Effect of Poly(acrylamide-acrylic acid) on the Fire Resistance and Anti-Aging Properties of Transparent Flame-Retardant Hydrogel Applied in Fireproof Glass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Transparent Flame-Retardant Hydrogel
2.3. Preparation of Fireproof Glass
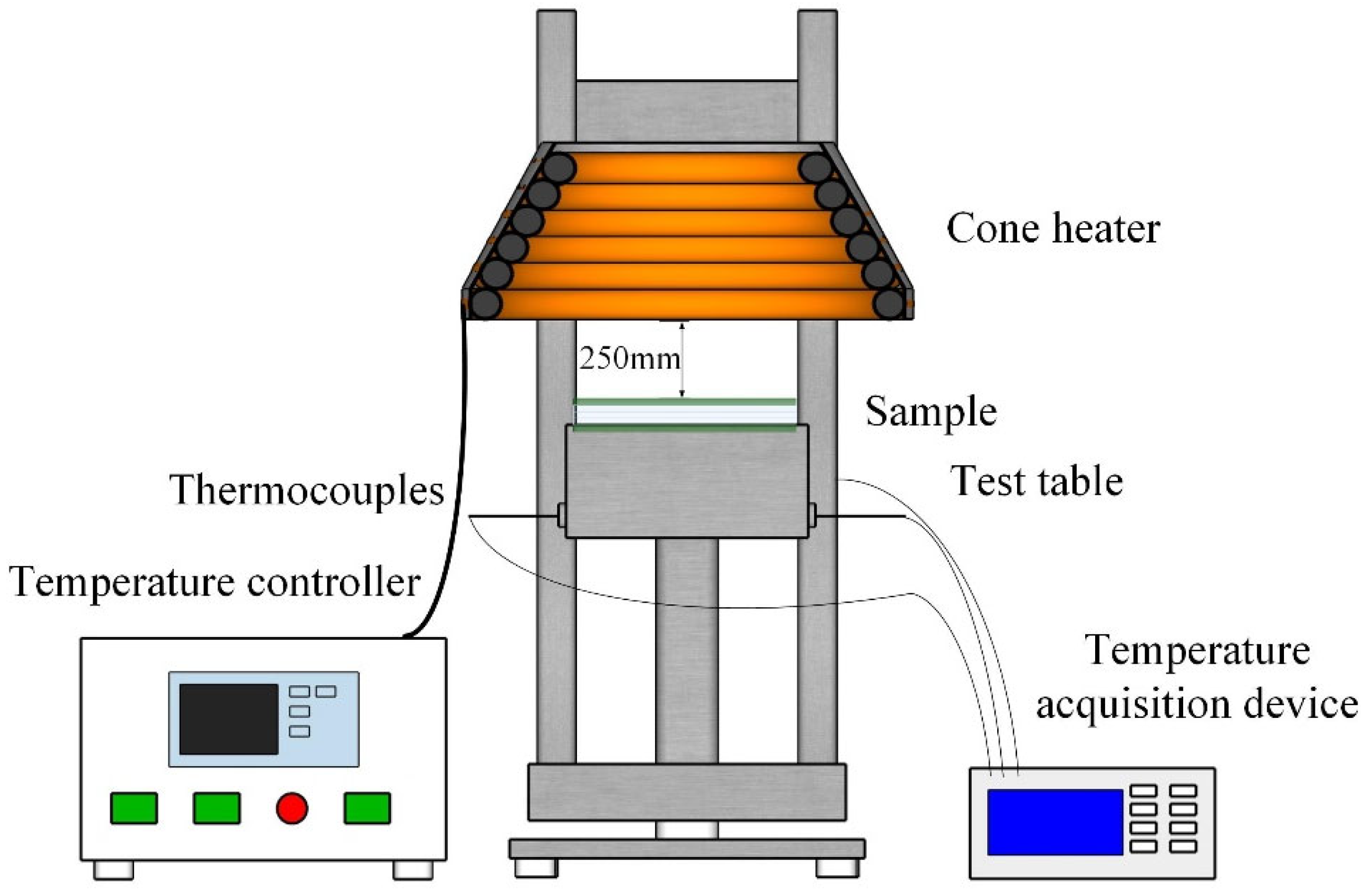
2.4. Measurements and Characterization
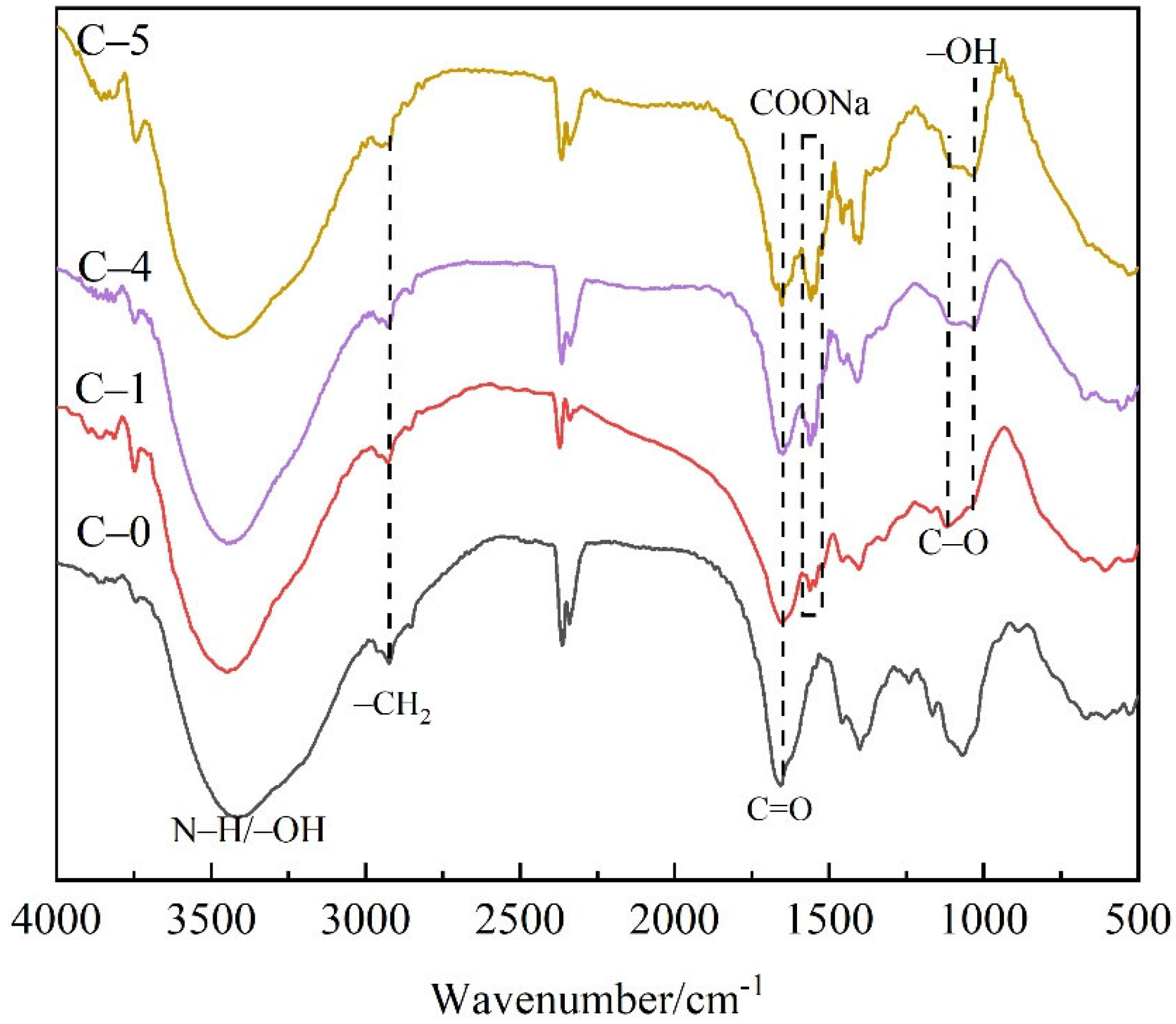
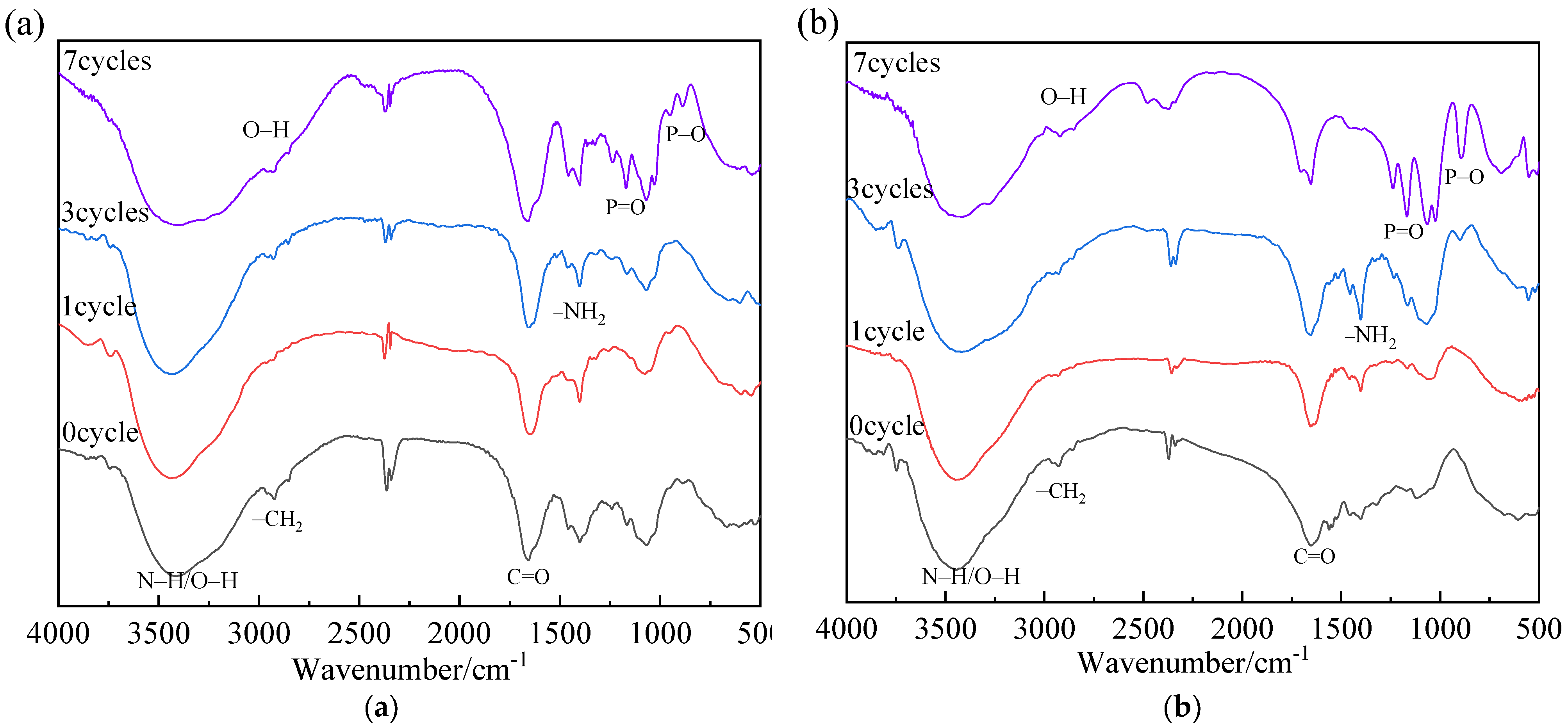
2.4.1. Fourier Transform Infrared (FTIR) Spectroscopy
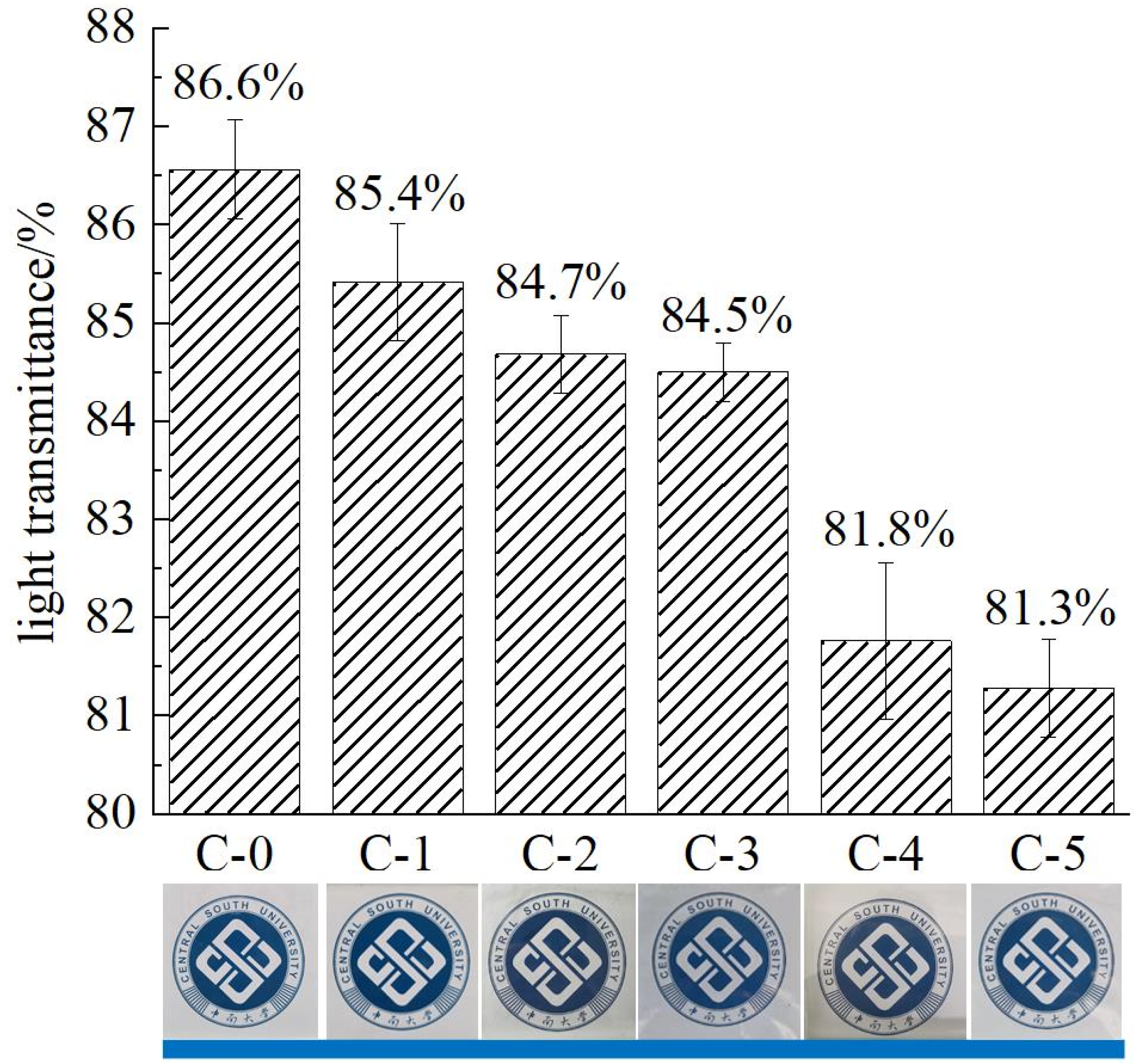
2.4.2. Optical Transparency Analysis
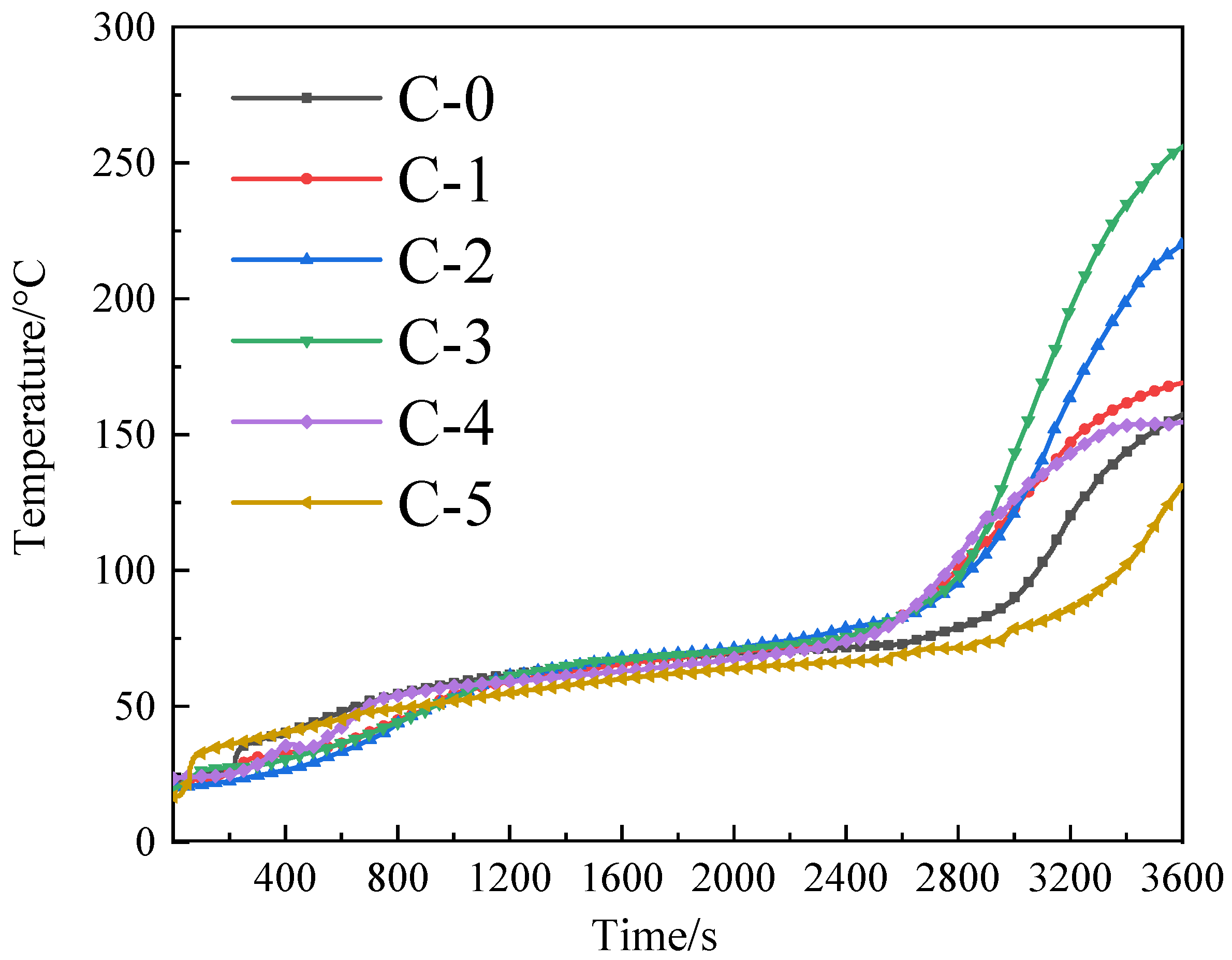
2.4.3. Heat Insulation Test
2.4.4. Thermogravimetric Analysis
2.4.5. Accelerated Aging Test
3. Results and Discussion
3.1. Characterization of Poly (acrylic acid-co-acrylamide)
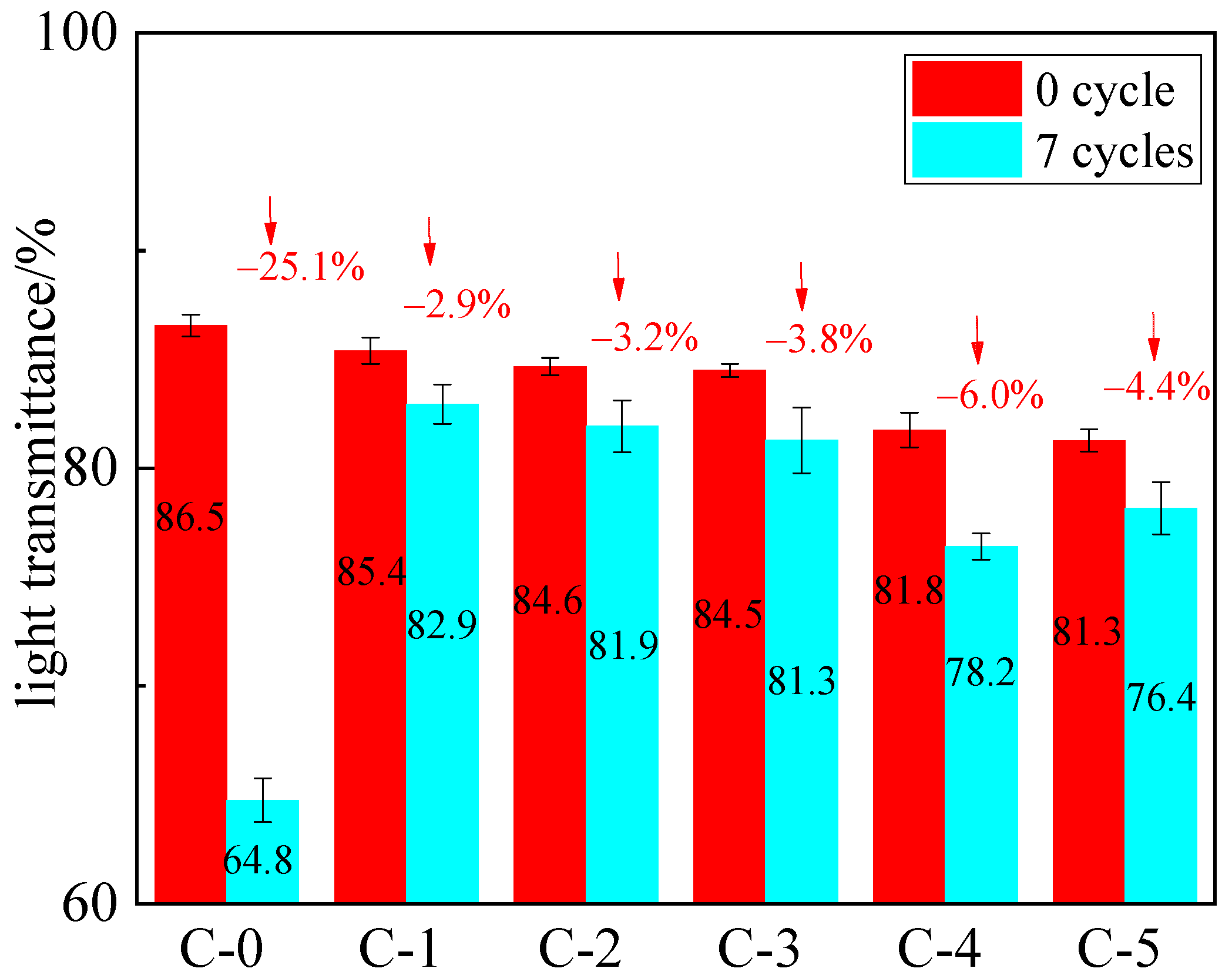
3.2. Optical Transparency Analysis
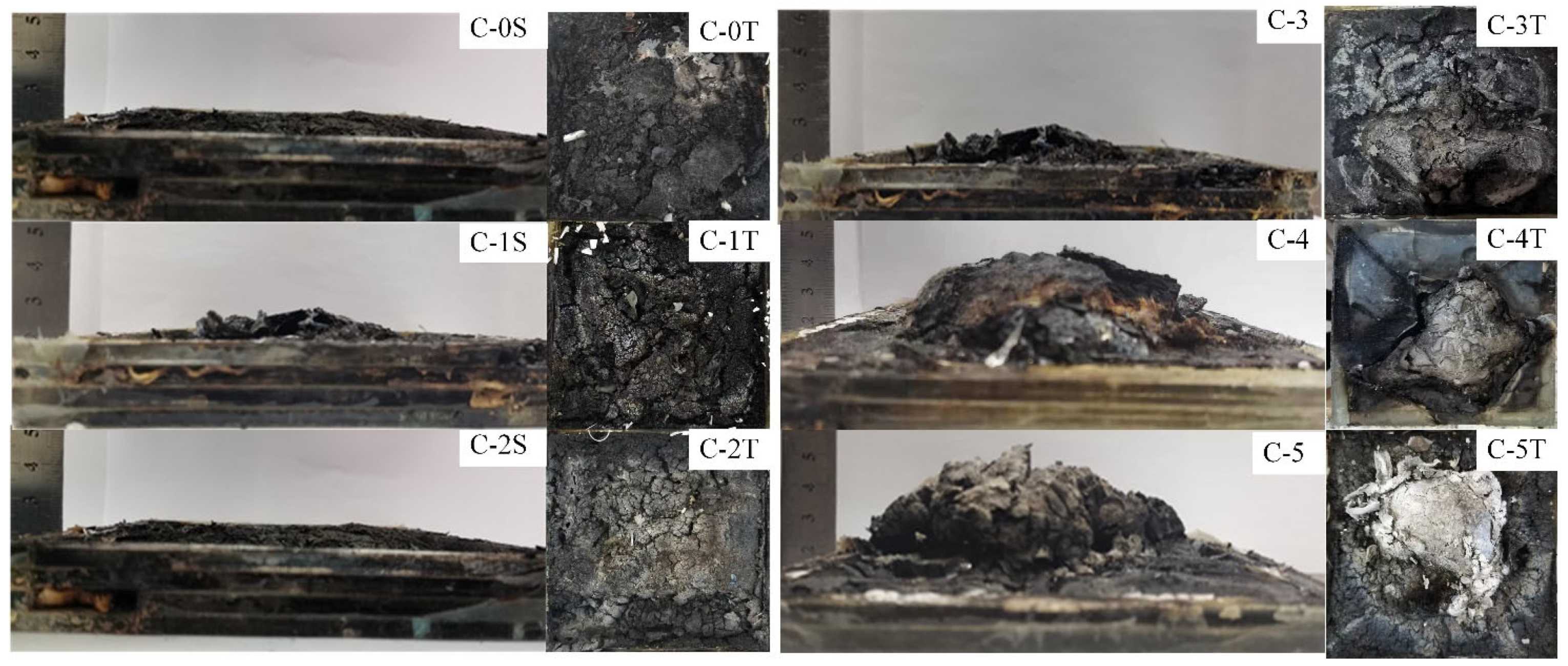
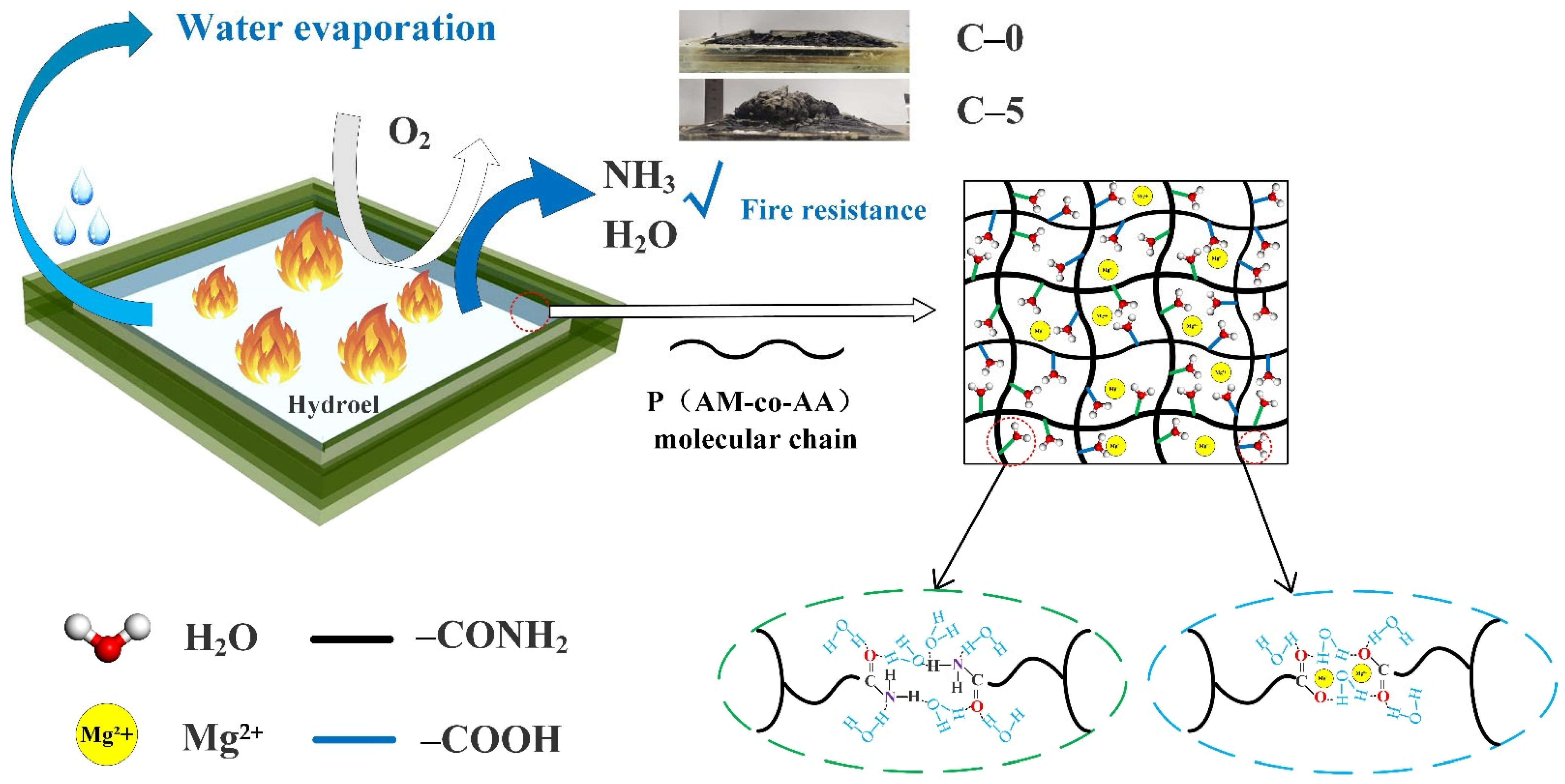
3.3. Fire Resistance Analysis
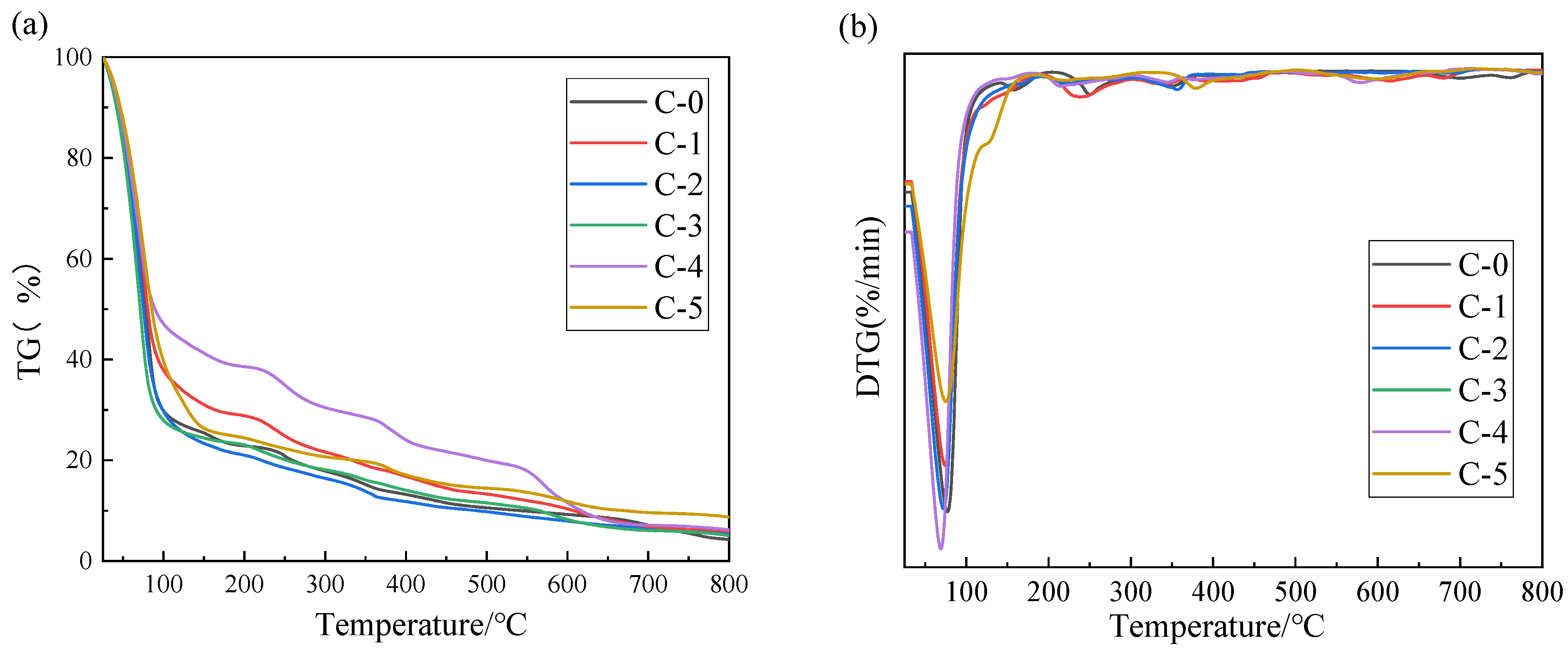
3.4. Thermal Stability Analysis
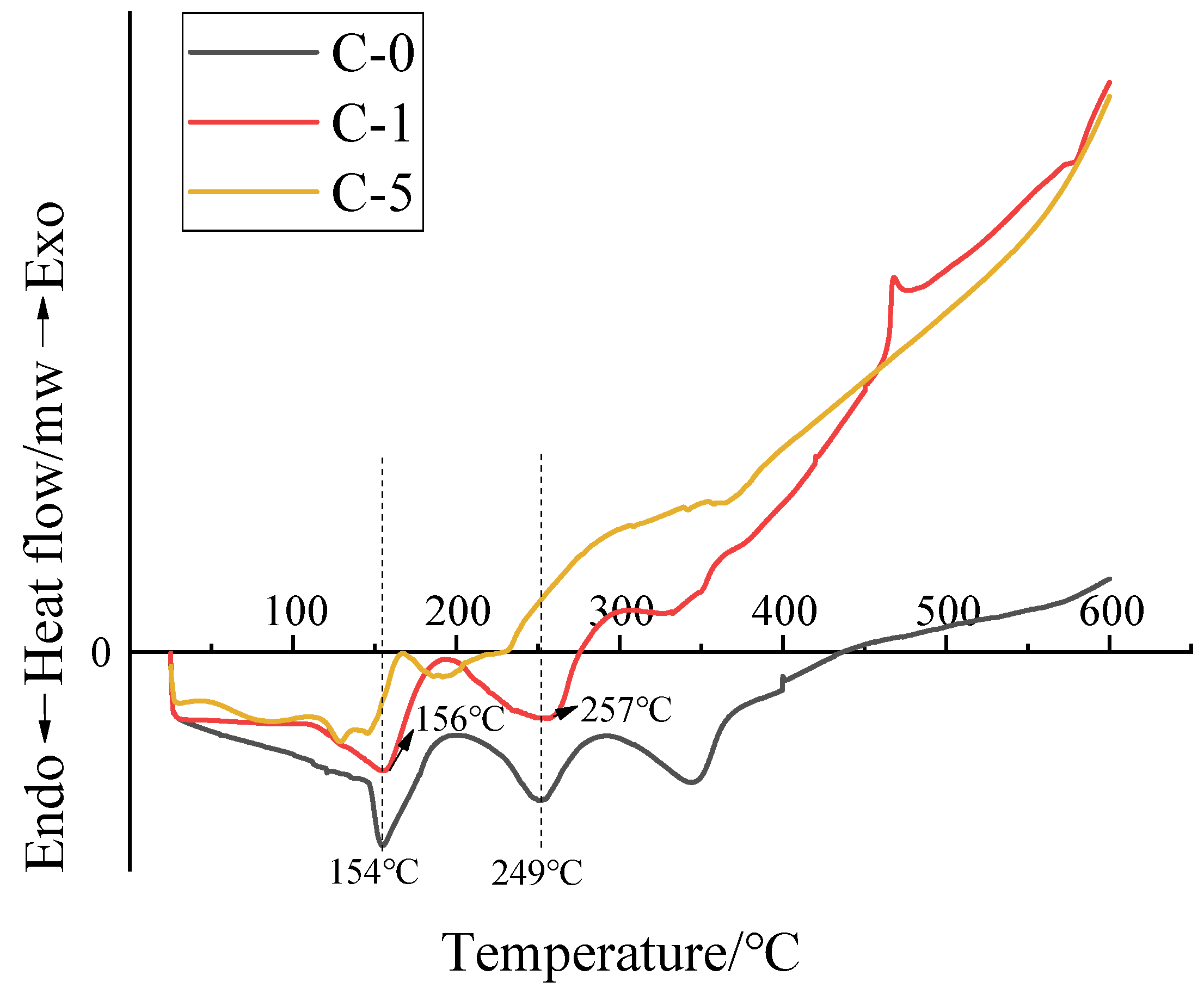
3.5. Anti-Aging Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fitzgerald, R.W. Fire defenses. In Building Fire Performance Analysis, 2nd ed.; John Wiley & Sons Ltd: Chichester, UK, 2004; pp. 11–18. [Google Scholar]
