Functionalization of Graphene Oxide with Polysilicone: Synthesis, Characterization, and Its Flame Retardancy in Epoxy Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
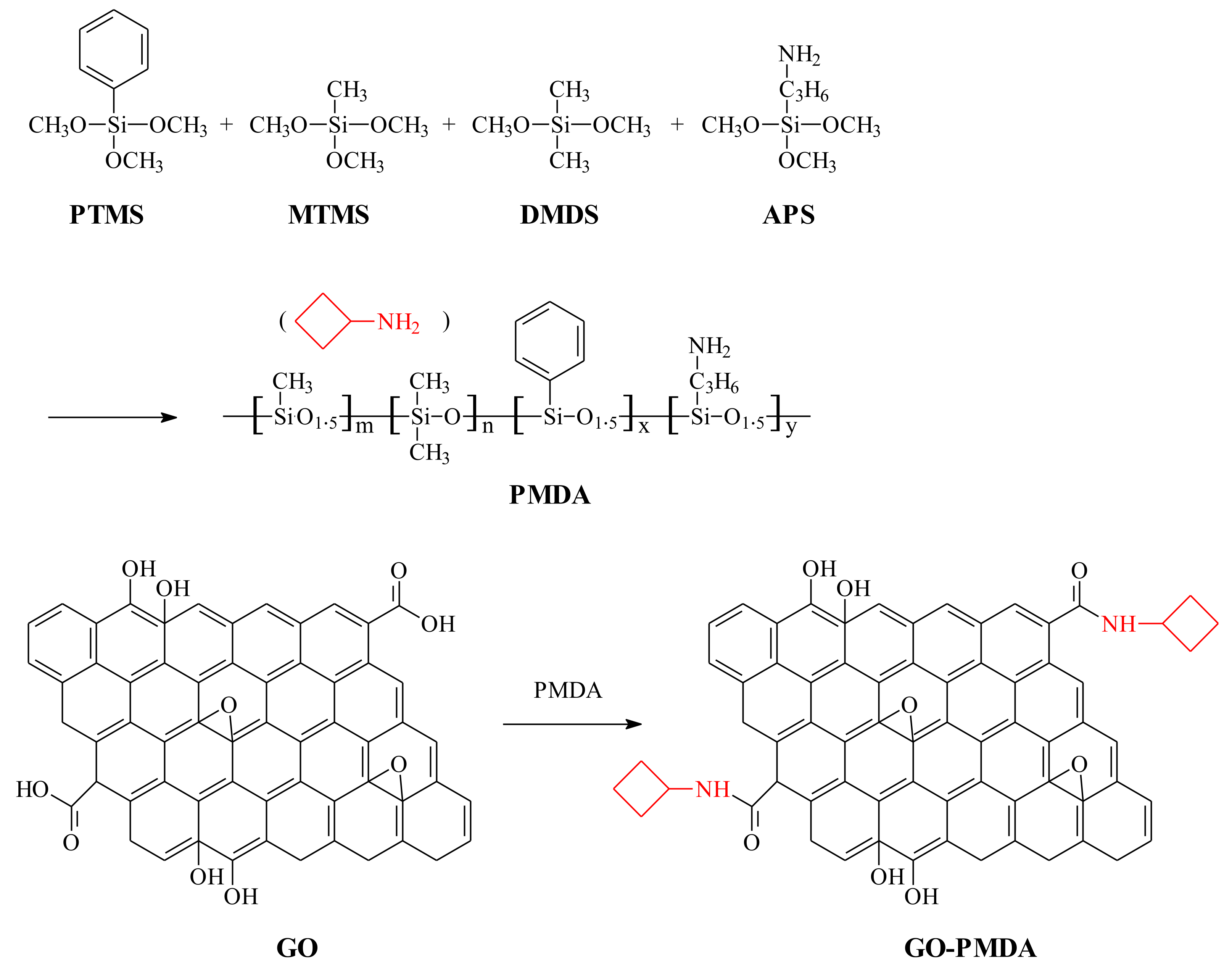
2.2. Synthesis of Polysilicone (PMDA)
2.3. Functionalization of Graphene Oxide (GO)
2.4. Preparation of Epoxy Composite
2.5. Characterization and Measurement
3. Results and Discussion
3.1. Structural Characterization
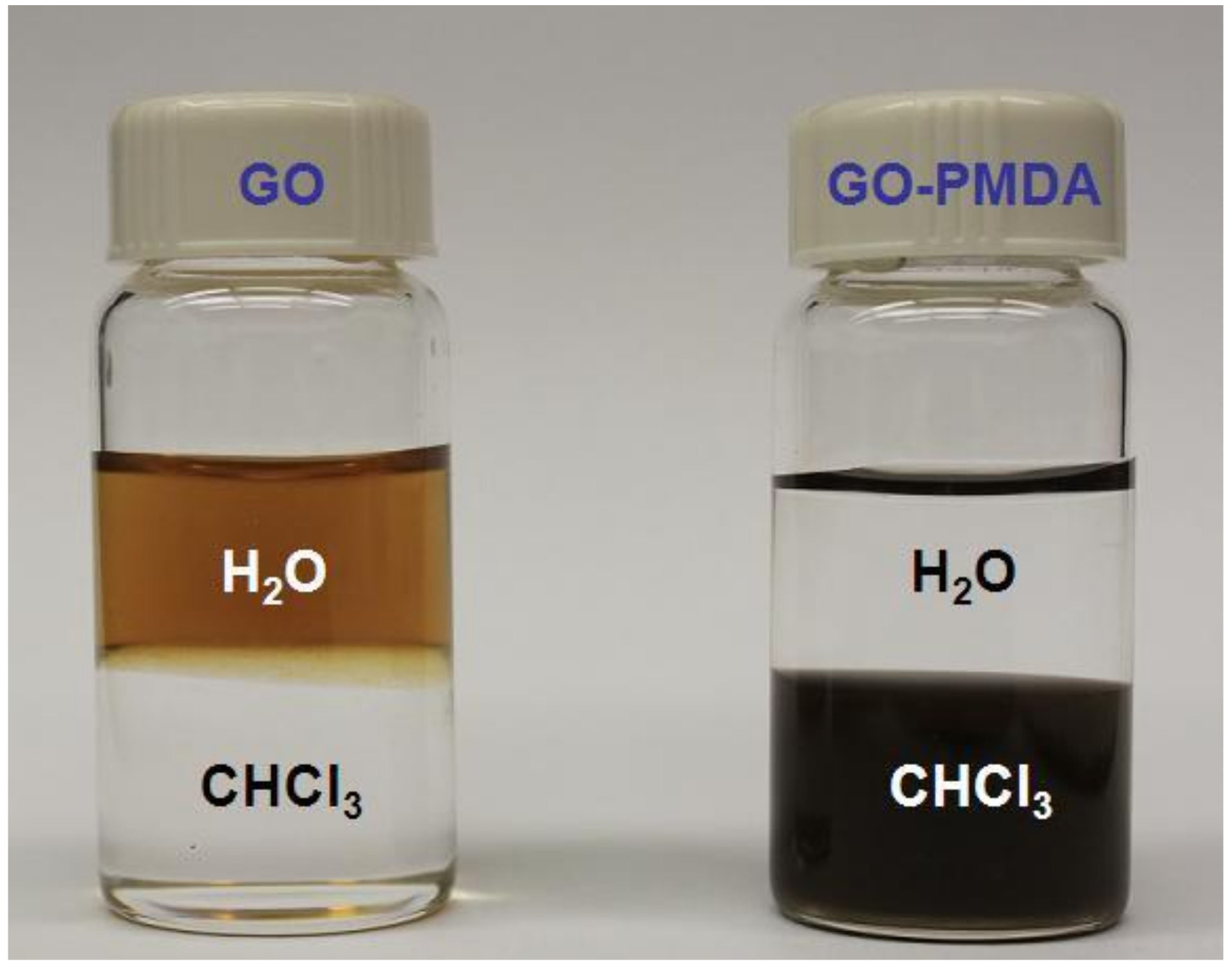
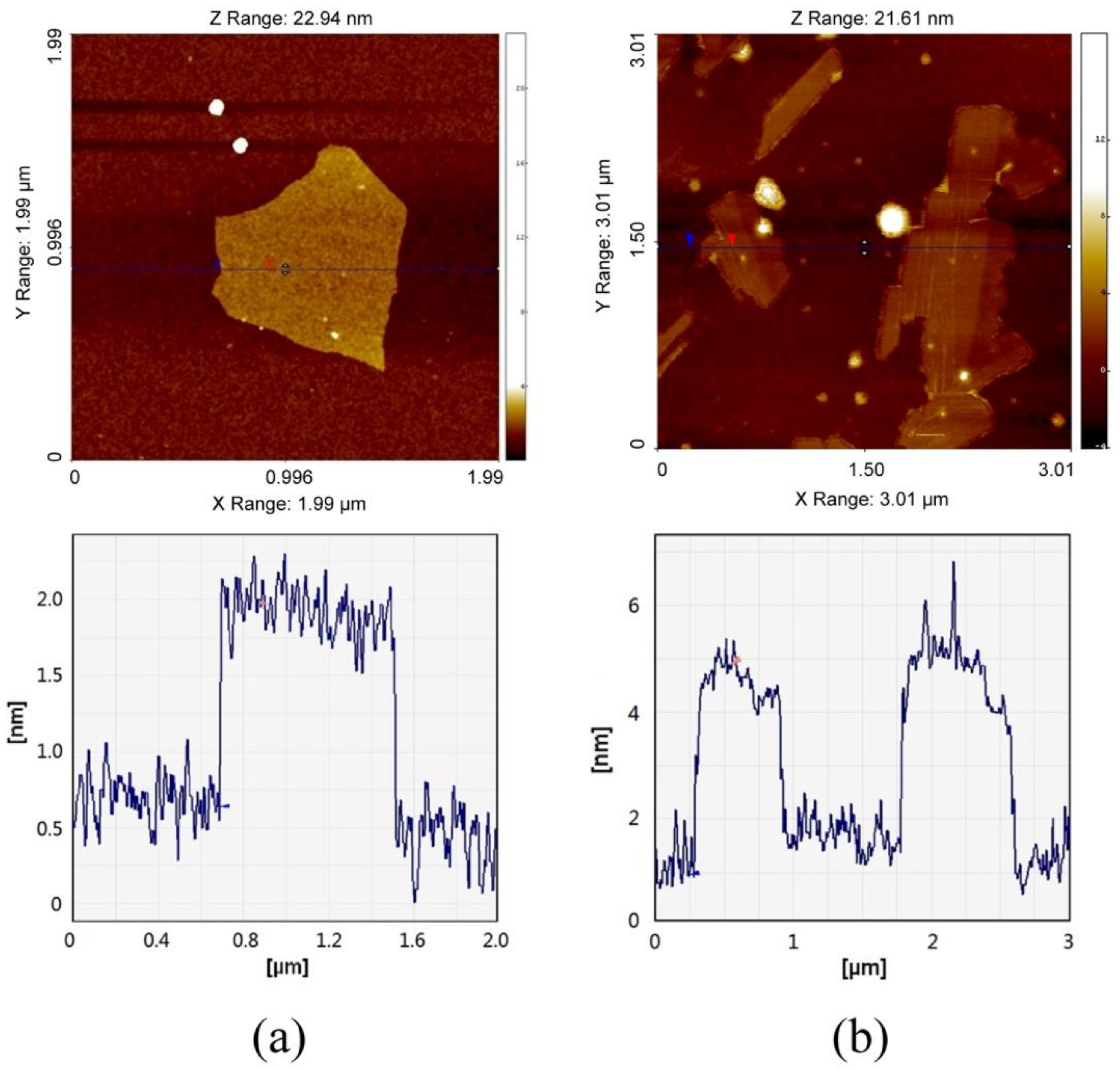
3.2. Dispersion
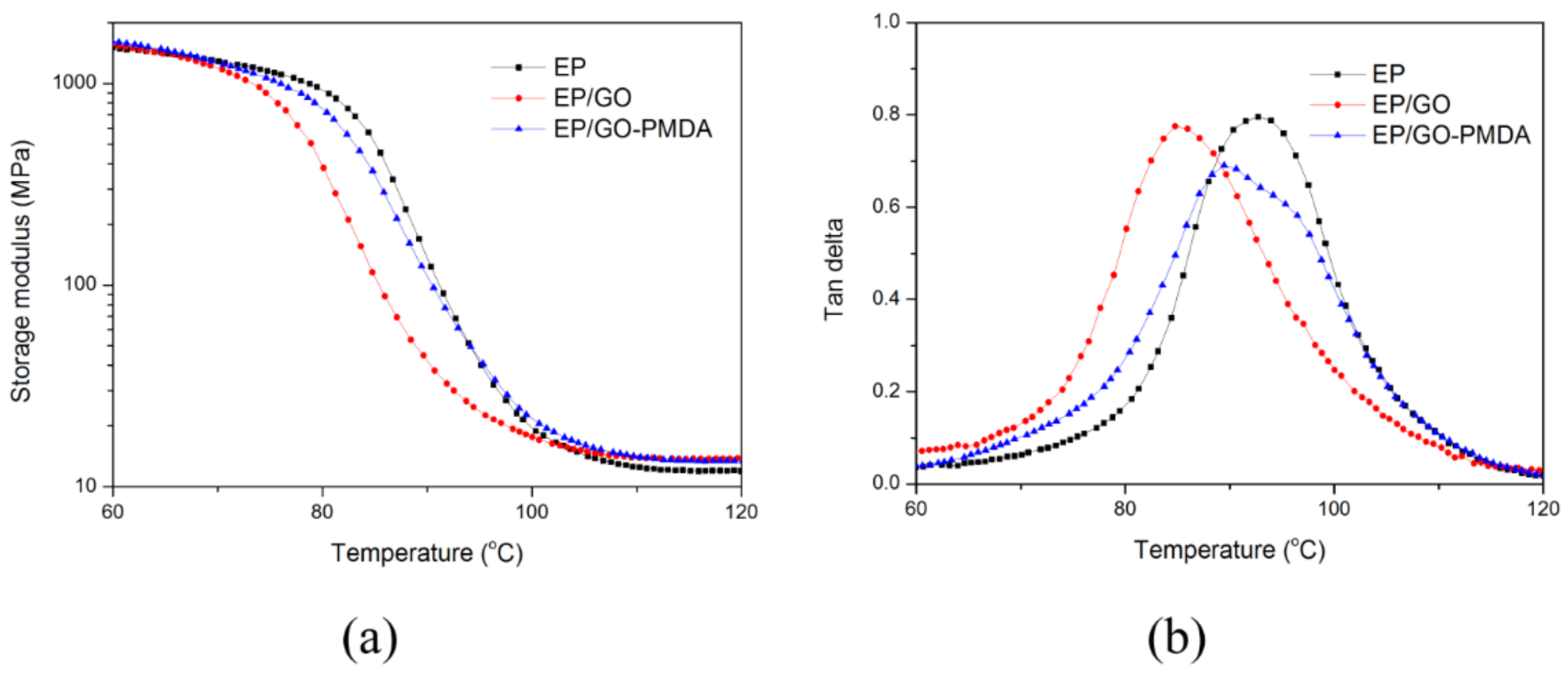
3.3. Thermal Stability
3.4. Flame Retardancy
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, H.; Ma, C.; Gu, J.; Guo, J.; Yan, X.; Huang, J.; Zhang, Q.; Guo, Z. An overview of multifunctional epoxy nanocomposites. J. Mater. Chem. C 2016, 4, 5890–5906. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, K.; Jiang, J.; Li, J.; Liu, Y. Highly dispersed melamine cyanurate flame-retardant epoxy resin composites. Polym. Int. 2017, 66, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Babu, H.; Llorca, J.; Wang, D. Impact of halogen-free flame retardant with varied phosphorus chemical surrounding on the properties of diglycidyl ether of bisphenol-A type epoxy resin: Synthesis, fire behaviour, flame-retardant mechanism and mechanical properties. RSC Adv. 2016, 6, 59226–59236. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Chen, W.; Duan, W.; Liu, Y.; Wang, Q. In situ synthesized and dispersed melamine polyphosphate flame retardant epoxy resin composites. J. Appl. Polym. Sci. 2019, 136, 47194. [Google Scholar] [CrossRef]
- Yu, P.; Manalo, A.; Ferdous, W.; Abousnina, R.; Schubel, P. Investigation on the physical, mechanical and microstructural properties of epoxy polymer matrix with crumb rubber and short fibres for composite railway sleepers. Constr. Build. Mater. 2021, 295, 123700. [Google Scholar] [CrossRef]
- Yan, W.; Yu, J.; Zhang, M.; Qin, S.; Wang, T.; Huang, W.; Long, L. Flame-retardant effect of a phenethyl-bridged DOPO derivative and layered double hydroxides for epoxy resin. RSC Adv. 2017, 7, 46236–46245. [Google Scholar] [CrossRef] [Green Version]
- Jian, R.; Wang, P.; Duan, W.; Wang, J.; Zheng, X.; Weng, J. Synthesis of a novel P/N/S-containing flame retardant and its application in epoxy resin: Thermal property, flame retardance, and pyrolysis behavior. Ind. Eng. Chem. Res. 2016, 55, 11520–11527. [Google Scholar] [CrossRef]
