Wound Healing with Electrical Stimulation Technologies: A Review
Abstract
1. Introduction
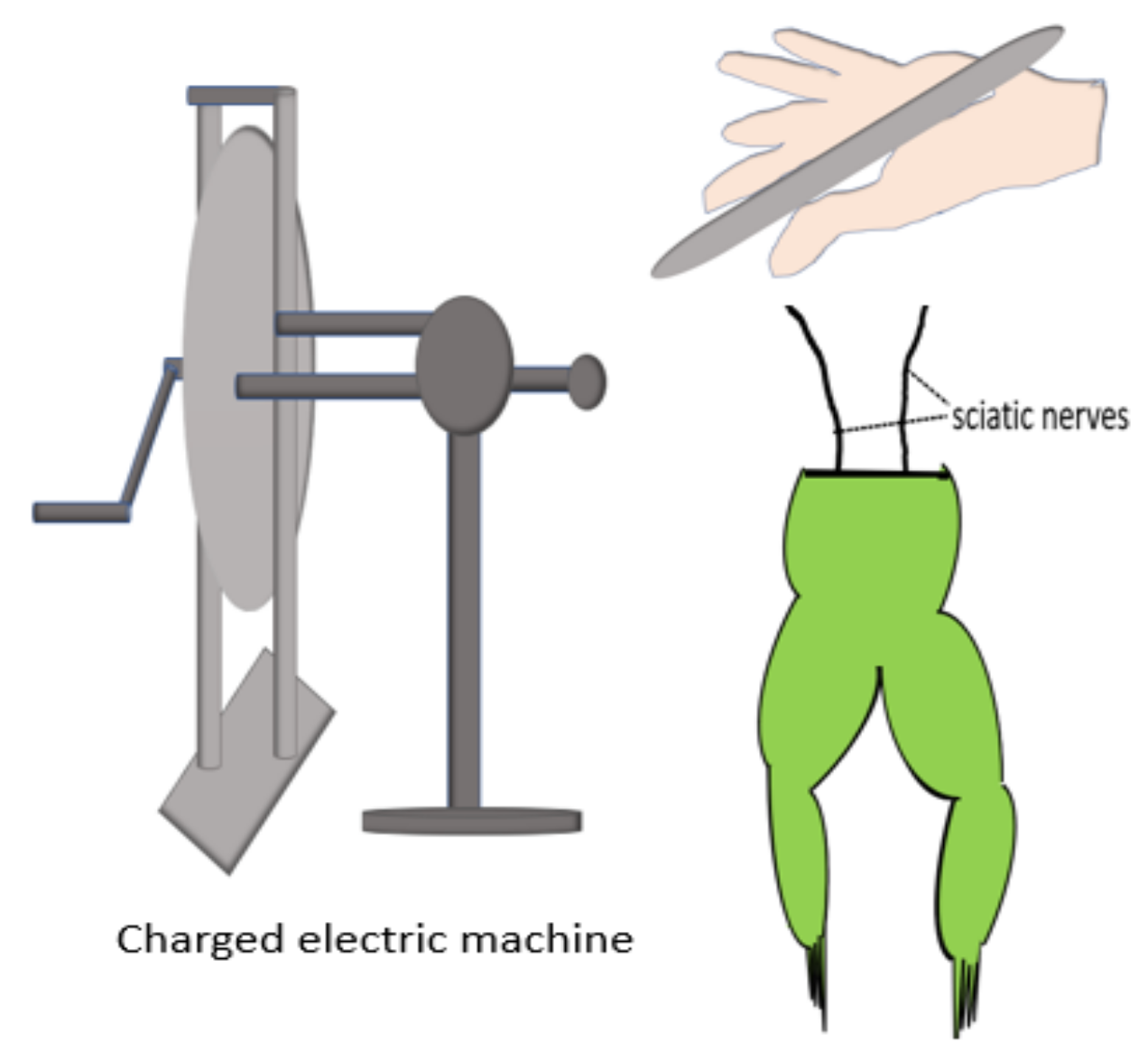
1.1. Skin as an Endogenous Battery
1.2. Role of an Endogenous Electric Field in Wound Repair
2. ES Approaches in Promoting Wound Healing
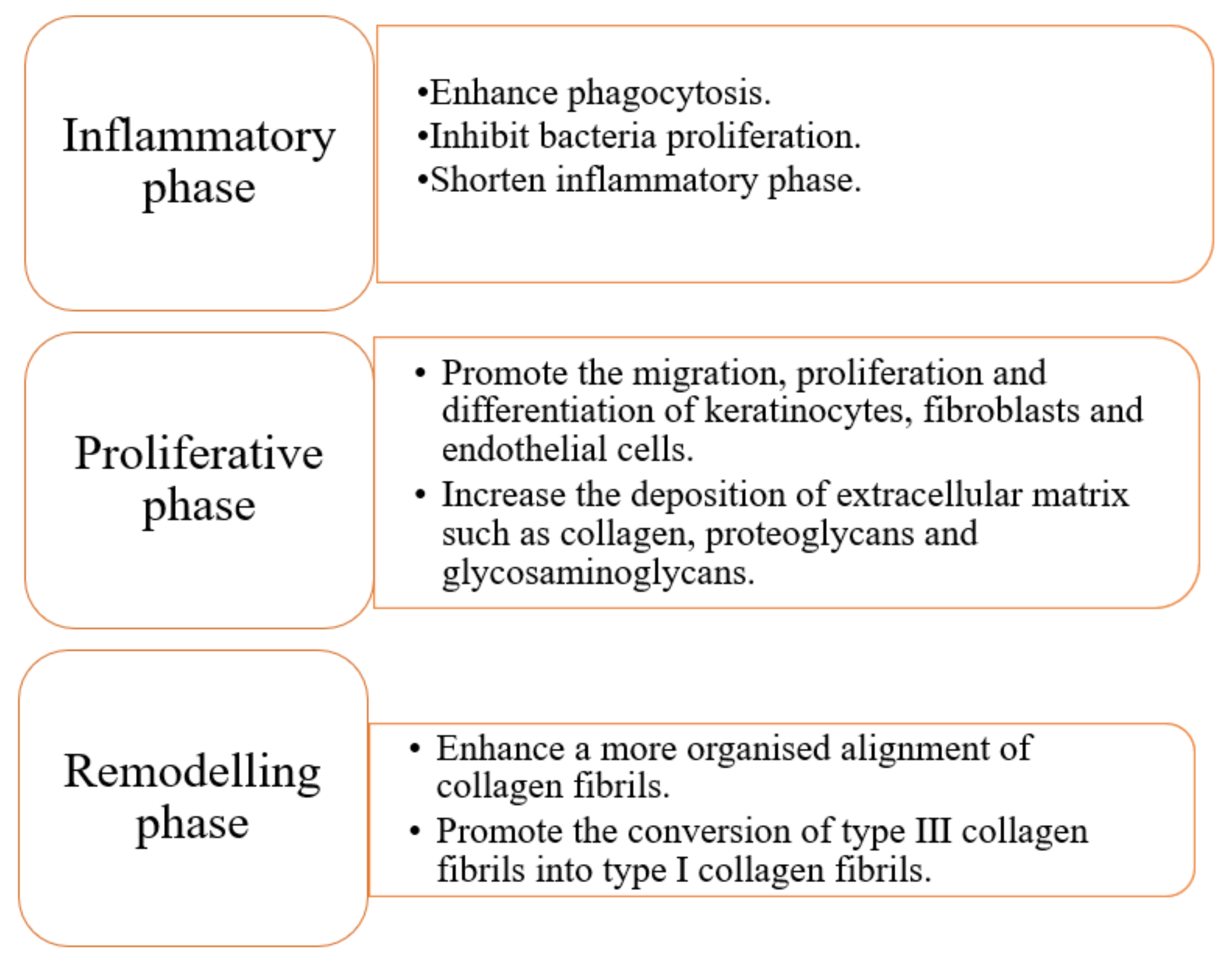
2.1. Inflammatory Phase
2.2. Proliferative Phase
2.3. Remodelling Phase
2.4. Other Aspects of Wound Healing Promoted by ES
3. Common Modes of ES
3.1. Direct Current (DC)
3.2. Pulsed Current (PC)
3.3. Alternating Current (AC)
- Period (s): time for a complete cycle including Ton and Toff.
