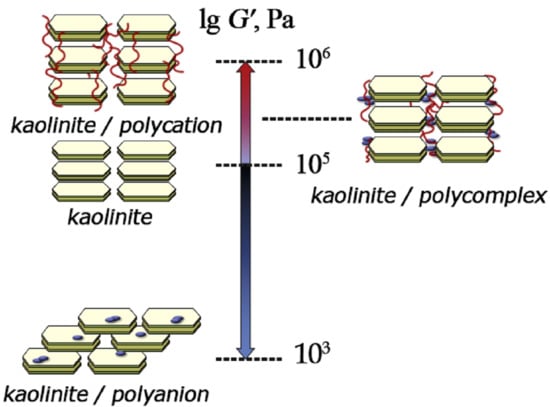
A Dramatic Change in Rheological Behavior of a Clay Material Caused by a Minor Addition of Hydrophilic and Amphiphilic Polyelectrolytes
Abstract
1. Introduction
2. Materials and Methods
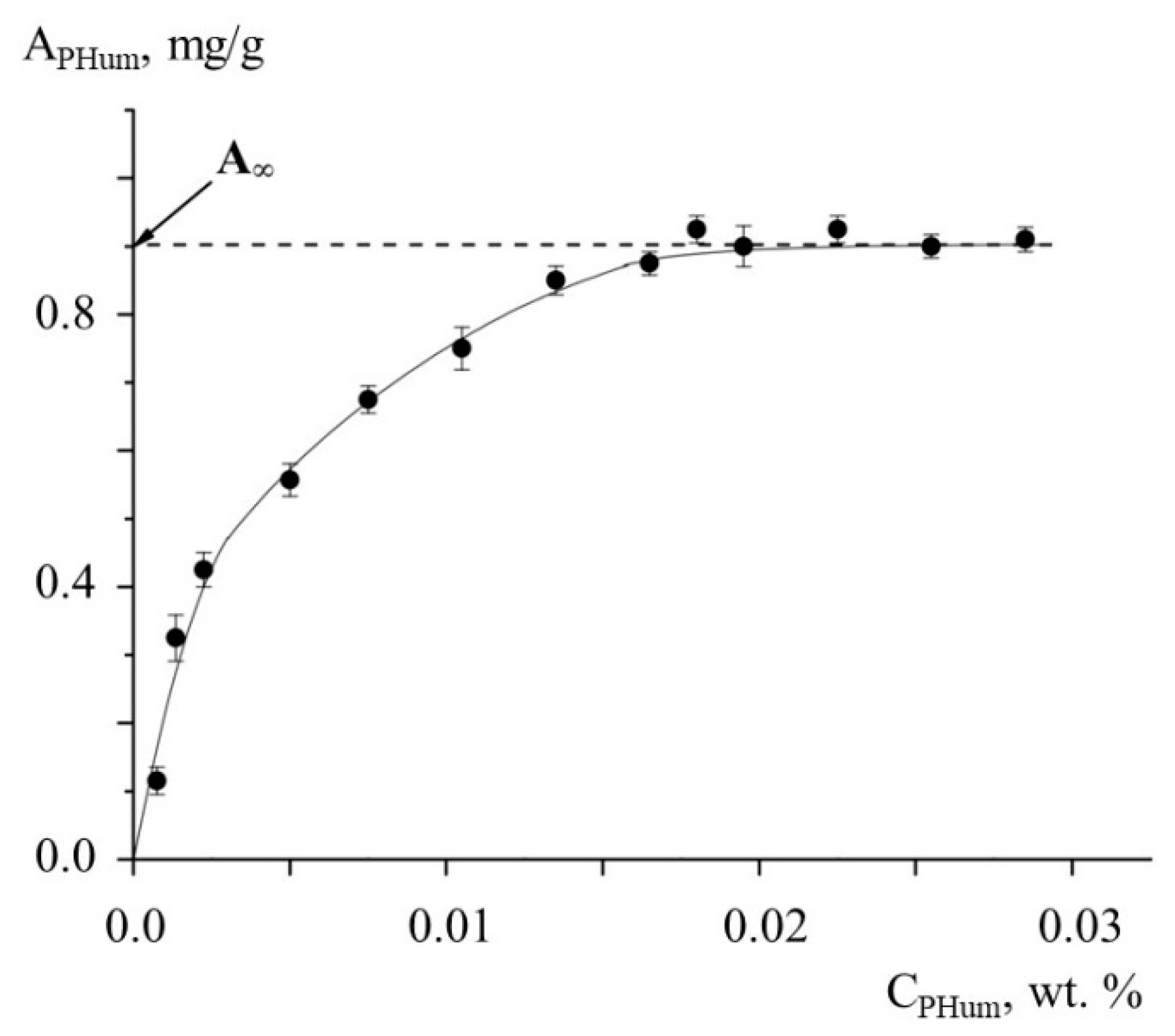
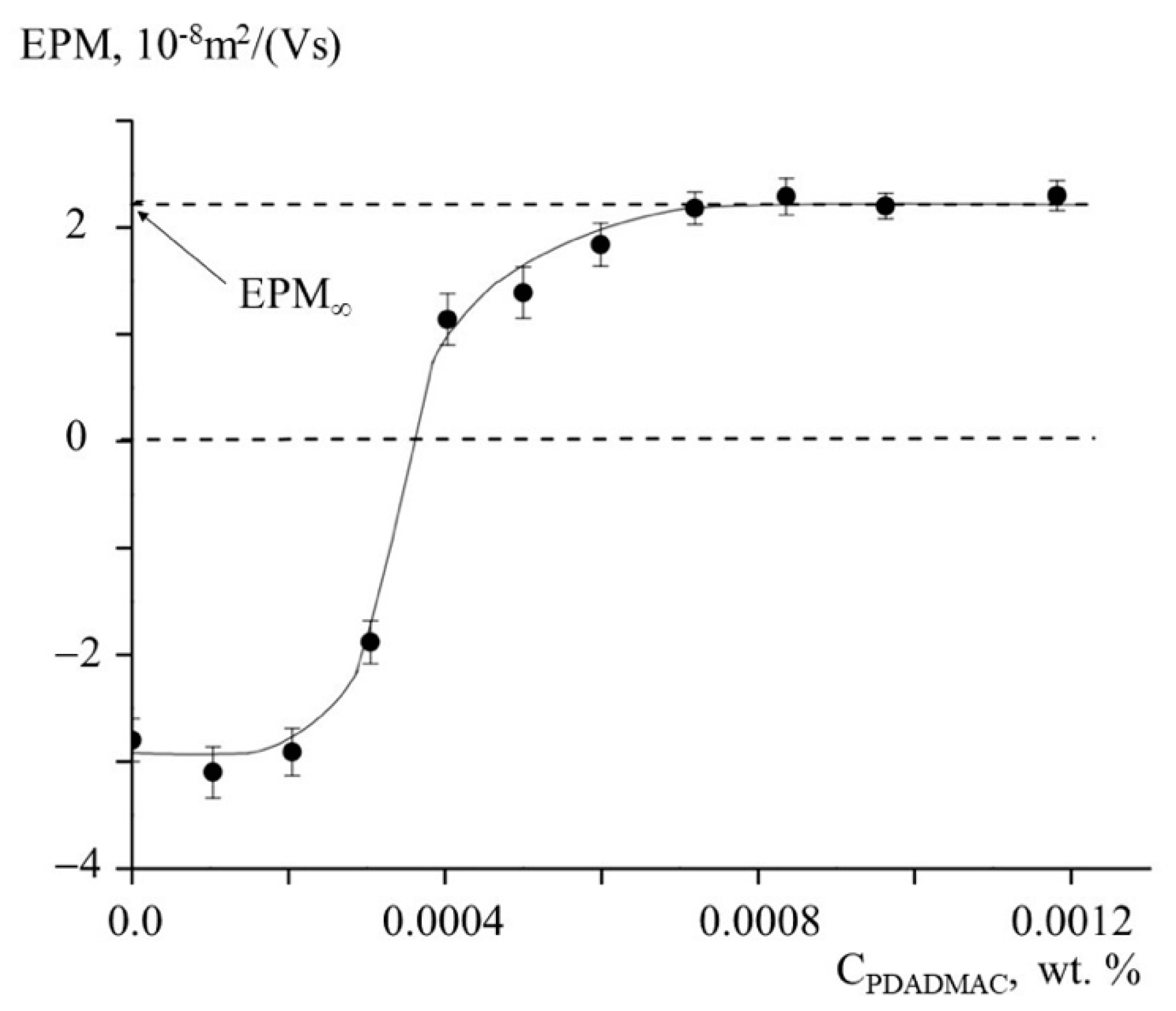
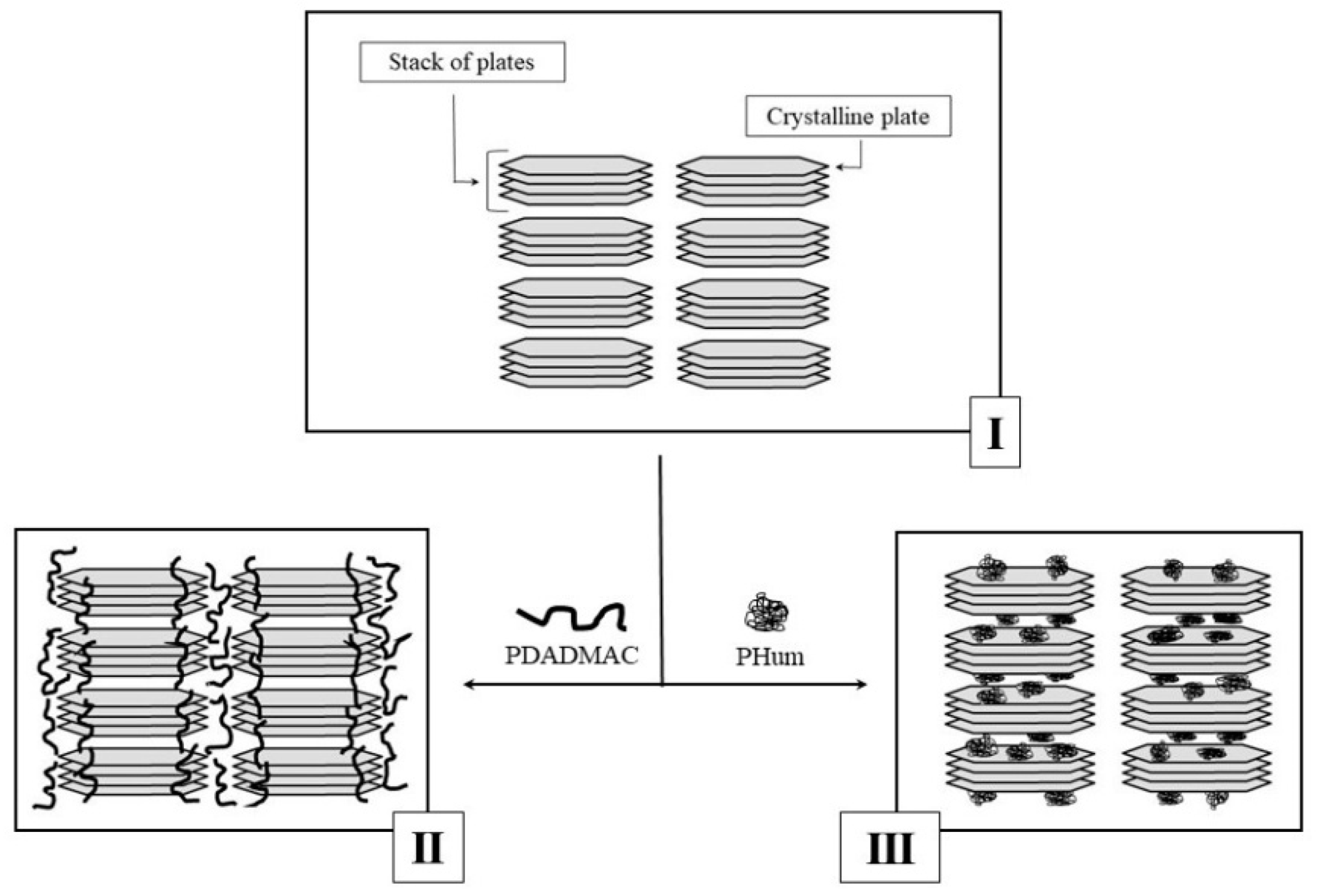
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kotal, M.; Bhowmick, A.K. Polymer nanocomposites from modified clays: Recent advances and challenges. Prog. Polym. Sci. 2015, 51, 127–187. [Google Scholar] [CrossRef]
- Khalifa, A.Z.; Cizer, Ö.; Pontikes, Y.; Heath, A.; Patureau, P.; Bernal, S.A.; Marsh, A.T. Advances in alkali-activation of clay minerals. Cem. Concr. Res. 2020, 132, 106050. [Google Scholar] [CrossRef]
- Rafati, R.; Smith, S.R.; Haddad, A.S.; Novara, R.; Hamidi, H. Effect of nanoparticles on the modifications of drilling fluids properties: A review of recent advances. J. Pet. Sci. Eng. 2018, 161, 61–76. [Google Scholar] [CrossRef]
- Vieira, C.M.F.; Pinheiro, R.M.; Rodriguez, R.J.S.; Candido, V.S.; Monteiro, S.N. Clay bricks added with effluent sludge from paper industry: Technical, economical and environmental benefits. Appl. Clay Sci. 2016, 132–133, 753–759. [Google Scholar] [CrossRef]
- Al Rashid, A.; Khan, S.A.; Al-Ghamdi, S.G.; Koç, M. Additive manufacturing: Technology, applications, markets, and opportunities for the built environment. Autom. Constr. 2020, 118, 103268. [Google Scholar] [CrossRef]