- Khotbehsara, M.; Manalo, A.; Aravinthan, T.; Turner, J.; Ferdous, W.; Hota, G. Effects of ultraviolet solar radiation on the properties of particulate-filled epoxy based polymer coating. Polym. Degrad. Stab. 2020, 181, 109352. [Google Scholar] [CrossRef]
- Hu, K.; Kulkarni, D.; Choi, I.; Tsukruk, V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Hersam, M. The reemergence of chemistry for post-graphene two-dimensional nanomaterials. ACS Nano 2015, 9, 4661–4663. [Google Scholar] [CrossRef]
- Guo, Y.; Bao, C.; Song, L.; Yuan, B.; Hu, Y. In situ polymerization of graphene, graphite oxide, and functionalized graphite oxide into epoxy resin and comparison study of on-the-flame behavior. Ind. Eng. Chem. Res. 2011, 50, 7772–7783. [Google Scholar] [CrossRef]
- Potts, J.; Dreyer, D.; Bielawski, C.; Ruoff, R. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar]
- Kim, H.; Abdala, A.; Macosko, C. Graphene/polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Sun, Y.; Li, C.; Xu, Y.; Bai, H.; Yao, Z.; Shi, G. Chemically converted graphene as substrate for immobilizing and enhancing the activity of a polymeric catalyst. Chem. Commun. 2010, 46, 4740–4742. [Google Scholar] [CrossRef] [PubMed]