- Frequency (Hz): number of complete cycles in a second.
- Pulse duration or pulse width (s): time for pulse of current to be on.
- Duty cycle (%) = × 100
3.4. Electrical Studies of In Vitro, In Vivo, and Clinical Trials
| Study Design | Type of ES | ES Field Strength | Exposure Duration | Model | Key Outcome | Reference |
|---|---|---|---|---|---|---|
| In vitro | PC | 1, 3, and 5 V | 15, 30, and 60 min | HDF |
| [52] |
| In vitro | PC | 200 μA Duty cycle: 0%, 10%, 50%, 90% | 24 h | HDF |
| [66] |
| In vivo | PC | 8 mA | 30 min | Rat |
| [90] |
| In vivo | PC | 50 μA | 11 days | Rat |
| [91] |
| RCT | DC | 1.48 ± 0.98 mA | An hour daily, 3 d/wk for 4 wk | Type 2 diabetic patients |
| [88] |
| Case series | PC | 1 V | 30 min each session, thrice daily, until wound closed | Chronic wounds of various aetiologies |
| [92] |
| RCT | HVMPC | 0.25 A | 30 min each session, 4 sessions given | Pressure ulcers |
| [93] |
| RCT | HVMPC | subjective dosage (minimum voltage of 100 V) | 50 min each session, until detachment of dressing | Burn wounds |
| [89] |
| RCT | PC | 20 mA | 30 min every other day for 4 weeks | Diabetic foot ulcers |
| [94] |
| Types of Cell | Direction | Optimal Range of Electric Field |
|---|---|---|
| Macrophage | Anode | DC/5–300 mV/mm [40] |
| Monocyte | Cathode | DC/150 mV/mm [40] |
| HUVEC | Cathode | DC/50–300 mV/mm [60] |
| HMEC | Cathode | DC/100–300 mV/mm [60] |
| Keratinocyte cell | Cathode | DC/50–200 mV/mm [95] |
| Fibroblast cell | Cathode | DC/50–200 mV/mm [55,96,97] |
4. ES in Relation to Tissue Engineering and Regenerative Medicine
4.1. Electroconductive Scaffold
4.1.1. Conducting Polymers (CPs)
4.1.2. Inorganic Conducting Materials
4.2. Nanogenerators (NG)
4.3. Stem Cell (SC) Therapy
4.4. Dielectrophoresis (DEP)
5. Conclusions
6. Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sen, C.K. Human wounds and its burden: An updated compendium of estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, M.N.; Yeung, J. Current status and future of skin substitutes for chronic wound healing. J. Cutan. Med. Surg. 2016, 21, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.F.; Fuller, G.W.; Vowden, P. Cohort study evaluating the burden of wounds to the UK’s National Health Service in 2017/2018: Update from 2012/2013. BMJ Open 2020, 10, e045253. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Heyer, K.; Herberger, K.; Protz, K.; Glaeske, G.; Augustin, M. Epidemiology of chronic wounds in Germany: Analysis of statutory health insurance data. Wound Repair Regen. 2015, 24, 434–442. [Google Scholar] [CrossRef]
- Turissini, J.D.; Elmarsafi, T.; Evans, K.K.; Kim, P.J. Major risk factors contributing to split thickness skin graft failure. Georget. Med. Rev. 2019, 3, 7755. [Google Scholar] [CrossRef]
- Guogienė, I.; Kievišas, M.; Varkalys, K.; Braziulis, K.; Rimdeika, R. Split-thickness skin grafting using grafts of different thickness. Eur. J. Plast. Surg. 2018, 41, 583–590. [Google Scholar] [CrossRef]
- Jones, R.E.; Foster, D.S.; Longaker, M.T. Management of chronic wounds—2018. JAMA 2018, 320, 1481–1482. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the treatment of chronic wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Isogai, Z.; Irisawa, R.; Otsuka, M.; Kadono, T.; Koga, M.; Hirosaki, K.; Asai, J.; Asano, Y.; Abe, M.; et al. Wound, pressure ulcer and burn guidelines—2: Guidelines for the diagnosis and treatment of pressure ulcers, second edition. J. Dermatol. 2018, 47, 929–978. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xu, Z.-X.; Hao, Y.-H.; Gao, Y.-B.; Yao, B.-W.; Zhang, J.; Wang, B.; Hu, Z.-Q.; Peng, R.-Y. A novel microcurrent dressing for wound healing in a rat skin defect model. Mil. Med. Res. 2019, 6, 1–9. [Google Scholar] [CrossRef]
- You, D.; Li, K.; Guo, W.; Zhao, G.; Fu, C. Poly (lactic-co-glycolic acid)/graphene oxide composites combined with electrical stimulation in wound healing: Preparation and characterization. Int. J. Nanomed. 2019, 14, 7039–7052. [Google Scholar] [CrossRef] [PubMed]
- Balakatounis, K.C.; Angoules, A. Low-intensity electrical stimulation in wound healing: Review of the efficacy of externally applied currents resembling the current of injury. Eplasty 2008, 8, 28. [Google Scholar]
- Zhao, M. Electrical fields in wound healing—An overriding signal that directs cell migration. Semin. Cell Dev. Biol. 2009, 20, 674–682. [Google Scholar] [CrossRef]
- Shen, Y.; Pfluger, T.; Ferreira, F.; Liang, J.; Navedo, M.F.; Zeng, Q.; Reid, B.; Zhao, M. Diabetic cornea wounds produce significantly weaker electric signals that may contribute to impaired healing. Sci. Rep. 2016, 6, 26525. [Google Scholar] [CrossRef]
- Wahlsten, O.; Skiba, J.B.; Makin, I.R.S.; Apell, S.P. Electrical field landscape of two electroceuticals. J. Electr. Bioimpedance 2016, 7, 13–19. [Google Scholar] [CrossRef]
- Galvani, L. De viribus electricitatis in motu musculari Commentarius. Bonoiensi Sci. Artium Intituo atque Acad. Comment. 1791, 7, 363-418.Piccolino, M. Luigi Galvani and animal electricity: Two centuries after the foundation of electrophysiology. Trends Neurosci. 1997, 20, 443–448. [Google Scholar] [CrossRef]
- Piccolino, M. Animal electricity and the birth of electrophysiology: The legacy of Luigi Galvani. Brain Res. Bull. 1998, 46, 381–407. [Google Scholar] [CrossRef]
- Cajavilca, C.; Varon, J.; Sternbach, G.L. Luigi Galvani and the foundations of electrophysiology. Resuscitation 2009, 80, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Du Bois-Reymond, E.H. Vorläufiger abriss einer untersuchung uber den sogenannten froschstrom und die electomotorischen fische. Ann. Phys. Chem. 1843, 58, 1–30. [Google Scholar] [CrossRef]