- Park, H.; Meacham, B.J.; Dembsey, N.A.; Goulthorpe, M. Integration of fire safety and building design. Build. Res. Inf. 2014, 42, 696–709. [Google Scholar] [CrossRef]
- Wang, Q. Study of Fire Control Safety of High-Rise Building and Countermeasures. Adv. Mater. Res. 2011, 418–420, 2308–2311. [Google Scholar] [CrossRef]
- Andreev, V.; Poliakova, T.; Grigoryan, M.; Matseevich, T.; Ter-Martirosyan, A.; Adamtsevich, A. Fire safety issues in the design and construction of high-rise buildings. MATEC Web Conf. 2018, 196, 418–420. [Google Scholar] [CrossRef]
- Bai, B.; Wang, Y.; Lu, L.; Wang, J.; Zhang, D. Fire Resistance and Heat Insulation Properties of Perfusion Type Fireproof Glass. IOP Conf. Ser. Mater. Sci. Eng. 2019, 493, 012009. [Google Scholar] [CrossRef]
- Kotlík, P.; Doubravová, K.; Horálek, J.; Kubáč, L.; Akrman, J. Acrylic copolymer coatings for protection against UV rays. J. Cult. Herit. 2014, 15, 44–48. [Google Scholar] [CrossRef]
- Cui, X.F.; Zhang, W.J.; Zou, W.; Liu, X.; Yang, H.; Yan, J.; Gao, Y. Water-retaining, tough and self-healing hydrogels and their uses as fire-resistant materials. Polym. Chem. 2019, 10, 5151–5158. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Nguyen, T.P.; Nguyen, T.D.; Rachid, E.A.; Trinh, V.T.; Decker, C. Accelerated degradation of water borne acrylic nanocomposites used in outdoor protective coatings. Polym. Degrad. Stab. 2016, 128, 65–76. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.K.; Seyfoddin, A.; Fatihhi, S.J. Acrylic acid/acrylamide-based hydrogels and its properties-A review. Polym. Degrad. Stab. 2020, 180, 308. [Google Scholar] [CrossRef]
- Yang, M.; Liu, C.; Li, Z.; Gao, G.; Liu, F. Temperature-Responsive Properties of Poly (acrylic acid-co-acrylamide) Hydrophobic Association Hydrogels with High Mechanical Strength. Macromolecules 2010, 43, 10645–10651. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Cheng, W.; Shao, Z.; Xue, D.; Zhao, Y.; Lu, W. A novel high-toughness, organic/inorganic double-network fire-retardant gel for coal-seam with high ground temperature. Fuel 2020, 263, 116779. [Google Scholar] [CrossRef]
- Cheng, W.; Hu, X.; Xie, J.; Zhao, Y. An intelligent gel designed to control the spontaneous combustion of coal: Fire prevention and extinguishing properties. Fuel 2017, 210, 826–835. [Google Scholar] [CrossRef]
- Xie, J.; Liu, X.; Liang, J.; Liang, J. Absorbency and adsorption of poly (acrylic acid-co-acrylamide) hydrogel. J. Appl. Polym. Sci. 2007, 106, 1606–1613. [Google Scholar] [CrossRef]
