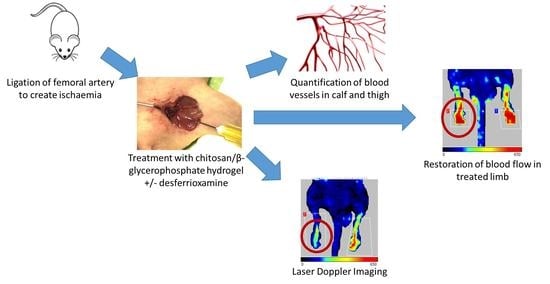
A Thermoresponsive Chitosan/β-Glycerophosphate Hydrogel for Minimally Invasive Treatment of Critical Limb Ischaemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
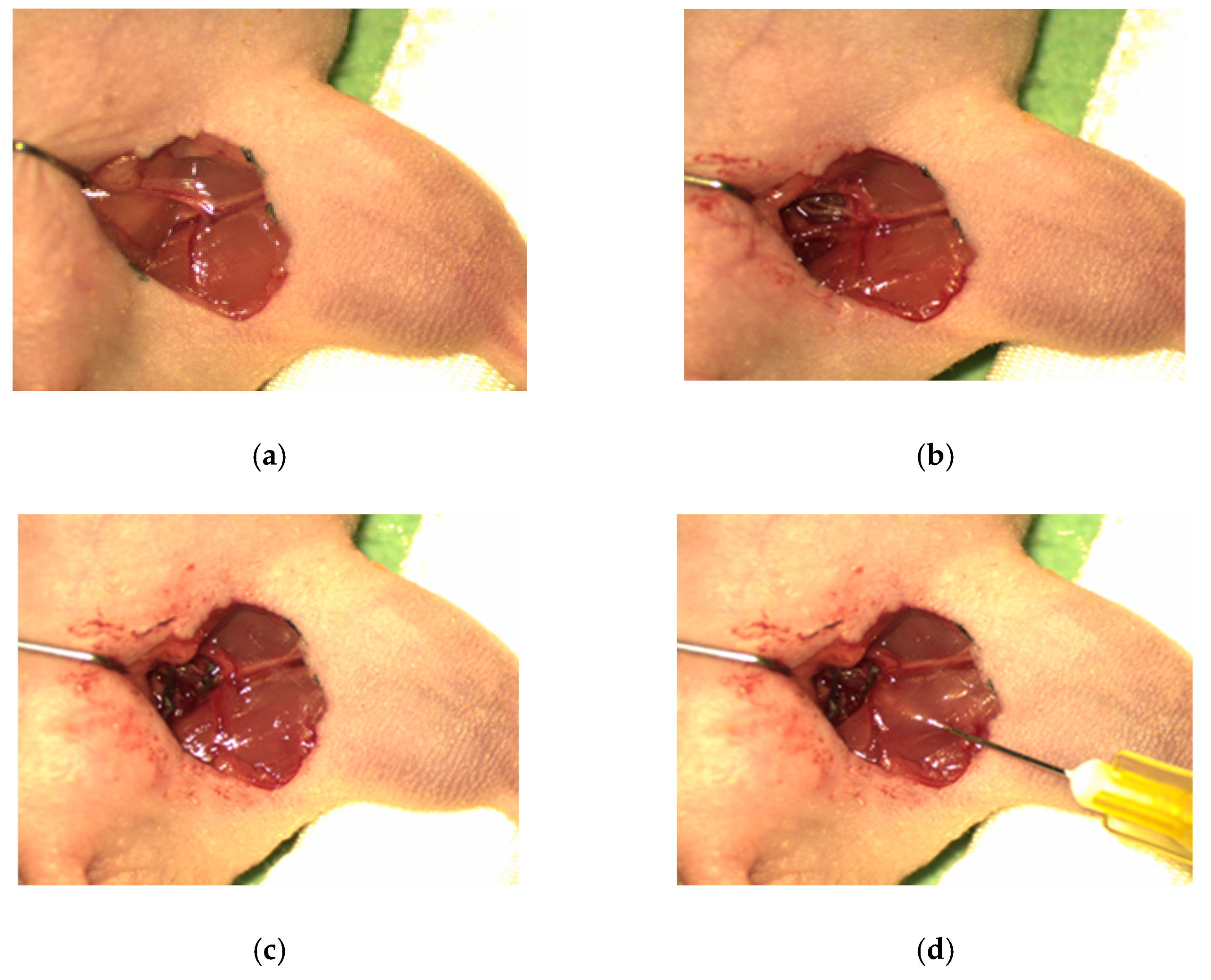
2.2. Animal Model
2.3. Preparation of Thermoresponsive Chitosan/β-GP Formulations
2.4. Assessment of Perfusion via Laser Doppler Imaging
2.5. Assessment of Ambulatory Impairment and Ischaemic Damage
2.6. Tissue Processing
2.7. Tissue Sectioning
2.8. Tissue Staining—CD31
2.9. Quantification of Capillary Density
2.10. Statistical Analysis
3. Results
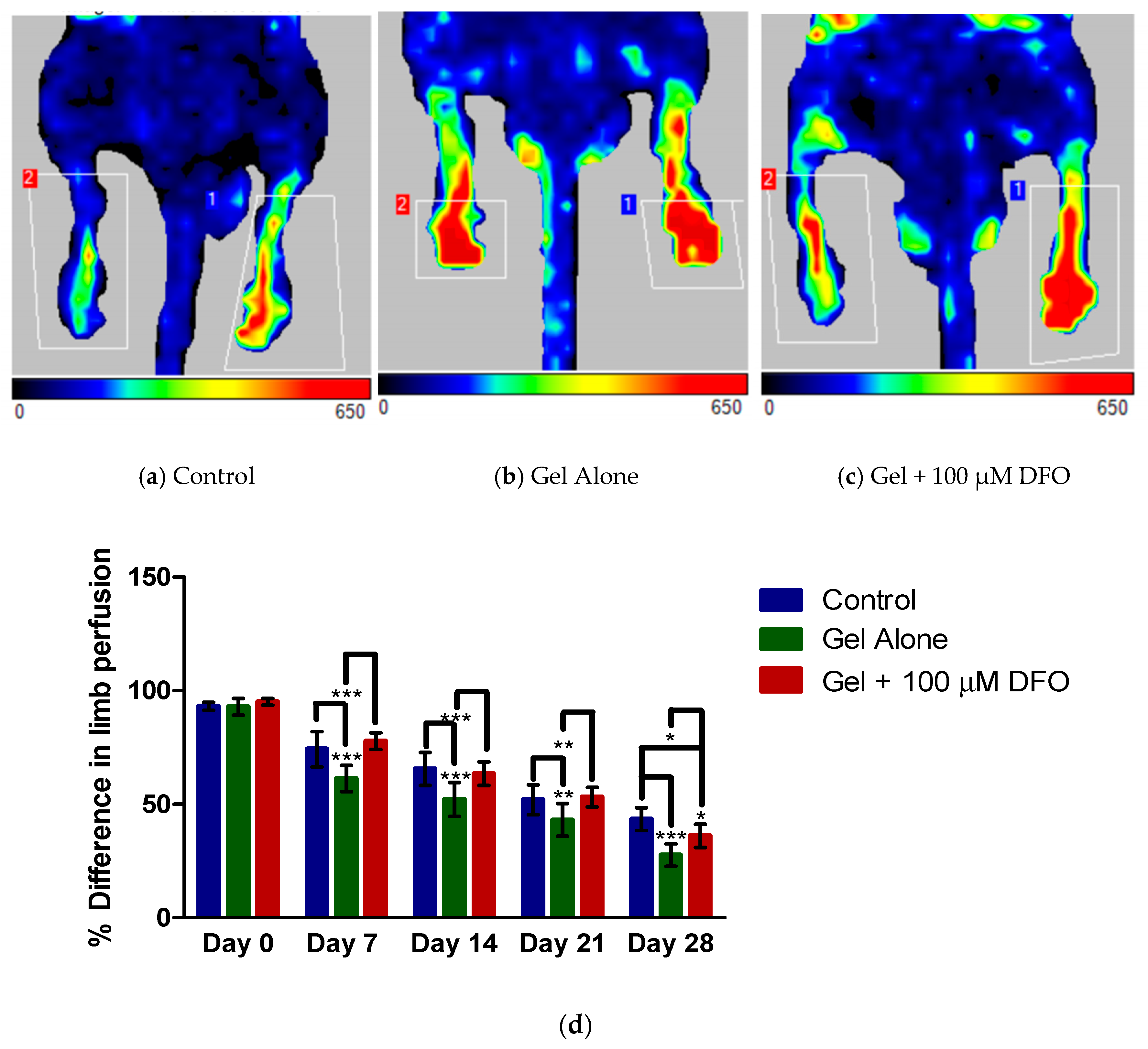
3.1. Laser Doppler Imaging: Assessment of Perfusion
3.2. Laser Doppler Imaging: Comparison between Groups
3.3. Assessment of Ambulatory Impairment and Ischaemic Damage
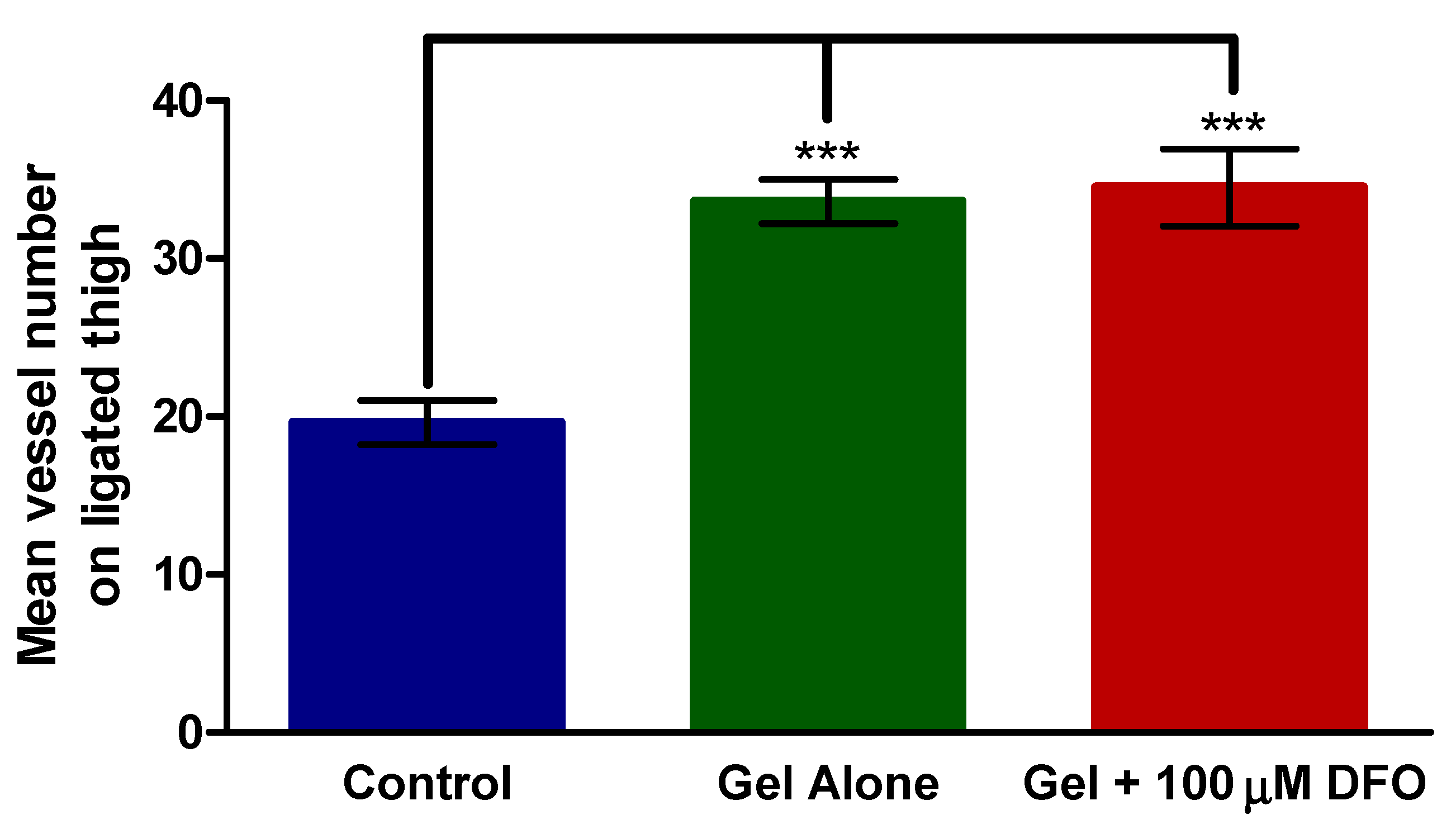
3.4. Histology—Thigh
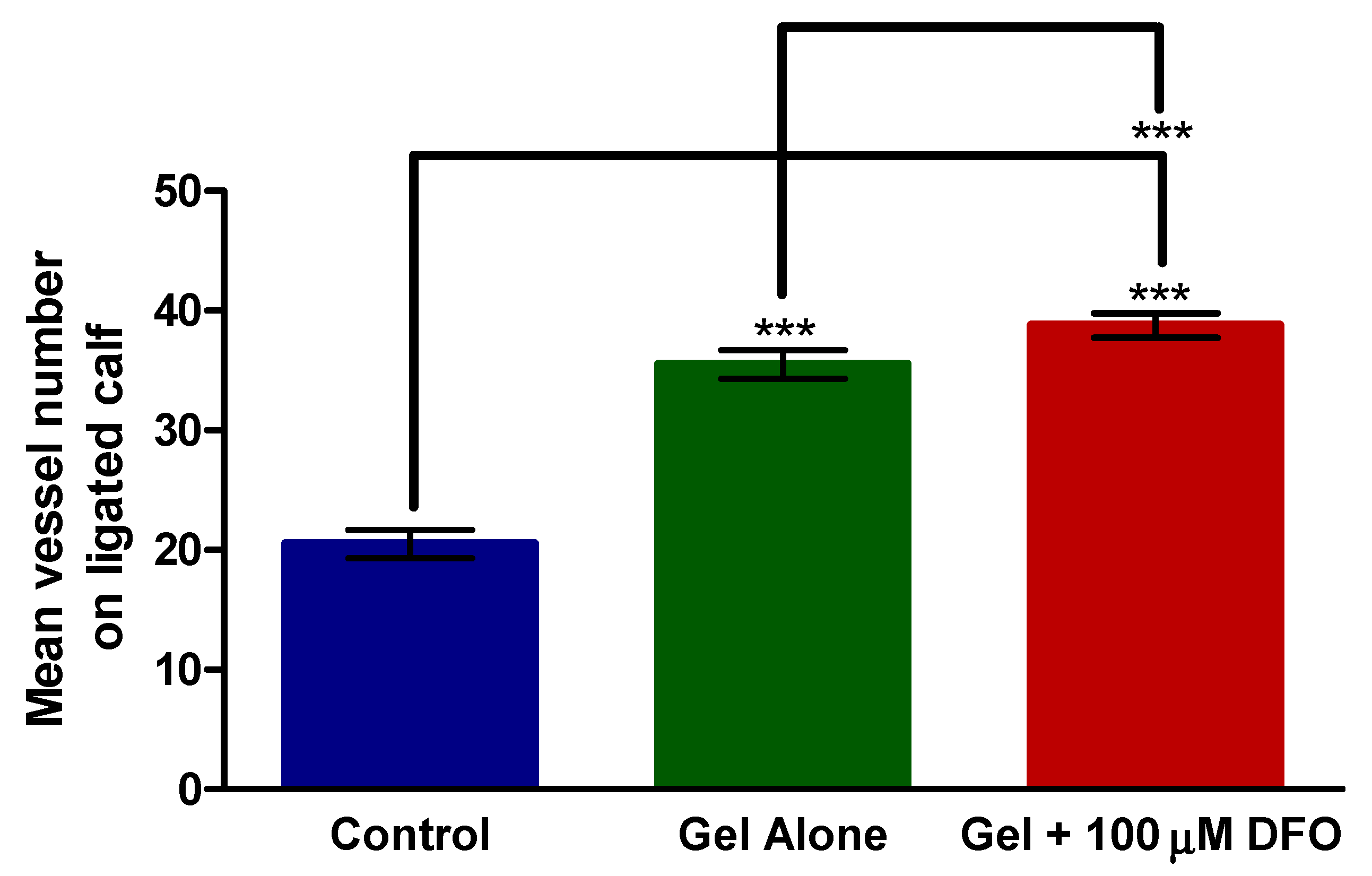
3.5. Histology—Calf
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakielski, P.; Pawłowska, S.; Rinoldi, C.; Ziai, Y.; De Sio, L.; Urbanek, O.; Zembrzycki, K.; Pruchniewski, M.; Lanzi, M.; Salatelli, E.; et al. Multifunctional Platform Based on Electrospun Nanofibers and Plasmonic Hydrogel: A Smart Nanostructured Pillow for Near-Infrared Light-Driven Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 54328–54342. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, X.; Chen, R.; Xue, H.; Lei, J.; Du, H.; Zhang, Z.; Chen, H. Universal Antibacterial Surfaces Fabricated from Quaternary Ammonium Salt-Based PNIPAM Microgels. ACS Appl. Mater. Interfaces 2020, 12, 19268–19276. [Google Scholar] [CrossRef]