- Moraes, J.D.D.; Bertolino, S.R.A.; Cuffini, S.L.; Ducart, D.F.; Bretzke, P.E.; Leonardi, G.R. Clay minerals: Properties and applications to dermocosmetic products and perspectives of natural raw materials for therapeutic purposes—A review. Int. J. Pharm. 2017, 534, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Ion, R.M.; Fierăscu, R.C.; Teodorescu, S.; Fierăscu, I.; Bunghez, I.R.; Ţurcanu-Caruţiu, D.; Ion, M.L. Ceramic materials based on clay minerals in cultural heritage study. Clays Clay Miner. Ceram. Mater. Based Clay Miner. 2016, 159–184. [Google Scholar] [CrossRef]
- Bergaya, F.; Theng, B.K.G.; Lagaly, G. Handbook of Clay Science. Developments in Clay Science 1; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Shaikh, S.; Nasser, M.S.; Hussein, I.; Benamor, A.; Onaizi, S.A.; Qiblawey, H. Influence of polyelectrolytes and other polymer complexes on the flocculation and rheological behaviors of clay minerals: A comprehensive review. Sep. Purif. Technol. 2017, 187, 137–161. [Google Scholar] [CrossRef]
- Arzhakov, M. Relaxation in Physical and Mechanical Behavior of Polymers; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: London, UK, 2019. [Google Scholar]
- Zadorozhnyy, V.; Gorshenkov, M.; Churyukanova, M.; Stepashkin, A.; Moskovskikh, D.; Ketov, S.; Zinnurova, L.; Sharma, A.; Louzguine-Luzgin, D.; Kaloshkin, S. Investigation of structure and thermal properties in composite materials based on metallic glasses with small addition of polytetrafluoroethylene. J. Alloys Compd. 2017, 707, 264–268. [Google Scholar] [CrossRef]
- De Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef]
