The Effect of the PVA/Chitosan/Citric Acid Ratio on the Hydrophilicity of Electrospun Nanofiber Meshes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Determination of the Viscosity of Polymer Solution Mixtures
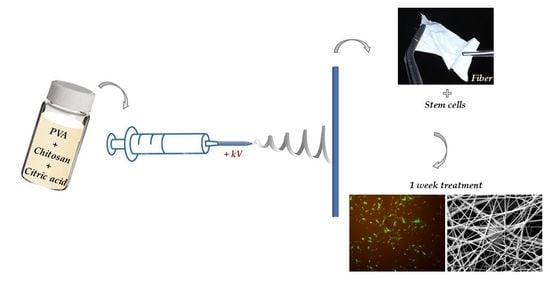
2.2.2. Electrospinning
2.2.3. Study of Composition and Monitoring of Cross-Linking Reactions via Infrared Spectroscopy
2.2.4. Morphological Study via Low-Vacuum Scanning Electron Microscopy
2.2.5. Morphological Study via Atomic Force Microscopy
2.2.6. Morphological Study via Contact Angle Measurements
2.2.7. Determination of the Swelling Ratio
2.2.8. Cell Culture and Viability Assay
2.2.9. Vitality Staining
2.2.10. Statistics
3. Results
3.1. Investigation of Initial Polymer Mixtures
