Combustion of a Solid Recovered Fuel (SRF) Produced from the Polymeric Fraction of Automotive Shredder Residue (ASR)
Abstract
:1. Introduction
2. Materials and Methods
2.1. ASR-Derived SRF Preparation and Characterization
2.2. Combustion Tests
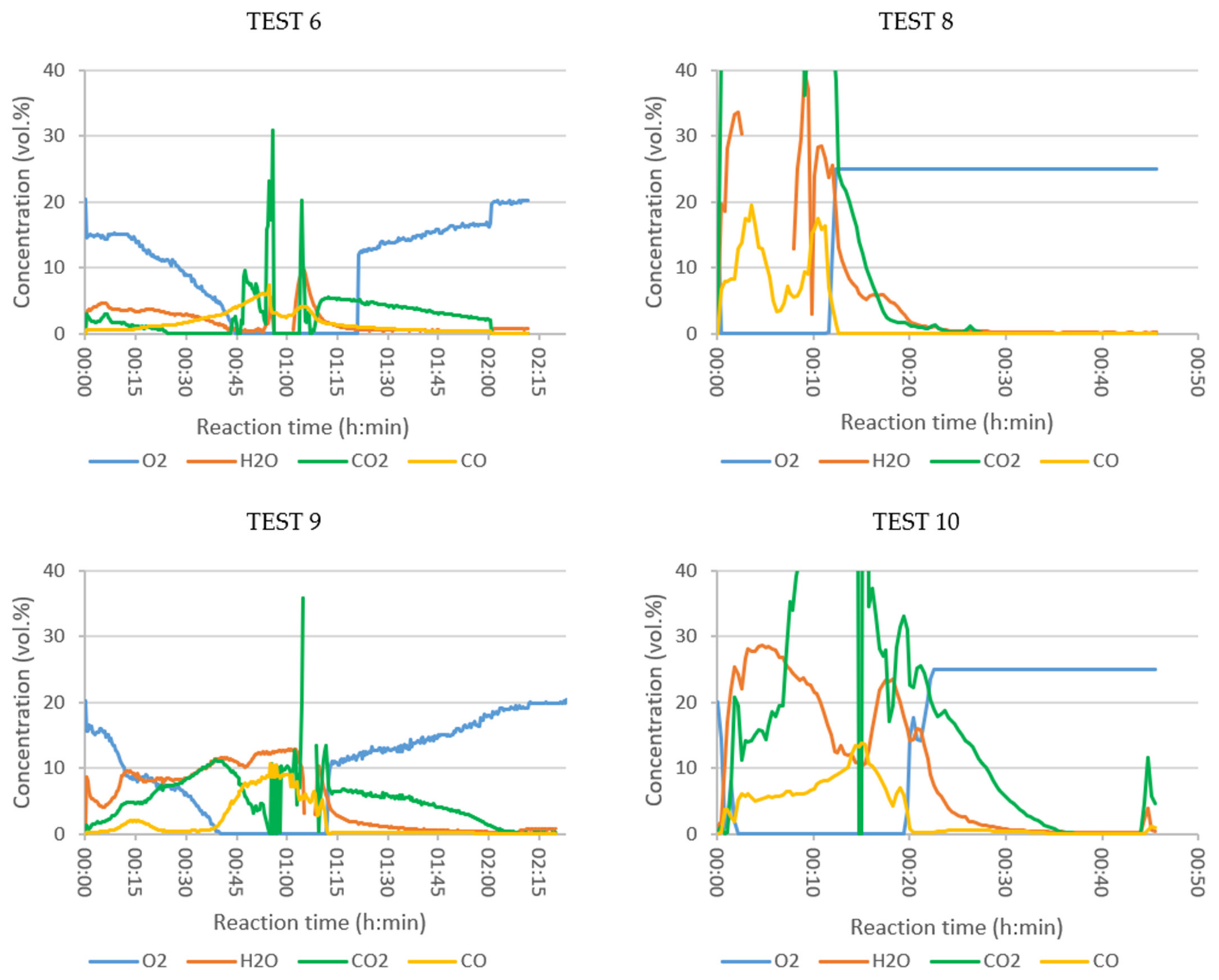
- The results are shown in concentration units and the combustion took place in a non-stationary state: the total gas volume varied during each test, as oxygen was consumed and combustion products were generated.
- The product concentration analysed at the process outlet at a given time resulted from a process in which the substances previously released from SRF (by thermal decomposition, partial or complete oxidation) were initially in contact with the oxidizer in the tank at the temperature reached at that time in the reactor and during the corresponding residence time, and, subsequently, in the tubular reactor at 550 °C or 900 °C during the residence time in this reactor.