- Panova, I.G.; Khaydapova, D.D.; Ilyasov, L.O.; Umarova, A.B.; Yaroslavov, A.A. Polyelectrolyte complexes based on natural macromolecules for chemical sand/soil stabilization. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 590, 124504. [Google Scholar] [CrossRef]
- Mezger, T. The Rheology Handbook; Vincentz Network: Hannover, Germany, 2020. [Google Scholar]
- Khaydapova, D.; Milanovskiy, E.; Shein, E. Rheological properties of different minerals and clay soils. Eurasian J. Soil Sci. 2015, 4, 198. [Google Scholar] [CrossRef][Green Version]
- Yu, J.; Wang, D.; Ge, X.; Yan, M.; Yang, M. Flocculation of kaolin particles by two typical polyelectrolytes: A comparative study on the kinetics and floc structures. Colloids Surf. A Physicochem. Eng. Asp. 2006, 290, 288–294. [Google Scholar] [CrossRef]
- Tian, B.; Ge, X.; Pan, G.; Fan, B.; Luan, Z. Adsorption and flocculation behaviors of polydiallyldimethylammonium (PDADMA) salts: Influence of counterion. Int. J. Miner. Process. 2006, 79, 209–216. [Google Scholar] [CrossRef]
- Sun, W.; Liu, W.-L.; Hu, Y.-H. FTIR analysis of adsorption of poly diallyl-dimethyl-ammonium chloride on kaolinite. J. Cent. South Univ. Technol. 2008, 15, 373–377. [Google Scholar] [CrossRef]
- Duman, O.; Tunç, S.; Çetinkaya, A. Electrokinetic and rheological properties of kaolinite in poly(diallyldimethylammonium chloride), poly(sodium 4-styrene sulfonate) and poly(vinyl alcohol) solutions. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 394, 23–32. [Google Scholar] [CrossRef]