- Metawea, O.R.M.; Abdelmoneem, M.A.; Haiba, N.S.; Khalil, H.H.; Teleb, M.; Elzoghby, A.O.; Khafaga, A.F.; Noreldin, A.E.; Albericio, F.; Khattab, S.N. A novel ‘smart’ PNIPAM-based copolymer for breast cancer targeted therapy: Synthesis, and characterization of dual pH/temperature-responsive lactoferrin-targeted PNIPAM-co-AA. Colloids Surf. B Biointerfaces 2021, 202, 111694. [Google Scholar] [CrossRef] [PubMed]
- Ruffolo, A.J.; Romano, M.; Ciapponi, A. Prostanoids for critical limb ischaemia. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef] [PubMed]
- Annex, B.H. Therapeutic angiogenesis for critical limb ischaemia. Nat. Rev. Cardiol. 2013, 10, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Levin, S.R.; Arinze, N.; Siracuse, J.J. Lower extremity critical limb ischemia: A review of clinical features and management. Trends Cardiovasc. Med. 2020, 30, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Varu, V.N.; Hogg, M.E.; Kibbe, M.R. Critical limb ischemia. J. Vasc. Surg. 2010, 51, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Farber, A.; Eberhardt, R.T. The Current State of Critical Limb Ischemia: A Systematic Review. JAMA Surg. 2016, 151, 1070–1077. [Google Scholar] [CrossRef]
- Torbjörnsson, E.; Fagerdahl, A.-M.; Blomgren, L.; Boström, L.; Ottosson, C.; Malmstedt, J. Risk factors for reamputations in patients amputated after revascularization for critical limb-threatening ischemia. J. Vasc. Surg. 2021, 73, 258–266. [Google Scholar] [CrossRef]
- Beegle, J.R.; Magner, N.L.; Kalomoiris, S.; Harding, A.; Zhou, P.; Nacey, C.; White, J.L.; Pepper, K.; Gruenloh, W.; Annett, G.; et al. Preclinical evaluation of mesenchymal stem cells overexpressing VEGF to treat critical limb ischemia. Mol. Ther. Methods Clin. Dev. 2016, 3, 16053. [Google Scholar] [CrossRef]
- Skóra, J.; Barć, P.; Pupka, A.; Dawiskiba, T.; Korta, K.; Albert, M.; Szyber, P. Transplantation of autologous bone marrow mononuclear cells with VEGF gene improves diabetic critical limb ischaemia. Endokrynol. Pol. 2013, 64, 129–138. [Google Scholar]