- Gui, H.; Xu, P.; Hu, Y.; Wang, J.; Yang, X.; Bahader, A.; Ding, Y. Synergistic effect of graphene and an ionic liquid containing phosphonium on the thermal stability and flame retardancy of polylactide. RSC Adv. 2015, 5, 27814–27822. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.; Stankovich, S.; Dikin, D.; Herrera-Alonso, M.; Piner, R.; Adamson, D.; Schniepp, H.; Chen, X.; Ruoff, R. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. Graphene production: New solutions to a new problem. Nat. Nanotechnol. 2008, 3, 528–529. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kuan, C.; Chen, C.; Kuan, H.; Yip, M.; Chiu, S.; Chiang, C. Preparation, thermal stability and electrical properties of PMMA functionalized graphene oxide nanosheets composites. Mater. Chem. Phys. 2012, 134, 677–695. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Zhang, P.; Song, L.; Yang, H.; Hu, Y. Covalent functionalization of graphene with organosilane and its use as a reinforcement in epoxy composites. Compos. Sci. Technol. 2012, 72, 737–743. [Google Scholar] [CrossRef]
- Qian, X.; Yu, B.; Bao, C.; Song, L.; Wang, B.; Xing, W.; Hu, Y.; Yuen, R. Silicon nanoparticle decorated graphene composites: Preparation and their reinforcement on the fire safety and mechanical properties of polyurea. J. Mater. Chem. A 2013, 1, 9827–9836. [Google Scholar] [CrossRef]