- Mietta, F.; Chassagne, C.; Winterwerp, J. Shear-induced flocculation of a suspension of kaolinite as function of pH and salt concentration. J. Colloid Interface Sci. 2009, 336, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Murray, H.H. Chapter 2 Structure and Composition of the Clay Minerals and their Physical and Chemical Properties. Dev. Clay Sci. 2006, 2, 7–31. [Google Scholar] [CrossRef]
- Feng, X.; Simpson, A.J.; Simpson, M. Chemical and mineralogical controls on humic acid sorption to clay mineral surfaces. Org. Geochem. 2005, 36, 1553–1566. [Google Scholar] [CrossRef]
- Trzciński, S.; Vårum, K.M.; Staszewska, D.U.; Smidsrød, O.; Bohdanecký, M. Comparative studies on molecular chain parameters of chitosans and poly(diallyldimethylammonium chloride): The stiffness B-parameter and the temperature coefficient of intrinsic viscosity. Carbohydr. Polym. 2002, 48, 171–178. [Google Scholar] [CrossRef]
- Aparicio, P.; Perez-Bernal, J.; Galán, E.; Bello, M.; Fernández, P.A. Kaolin fractal dimension. Comparison with other properties. Clay Miner. 2004, 39, 75–84. [Google Scholar] [CrossRef][Green Version]
- Piccolo, A. The Supramolecular Structure of Humic Substances. Soil Sci. 2001, 166, 810–832. [Google Scholar] [CrossRef]
- Panova, I.; Drobyazko, A.; Spiridonov, V.; Sybachin, A.; Kydralieva, K.; Jorobekova, S.; Yaroslavov, A. Humics-based interpolyelectrolyte complexes for antierosion protection of soil: Model investigation. Land Degrad. Dev. 2018, 30, 337–347. [Google Scholar] [CrossRef]
- Hillel, D. Introduction to Soil Physics; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]

| No. | Sample | εin, % | |||
|---|---|---|---|---|---|
| 1 | Kaolinite | 212.0 ± 10.0 | 70.0 ± 5.0 | 0.52 ± 0.04 | 9.8 ± 0.8 |