- Ariaeenejad, S.; Hosseini, E.; Motamedi, E.; Moosavi-Movahedi, A.A.; Salekdeh, G.H. Application of carboxymethyl cellulose-g-poly (acrylic acid-co-acrylamide) hydrogel sponges for improvement of efficiency, reusability and thermal stability of a recombinant xylanase. Chem. Eng. J. 2019, 375, 122022. [Google Scholar] [CrossRef]
- Zahra, Q.; Minhas, M.U.; Khan, S.; Wu, P.; Suhail, M.; Iqbal, R.; Bashir, M. Fabrication of polyethylene glycol hydrogels with enhanced swelling; loading capacity and release kinetics. Polym. Bull. 2021. [Google Scholar] [CrossRef]
- Mehra, S.; Nisar, S.; Chauhan, S.; Singh, V.; Rattan, S. Soy Protein-Based Hydrogel under Microwave-Induced Grafting of Acrylic Acid and 4-(4-Hydroxyphenyl) butanoic Acid: A Potential Vehicle for Controlled Drug Delivery in Oral Cavity Bacterial Infections. ACS Omega 2020, 5, 21610–21622. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.S.; Ibrahim, H.M.M. Characterization and antimicrobial properties of metal complexes of polypropylene fibers grafted with acrylic acid using gamma irradiation. Polym. Adv. Technol. 2016, 27, 532–541. [Google Scholar] [CrossRef]