- Katagiri, T.; Kondo, K.; Shibata, R.; Hayashida, R.; Shintani, S.; Yamaguchi, S.; Shimizu, Y.; Unno, K.; Kikuchi, R.; Kodama, A.; et al. Therapeutic angiogenesis using autologous adipose-derived regenerative cells in patients with critical limb ischaemia in Japan: A clinical pilot study. Sci. Rep. 2020, 10, 16045. [Google Scholar] [CrossRef] [PubMed]
- Norgren, L.; Weiss, N.; Nikol, S.; Hinchliffe, R.J.; Lantis, J.C.; Patel, M.R.; Reinecke, H.; Ofir, R.; Rosen, Y.; Peres, D.; et al. PLX-PAD Cell Treatment of Critical Limb Ischaemia: Rationale and Design of the PACE Trial. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2019, 57, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Belch, J.; Hiatt, W.R.; Baumgartner, I.; Driver, I.V.; Nikol, S.; Norgren, L.; Van Belle, E. Effect of fibroblast growth factor NV1FGF on amputation and death: A randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet 2011, 377, 1929–1937. [Google Scholar] [CrossRef]
- Henry, T.D.; Hirsch, A.T.; Goldman, J.; Wang, Y.L.; Lips, D.L.; McMillan, W.D.; Duval, S.; Biggs, T.A.; Keo, H.H. Safety of a non-viral plasmid-encoding dual isoforms of hepatocyte growth factor in critical limb ischemia patients: A phase I study. Gene Ther. 2011, 18, 788–794. [Google Scholar] [CrossRef]
- Morishita, R.; Shimamura, M.; Takeya, Y.; Nakagami, H.; Chujo, M.; Ishihama, T.; Yamada, E.; Rakugi, H. Combined Analysis of Clinical Data on HGF Gene Therapy to Treat Critical Limb Ischemia in Japan. Curr. Gene Ther. 2020, 20, 25–35. [Google Scholar] [CrossRef]
