Influence of TiO2 and ZrO2 Nanoparticles on Adhesive Bond Strength and Viscosity of Dentin Polymer: A Physical and Chemical Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the EA and Its Reinforcement with Filler Nanoparticles
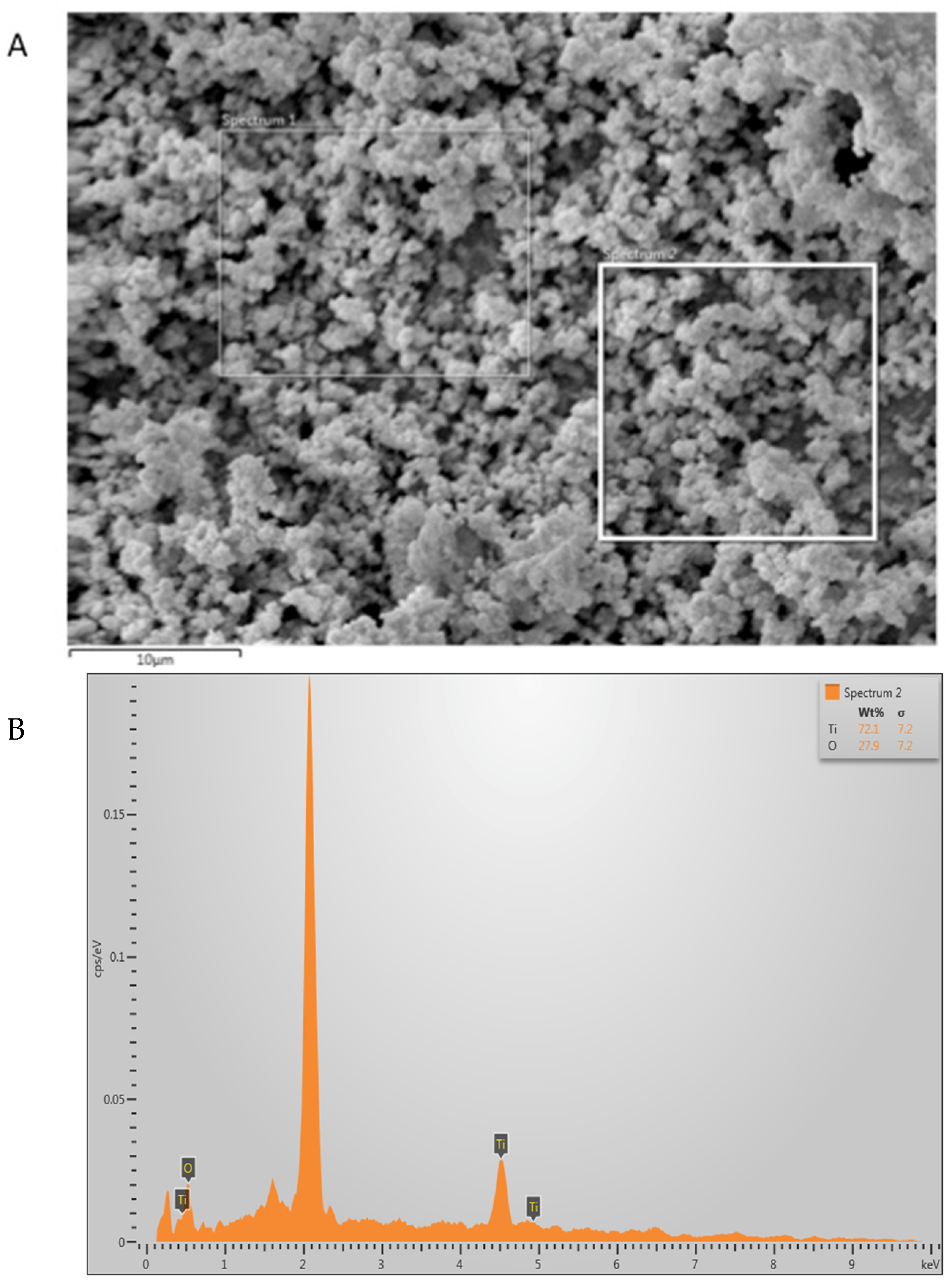
2.2. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray (EDX) Analysis
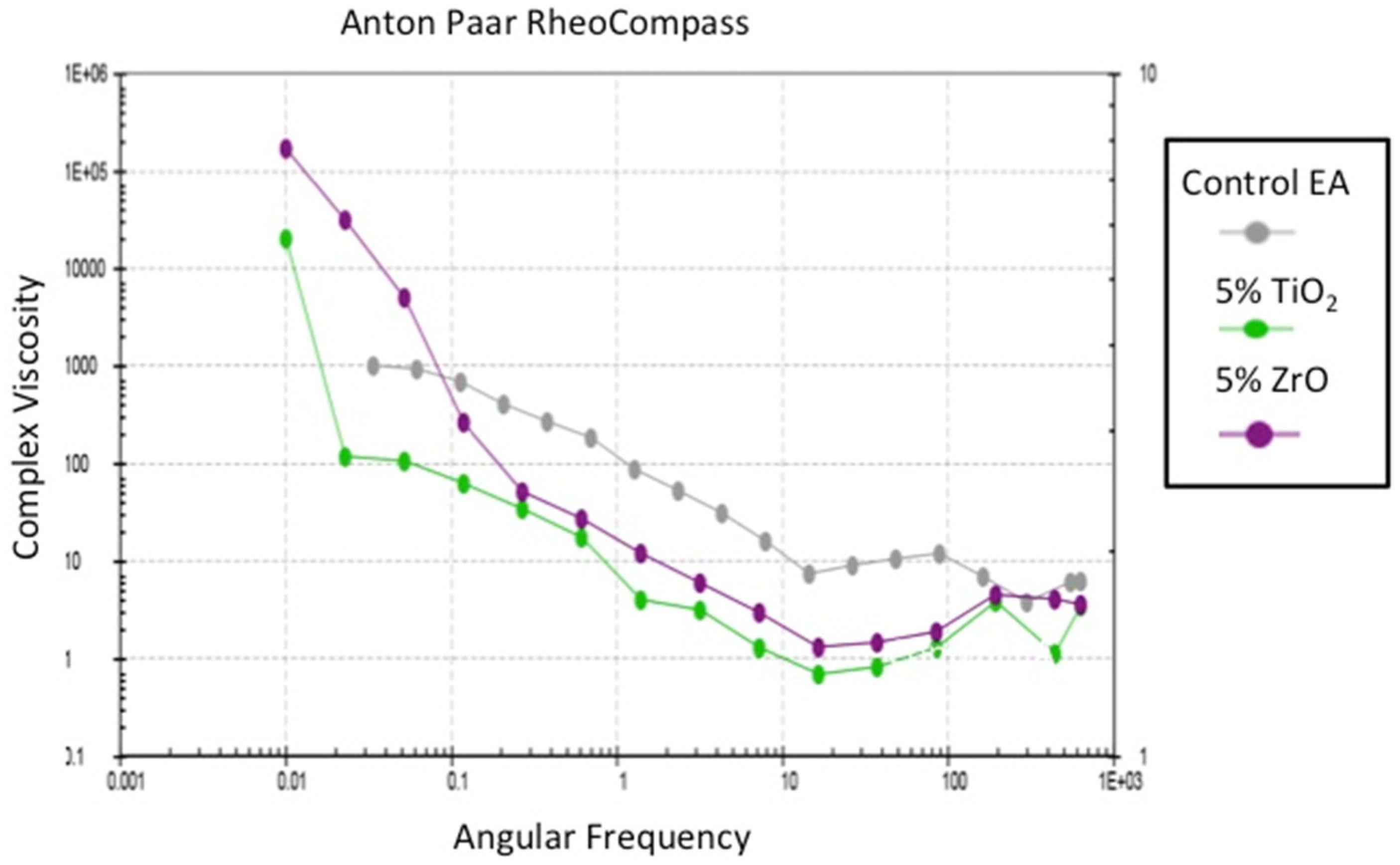
2.3. Assessment of the Rheological Properties of the Adhesives
2.4. SBS Testing and Interfacial Fracture Types of the Adhesives
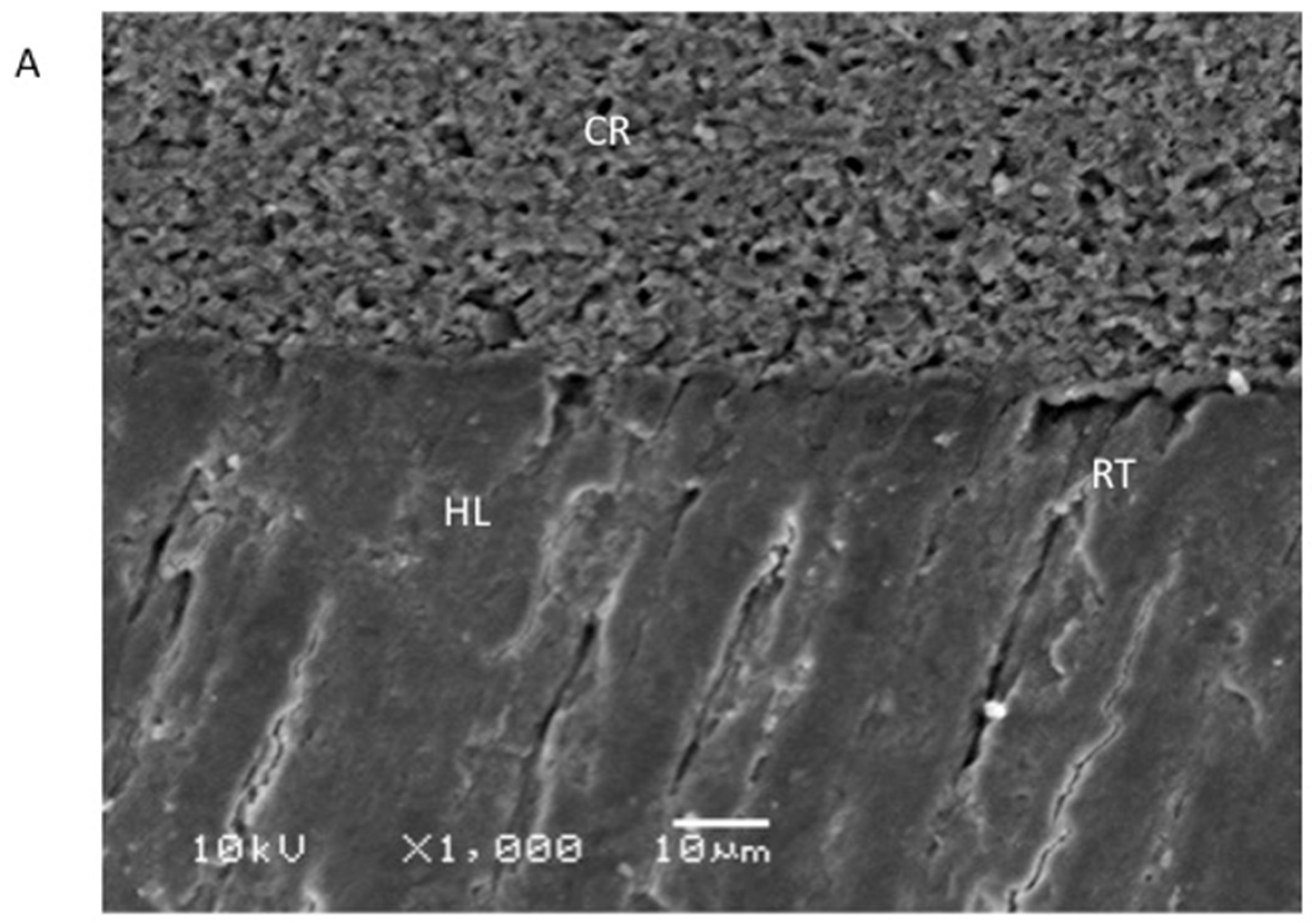
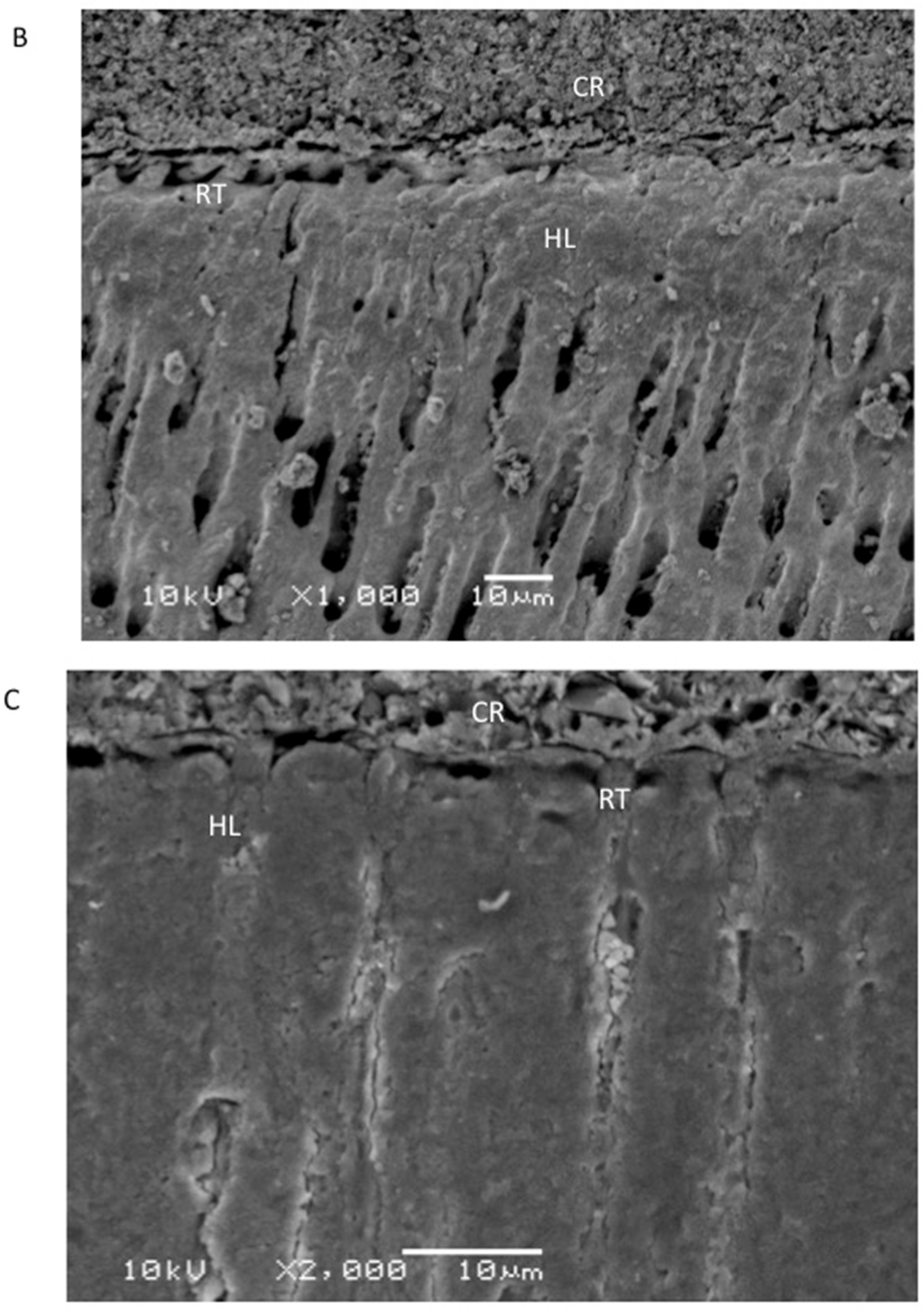
2.5. Evaluation of the Resin–Dentin Interface
2.6. Statistical Analysis
3. Results
3.1. Outcomes of the Characterization of the Filler Nanoparticles
3.2. Outcomes of the Assessment of the Rheological Properties
3.3. Outcomes of the SBS Testing and Failure Types Investigation
3.4. Outcomes of the Evaluation of the Resin–Dentin Interface
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.W.Q.; Zhao, J.; Zhao, B.; Ma, Z.; Zhang, C. Adhesion in teeth. Front. Mater. 2021, 7, 615225. [Google Scholar] [CrossRef]
- Perdigao, J. Current perspectives on dental adhesion: (1) Dentin adhesion—Not there yet. Jpn. Dent. Sci. Rev. 2020, 56, 190–207. [Google Scholar] [CrossRef] [PubMed]
- FDI, World Dental Federation. FDI policy statement on Minimal Intervention Dentistry (MID) for managing dental caries: Adopted by the General Assembly: September 2016, Poznan, Poland. Int. Dent. J. 2017, 67, 6–7. [Google Scholar] [CrossRef]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol. 2017, 8, 1–17. [Google Scholar]
- Ferracane, J.L. Models of Caries Formation around Dental Composite Restorations. J. Dent. Res. 2017, 96, 364–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iliev, G.; Hardan, L.; Kassis, C.; Bourgi, R.; Cuevas-Suarez, C.E.; Lukomska-Szymanska, M.; Mancino, D.; Haikel, Y.; Kharouf, N. Shelf Life and Storage Conditions of Universal Adhesives: A Literature Review. Polymers 2021, 13, 2708. [Google Scholar] [CrossRef] [PubMed]
