromolecules in Protein@ZIF-8 Biocomposites. Chem. Mater. 2018, 30, 1069–1077. [Google Scholar] [CrossRef] [Green Version]
- Liang, K.; Ricco, R.; Doherty, C.M.; Styles, M.J.; Bell, S.; Kirby, N.; Mudie, S.; Haylock, D.; Hill, A.J.; Doonan, C.J.; et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 2015, 6, 7240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maddigan, N.K.; Tarzia, A.; Huang, D.M.; Sumby, C.J.; Bell, S.G.; Falcaro, P.; Doonan, C.J. Protein surface functionalisation as a general strategy for facilitating biomimetic mineralisation of ZIF-8. Chem. Sci. 2018, 9, 4217–4223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalisz, H.M.; Schmid, R.D. Effect of Deglycosylation on the Activity and Stability of Glucose Oxidase From Aspergillus Niger. In Flavins and Flavoproteins 1990: Proceedings of the Tenth International Symposium, Como, Italy, 15–20 July 1990; Curti, B., Ronchi, S., Zanetti, G., Eds.; De Gruyter: Berlin, Germany, 2019; pp. 205–208. [Google Scholar]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Hayashi, S.; Nakamura, S. Multiple forms of glucose oxidase with different carbohydrate compositions. Biochim. Biophys. Acta Enzymol. 1981, 657, 40–51. [Google Scholar] [CrossRef]
- Schmid, A.; Dordick, J.S.; Hauer, B.; Kiener, A.; Wubbolts, M.; Witholt, B. Industrial biocatalysis today and tomorrow. Nature 2001, 409, 258–268. [Google Scholar] [CrossRef]
- Nakamura, S.; Hayashi, S.; Koga, K. Effect of periodate oxidation on the structure and properties of glucose oxidase. Biochim. Biophys. Acta Enzymol. 1976, 445, 294–308. [Google Scholar] [CrossRef]
- Kalisz, H.M.; Hecht, H.J.; Schomburg, D.; Schmid, R.D. Effects of carbohydrate depletion on the structure, stability and activity of glucose oxidase from Aspergillus niger. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1991, 1080, 138–142. [Google Scholar] [CrossRef]
- Hunter, R. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: New York, NY, USA, 1981. [Google Scholar]
- Li, Z.; Zhou, G.; Dai, H.; Yang, M.; Fu, Y.; Ying, Y.; Li, Y. Biomineralization-mimetic preparation of hybrid membranes with ultra-high loading of pristine metal–organic frameworks grown on silk nanofibers for hazard collection in water. J. Mater. Chem. A 2018, 6, 3402–3413. [Google Scholar] [CrossRef]
- Jin, W.; Jiang, S.; Pan, H.; Tang, R. Amorphous Phase Mediated Crystallization: Fundamentals of Biomineralization. Crystals 2018, 8, 48. [Google Scholar] [CrossRef] [Green Version]
- Illanes, A. Enzyme Biocatalysis: Principles and Applications; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Chen, G.; Kou, X.; Huang, S.; Tong, L.; Shen, Y.; Zhu, W.; Zhu, F.; Ouyang, G. Modulating the Biofunctionality of Metal–Organic-Framework-Encapsulated Enzymes through Controllable Embedding Patterns. Angew. Chemie Int. Ed. 2020, 59, 2867–2874. [Google Scholar] [CrossRef]