- Muona, K.; Mäkinen, K.; Hedman, M.; Manninen, H.; Ylä-Herttuala, S. 10-year safety follow-up in patients with local VEGF gene transfer to ischemic lower limb. Gene Ther. 2012, 19, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Duffy, G.P.; Herron, C.C. Mesenchymal stem cells to augment therapeutic angiogenesis in hind-limb ischemia models: How important is their source? Stem Cell Res. Ther. 2013, 4, 131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marui, A.; Tabata, Y.; Kojima, S.; Yamamoto, M.; Tambara, K.; Nishina, T.; Saji, Y.; Inui, K.; Hashida, T.; Yokoyama, S.; et al. A Novel Approach to Therapeutic Angiogenesis for Patients with Critical Limb Ischemia by Sustained Release of Basic Fibroblast Growth Factor Using Biodegradable Gelatin Hydrogel. Circ. J. 2007, 71, 5. [Google Scholar] [CrossRef]
- Marsico, G.; Jin, C.; Abbah, S.A.; Brauchle, E.M.; Thomas, D.; Rebelo, A.L.; Orbanić, D.; Chantepie, S.; Contessotto, P.; Papy-Garcia, D.; et al. Elastin-like hydrogel stimulates angiogenesis in a severe model of critical limb ischemia (CLI): An insight into the glyco-host response. Biomaterials 2021, 269, 120641. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Cheng, N.-C.; Lin, W.-J.; Ling, T.-Y.; Young, T.-H. Sustained release of adipose-derived stem cells by thermosensitive chitosan/gelatin hydrogel for therapeutic angiogenesis. Acta Biomater. 2017, 51, 258–267. [Google Scholar] [CrossRef]
- Mohandas, A.; Anisha, B.S.; Chennazhi, K.P.; Jayakumar, R. Chitosan–hyaluronic acid/VEGF loaded fibrin nanoparticles composite sponges for enhancing angiogenesis in wounds. Colloids Surf. B Biointerfaces 2015, 127, 105–113. [Google Scholar] [CrossRef]