- Farooq, I.; Ali, S.; Al-Saleh, S.; AlHamdan, E.M.; AlRefeai, M.H.; Abduljabbar, T.; Vohra, F. Synergistic Effect of Bioactive Inorganic Fillers in Enhancing Properties of Dentin Adhesives-A Review. Polymers 2021, 13, 2169. [Google Scholar] [CrossRef]
- AlFawaz, Y.F.; Almutairi, B.; Kattan, H.F.; Zafar, M.S.; Farooq, I.; Naseem, M.; Vohra, F.; Abduljabbar, T. Dentin Bond Integrity of Hydroxyapatite Containing Resin Adhesive Enhanced with Graphene Oxide Nano-Particles-An SEM, EDX, Micro-Raman, and Microtensile Bond Strength Study. Polymers 2020, 12, 2978. [Google Scholar] [CrossRef] [PubMed]
- Alhenaki, A.M.; Attar, E.A.; Alshahrani, A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Dentin Bond Integrity of Filled and Unfilled Resin Adhesive Enhanced with Silica Nanoparticles-An SEM, EDX, Micro-Raman, FTIR and Micro-Tensile Bond Strength Study. Polymers 2021, 13, 1093. [Google Scholar] [CrossRef]
- Almutairi, B.; Kattan, H.F.; BinMahfooz, A.M.; Qutub, O.A.; Basunbul, G.; ArRejaie, A.S.; Farooq, I.; Vohra, F.; Abduljabbar, T. Synergistic effect of graphene oxide/calcium phosphate nanofiller in a dentin adhesive on its dentin bond integrity and degree of conversion. A scanning electron microscopy, energy dispersive X-ray spectroscopy, Fourier transform infrared, micro-Raman, and bond strength study. Microsc. Res. Tech. 2021, 84, 2082–2094. [Google Scholar]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [Green Version]
- Musial, J.; Krakowiak, R.; Mlynarczyk, D.T.; Goslinski, T.; Stanisz, B.J. Titanium Dioxide Nanoparticles in Food and Personal Care Products-What Do We Know about Their Safety? Nanomaterials 2020, 10, 1110. [Google Scholar] [CrossRef]