- Barker, A.T.; Jaffe, L.F.; Vanable, J.W. The glabrous epidermis of cavies contains a powerful battery. Am. J. Physiol. Integr. Comp. Physiol. 1982, 242, R358–R366. [Google Scholar] [CrossRef] [PubMed]
- Foulds, I.S.; Barker, A.T. Human skin battery potentials and their possible role in wound healing. Br. J. Dermatol. 1983, 109, 515–522. [Google Scholar] [CrossRef]
- Zhao, M.; Song, B.; Pu, J.; Wada, T.; Reid, B.; Tai, G.; Wang, F.; Guo, A.; Walczysko, P.; Gu, Y.; et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-γ and PTEN. Nature 2006, 442, 457–460. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, M.; Vanable, J.W., Jr. Electrical fields in Notophthalmus viridescens limb stumps. Dev. Biol. 1986, 116, 184–193. [Google Scholar] [CrossRef]
- Vieira, A.C.; Reid, B.; Cao, L.; Mannis, M.J.; Schwab, I.R.; Zhao, M. Ionic components of electric current at rat corneal wounds. PLoS ONE 2011, 6, e17411. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.R.; Nuccitelli, R.; Zhao, M. Non-invasive measurement of bioelectric currents with a vibrating probe. Nat. Protoc. 2007, 2, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.; Song, B.; McCaig, C.D.; Zhao, M. Wound healing in rat cornea: The role of electric currents. FASEB J. 2004, 19, 379–386. [Google Scholar] [CrossRef]
- Nuccitelli, R.; Nuccitelli, P.; Ramlatchan, S.; Sanger, R.; Smith, P.J. Imaging the electric field associated with mouse and human skin wounds. Wound Repair Regen. 2008, 16, 432–441. [Google Scholar] [CrossRef]
- Jaffe, L.F.; Vanable, J.W. Electric fields and wound healing. Clin. Dermatol. 1984, 2, 34–44. [Google Scholar] [CrossRef]
- Nuccitelli, R. Endogenous electric fields in embryos during development, regeneration and wound healing. Radiat. Prot. Dosim. 2003, 106, 375–383. [Google Scholar] [CrossRef]
- Guo, A.; Song, B.; Reid, B.; Gu, Y.; Forrester, J.V.; Jahoda, C.A.; Zhao, M. Effects of physiological electric fields on migration of human dermal fibroblasts. J. Investig. Dermatol. 2010, 130, 2320–2327. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Luo, Y.; Wang, T.; Wan, C.; Pan, L.; Pan, S.; He, K.; Neo, A.; Chen, X. Artificial skin perception. Adv. Mater. 2021, 33, 1–20. [Google Scholar] [CrossRef]
- Ashrafi, M.; Alonso-Rasgado, T.; Baguneid, M.; Bayat, A. The efficacy of electrical stimulation in experimentally induced cutaneous wounds in animals. Vet. Dermatol. 2016, 27, 235-e57. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Souza, A.K.; Souza, T.R.; das Neves, L.M.S.; Ferreira Leite, G.d.P.M.; Garcia, S.B.; de Jesus Guirro, R.R.; Barbosa, R.I.; de Oliveira Guirro, E.C. Effect of high voltage pulsed current on the integration of total skin grafts in rats submitted to nicotine action. J. Tissue Viability 2019, 28, 161–166. [Google Scholar] [CrossRef]
- Wang, K.; Parekh, U.; Ting, J.K.; Yamamoto, N.A.D.; Zhu, J.; Costantini, T.; Arias, A.C.; Eliceiri, B.P.; Ng, T.N. A platform to study the effects of electrical stimulation on immune cell activation during wound healing. Adv. Biosyst. 2019, 3, e1900106. [Google Scholar] [CrossRef] [PubMed]
- Lévêque, M.; Penna, A.; le Trionnaire, S.; Belleguic, C.; Desrues, B.; Brinchault, G.; Jouneau, S.; Lagadic-Gossmann, D.; Martin-Chouly, C. Phagocytosis depends on TRPV2-mediated calcium influx and requires TRPV2 in lipids rafts: Alteration in macrophages from patients with cystic fibrosis. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hoare, J.I.; Rajnicek, A.M.; McCaig, C.D.; Barker, R.N.; Wilson, H.M. Electric fields are novel determinants of human macrophage functions. J. Leukoc. Biol. 2016, 99, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Rowley, B.A. Electrical current effects on E. coil growth rates. Exp. Biol. Med. 1972, 139, 929–934. [Google Scholar] [CrossRef]
- Barranco, S.D.; Spadaro, J.A.; Berger, T.J.; Becker, R.O. In vitro effect of weak direct current on Staphylococcus aureus. Clin. Orthop. Relat. Res. 1974, 100, 250–255. [Google Scholar] [CrossRef]
- Dauben, T.J.; Ziebart, J.; Bender, T.; Zaatreh, S.; Kreikemeyer, B.; Bader, R. A novel in vitro system for comparative analyses of bone cells and bacteria under electrical stimulation. BioMed Res. Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthi, V.R.; Rogers, A.; Peifer, J.; Niyonshuti, I.I.; Chen, J.; Wang, Y. Microampere electric current causes bacterial membrane damage and two-way leakage in a short period of time. Appl. Environ. Microbiol. 2020, 86, 01015–01020. [Google Scholar] [CrossRef] [PubMed]
- Petrofsky, J.; Laymon, M.; Chung, W.; Collins, K.; Yang, T.-N. Effect of Electrical Stimulation on Bacterial Growth. J. Orthop. Neurol. Surg. 2008, 31, 43. [Google Scholar]
- Rouabhia, M.; Park, H.J.; Abedin-Do, A.; Douville, Y.; Methot, M.; Zhang, Z. Electrical stimulation promotes the proliferation of human keratinocytes, increases the production of keratin 5 and 14, and increases the phosphorylation of ERK1/2 and p38 MAP kinases. J. Tissue Eng. Regen. Med. 2020, 14, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Hart, F.X.; Laird, M.; Riding, A.; Pullar, C.E. Keratinocyte galvanotaxis in combined DC and AC electric fields supports an electromechanical transduction sensing mechanism. Bioelectromagnetics 2013, 34, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Sun, H.; Liu, J.; Guo, X.; Huang, J.; Jiang, X.; Zhang, Y.; Huang, Y.; Fan, D.; Zhang, J. Keratinocyte electrotaxis induced by physiological pulsed direct current electric fields. Bioelectrochemistry 2019, 127, 113–124. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Sebastian, A.; Iqbal, S.A.; Colthurst, J.; Volk, S.W.; Bayat, A. Electrical stimulation enhances epidermal proliferation in human cutaneous wounds by modulating p53–SIVA1 interaction. J. Investig. Dermatol. 2015, 135, 1166–1174. [Google Scholar] [CrossRef]