- The gas analyser used was a standard stack emissions test equipment, calibrated to measure concentration of pollutants within typical emission ranges in the flue gases of industrial installations, after all the gas cleaning units. In the combustion tests presented in this work, the analyser measured the “raw combustion gases”. For some compounds, the concentration values were almost permanently above the calibration limits, but still within the measuring range of the analyser. Although exact concentration values could not be determined, important qualitative trend information could be obtained. The upper concentration limits of the analyser for the compounds, or groups of compounds, are given in the Supplementary Material (Table S2).
- When the concentration values were out of range for the instrument, no results were shown in the concentration vs. time plots.
3. Results and Discussion
3.1. Characterization of the Prepared SRF
- Ash (550 °C) = 21.0 wt.% (LoQ = 0.001%);
- Dry matter (ar) = 97.9 wt.% (LoQ = 0.1%);
- GCV (d) = 27.9 MJ/kg (LoQ = 0.500 MJ/kg);
- NCV (d) = 26.3 MJ/kg (LoQ = 0.500 MJ/kg);
- NCV (ar) = 25.7 MJ/kg.
3.2. Thermal Analysis of SRF
3.3. Optimization of the Flow and Residence Time in Combustion Tests
3.4. Combustion Tests with Online Analysis of Vapors
3.4.1. Influence of the Tubular Reactor Temperature
3.4.2. Influence of Oxidizer
3.5. Liquid Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bratu, V.; Gaba, A.; Elena Valentina, S.; Anghelina, F. Natural Gas Consumption Reducing in Aluminum Melting Furnaces by Heat Recovery of Flue. Sci. Bull. VALAHIA Univ.–Mater. Mech. 2016, 14, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Capuzzi, S.; Timelli, G. Preparation and Melting of Scrap in Aluminum Recycling: A Review. Metals 2018, 8, 249. [Google Scholar] [CrossRef] [Green Version]
- Brough, D.; Jouhara, H. The Aluminium Industry: A Review on State-of-the-Art Technologies, Environmental Impacts and Possibilities for Waste Heat Recovery. Int. J. Thermofluids 2020, 1, 100007. [Google Scholar] [CrossRef]
- Arink, T.; Hassan, M.I. Metal Scrap Preheating using Flue Gas Waste Heat. Energy Procedia 2017, 105, 4788–4795. [Google Scholar] [CrossRef]