- Besinis, A.; De Peralta, T.; Handy, R.D. The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology 2014, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Jowkar, Z.; Hamidi, S.A.; Shafiei, F.; Ghahramani, Y. The Effect of Silver, Zinc Oxide, and Titanium Dioxide Nanoparticles Used as Final Irrigation Solutions on the Fracture Resistance of Root-Filled Teeth. Clin. Cosmet. Investig. Dent. 2020, 12, 141–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef]
- Sun, J.; Petersen, E.J.; Watson, S.S.; Sims, C.M.; Kassman, A.; Frukhtbeyn, S.; Skrtic, D.; Ok, M.T.; Jacobs, D.S.; Reipa, V.; et al. Biophysical characterization of functionalized titania nanoparticles and their application in dental adhesives. Acta Biomater. 2017, 53, 585–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteban Florez, F.L.; Kraemer, H.; Hiers, R.D.; Sacramento, C.M.; Rondinone, A.J.; Silverio, K.G.; Khajotia, S.S. Sorption, solubility and cytotoxicity of novel antibacterial nanofilled dental adhesive resins. Sci. Rep. 2020, 10, 13503. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Tonello, C.M.; Lisboa-Filho, P.N.; Arruda, L.B.; Tokuhara, C.K.; Oliveira, R.C.; Furuse, A.Y.; Rubo, J.H.; Borges, A.F.S. Titanium dioxide nanotubes addition to self-adhesive resin cement: Effect on physical and biological properties. Dent. Mater. 2017, 33, 866–875. [Google Scholar] [CrossRef] [Green Version]
- Skocaj, M.; Filipic, M.; Petkovic, J.; Novak, S. Titanium dioxide in our everyday life; is it safe? Radiol. Oncol. 2011, 45, 227–247. [Google Scholar] [CrossRef] [Green Version]
- Madfa, A.A.A.-S.F.A.; Al-Quadami, N.H.; Al-Sanabani, J.S.; Amran, A.G. Use of Zirconia in Dentistry: An Overview. Open Biomat. J. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Grech, J.A.E. Zirconia in dental prosthetics: A literature review. J. Mater. Res. Technol. 2019, 8, 4956–4964. [Google Scholar] [CrossRef]