- Wang, X.; Song, L.; Yang, H.; Xing, W.; Kandola, B.; Hu, Y. Simultaneous reduction and surface functionalization of graphene oxide with POSS for reducing fire hazards in epoxy composites. J. Mater. Chem. 2012, 22, 22037–22043. [Google Scholar] [CrossRef]
- Hu, W.; Zhan, J.; Wang, X. Effect of Functionalized graphene oxide with hyper-branched flame retardant on flammability and thermal stability of cross-linked polyethylene. Ind. Eng. Chem. Res. 2014, 53, 3073–3083. [Google Scholar] [CrossRef]
- Attia, N.; Abd El-Aal, N.; Hassan, M. Facile synthesis of graphene sheets decorated nanoparticles and flammability of their polymer nanocomposites. Polym. Degrad. Stab. 2016, 126, 65–74. [Google Scholar] [CrossRef]
- Liao, S.; Liu, P.; Hsiao, M.; Teng, C.; Wang, C.; Ger, M.; Chiang, C. One-step reduction and functionalization of graphene oxide with phosphorus-based compound to produce flame-retardant epoxy nanocomposite. Ind. Eng. Chem. Res. 2012, 51, 4573–4581. [Google Scholar] [CrossRef]
- Yu, B.; Shi, Y.; Yuan, B.; Qiu, S.; Xing, W.; Hu, W.; Song, L.; Lo, S.; Hu, Y. Enhanced thermal and flame retardant properties of flame-retardant-wrapped graphene/epoxy resin nanocomposites. J. Mater. Chem. A 2015, 3, 8034–8044. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.; Chandra, V.; Kim, N.; Kemp, K.; Hobza, P.; Zboril, R.; Kim, K. Functionalization of graphene: Covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [PubMed]
- Xue, Y.; Liu, Y.; Lu, F.; Qu, J.; Chen, H.; Dai, L. Functionalization of graphene oxide with polyhedral oligomeric silsesquioxane (POSS) for multifunctional applications. J. Phys. Chem. Lett. 2012, 3, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Flame retardancy and dispersion of functionalized carbon nanotubes in thiol-ene nanocomposites. Polymers 2021, 13, 3308. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Wu, F.; Wang, J. Thermal degradation behavior of epoxy resin containing modified carbon nanotubes. Polymers 2021, 13, 3332. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.; Kosynkin, D.; Berlin, J.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.; Lu, W.; Tour, J. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wei, P.; Qian, Y.; Liu, J. The synthesis of a novel graphene-based inorganic–organic hybrid flame retardant and its application in epoxy resin. Compos. Part B Eng. 2014, 60, 341–349. [Google Scholar] [CrossRef]