| 2 | Kaolinite/PDADMAC | 2080.0 ± 24.0 | 352.0 ± 69.0 | 115.0 ± 8.0 | 0.75 ± 0.14 |
| 3 | Kaolinite/IPEC(+) | 664.0 ± 62.0 | 182.0 ± 12.0 | 6.4 ± 0.5 | 3.8 ± 0.32 |
| 4 | Kaolinite/IPEC(−) | 410.0 ± 26.0 | 127.0 ± 10.0 | 1.67 ± 0.14 | 5.2 ± 0.3 |
| 5 | Kaolinite/PHum | 3.5 ± 0.8 | 0.67 ± 0.06 | 0.027 ± 0.003 | 73.6 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panova, I.G.; Kiushov, A.A.; Khaydapova, D.D.; Zezin, S.B.; Arzhakov, M.S.; Yaroslavov, A.A. A Dramatic Change in Rheological Behavior of a Clay Material Caused by a Minor Addition of Hydrophilic and Amphiphilic Polyelectrolytes. Polymers 2021, 13, 3662. https://doi.org/10.3390/polym13213662
Panova IG, Kiushov AA, Khaydapova DD, Zezin SB, Arzhakov MS, Yaroslavov AA. A Dramatic Change in Rheological Behavior of a Clay Material Caused by a Minor Addition of Hydrophilic and Amphiphilic Polyelectrolytes. Polymers. 2021; 13(21):3662. https://doi.org/10.3390/polym13213662
Chicago/Turabian StylePanova, Irina G., Alexander A. Kiushov, Dolgor D. Khaydapova, Sergey B. Zezin, Maxim S. Arzhakov, and Alexander A. Yaroslavov. 2021. "A Dramatic Change in Rheological Behavior of a Clay Material Caused by a Minor Addition of Hydrophilic and Amphiphilic Polyelectrolytes" Polymers 13, no. 21: 3662. https://doi.org/10.3390/polym13213662
APA StylePanova, I. G., Kiushov, A. A., Khaydapova, D. D., Zezin, S. B., Arzhakov, M. S., & Yaroslavov, A. A. (2021). A Dramatic Change in Rheological Behavior of a Clay Material Caused by a Minor Addition of Hydrophilic and Amphiphilic Polyelectrolytes. Polymers, 13(21), 3662. https://doi.org/10.3390/polym13213662