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact. Mater. 2020, 5, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Abd Halim, A.A.; Rageh Al-Maleki, A.; Shahzadi, L.; Zubairi, W. Novel chitosan derivative based composite scaffolds with enhanced angiogenesis; potential candidates for healing chronic non-healing wounds. J. Mater. Sci. Mater. Med. 2019, 30, 72. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Li, F.; Griffith, M.; Ruel, M.; Suuronen, E.J. Application of Chitosan-Based Biomaterials for Blood Vessel Regeneration. Macromol. Symp. 2010, 297, 138–146. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, P.; Vulesevic, B.; Kuraitis, D.; Li, F.; Fook Yang, A.; Griffith, M.; Ruel, M.; Suuronen, E.J. A Collagen–Chitosan Hydrogel for Endothelial Differentiation and Angiogenesis. Tissue Eng. Part A 2010, 16, 3099–3109. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.; Burns, A.J.; de Bruijn, J.D. Chitosan-based hydrogels do not induce angiogenesis. J. Tissue Eng. Regen. Med. 2010, 4, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.-H.; Vo, T.-S.; Ngo, D.-N.; Kang, K.-H.; Je, J.-Y.; Pham, H.N.-D.; Byun, H.-G.; Kim, S.-K. Biological effects of chitosan and its derivatives. Food Hydrocoll. 2015, 51, 200–216. [Google Scholar] [CrossRef]
- Hastings, C.L.; Roche, E.T.; Ruiz-Hernandez, E.; Schenke-Layland, K.; Walsh, C.J.; Duffy, G.P. Drug and cell delivery for cardiac regeneration. Adv. Drug Deliv. Rev. 2015, 84, 85–106. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Jiang, L.J.; Cao, P.P.; Li, J.B.; Chen, X.G. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr. Polym. 2015, 117, 524–536. [Google Scholar] [CrossRef]
- Wang, R.; Yao, X.; Li, T.; Li, X.; Jin, M.; Ni, Y.; Yuan, W.; Xie, X.; Lu, L.; Li, M. Reversible Thermoresponsive Hydrogel Fabricated from Natural Biopolymer for the Improvement of Critical Limb Ischemia by Controlling Release of Stem Cells. Adv. Healthc. Mater. 2019, 8, 1900967. [Google Scholar] [CrossRef]