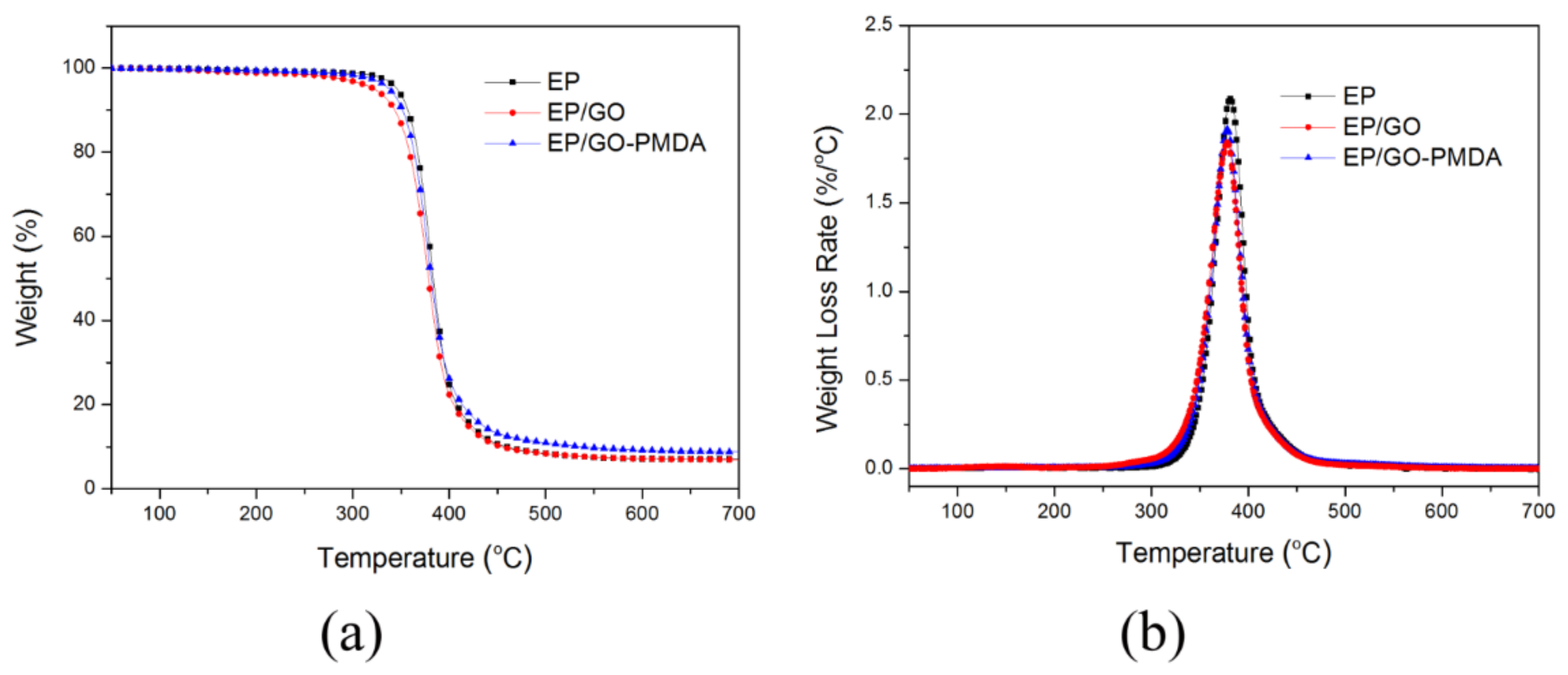

| Sample | Temperature (°C) | Peak Rate (wt%/°C) | Residues (wt%) | ||
|---|---|---|---|---|---|
| T5wt% | T50wt% | Tmax | |||
| EP | 346.0 | 383.5 | 381.4 | 2.09 | 7.02 |
| EP/GO | 322.5 | 378.6 | 378.2 | 1.84 | 6.95 |
| EP/GO-PMDA | 337.9 | 381.3 | 378.2 | 1.92 | 8.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J. Functionalization of Graphene Oxide with Polysilicone: Synthesis, Characterization, and Its Flame Retardancy in Epoxy Resin. Polymers 2021, 13, 3857. https://doi.org/10.3390/polym13213857
Wang J. Functionalization of Graphene Oxide with Polysilicone: Synthesis, Characterization, and Its Flame Retardancy in Epoxy Resin. Polymers. 2021; 13(21):3857. https://doi.org/10.3390/polym13213857
Chicago/Turabian StyleWang, Jiangbo. 2021. "Functionalization of Graphene Oxide with Polysilicone: Synthesis, Characterization, and Its Flame Retardancy in Epoxy Resin" Polymers 13, no. 21: 3857. https://doi.org/10.3390/polym13213857
APA StyleWang, J. (2021). Functionalization of Graphene Oxide with Polysilicone: Synthesis, Characterization, and Its Flame Retardancy in Epoxy Resin. Polymers, 13(21), 3857. https://doi.org/10.3390/polym13213857