- Apratim, A.; Eachempati, P.; Krishnappa Salian, K.K.; Singh, V.; Chhabra, S.; Shah, S. Zirconia in dental implantology: A review. J. Int. Soc. Prev. Community Dent. 2015, 5, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Arena, A.; Prete, F.; Rambaldi, E.; Bignozzi, M.C.; Monaco, C.; Di Fiore, A.; Chevalier, J. Nanostructured Zirconia-Based Ceramics and Composites in Dentistry: A State-of-the-Art Review. Nanomaterials 2019, 9, 1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halder, S.; Ahemad, S.; Das, S.; Wang, J. Epoxy/Glass Fiber Laminated Composites Integrated with Amino Functionalized ZrO22 for Advanced Structural Applications. ACS Appl. Mater. Interfaces 2016, 8, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Lohbauer, U.; Wagner, A.; Belli, R.; Stoetzel, C.; Hilpert, A.; Kurland, H.D.; Grabow, J.; Muller, F.A. Zirconia nanoparticles prepared by laser vaporization as fillers for dental adhesives. Acta Biomater. 2010, 6, 4539–4546. [Google Scholar] [CrossRef]
- Cionca, N.; Hashim, D.; Mombelli, A. Zirconia dental implants: Where are we now, and where are we heading? Periodontol. 2000. 2017, 73, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Lughi, V.; Sergo, V. Low temperature degradation aging of zirconia: A critical review of the relevant aspects in dentistry. Dent. Mater. 2010, 26, 807–820. [Google Scholar] [CrossRef]
- Kensche, A.; Dahne, F.; Wagenschwanz, C.; Richter, G.; Viergutz, G.; Hannig, C. Shear bond strength of different types of adhesive systems to dentin and enamel of deciduous teeth in vitro. Clin. Oral. Investig. 2016, 20, 831–840. [Google Scholar] [CrossRef]
- Al-Hamdan, R.S.; Almutairi, B.; Kattan, H.F.; Alsuwailem, N.A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of Hydroxyapatite Nanospheres in Dentin Adhesive on the Dentin Bond Integrity and Degree of Conversion: A Scanning Electron Microscopy (SEM), Raman, Fourier Transform-Infrared (FTIR), and Microtensile Study. Polymers 2020, 12, 2948. [Google Scholar] [CrossRef]