- Codd, R.; Richardson-Sanchez, T.; Telfer, T.J.; Gotsbacher, M.P. Advances in the Chemical Biology of Desferrioxamine B. ACS Chem. Biol. 2018, 13, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Wu, Z.; Zhao, H.; Cui, H.; Shen, J.; Chang, J.; Li, H.; He, Y. Bioactive Injectable Hydrogels Containing Desferrioxamine and Bioglass for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 30103–30114. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, M.; Wang, L.; Jiao, B. HIFs, angiogenesis, and cancer. J. Cell. Biochem. 2013, 114, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jia, P.; Kang, H.; Zhang, H.; Liu, Y.; Yang, P.; Yan, Y.; Zuo, G.; Guo, L.; Jiang, M.; et al. Upregulating Hif-1α by Hydrogel Nanofibrous Scaffolds for Rapidly Recruiting Angiogenesis Relative Cells in Diabetic Wound. Adv. Healthc. Mater. 2016, 5, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cheng, R.; Zhao, X.; Zhang, Y.; Tam, A.; Yan, Y.; Shen, H.; Zhang, Y.S.; Qi, J.; Feng, Y.; et al. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia Mater. 2019, 11, 3. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, Y.; Guo, P.; Duan, T.; Cheng, W.; Guo, Y.; Zheng, X.; Lu, G.; Lu, Q.; Kaplan, D.L. Injectable Desferrioxamine-Laden Silk Nanofiber Hydrogels for Accelerating Diabetic Wound Healing. ACS Biomater. Sci. Eng. 2021, 7, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Limbourg, A.; Korff, T.; Napp, L.C.; Schaper, W.; Drexler, H.; Limbourg, F.P. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat. Protoc. 2009, 4, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Goggi, J.L.; Haslop, A.; Boominathan, R.; Chan, K.; Soh, V.; Cheng, P.; Robins, E.G.; Bhakoo, K.K. Imaging the Proangiogenic Effects of Cardiovascular Drugs in a Diabetic Model of Limb Ischemia. Contrast Media Mol. Imaging 2019, 2019, 2538909. [Google Scholar] [CrossRef]