- Wu, X.; Yang, C.; Ge, J.; Liu, Z. Polydopamine tethered enzyme/metal–organic framework composites with high stability and reusability. Nanoscale 2015, 7, 18883–18886. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.-L.; Xu, H.; Lai, L.-H.; Gu, W.-M.; Xu, P.; Xiong, J.; Yin, H.; Li, X.-H.; Ma, Y.-Z.; Zhou, J.; et al. Magnetic ZIF-8/cellulose/Fe3O4 nanocomposite: Preparation, characterization, and enzyme immobilization. Bioresour. Bioprocess. 2017, 4, 56. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Yue, H.; Zhang, Y.; Gao, X.; Li, X.; Wang, L.; Cao, Y.; Hou, M.; An, H.; Zhang, L.; et al. Packaging and delivering enzymes by amorphous metal-organic frameworks. Nat. Commun. 2019, 10, 5165. [Google Scholar] [CrossRef]


| Sample | Zeta Potential (mV) |
|---|---|
| GOx | −10.2 ± 4.6 |
| 2.5-oxGOx | −36.9 ± 5.6 |
| 5-oxGOx | −32.7 ± 4.4 |
| 50-oxGOx | −29.5 ± 5.2 |
| GOx in Zn(II) solution | −1.95 ± 3.8 |
| GOx in HmIM solution | −13.1 ± 3.6 |
| 2.5-oxGOx in Zn(II) solution | −1.93 ± 4.2 |
| 2.5-oxGOx in HmIM solution | −23.6 ± 4.9 |
| Parameter | GOx-MX1 | oxGOx-MX1 | GOx-MX2 | oxGOx-MX2 |
|---|---|---|---|---|
| YA | 0.82 | 0.99 | 0.95 | 0.99 |
| YP | 0.52 | 0.56 | 0.40 | 0.63 |
| Ploading (mg/gcarrier) | 41.25 | 24.14 | 37.47 | 36.56 |
| Specific activity (U/gbiocomposite) | 1591.25 | 855.84 | 815.02 | 873.51 |
| Specific activity (U/mgenzyme bound) | 39.18 | 25.61 | 33.51 | 28.83 |
| η × 100 (%) | 22 | 22 | 19 | 24 |
| Parameter | GOx-MX1-SDS | oxGOx-MX1-SDS | GOx-MX2-SDS | oxGOx-MX2-SDS |
|---|---|---|---|---|
| YA | 0.28 | 0.50 | 0.26 | 0.55 |
| YP | 24.40 | 28.90 | 22.72 | 32.72 |
| Ploading (mg/gcarrier) | 52.55 | 534.55 | 15.88 | 338.90 |
| Specific activity (U/gbiocomposite) | 2.32 | 20.46 | 2.45 | 22.59 |
| Specific activity (U/mgenzyme bound) | 1.3 | 17 | 3 | 19 |
| η × 100 (%) | 0.28 | 0.50 | 0.26 | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanišić, M.D.; Popović Kokar, N.; Ristić, P.; Balaž, A.M.; Senćanski, M.; Ognjanović, M.; Đokić, V.R.; Prodanović, R.; Todorović, T.R. Chemical Modification of Glycoproteins’ Carbohydrate Moiety as a General Strategy for the Synthesis of Efficient Biocatalysts by Biomimetic Mineralization: The Case of Glucose Oxidase. Polymers 2021, 13, 3875. https://doi.org/10.3390/polym13223875
Stanišić MD, Popović Kokar N, Ristić P, Balaž AM, Senćanski M, Ognjanović M, Đokić VR, Prodanović R, Todorović TR. Chemical Modification of Glycoproteins’ Carbohydrate Moiety as a General Strategy for the Synthesis of Efficient Biocatalysts by Biomimetic Mineralization: The Case of Glucose Oxidase. Polymers. 2021; 13(22):3875. https://doi.org/10.3390/polym13223875
Chicago/Turabian StyleStanišić, Marija D., Nikolina Popović Kokar, Predrag Ristić, Ana Marija Balaž, Milan Senćanski, Miloš Ognjanović, Veljko R. Đokić, Radivoje Prodanović, and Tamara R. Todorović. 2021. "Chemical Modification of Glycoproteins’ Carbohydrate Moiety as a General Strategy for the Synthesis of Efficient Biocatalysts by Biomimetic Mineralization: The Case of Glucose Oxidase" Polymers 13, no. 22: 3875. https://doi.org/10.3390/polym13223875
APA StyleStanišić, M. D., Popović Kokar, N., Ristić, P., Balaž, A. M., Senćanski, M., Ognjanović, M., Đokić, V. R., Prodanović, R., & Todorović, T. R. (2021). Chemical Modification of Glycoproteins’ Carbohydrate Moiety as a General Strategy for the Synthesis of Efficient Biocatalysts by Biomimetic Mineralization: The Case of Glucose Oxidase. Polymers, 13(22), 3875. https://doi.org/10.3390/polym13223875