- Lubojanski, A.; Dobrzynski, M.; Nowak, N.; Rewak-Soroczynska, J.; Sztyler, K.; Zakrzewski, W.; Dobrzynski, W.; Szymonowicz, M.; Rybak, Z.; Wiglusz, K.; et al. Application of Selected Nanomaterials and Ozone in Modern Clinical Dentistry. Nanomaterials 2021, 11, 259. [Google Scholar] [CrossRef]
- Sturmer, M.; Garcia, I.M.; Souza, V.S.; Visioli, F.; Scholten, J.D.; Samuel, S.M.W.; Leitune, V.C.B.; Collares, F.M. Titanium dioxide nanotubes with triazine-methacrylate monomer to improve physicochemical and biological properties of adhesives. Dent. Mater. 2021, 37, 223–235. [Google Scholar] [CrossRef]
- Yousefi, K.; Manesh, H.D.; Khalifeh, A.R.; Gholami, A. Fabrication and Characterization of a Nanofast Cement for Dental Restorations. Biomed. Res. Int. 2021, 2021, 7343147. [Google Scholar] [CrossRef]
- Kobayashi, E.; Matsumoto, S.; Doi, H.; Yoneyama, T.; Hamanaka, H. Mechanical properties of the binary titanium-zirconium alloys and their potential for biomedical materials. J. Biomed. Mater. Res. 1995, 29, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Bona, A.D.; Pecho, O.E.; Alessandretti, R. Zirconia as a Dental Biomaterial. Materials 2015, 8, 4978–4991. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, K.; Davoren, M.; Boertz, J.; Schins, R.P.; Hoffmann, E.; Dopp, E. Titanium dioxide nanoparticles induce oxidative stress and DNA-adduct formation but not DNA-breakage in human lung cells. Part. Fibre Toxicol. 2009, 6, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behbahani, A.R.S.; Esmaeilifar, A. Hydrothermal synthesis of zirconia nanoparticles from commercial zirconia. Proc. Eng. 2012, 42, 908–917. [Google Scholar] [CrossRef] [Green Version]
- Majedi, A.A.A.; Davar, F. Green synthesis of zirconia nanoparticles using the modified Pechini method and characterization of its optical and electrical properties. J. Sol.-Gel. Sci. Tech. 2016, 77, 542–552. [Google Scholar] [CrossRef]