- Diop, M.A.; Shi, Z.; Fafard, M.; Bousso, S.A.; Wenju, T.; Wang, Z. Green Power Furnaces in Aluminum Cast House for Scrap Preheating Using CO2-Flue Gas. J. Sustain. Metall. 2021, 7, 46–59. [Google Scholar] [CrossRef]
- Chatziaras, N.; Psomopoulos, C.; Themelis, N. Use of waste derived fuels in cement industry: A review. Manag. Environ. Qual. Int. J. 2016, 27, 178–193. [Google Scholar] [CrossRef]
- Scherz, P. Combustion of Waste-Derived Fuels—An Investigation for STENA Metall’s Aluminum Recycling Plant. Master’s Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2016. [Google Scholar]
- Roh, S.A.; Kim, W.H.; Yun, J.H.; Min, T.J.; Kwak, Y.H.; Seo, Y.C. Pyrolysis and gasification-melting of automobile shredder residue. J. Air Waste Manag. Assoc. 2013, 63, 1137–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilber, T.; Maier, J.; Scheffknecht, G.; Agraniotis, M.; Grammelis, P.; Kakaras, E.; Glorius, T.; Becker, U.; Derichs, W.; Schiffer, H.-P.; et al. Advantages and Possibilities of Solid Recovered Fuel Cocombustion in the European Energy Sector. J. Air Waste Manag. Assoc. 2007, 57, 1178–1189. [Google Scholar] [CrossRef] [Green Version]
- Stenseng, M.; Zolin, A.; Cenni, R.; Frandsen, F.; Jensen, A.; Dam-Johansen, K. Thermal Analysis in Combustion Research. J. Therm. Anal. Calorim. 2001, 64, 1325–1334. [Google Scholar] [CrossRef]
- Szűcs, T.; Szentannai, P.; Szilágyi, I.M.; Bakos, L.P. Comparing different reaction models for combustion kinetics of solid recovered fuel. J. Therm. Anal. Calorim. 2020, 139, 555–565. [Google Scholar] [CrossRef] [Green Version]
- Gunaratne, T.; Krook, J.; Andersson, H.; Eklund, M. Potential valorisation of shredder fines: Towards integrated processes for material upgrading and resource recovery. Resour. Conserv. Recycl. 2020, 154, 104590. [Google Scholar] [CrossRef]
- Hyks, J.; Oberender, A.; Hjelmar, O.; Cimpan, C.; Wenzel, H.; Hu, G.; Cramer, J. Shredder Residues: Problematic Substances in Relation to Resource Recovery; Miljøstyrelsen: København, Denmark, 2014. [Google Scholar]