- Xu, Z.; Xie, X.; Yan, L.; Feng, Y. Fabrication of organophosphate-grafted kaolinite and its effect on the fire-resistant and anti-aging properties of amino transparent fire-retardant coatings. Polym. Degrad. Stab. 2021, 188, 109589. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, Z.; Zhao, T.; Liu, M.; Hu, M.; Li, J. Synthesis, characterization, and swelling behaviors of salt-sensitive maize bran-poly(acrylic acid) superabsorbent hydrogel. J. Agric. Food Chem 2014, 62, 8867–8874. [Google Scholar] [CrossRef]
- Gong, Z.; Niu, F.; Zhang, G.; Li, J.; Li, G.; Huang, W.; Deng, H.; Sun, R.; Wong, C. Effects of composition on the properties of dual physically cross-linked hydrogel composed of polyvinyl alcohol and poly (acrylamide-co-acrylic acid). J. Polym. Res. 2017, 24, 127. [Google Scholar] [CrossRef]
- Loste, J.; Lopez-Cuesta, J.-M.; Billon, L.; Garay, H.; Save, M. Transparent polymer nanocomposites: An overview on their synthesis and advanced properties. Prog. Polym. Sci. 2019, 89, 133–158. [Google Scholar] [CrossRef]
- Mastalska-Popławska, J.; Izak, P.; Wójcik, Ł.; Stempkowska, A.; Góral, Z.; Krzyżak, A.; Habina, I. Synthesis and characterization of cross-linked poly (sodium acrylate)/sodium silicate hydrogels. Polym. Eng. Sci. 2019, 59, 1279–1287. [Google Scholar] [CrossRef]
- Mostovoy, A.S.; Nurtazina, A.S.; Kadykova, Y.; Bekeshev, A.Z. Highly Efficient Plasticizers-Antipirenes for Epoxy Polymers. Inorg. Mater. Appl. Res. 2019, 10, 1135–1139. [Google Scholar] [CrossRef]