- Casagrande, S.M.; Biondo-Simões, M.D.L.P.; Ioshii, S.; Robes, R.R.; Biondo-Simões, R.; Boeno, B.R.D.O. Histological evaluation of the effect of low-frequency electric stimulation on healing Achilles tendons in rats. Acta Cir. Bras. 2021, 36, e360103. [Google Scholar] [CrossRef]
- Urabe, H.; Akimoto, R.; Kamiya, S.; Hosoki, K.; Ichikawa, H.; Nishiyama, T. Effects of pulsed electrical stimulation on growth factor gene expression and proliferation in human dermal fibroblasts. Mol. Cell. Biochem. 2021, 476, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Rouabhia, M.; Park, H.J.; Zhang, Z. Electrically activated primary human fibroblasts improve in vitro and in vivo skin regeneration. J. Cell. Physiol. 2016, 231, 1814–1821. [Google Scholar] [CrossRef]
- Wang, Y.; Rouabhia, M.; Zhang, Z. Pulsed electrical stimulation benefits wound healing by activating skin fibroblasts through the TGFβ1/ERK/NF-κB axis. Biochim. Biophys. Acta BBA—Gen. Subj. 2016, 1860, 1551–1559. [Google Scholar] [CrossRef]
- Rouabhia, M.; Park, H.; Meng, S.; Derbali, H.; Zhang, Z. Electrical stimulation promotes wound healing by enhancing dermal fibroblast activity and promoting myofibroblast transdifferentiation. PLoS ONE 2013, 8, e71660. [Google Scholar] [CrossRef]
- Snyder, S.; DeJulius, C.; Willits, R.K. Electrical stimulation increases random migration of human dermal fibroblasts. Ann. Biomed. Eng. 2017, 45, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Benington, L.; Rajan, G.; Locher, C.; Lim, L.Y. Fibroblast growth factor 2—A review of stabilisation approaches for clinical applications. Pharmaceutics 2020, 12, 508. [Google Scholar] [CrossRef]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020, 5, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M.; Maeshige, N.; Koga, Y.; Ishikawa-Aoyama, M.; Miyoshi, M.; Sugimoto, M.; Terashi, H.; Usami, M. Monophasic pulsed 200-μA current promotes galvanotaxis with polarization of actin filament and integrin α2β1 in human dermal fibroblasts. Eplasty 2016, 16, 6. [Google Scholar]
- Cunha, F.; Rajnicek, A.M.; McCaig, C.D. Electrical stimulation directs migration, enhances and orients cell division and upregulates the chemokine receptors CXCR4 and CXCR2 in endothelial cells. J. Vasc. Res. 2019, 56, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Geng, K.; Wang, J.; Liu, P.; Tian, X.; Liu, H.; Wang, X.; Hu, C.; Yan, H. Electrical stimulation facilitates the angiogenesis of human umbilical vein endothelial cells through MAPK/ERK signaling pathway by stimulating FGF2 secretion. Am. J. Physiol.—Cell Physiol. 2019, 317, C277–C286. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Forrester, J.V.; Zhao, M. DC electric stimulation upregulates angiogenic factors in endothelial cells through activation of VEGF receptors. Cytokine 2011, 55, 110–115. [Google Scholar] [CrossRef]
- Shoeibi, S.; Mozdziak, P.; Mohammadi, S. Important signals regulating coronary artery angiogenesis. Microvasc. Res. 2018, 117, 1–9. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Teleanu, D.M. Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. J. Clin. Med. 2019, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Shu, L.; Xu, P.; Bai, S. Mesenchymal stem cells attract endothelial progenitor cells via a positive feedback loop between CXCR2 and CXCR4. Stem Cells Int. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M.; Sugimoto, M.; Yoshikawa, Y.; Hiramatsu, T.; Inoue, T. Monophasic pulsed current stimulation of duty cycle 10% promotes differentiation of human dermal fibroblasts into myofibroblasts. Phys. Ther. Res. 2021, 24, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Volk, S.W.; Halai, P.; Colthurst, J.; Paus, R.; Bayat, A. Enhanced neurogenic biomarker expression and reinnervation in human acute skin wounds treated by electrical stimulation. J. Investig. Dermatol. 2017, 137, 737–747. [Google Scholar] [CrossRef]
- Keast, D.H.; Moffatt, C.; Janmohammad, A. Lymphedema impact and prevalence international study: The Canadian data. Lymphat. Res. Biol. 2019, 17, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Bjork, R.; Hettrick, H. Lymphedema: New concepts in diagnosis and treatment. Curr. Dermatol. Rep. 2019, 8, 190–198. [Google Scholar] [CrossRef]
- Kajiya, K.; Matsumoto-Okazaki, Y.; Sawane, M.; Fukada, K.; Takasugi, Y.; Akai, T.; Saito, N.; Mori, Y. Electric current-induced lymphatic activation. Exp. Dermatol. 2014, 23, 936–938. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yang, K.; Browne, M.; Bostan, L.; Worsley, P. Wearable electrical stimulation to improve lymphatic function. IEEE Sens. Lett. 2019, 3, 1–4. [Google Scholar] [CrossRef]
- Baglivo, M.; Martelli, F.; Paolacci, S.; Manara, E.; Michelini, S.; Bertelli, M. Electrical stimulation in the treatment of lymphedema and associated skin ulcers. Lymphat. Res. Biol. 2020, 18, 270–276. [Google Scholar] [CrossRef] [PubMed]
- AlGhatrif, M.; Lindsay, J. A brief review: History to understand fundamentals of electrocardiography. J. Community Hosp. Intern. Med. Perspect. 2012, 2, 14383. [Google Scholar] [CrossRef] [PubMed]
- Baloglu, U.B.; Talo, M.; Yildirim, O.; Tan, R.S.; Acharya, U.R. Classification of myocardial infarction with multi-lead ECG signals and deep CNN. Pattern Recognit. Lett. 2019, 122, 23–30. [Google Scholar] [CrossRef]
- Ball, C.M.; Featherstone, P.J. The early history of cardiac pacing. Anaesth. Intensiv. Care 2019, 47, 320–321. [Google Scholar] [CrossRef]