- Beckmann, M.; Pohl, M.; Bernhardt, D.; Gebauer, K. Criteria for solid recovered fuels as a substitute for fossil fuels—A review. Waste Manag. Res. 2012, 30, 354–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conesa, J.A.; Rey, L.; Aracil, I. Modeling the thermal decomposition of automotive shredder residue. J. Therm. Anal. Calorim. 2016, 124, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Quan, C.; Li, A.; Gao, N. Combustion and Pyrolysis of Electronic Waste: Thermogravimetric Analysis and Kinetic Model. Procedia Environ. Sci. 2013, 18, 776–782. [Google Scholar] [CrossRef] [Green Version]
- Williams, K.S.; Khodier, A. Meeting EU ELV targets: Pilot-scale pyrolysis automotive shredder residue investigation of PAHs, PCBs and environmental contaminants in the solid residue products. Waste Manag. 2020, 105, 233–239. [Google Scholar] [CrossRef]
- Mancini, G.; Viotti, P.; Luciano, A.; Raboni, M.; Fino, D. Full scale treatment of ASR wastes in a modified rotary kiln. Waste Manag. 2014, 34, 2347–2354. [Google Scholar] [CrossRef] [PubMed]
- De Marco, I.; Caballero, B.M.; Cabrero, M.A.; Laresgoiti, M.F.; Torres, A.; Chomon, M.J. Recycling of automobile shredder residues by means of pyrolysis. J. Anal. Appl. Pyrolysis 2007, 79, 403–408. [Google Scholar] [CrossRef]
- Chae, J.S.; Kim, S.W.; Ohm, T.I. Combustion characteristics of solid refuse fuels from different waste sources. J. Renew. Mater. 2020, 8, 789–799. [Google Scholar] [CrossRef]
- Edo-Alcón, N.; Gallardo, A.; Colomer-Mendoza, F.J. Characterization of SRF from MBT plants: Influence of the input waste and of the processing technologies. Fuel Process. Technol. 2016, 153, 19–27. [Google Scholar] [CrossRef]
- Strobel, R.; Waldner, M.H.; Gablinger, H. Highly efficient combustion with low excess air in a modern energy-from-waste (EfW) plant. Waste Manag. 2018, 73, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Mancini, G.; Viotti, P.; Luciano, A.; Fino, D. On the ASR and ASR thermal residues characterization of full scale treatment plant. Waste Manag. 2014, 34, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Rey, L.; Conesa, J.A.; Aracil, I.; Garrido, M.A.; Ortuño, N. Pollutant formation in the pyrolysis and combustion of Automotive Shredder Residue. Waste Manag. 2016, 56, 376–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Environment Agency. Municipal Waste Incineration; Guidebook 2019; European Environment Agency: København, Denmark, 2019. [Google Scholar]