- Zinatloo-Ajabshir, S.S.-N.M. Zirconia Nanostructures: Novel Facile Surfactant-Free Preparation and Characterization. App. Ceram. Technol. 2016, 13, 108–115. [Google Scholar] [CrossRef] [Green Version]
- AlHamdan, E.M.; Al-Saleh, S.; AlRefeai, M.H.; Farooq, I.; Abrar, E.; Vohra, F.; Abduljabbar, T. Adhesive bond integrity of Y-TZP post with calcium fluoride infiltrated resin dentin adhesive: An SEM, EDX, FTIR and micro-Raman study. Surf. Interface Anal. 2021, 53, 956–962. [Google Scholar] [CrossRef]
- Alqarawi, F.K.; Alkahtany, M.F.; Almadi, K.H.; Ben Gassem, A.A.; Alshahrani, F.A.; AlRefeai, M.H.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of Different Conditioning Treatments on the Bond Integrity of Root Dentin to rGO Infiltrated Dentin Adhesive. SEM, EDX, FTIR and MicroRaman Study. Polymers 2021, 13, 1555. [Google Scholar] [CrossRef]
- Lee, J.H.; Um, C.M.; Lee, I.B. Rheological properties of resin composites according to variations in monomer and filler composition. Dent. Mater. 2006, 22, 515–526. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. ISO/TS 11405 Dentistry—Testing of Adhesion to Tooth Structure, 3rd ed.; International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- Helvatjoglu-Antoniades, M.; Koliniotou-Kubia, E.; Dionyssopoulos, P. The effect of thermal cycling on the bovine dentine shear bond strength of current adhesive systems. J. Oral Rehabil. 2004, 31, 911–917. [Google Scholar] [CrossRef]
- Felemban, N.H.; Ebrahim, M.I. The influence of adding modified zirconium oxide-titanium dioxide nano-particles on mechanical properties of orthodontic adhesive: An in vitro study. BMC Oral Health 2017, 17, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Zhang, F.; Xie, H.; Gu, N. Nanoparticle-reinforced resin-based dental composites. J. Dent. 2008, 36, 450–455. [Google Scholar] [CrossRef]