- Burmistrov, I.; Vikulova, M.; Panova, L.; Yudintseva, T. Development of acrylate-based polymeric layers for fireproof laminated glass. Prospect. Fundam. Sci. Dev. 2017, 1899, 9828. [Google Scholar] [CrossRef]
- Li, Z.; Wei, P.; Yang, Y.; Yan, Y.; Shi, D. Synthesis of a hyperbranched poly (phosphamide ester) oligomer and its high-effective flame retardancy and accelerated nucleation effect in polylactide composites. Polym. Degrad. Stab. 2014, 110, 104–112. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Wang, X.; Wang, X. Synergistic flame-retardant and smoke suppression effects of zinc borate in transparent intumescent fire-retardant coatings applied on wood substrates. J. Therm. Anal. Calorim. 2018, 136, 1563–1574. [Google Scholar] [CrossRef]
- Lizárraga-Laborín, L.L.; Quiroz-Castillo, J.M.; Encinas-Encinas, J.C.; Castillo-Ortega, M.M.; Burruel-Ibarra, S.E.; Romero-García, J.; Torres-Ochoa, J.A.; Cabrera-Germán, D.; Rodríguez-Félix, D.E. Accelerated weathering study of extruded polyethylene/poly (lactic acid)/chitosan films. Polym. Degrad. Stab. 2018, 155, 43–51. [Google Scholar] [CrossRef]