- Donnelly, C.; Stegmüller, J.; Blazevich, A.J.; von Roten, F.C.; Kayser, B.; Neyroud, D.; Place, N. Modulation of torque evoked by wide-pulse, high-frequency neuromuscular electrical stimulation and the potential implications for rehabilitation and training. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Gibson, W.; Wand, B.M.; Meads, C.; Catley, M.J.; O’Connell, N.E. Transcutaneous electrical nerve stimulation (TENS) for chronic pain—An overview of Cochrane reviews. Cochrane Database Syst. Rev. 2019, 1–27. [Google Scholar] [CrossRef]
- Li, M.; Yao, X.; Sun, L.; Zhao, L.; Xu, W.; Zhao, H.; Zhao, F.; Zou, X.; Cheng, Z.; Li, B.; et al. Effects of electroconvulsive therapy on depression and its potential mechanism. Front. Psychol. 2020, 11, 80. [Google Scholar] [CrossRef]
- Weiner, R.D.; Reti, I.M. Key updates in the clinical application of electroconvulsive therapy. Int. Rev. Psychiatry 2017, 29, 54–62. [Google Scholar] [CrossRef]
- Little, S.; Brown, P. Debugging adaptive deep brain stimulation for Parkinson’s disease. Mov. Disord. 2020, 35, 555–561. [Google Scholar] [CrossRef]
- Ryan, C.N.M.; Doulgkeroglou, M.N.; Zeugolis, D.I. Electric field stimulation for tissue engineering applications. BMC Biomed. Eng. 2021, 3, 1. [Google Scholar] [CrossRef]
- Ojingwa, J.C.; Isseroff, R.R. Electrical stimulation of wound healing. J. Investig. Dermatol. 2003, 121, 1–12. [Google Scholar] [CrossRef]
- Kloth, L.C. Electrical stimulation for wound healing: A review of evidence from in vitro studies, animal experiments, and clinical trials. Int. J. Low. Extrem. Wounds 2005, 4, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Polak, A.; Kloth, L.C.; Blaszczak, E.; Taradaj, J.; Nawrat-Szołtysik, A.; Walczak, A.; Bialek, L.; Paczula, M.; Franek, A.; Kucio, C. Evaluation of the healing progress of pressure ulcers treated with cathodal high-voltage monophasic pulsed current: Results of a prospective, double-blind, randomized clinical trial. Adv. Skin Wound Care 2016, 29, 447–459. [Google Scholar] [CrossRef]
- Polak, A.; Kucio, C.; Kloth, L.; Paczula, M.; Hordynska, E.; Ickowicz, T.; Blaszczak, E.; Kucio, E.; Oleszczyk, K.; Ficek, K.; et al. A randomized, controlled clinical study to assess the effect of anodal and cathodal electrical stimulation on periwound skin blood flow and pressure ulcer size reduction in persons with neurological injuries. Ostomy Wound Manag. 2018, 64, 10–29. [Google Scholar] [CrossRef]
- Polak, A.; Kloth, L.C.; Blaszczak, E.; Taradaj, J.; Nawrat-Szoltysik, A.; Ickowicz, T.; Hordynska, E.; Franek, A.; Kucio, C. The efficacy of pressure ulcer treatment with cathodal and cathodal-anodal high-voltage monophasic pulsed current: A prospective, randomized, controlled clinical trial. Phys. Ther. 2017, 97, 777–789. [Google Scholar] [CrossRef]
- Wang, X.-F.; Li, M.-L.; Fang, Q.-Q.; Zhao, W.-Y.; Lou, D.; Hu, Y.-Y.; Chen, J.; Tan, W.-Q. Flexible electrical stimulation device with Chitosan-Vaseline® dressing accelerates wound healing in diabetes. Bioact. Mater. 2021, 6, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri-Tehrani, M.R.; Nasiripoor, F.; Torkaman, G.; Hedayati, M.; Annabestani, Z.; Asadi, M.R. Effect of low-intensity direct current on expression of vascular endothelial growth factor and nitric oxide in diabetic foot ulcers. J. Rehabil. Res. Dev. 2014, 51, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.C.; Guirro, E.C.; Gonçalves, A.C.; Farina, J.A., Jr.; Murta, L.O., Jr.; Guirro, R.R. High-voltage electric stimulation of the donor site of skin grafts accelerates the healing process. A randomized blinded clinical trial. Burns 2018, 44, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Maeshige, N.; Honda, H.; Yoshikawa, Y.; Uemura, M.; Yamamoto, M.; Terashi, H. Optimum microcurrent stimulation intensity for galvanotaxis in human fibroblasts. J. Wound Care 2012, 21, 5–10. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, M.H.; Kwon, B.-J.; Seo, H.J.; Koo, M.-A.; You, K.E.; Kim, D.; Park, J.-C. Control of neonatal human dermal fibroblast migration on poly (lactic-co-glycolic acid)-coated surfaces by electrotaxis. J. Tissue Eng. Regen. Med. 2017, 11, 862–868. [Google Scholar] [CrossRef]
- Wang, L.; Mao, L.; Qi, F.; Li, X.; Ullah, M.W.; Zhao, M.; Shi, Z.; Yang, G. Synergistic effect of highly aligned bacterial cellulose/gelatin membranes and electrical stimulation on directional cell migration for accelerated wound healing. Chem. Eng. J. 2021, 424, 130563. [Google Scholar] [CrossRef]
- Oliveira, K.M.C.; Barker, J.H.; Berezikov, E.; Pindur, L.; Kynigopoulos, S.; Eischen-Loges, M.; Han, Z.; Bhavsar, M.B.; Henrich, D.; Leppik, L. Electrical stimulation shifts healing/scarring towards regeneration in a rat limb amputation model. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.R.; Torkaman, G.; Hedayati, M.; Mofid, M. Role of sensory and motor intensity of electrical stimulation on fibroblastic growth factor-2 expression, inflammation, vascularization, and mechanical strength of full-thickness wounds. J. Rehabil. Res. Dev. 2013, 50, 489. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, X.; Rajagopalan, P.; Zhang, L.; Zhan, S.; Huang, S.; Li, W.; Zeng, X.; Ye, Q.; Liu, Y.; et al. Toward controlled electrical stimulation for wound healing based on a precision layered skin model. ACS Appl. Bio Mater. 2020, 3, 8901–8910. [Google Scholar] [CrossRef]
- Abedin-Do, A.; Zhang, Z.; Douville, Y.; Méthot, M.; Rouabhia, M. Effect of electrical stimulation on diabetic human skin fibroblast growth and the secretion of cytokines and growth factors involved in wound healing. Biology 2021, 10, 641. [Google Scholar] [CrossRef] [PubMed]