- Garcia-Contreras, R.; Scougall-Vilchis, R.J.; Contreras-Bulnes, R.; Sakagami, H.; Morales-Luckie, R.A.; Nakajima, H. Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. J. Appl. Oral Sci. 2015, 23, 321–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.G.R.; Gordaninejad, F.; Suhr, J. Energy absorption capability of nanocomposites: A review. CompSci Tech. 2009, 69, 2392–2409. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Peng, W.; Zare, Y.; Rhee, K.Y. Effects of Size and Aggregation/Agglomeration of Nanoparticles on the Interfacial/Interphase Properties and Tensile Strength of Polymer Nanocomposites. Nanoscale Res. Lett. 2018, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Zare, Y.; Rhee, K.Y. Dependence of Z Parameter for Tensile Strength of Multi-Layered Interphase in Polymer Nanocomposites to Material and Interphase Properties. Nanoscale Res. Lett. 2017, 12, 42. [Google Scholar] [CrossRef] [Green Version]
- Peumans, M.; Kanumilli, P.; De Munck, J.; Van Landuyt, K.; Lambrechts, P.; Van Meerbeek, B. Clinical effectiveness of contemporary adhesives: A systematic review of current clinical trials. Dent. Mater. 2005, 21, 864–881. [Google Scholar] [CrossRef]
- Kharouf, N.A.T.; Eid, A.; Maguina, L.; Zghal, J.; Sekayan, N.; Bourgi, R.; Hardan, L.; Sauro, S.; Haikel, Y.; Mancino, D. Does Adhesive Layer Thickness and Tag Length Influence Short/Long-Term Bond Strength of Universal Adhesive Systems? An In Vitro Study. Appl. Sci. 2021, 11, 2635. [Google Scholar] [CrossRef]
- Anchieta, R.B.; Oliveira, F.G.; Sundfeld, R.H.; Rahal, V.; Machado, L.S.; Alexandre, R.S.; Sundefeld, M.L.; Rocha, E.P. Analysis of hybrid layer thickness, resin tag length and their correlation with microtensile bond strength using a total etch adhesive to intact dentin. Acta Odontol. Latinoam. 2011, 24, 272–278. [Google Scholar] [PubMed]

| SBS (MPa) (Mean ± SD) | Failure Mode Analysis (%) | |||||