- Grabmayer, K.; Wallner, G.M.; Beißmann, S.; Braun, U.; Steffen, R.; Nitsche, D.; Röder, B.; Buchberger, W.; Lang, R.W. Accelerated aging of polyethylene materials at high oxygen pressure characterized by photoluminescence spectroscopy and established aging characterization methods. Polym. Degrad. Stab. 2014, 109, 40–49. [Google Scholar] [CrossRef]



| Samples | AM/% | AA/% | MBA/% | Initiator/% | Flame Retardants /% | Deionized Water/% |
|---|---|---|---|---|---|---|
| C-0 | 9 | 0 | 0.075 | 0.09 | 13 | 78 |
| C-1 | 7.2 | 1.8 | 0.075 | 0.09 | 13 | 78 |
| C-2 | 6.75 | 2.25 | 0.075 | 0.09 | 13 | 78 |
| C-3 | 6 | 3 | 0.075 | 0.09 | 13 | 78 |
| C-4 | 4.5 | 4.5 | 0.075 | 0.09 | 13 | 78 |
| C-5 | 3 | 6 | 0.075 | 0.09 | 13 | 78 |
| Samples | Before Aging Test | After Aging Test | ||||
|---|---|---|---|---|---|---|
| Temperature at 3600 s | Temperature Range in Stable Stage | Temperature at 3600 s | Changes | Temperature Range in Stable Stage | Changes | |
| C-0 | 158.0 °C | 80–100 °C | 485.9 °C | 327.9 °C | 100–120 °C | Increased 20 °C |
| C-1 | 169.1 °C | 173.1 °C | 4 °C | |||
| C-2 | 220.1 °C | 335.4 °C | 115.4 °C | |||
| C-3 | 255.2 °C | 395.7 °C | 140.7 °C | |||
| C-4 | 154.5 °C | 170.6 °C | 16.1 °C | |||
| C-5 | 131.4 °C | 152.0 °C | 20.6 °C | |||
| IR Band cm−1 | Functional Groups | After 7 Cycles | Observations | |
|---|---|---|---|---|
| Intensity | Changes | |||
| 2920 | C–H stretching in alkane | − | Weak | Significantly decreased |