- Ramadhinara, A.; Poulas, K. Use of wireless microcurrent stimulation for the treatment of diabetes-related wounds: 2 case reports. Adv. Skin Wound Care 2013, 26, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Recio, A.C.; Felter, C.E.; Schneider, A.C.; McDonald, J.W. High-voltage electrical stimulation for the management of stage III and IV pressure ulcers among adults with spinal cord injury: Demonstration of its utility for recalcitrant wounds below the level of injury. J. Spinal Cord Med. 2012, 35, 58–63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ibrahim, Z.M.; Waked, I.S.; Ibrahim, O. Negative pressure wound therapy versus microcurrent electrical stimulation in wound healing in burns. J. Wound Care 2019, 28, 214–219. [Google Scholar] [CrossRef]
- Borba, G.C.; Hochman, B.; Liebano, R.E.; Enokihara, M.M.; Ferreira, L.M. Does preoperative electrical stimulation of the skin alter the healing process? J. Surg. Res. 2011, 166, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Sari, Y.; Hartono; Sutrisna, E.; Saryono. The effect of short duration of electrical stimulation on wound healing in acute wound in a rat model. Wound Med. 2019, 24, 36–44. [Google Scholar] [CrossRef]
- Fraccalvieri, M.; Salomone, M.; Zingarelli, E.M.; Rivarossa, F.; Bruschi, S. Electrical stimulation for difficult wounds: Only an alternative procedure? Int. Wound J. 2014, 12, 669–673. [Google Scholar] [CrossRef]
- Ud-Din, S.; Sebastian, A.; Giddings, P.; Colthurst, J.; Whiteside, S.; Morris, J.; Nuccitelli, R.; Pullar, C.; Baguneid, M.; Bayat, A. Angiogenesis is induced and wound size is reduced by electrical stimulation in an acute wound healing model in human skin. PLoS ONE 2015, 10, e0124502. [Google Scholar] [CrossRef]
- Petrofsky, J.S.; Lawson, D.; Berk, L.; Suh, H. Enhanced healing of diabetic foot ulcers using local heat and electrical stimulation for 30 min three times per week. J. Diabetes 2010, 2, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.M.A.; Fauzi, M.B.; Nordin, A.; Hiraoka, Y.; Tabata, Y.; Yunus, M.H.M. Fabrication of bio-based gelatin sponge for potential use as a functional acellular skin substitute. Polymers 2020, 12, 2678. [Google Scholar] [CrossRef] [PubMed]
- Ooi, K.S.; Haszman, S.; Wong, Y.N.; Soidin, E.; Hesham, N.; Mior, M.A.A.; Tabata, Y.; Ahmad, I.; Fauzi, M.B.; Yunus, M.H. Physicochemical characterization of bilayer hybrid nanocellulose-collagen as a potential wound dressing. Materials 2020, 13, 4352. [Google Scholar] [CrossRef]
- Busra, F.M.; Rajab, N.F.; Tabata, Y.; Bin Saim, A.; Idrus, R.B.; Chowdhury, S.R. Rapid treatment of full-thickness skin loss using ovine tendon collagen type I scaffold with skin cells. J. Tissue Eng. Regen. Med. 2019, 13, 874–891. [Google Scholar] [CrossRef]
- Park, S.-B.; Lih, E.; Park, K.-S.; Joung, Y.K.; Han, D.K. Biopolymer-based functional composites for medical applications. Prog. Polym. Sci. 2017, 68, 77–105. [Google Scholar] [CrossRef]
- Zare, E.N.; Agarwal, T.; Zarepour, A.; Pinelli, F.; Zarrabi, A.; Rossi, F.; Ashrafizadeh, M.; Maleki, A.; Shahbazi, M.-A.; Maiti, T.K.; et al. Electroconductive multi-functional polypyrrole composites for biomedical applications. Appl. Mater. Today 2021, 24, 101117. [Google Scholar] [CrossRef]
- Levi, B.G. Nobel Prize in chemistry salutes the discovery of conducting polymers. Phys. Today 2000, 53, 19–22. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH) x. J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Shirakawa, H.; McDiarmid, A.; Heeger, A. Focus article: Twenty-five years of conducting polymers. Chem. Commun. 2003, 1, 1–4. [Google Scholar] [CrossRef]
- Tang, X.; Saveh-Shemshaki, N.; Kan, H.-M.; Khan, Y.; Laurencin, C.T. Biomimetic electroconductive nanofibrous matrices for skeletal muscle regenerative engineering. Regen. Eng. Transl. Med. 2020, 6, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Athukorala, S.S.; Tran, T.S.; Balu, R.; Truong, V.K.; Chapman, J.; Dutta, N.K.; Choudhury, R.N. 3D printable electrically conductive hydrogel scaffolds for biomedical applications: A review. Polymers 2021, 13, 474. [Google Scholar] [CrossRef]
- Wijsboom, Y.H.; Patra, A.; Zade, S.S.; Sheynin, Y.; Li, M.; Shimon, L.J.W.; Bendikov, M. Controlling rigidity and planarity in conjugated polymers: Poly (3,4-ethylenedithioselenophene). Angew. Chem. Int. Ed. 2009, 48, 5443–5447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-S.; Yan, Y.-H.; Li, S.-P.; Feng, T. Synthesis of a novel biodegradable and electroactive polyphosphazene for biomedical application. Biomed. Mater. 2009, 4, 035008. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, S. Synthesis and evaluation of chitosan-polyaniline copolymer in presence of ammonium persulfate as initiator. J. Appl. Chem. Res. 2014, 8, 47–54. [Google Scholar]
- Hardy, J.G.; Lee, J.Y.; Schmidt, C.E. Biomimetic conducting polymer-based tissue scaffolds. Curr. Opin. Biotechnol. 2013, 24, 847–854. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Ahearne, M. Fabrication and biocompatibility of electroconductive silk fibroin/PEDOT: PSS composites for corneal epithelial regeneration. Polymers 2020, 12, 3028. [Google Scholar] [CrossRef]
- Distler, T.; Polley, C.; Shi, F.; Schneidereit, D.; Ashton, M.D.; Friedrich, O.; Kolb, J.F.; Hardy, J.G.; Detsch, R.; Seitz, H.; et al. Electrically conductive and 3D-printable oxidized alginate-gelatin polypyrrole: PSS hydrogels for tissue engineering. Adv. Health Mater. 2021, 10, 2001876. [Google Scholar] [CrossRef]