|---|---|---|---|---|---|---|
| Group (n = 10) | NTC | TC | p-Value * | Adhesive | Cohesive | Mixed |
| Control EA (0% particles) | 21.03 ± 2.44 a,A | - | <0.01 | 100 | 0 | 0 |
| - | 17.62 ± 1.70 a,B | 100 | 0 | 0 | ||
| 5% TiO2 | 25.35 ± 1.53 b,A | 80 | 0 | 20 | ||
| - | 23.89 ± 1.95 b,B | 100 | 0 | 0 | ||
| 5% ZrO2 | 23.10 ± 2.22 b,A | 70 | 10 | 20 | ||
| - | 20.72 ± 1.32 b,B | 80 | 0 | 20 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Saleh, S.; Alateeq, A.; Alshaya, A.H.; Al-Qahtani, A.S.; Tulbah, H.I.; Binhasan, M.; Shabib, S.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of TiO2 and ZrO2 Nanoparticles on Adhesive Bond Strength and Viscosity of Dentin Polymer: A Physical and Chemical Evaluation. Polymers 2021, 13, 3794. https://doi.org/10.3390/polym13213794
Al-Saleh S, Alateeq A, Alshaya AH, Al-Qahtani AS, Tulbah HI, Binhasan M, Shabib S, Farooq I, Vohra F, Abduljabbar T. Influence of TiO2 and ZrO2 Nanoparticles on Adhesive Bond Strength and Viscosity of Dentin Polymer: A Physical and Chemical Evaluation. Polymers. 2021; 13(21):3794. https://doi.org/10.3390/polym13213794
Chicago/Turabian StyleAl-Saleh, Samar, Abdullah Alateeq, Abdulaziz H. Alshaya, Amal S. Al-Qahtani, Huda I. Tulbah, Mashael Binhasan, Sara Shabib, Imran Farooq, Fahim Vohra, and Tariq Abduljabbar. 2021. "Influence of TiO2 and ZrO2 Nanoparticles on Adhesive Bond Strength and Viscosity of Dentin Polymer: A Physical and Chemical Evaluation" Polymers 13, no. 21: 3794. https://doi.org/10.3390/polym13213794
APA StyleAl-Saleh, S., Alateeq, A., Alshaya, A. H., Al-Qahtani, A. S., Tulbah, H. I., Binhasan, M., Shabib, S., Farooq, I., Vohra, F., & Abduljabbar, T. (2021). Influence of TiO2 and ZrO2 Nanoparticles on Adhesive Bond Strength and Viscosity of Dentin Polymer: A Physical and Chemical Evaluation. Polymers, 13(21), 3794. https://doi.org/10.3390/polym13213794