- Ma, W.; Wenga, T.; Frandsen, F.J.; Yan, B.; Chen, G. The fate of chlorine during MSW incineration: Vaporization, transformation, deposition, corrosion and remedies. Prog. Energy Combust. Sci. 2020, 76, 100789. [Google Scholar] [CrossRef]
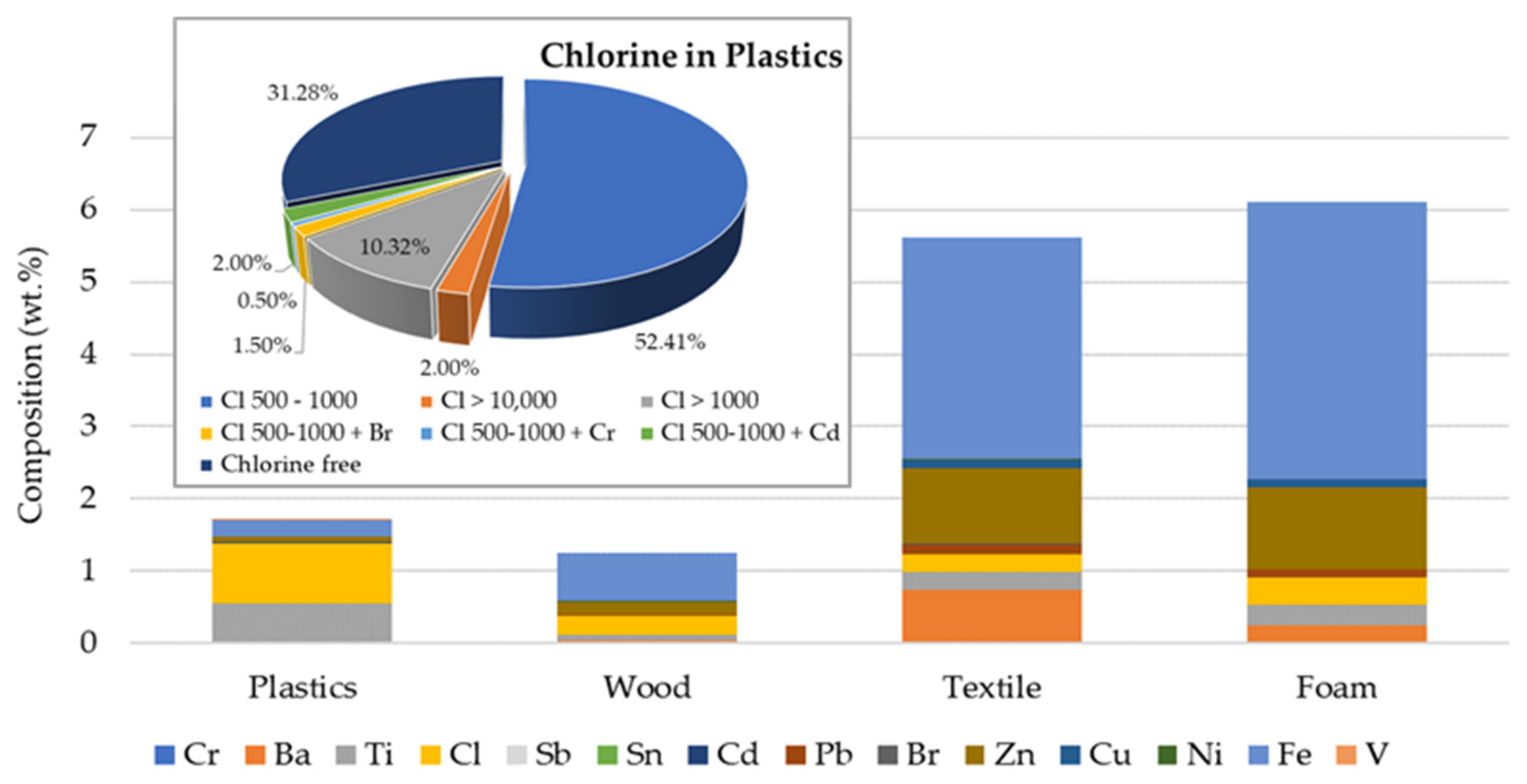
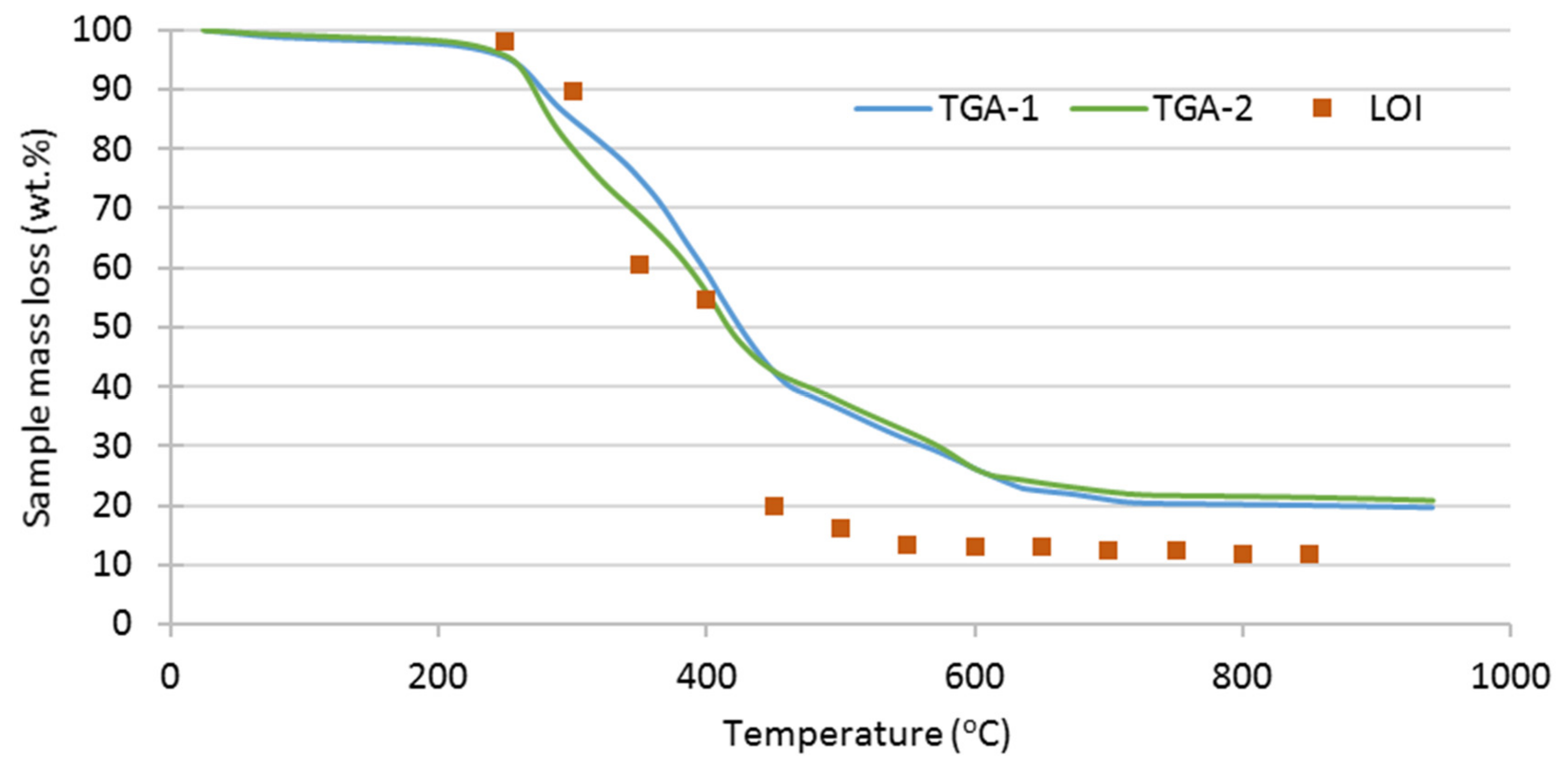


| Test | Oxidizer | Oxidizer Flow (NL/min) | Tubular Reactor T (°C) | Gases Analysis |
|---|---|---|---|---|
| 1 | Air | 4.7 | - 2 | No |
| 2 | Air | 4.7 | 550 | No |
| 3 | Air | 3.3 | 550 | No |
| 4 | Air | 1.3 | 550 | No |
| 5 | Air | 2.2 | 550 | No |
| 6 | Air | 2.2 | 550 | Yes |
| 7 | Oxygen | 2.2 | 900 | No |
| 8 | Oxygen | 2.2 | 900 | Yes |
| 9 | Air | 2.2 | 900 | Yes |
| 10 | Enriched air | 3.5 1 | 900 | Yes |
| Parameter | Value | Unit | Uncertainty (%) |
|---|---|---|---|
| Flash point | >150 | °C | 30 |
| Sb | 0.44·10−2 | wt.% | 32 |
| As | 0.06·10−2 | wt.% | 24 |
| Pb | 5.16·10−2 | wt.% | 32 |
| Cr | 0.85·10−2 | wt.% | 39 |
| Co | 0.15·10−2 | wt.% | 25 |
| Cu | 3.39·10−2 | wt.% | 28 |
| Mn | 2.72·10−2 | wt.% | 39 |
| Ni | 0.60·10−2 | wt.% | 30 |
| V | 0.10·10−2 | wt.% | 31 |
| Hg | 0.01·10−2 | wt.% | 28 |
| Cd | 0.07·10−2 | wt.% | 27 |
| Tl | <0.5 10−4 | wt.% | 31 |
| Hg + Cd + Tl | 0.08·10−2 | wt.% | 31 |
| Sb + As + Pb + Cr + Co + Cu + Mn + Ni + V | 13.47·10−2 | wt.% | 39 |
| total S | 0.71 | wt.% | 20 |
| total Br | 0.06 | wt.% | 33 |
| total Cl * | 0.77 | wt.% | 23 |
| total F | 0.07 | wt.% | 29 |
| total I | 0.02 | wt.% | 50 |
| total halogen | 0.92 | wt.% | 50 |
| PCBs (sum max. 7 compounds) | 0.565 | mg/kg | 26 |
| Proximate and Elemental Analysis (As Received, wt.%) | |||
|---|---|---|---|
| Moisture | 1.5 ± 0.1 | ||
| Organic content | 85.2 ± 0.8 79.8 volatile material 5.4 fixed carbon 2 | C | 65.2 ± 1.0 |
| H | 8.1 ± 0.2 | ||
| N | 1.5 ± 0.3 | ||
| O | 4.8 ± 1.0 | ||
| S | 0.5 ± 0.1 | ||
| Cl | 1.0 ± 0.2 | ||
| Br 1 | 2.44·10−2 ± 1.34·10−2 | ||
| Others 2 | ≈4.1 | ||
| Ashes | 13.3 ± 0.9 | ||
| Calorific Value (MJ/kg) | |||
| GCV (dry) | 30.8 ± 0.1 | ||
| NCV (dry) | 28.9 ± 0.1 | ||
| NCV (as received) | 28.4 ± 0.1 | ||