- Manzari-Tavakoli, A.; Tarasi, R.; Sedghi, R.; Moghimi, A.; Niknejad, H. Fabrication of nanochitosan incorporated polypyrrole/alginate conducting scaffold for neural tissue engineering. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pasela, B.R.; Castillo, A.P.; Simon, R.; Pulido, M.T.; Mana-Ay, H.; Abiquibil, M.R.; Montecillo, R.; Thumanu, K.; von Tumacder, D.; Taaca, K.L. Synthesis and characterization of acetic acid-doped polyaniline and polyaniline–chitosan composite. Biomimetics 2019, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.U.; Bilal, S.; Ali Shah, A.U.H. Synthesis and characterization of polyaniline-chitosan patches with enhanced stability in physiological conditions. Polymers 2020, 12, 2870. [Google Scholar] [CrossRef]
- Ma, C.; Jiang, L.; Wang, Y.; Gang, F.; Xu, N.; Li, T.; Liu, Z.; Chi, Y.; Wang, X.; Zhao, L.; et al. 3D printing of conductive tissue engineering scaffolds containing polypyrrole nanoparticles with different morphologies and concentrations. Materials 2019, 12, 2491. [Google Scholar] [CrossRef] [PubMed]
- Garrudo, F.; Chapman, C.A.; Hoffman, P.R.; Udangawa, R.; Silva, J.C.; Mikael, P.E.; Rodrigues, C.A.; Cabral, J.M.; Morgado, J.; Ferreira, F.C.; et al. Polyaniline-polycaprolactone blended nanofibers for neural cell culture. Eur. Polym. J. 2019, 117, 28–37. [Google Scholar] [CrossRef]
- Korupalli, C.; Li, H.; Nguyen, N.; Mi, F.; Chang, Y.; Lin, Y.; Sung, H. Conductive materials for healing wounds: Their incorporation in electroactive wound dressings, characterization, and perspectives. Adv. Health Mater. 2021, 10, e2001384. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Y.; Song, X.; Dubal, D.P.; Zhao, X.; Zhang, D. Review on carbon/polyaniline hybrids: Design and synthesis for supercapacitor. Molecules 2019, 24, 2263. [Google Scholar] [CrossRef]
- MacDiarmid, A.G.; Chiang, J.; Halpern, M.; Huang, W.; Mu, S.; Nanaxakkara, L.; Wu, S.W.; Yaniger, S.I. “ Polyaniline ”: Interconversion of Metallic and Insulating Forms. Mol. Cryst. Liq. Cryst. 1985, 121, 173–180. [Google Scholar] [CrossRef]
- Saberi, A.; Jabbari, F.; Zarrintaj, P.; Saeb, M.R.; Mozafari, M. Electrically conductive materials: Opportunities and challenges in tissue engineering. Biomolecules 2019, 9, 448. [Google Scholar] [CrossRef]
- Borriello, A.; Guarino, V.; Schiavo, L.; Alvarez-Perez, M.A.; Ambrosio, L. Optimizing PANi doped electroactive substrates as patches for the regeneration of cardiac muscle. J. Mater. Sci. Mater. Electron. 2011, 22, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Mitriashkin, A.; Lim, T.T.; Goh, J.C.-H. Conductive polypyrrole-encapsulated silk fibroin fibers for cardiac tissue engineering. Biomaterials 2021, 276, 121008. [Google Scholar] [CrossRef]
- Gotovtsev, P.M.; Badranova, G.U.; Zubavichus, Y.V.; Chumakov, N.K.; Antipova, C.G.; Kamyshinsky, R.; Presniakov, M.Y.; Tokaev, K.V.; Grigoriev, T. Electroconductive PEDOT: PSS-based hydrogel prepared by freezing-thawing method. Heliyon 2019, 5, e02498. [Google Scholar] [CrossRef] [PubMed]
- Sordini, L.; Silva, J.C.; Garrudo, F.F.F.; Rodrigues, C.A.V.; Marques, A.C.; Linhardt, R.J.; Cabral, J.M.S.; Morgado, J.; Ferreira, F.C. PEDOT: PSS-coated polybenzimidazole electroconductive nanofibers for biomedical applications. Polymers 2021, 13, 2786. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Sun, T.; Sultana, N.; Lim, M.M.; Khan, T.H.; Ismail, A.F. Conductive PEDOT: PSS coated polylactide (PLA) and poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) electrospun membranes: Fabrication and characterization. Mater. Sci. Eng. C 2016, 61, 396–410. [Google Scholar] [CrossRef]
- Niu, X.; Rouabhia, M.; Chiffot, N.; King, M.W.; Zhang, Z. An electrically conductive 3D scaffold based on a nonwoven web of poly(l-lactic acid) and conductive poly(3,4-ethylenedioxythiophene). J. Biomed. Mater. Res. Part A 2015, 103, 2635–2644. [Google Scholar] [CrossRef]
- Mantione, D.; del Agua, I.; Sanchez-Sanchez, A.; Mecerreyes, D. Poly(3,4-ethylenedioxythiophene) (PEDOT) derivatives: Innovative conductive polymers for bioelectronics. Polymers 2017, 9, 354. [Google Scholar] [CrossRef]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.; Shin, S.R.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Carbon-based nanomaterials: Multifunctional materials for biomedical engineering. ACS Nano 2013, 7, 2891–2897. [Google Scholar] [CrossRef] [PubMed]
- Fal, J.; Bulanda, K.; Oleksy, M.; Sobczak, J.; Shi, J.; Liu, M.; Boncel, S.; Żyła, G. High AC and DC Electroconductivity of Scalable and Economic Graphite—Diamond Polylactide Nanocomposites. Materials 2021, 14, 2835. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Gensini, L.; Serna, J.A.; Cifuentes, J.; Cruz, J.C.; Muñoz-Camargo, C. Graphene oxide-embedded extracellular matrix-derived hydrogel as a multiresponsive platform for 3D bioprinting applications. Int. J. Bioprinting 2021, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.; Abdellaoui, S.; Minteer, S.D. Enzymatic biofuel cells: 30 years of critical advancements. Biosens. Bioelectron. 2016, 76, 91–102. [Google Scholar] [CrossRef]
- Miyake, T.; Haneda, K.; Yoshino, S.; Nishizawa, M. Flexible, layered biofuel cells. Biosens. Bioelectron. 2013, 40, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Kai, H.; Yamauchi, T.; Ogawa, Y.; Tsubota, A.; Magome, T.; Miyake, T.; Yamasaki, K.; Nishizawa, M. Accelerated wound healing on skin by electrical stimulation with a bioelectric plaster. Adv. Health Mater. 2017, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.; Li, Y.; Wang, Y.; Wei, G. Recent advance in the fabrication of 2D and 3D metal carbides-based nanomaterials for energy and environmental applications. Nanomaterials 2021, 11, 246. [Google Scholar] [CrossRef] [PubMed]