- Tripodo, G.; Trapani, A.; Rosato, A.; Di Franco, C.; Tamma, R.; Trapani, G.; Ribatti, D.; Mandracchia, D. Hydrogels for biomedical applications from glycol chitosan and PEG diglycidyl ether exhibit pro-angiogenic and antibacterial activity. Carbohydr. Polym. 2018, 198, 124–130. [Google Scholar] [CrossRef] [PubMed]
- McBane, J.E.; Vulesevic, B.; Padavan, D.T.; McEwan, K.A.; Korbutt, G.S.; Suuronen, E.J. Evaluation of a Collagen-Chitosan Hydrogel for Potential Use as a Pro-Angiogenic Site for Islet Transplantation. PLoS ONE 2013, 8, e77538. [Google Scholar] [CrossRef]
- Ferraro, B.; Cruz, Y.L.; Baldwin, M.; Coppola, D.; Heller, R. Increased perfusion and angiogenesis in a hindlimb ischemia model with plasmid FGF-2 delivered by noninvasive electroporation. Gene Ther. 2010, 17, 763–769. [Google Scholar] [CrossRef]
- Ruvinov, E.; Leor, J.; Cohen, S. The effects of controlled HGF delivery from an affinity-binding alginate biomaterial on angiogenesis and blood perfusion in a hindlimb ischemia model. Biomaterials 2010, 31, 4573–4582. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.A.; Distasi, M.R.; Bills, R.G.; Miller, S.J.; Alloosh, M.; Murphy, M.P.; Akingba, A.G.; Sturek, M.; Dalsing, M.C.; Unthank, J.L. Marvels, mysteries, and misconceptions of vascular compensation to peripheral artery occlusion. Microcirculation 2010, 17, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Murrant, C.L. Structural and functional limitations of the collateral circulation in peripheral artery disease. J. Physiol. 2008, 586, 5845. [Google Scholar] [CrossRef] [PubMed]

| Group Name | Treatment |
|---|---|
| Control | None |
| Chitosan/β-GP gel alone | 300 µL chitosan/β-glycerophosphate hydrogel |
| Chitosan/β-GP gel + DFO | 300 µL chitosan/β-glycerophosphate hydrogel loaded with 100 µM DFO |