| 1672 | C=O stretching in acrylamide | + | Strong | Significantly Increased |
| 1150 | C–O stretching in ester group | + | Strong | Slightly Increased |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Cai, M.; Yan, L. Effect of Poly(acrylamide-acrylic acid) on the Fire Resistance and Anti-Aging Properties of Transparent Flame-Retardant Hydrogel Applied in Fireproof Glass. Polymers 2021, 13, 3668. https://doi.org/10.3390/polym13213668
Wang F, Cai M, Yan L. Effect of Poly(acrylamide-acrylic acid) on the Fire Resistance and Anti-Aging Properties of Transparent Flame-Retardant Hydrogel Applied in Fireproof Glass. Polymers. 2021; 13(21):3668. https://doi.org/10.3390/polym13213668
Chicago/Turabian StyleWang, Feiyue, Mengtao Cai, and Long Yan. 2021. "Effect of Poly(acrylamide-acrylic acid) on the Fire Resistance and Anti-Aging Properties of Transparent Flame-Retardant Hydrogel Applied in Fireproof Glass" Polymers 13, no. 21: 3668. https://doi.org/10.3390/polym13213668
APA StyleWang, F., Cai, M., & Yan, L. (2021). Effect of Poly(acrylamide-acrylic acid) on the Fire Resistance and Anti-Aging Properties of Transparent Flame-Retardant Hydrogel Applied in Fireproof Glass. Polymers, 13(21), 3668. https://doi.org/10.3390/polym13213668