- Rasool, K.; Helal, M.; Ali, A.; Ren, C.E.; Gogotsi, Y.; Mahmoud, K.A. Antibacterial activity of Ti3C2Tx MXene. ACS Nano 2016, 10, 3674–3684. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Hu, S.; Gao, Y.; Wang, L.; Zhao, W.; Fu, L.; Cheng, H.; Xia, L.; Xie, S.; Ye, W.; et al. Biodegradable and electroactive regenerated bacterial cellulose/MXene (Ti3C2Tx) composite hydrogel as wound dressing for accelerating skin wound healing under electrical stimulation. Adv. Health Mater. 2020, 9, 1–13. [Google Scholar] [CrossRef]
- Sappati, K.K.; Bhadra, S. Piezoelectric polymer and paper substrates: A review. Sensors 2018, 18, 3605. [Google Scholar] [CrossRef]
- Mahapatra, B.; Patel, K.K.; Patel, P.K. A review on recent advancement in materials for piezoelectric/triboelectric nanogenerators. Mater. Today Proc. 2021, 46, 5523–5529. [Google Scholar] [CrossRef]
- Long, Y.; Wei, H.; Li, J.; Yao, G.; Yu, B.; Ni, D.; Gibson, A.L.; Lan, X.; Jiang, Y.; Cai, W.; et al. Effective wound healing enabled by discrete alternative electric fields from wearable nanogenerators. ACS Nano 2018, 12, 12533–12540. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Lee, Y.; Lee, M.-G.; Song, W.J.; Park, J.-U.; Sun, J.-Y. Accelerated wound healing with an ionic patch assisted by a triboelectric nanogenerator. Nano Energy 2021, 79, 105463. [Google Scholar] [CrossRef]
- Li, Q.; Xing, J.; Shang, D.; Wang, Y. A flow velocity measurement method based on a PVDF piezoelectric sensor. Sensors 2019, 19, 1657. [Google Scholar] [CrossRef]
- Du, S.; Zhou, N.; Gao, Y.; Xie, G.; Du, H.; Jiang, H.; Zhang, L.; Tao, J.; Zhu, J. Bioinspired hybrid patches with self-adhesive hydrogel and piezoelectric nanogenerator for promoting skin wound healing. Nano Res. 2020, 13, 2525–2533. [Google Scholar] [CrossRef]
- Riha, S.; Maarof, M.; Fauzi, M. Synergistic effect of biomaterial and stem cell for skin tissue engineering in cutaneous wound healing: A concise review. Polymers 2021, 13, 1546. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, Q.; Wang, B.; Luo, R.; Xiao, X.; Zhang, Y.; Ma, L.; Feng, X.; Huang, J.; Sun, X.; et al. Rejuvenation of senescent bone marrow mesenchymal stromal cells by pulsed triboelectric stimulation. Adv. Sci. 2021, 8, e2100964. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.-Y.; Mun, S.; Ng, W.B.; Kang, K.; Han, K.; Hwang, S.; Kim, H.-H.; Lee, J.H. Modulation of human mesenchymal stem cells by electrical stimulation using an enzymatic biofuel cell. Catalysts 2021, 11, 62. [Google Scholar] [CrossRef]
- Bicer, M.; Sheard, J.; Iandolo, D.; Boateng, S.Y.; Cottrell, G.S.; Widera, D. Electrical stimulation of adipose-derived stem cells in 3D nanofibrillar cellulose increases their osteogenic potential. Biomolecules 2020, 10, 1696. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Huang, Y.; Chen, W.; Che, J.; Liu, T.; Na, J.; Wang, R.; Fan, Y. Cyclic strain and electrical co-stimulation improve neural differentiation of marrow-derived mesenchymal stem cells. Front. Cell Dev. Biol. 2021, 9, 1–15. [Google Scholar] [CrossRef]
- Kämmerer, P.W.; Engel, V.; Plocksties, F.; Jonitz-Heincke, A.; Timmermann, D.; Engel, N.; Frerich, B.; Bader, R.; Thiem, D.G.E.; Skorska, A.; et al. Continuous electrical stimulation affects initial growth and proliferation of adipose-derived stem cells. Biomedicines 2020, 8, 482. [Google Scholar] [CrossRef]
- Sarno, B.; Heineck, D.; Heller, M.J.; Ibsen, S.D. Dielectrophoresis: Developments and applications from 2010 to 2020. Electrophoresis 2021, 42, 539–564. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.A.; Ibrahim, F.; Yafouz, B. Dielectrophoresis for biomedical sciences applications: A review. Sensors 2017, 17, 449. [Google Scholar] [CrossRef]
- Buyong, M.R.; Larki, F.; Faiz, M.S.; Hamzah, A.A.; Yunas, J.; Majlis, B.Y. A tapered aluminium microelectrode array for improvement of dielectrophoresis-based particle manipulation. Sensors 2015, 15, 10973–10990. [Google Scholar] [CrossRef]
- Agarwal, T.; Maiti, T.K. Dielectrophoresis-Based Devices for Cell Patterning. In Bioelectronics and Medical Devices; Elservier Ltd.: Amsterdam, The Netherlands, 2019; pp. 493–511. ISBN 9780081024201. [Google Scholar]
- Buyong, M.R.; Kayani, A.A.; Hamzah, A.A.; Majlis, B.Y. Dielectrophoresis manipulation: Versatile lateral and vertical mechanisms. Biosensors 2019, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Buyong, M.R.; Larki, F.; Takamura, Y.; Majlis, B.Y. Tapered microelectrode array system for dielectrophoretically filtration: Fabrication, characterization, and simulation study. J. Micro/Nanolithogr. MEMS MOEMS 2017, 16, 044501. [Google Scholar] [CrossRef]
- Deivasigamani, R.; Maidin, N.; Wee, M.; Mohamed, M.; Buyong, M. Dielectrophoresis prototypic polystyrene particle synchronization toward alive keratinocyte cells for rapid chronic wound healing. Sensors 2021, 21, 3007. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheah, Y.J.; Buyong, M.R.; Mohd Yunus, M.H. Wound Healing with Electrical Stimulation Technologies: A Review. Polymers 2021, 13, 3790. https://doi.org/10.3390/polym13213790
Cheah YJ, Buyong MR, Mohd Yunus MH. Wound Healing with Electrical Stimulation Technologies: A Review. Polymers. 2021; 13(21):3790. https://doi.org/10.3390/polym13213790
Chicago/Turabian StyleCheah, Yt Jun, Muhamad Ramdzan Buyong, and Mohd Heikal Mohd Yunus. 2021. "Wound Healing with Electrical Stimulation Technologies: A Review" Polymers 13, no. 21: 3790. https://doi.org/10.3390/polym13213790
APA StyleCheah, Y. J., Buyong, M. R., & Mohd Yunus, M. H. (2021). Wound Healing with Electrical Stimulation Technologies: A Review. Polymers, 13(21), 3790. https://doi.org/10.3390/polym13213790