| Score | Assessment of Ambulatory Impairment of the Ischaemic Limb |
|---|---|
| 0 | Flexing the toes to resist traction on the tail, as for the non-operated foot |
| 1 | Plantar flexion |
| 2 | No dragging but no plantar flexion |
| 3 | Dragging of the foot |
| 4 | Non-weight bearing |
| 5 | Immobile |
| Score | Assessment of Ischaemic Damage to Limb |
|---|---|
| 0 | Plantar flexion, no discolouration |
| 1 | Plantar flexion, mild discolouration |
| 2 | No plantar flexion, mild discolouration |
| 3 | No plantar flexion, moderate to severe discolouration |
| 4 | Any necrosis of the foot |
| 5 | Amputation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herron, C.; Hastings, C.L.; Herron-Rice, C.; Kelly, H.M.; O’Dwyer, J.; Duffy, G.P. A Thermoresponsive Chitosan/β-Glycerophosphate Hydrogel for Minimally Invasive Treatment of Critical Limb Ischaemia. Polymers 2021, 13, 3568. https://doi.org/10.3390/polym13203568
Herron C, Hastings CL, Herron-Rice C, Kelly HM, O’Dwyer J, Duffy GP. A Thermoresponsive Chitosan/β-Glycerophosphate Hydrogel for Minimally Invasive Treatment of Critical Limb Ischaemia. Polymers. 2021; 13(20):3568. https://doi.org/10.3390/polym13203568
Chicago/Turabian StyleHerron, Caroline, Conn L. Hastings, Clodagh Herron-Rice, Helena M. Kelly, Joanne O’Dwyer, and Garry P. Duffy. 2021. "A Thermoresponsive Chitosan/β-Glycerophosphate Hydrogel for Minimally Invasive Treatment of Critical Limb Ischaemia" Polymers 13, no. 20: 3568. https://doi.org/10.3390/polym13203568
APA StyleHerron, C., Hastings, C. L., Herron-Rice, C., Kelly, H. M., O’Dwyer, J., & Duffy, G. P. (2021). A Thermoresponsive Chitosan/β-Glycerophosphate Hydrogel for Minimally Invasive Treatment of Critical Limb Ischaemia. Polymers, 13(20), 3568. https://doi.org/10.3390/polym13203568