| Test | Ash (wt.% SRF) | C Content (wt.% ash) |
|---|---|---|
| 1 | 18.6 | 2.9 ± 0.3 |
| 2 | 26.0 | 2.8 ± 0.1 |
| 3 | 15.0 | 2.8 ± 0.4 |
| 4 | 14.0 | 14.2 ± 2.1 |
| 5 | 19.0 | 2.5 ± 0.2 |
| 6 | 34.0 | 7.6 ± 1.3 |
| 7 | 30.0 | 2.1 ± 0.2 |
| 8 | 24.0 | 2.2 ± 0.4 |
| 9 | 14.0 | 3.0 ± 0.1 |
| 10 | 20.0 | 2.2 ± 0.3 |
| Test | Water (Area %) | Other Organic Compounds (Area %) |
|---|---|---|
| 1 | 48.7 | 43.2 |
| 2 | 50.4 | 45.3 |
| 3 | 81.5 | 12.9 |
| 4 | 77.9 | 19.5 |
| 5 | n.c. * | n.c. * |
| 6 | 36.1 | 58.4 |
| 7 | 69.1 | 11.5 |
| 8 | 92.2 | 7.8 |
| 9 | 68.8 | 7.4 |
| 10 | 99.2 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acha, E.; Lopez-Urionabarrenechea, A.; Delgado, C.; Martinez-Canibano, L.; Perez-Martinez, B.B.; Serras-Malillos, A.; Caballero, B.M.; Unamunzaga, L.; Dosal, E.; Montes, N.; et al. Combustion of a Solid Recovered Fuel (SRF) Produced from the Polymeric Fraction of Automotive Shredder Residue (ASR). Polymers 2021, 13, 3807. https://doi.org/10.3390/polym13213807
Acha E, Lopez-Urionabarrenechea A, Delgado C, Martinez-Canibano L, Perez-Martinez BB, Serras-Malillos A, Caballero BM, Unamunzaga L, Dosal E, Montes N, et al. Combustion of a Solid Recovered Fuel (SRF) Produced from the Polymeric Fraction of Automotive Shredder Residue (ASR). Polymers. 2021; 13(21):3807. https://doi.org/10.3390/polym13213807
Chicago/Turabian StyleAcha, Esther, Alexander Lopez-Urionabarrenechea, Clara Delgado, Lander Martinez-Canibano, Borja Baltasar Perez-Martinez, Adriana Serras-Malillos, Blanca María Caballero, Lucía Unamunzaga, Elena Dosal, Noelia Montes, and et al. 2021. "Combustion of a Solid Recovered Fuel (SRF) Produced from the Polymeric Fraction of Automotive Shredder Residue (ASR)" Polymers 13, no. 21: 3807. https://doi.org/10.3390/polym13213807
APA StyleAcha, E., Lopez-Urionabarrenechea, A., Delgado, C., Martinez-Canibano, L., Perez-Martinez, B. B., Serras-Malillos, A., Caballero, B. M., Unamunzaga, L., Dosal, E., Montes, N., & Barrenetxea-Arando, J. (2021). Combustion of a Solid Recovered Fuel (SRF) Produced from the Polymeric Fraction of Automotive Shredder Residue (ASR). Polymers, 13(21), 3807. https://doi.org/10.3390/polym13213807