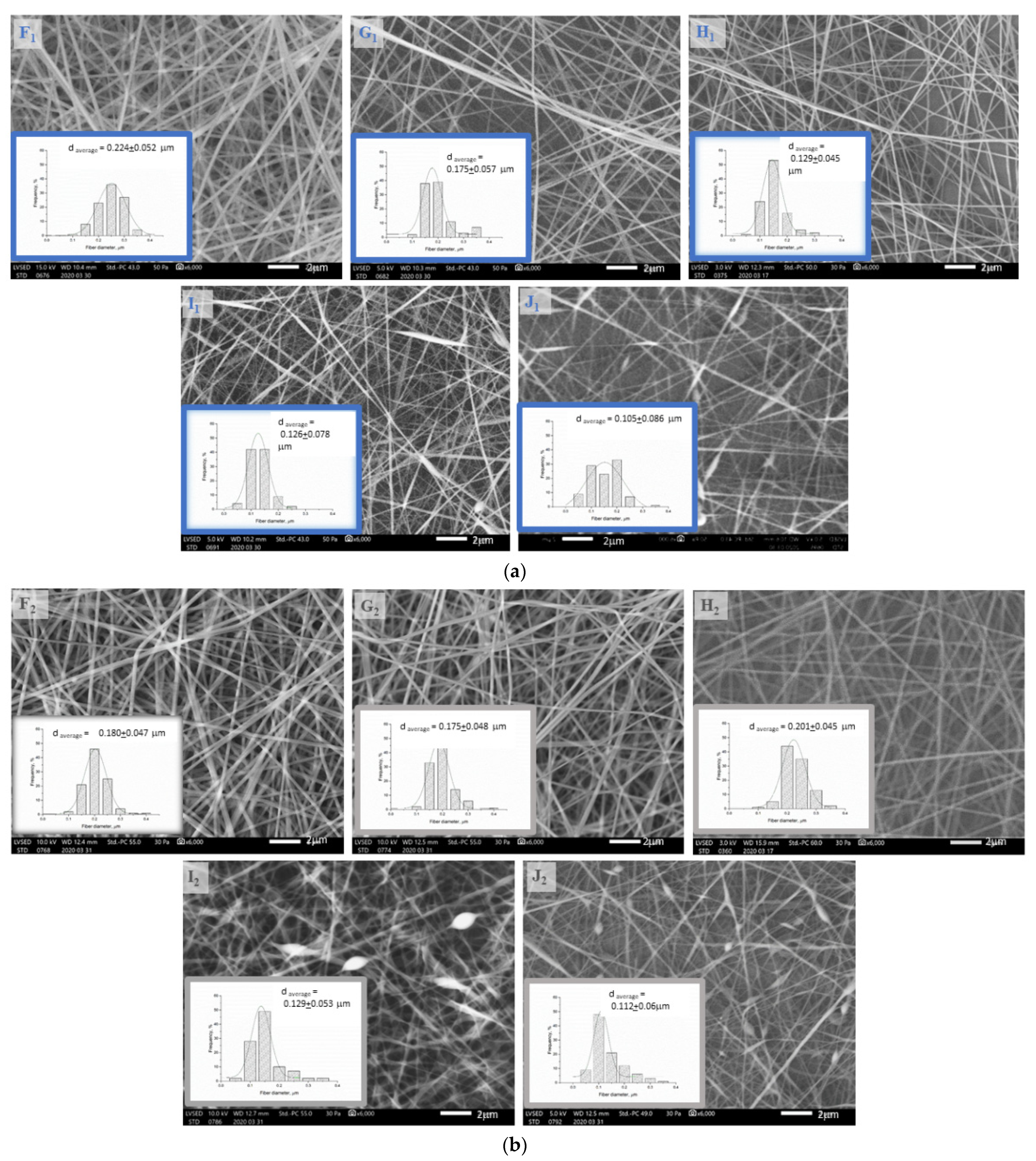
3.2. Investigation of the Structure of the Fiber Network via Scanning Electron Microscopy
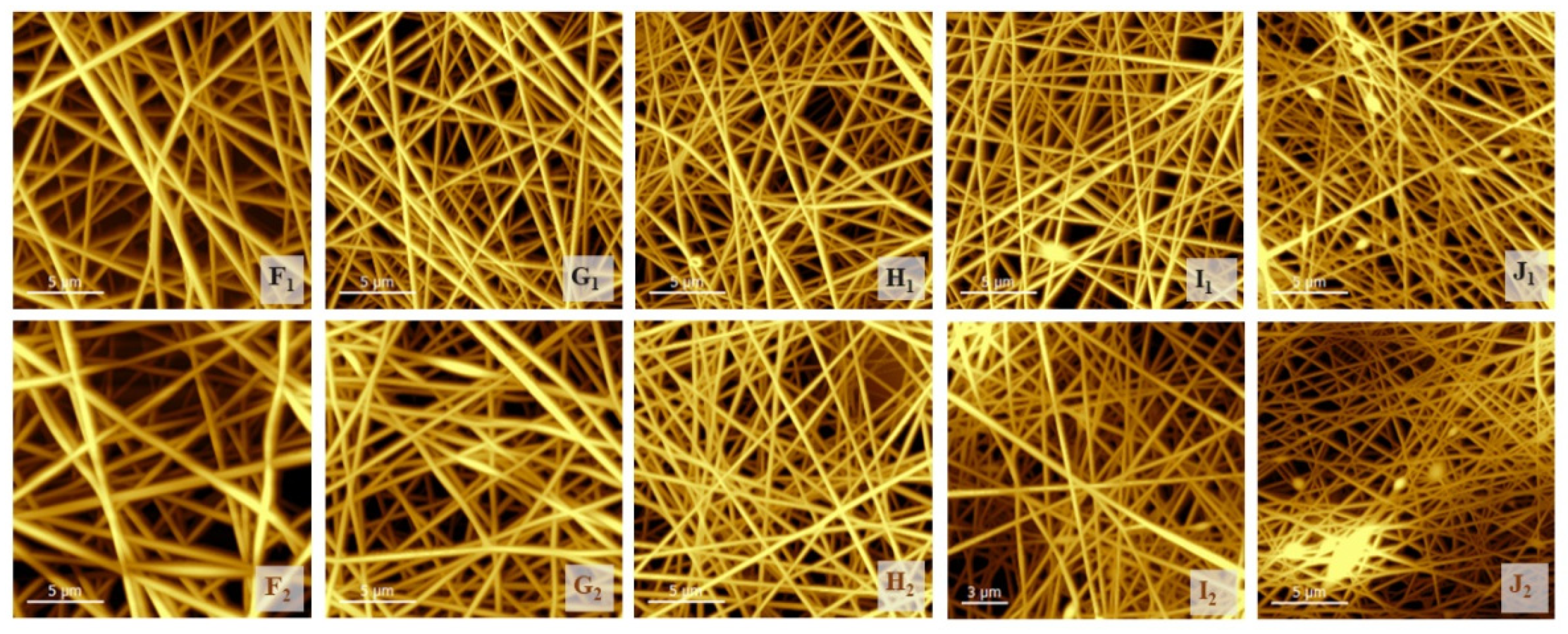
3.3. Investigation of the Structure of the Fiber Network via Atomic Force Microscopy
3.4. Interpretation of Contact Angle Measurements
3.5. Infrared Spectroscopy Measurements
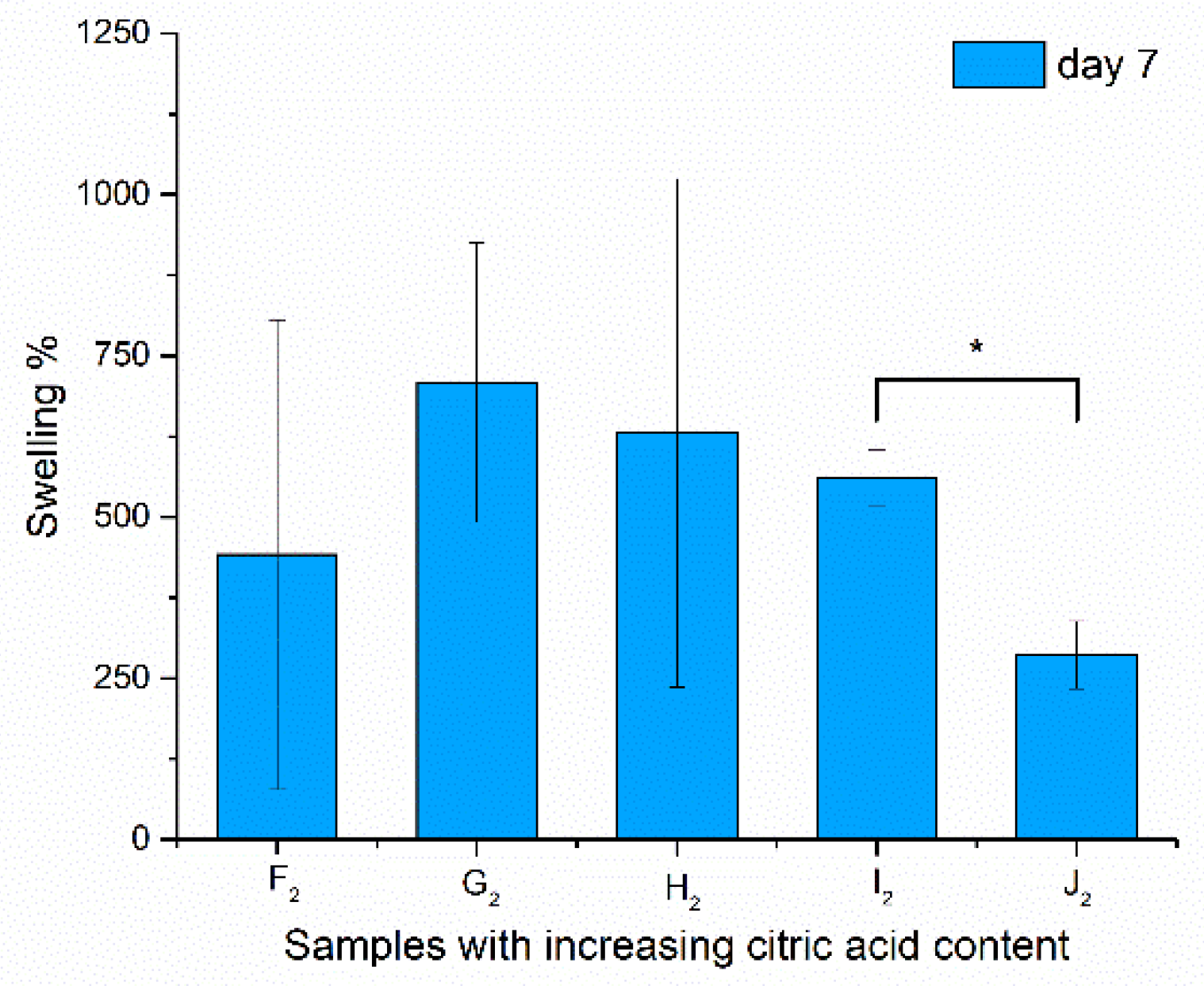
3.6. Swelling Studies
3.7. Biocompatibility and Viability Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | atomic force microscopy |
| ATR | attenuated total reflectance |
| DPSCs | dental pulp stem cells |
| ECM | extracellular matrix |
| FDA | fluorescein diacetate |
| PVA | polyvinyl alcohol |
| GA | glutaraldehyde |
| IR | infrared spectroscopy |
| LV-SEM | low-vacuum scanning electron microscopy |
References
- Hussin, M.S.F.; Serah, A.M.; Azlan, K.A.; Abdullah, H.Z.; Idris, M.I.; Ghazali, I.; Shariff, Amir Husni Mohd Huda, N.; Zakaria, A.A. A Bibliometric Analysis of the Global Trend of Using Alginate, Gelatine, and Hydroxyapatite for Bone Tissue Regeneration Applications. Polym. Bone 2021, 13, 647. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Janowski, G.M. A Novel Spatially Designed and Functionally Graded Electrospun Membrane for Periodontal Regeneration. Acta Biomater. 2011, 7, 216–224. [Google Scholar] [CrossRef]
- Pelipenko, J.; Kocbek, P.; Kristl, J. Critical Attributes of Nanofibers: Preparation, Drug Loading, and Tissue Regeneration. Int. J. Pharm. 2015, 2, 57–74. [Google Scholar] [CrossRef]
- Pilipchuk, S.P.; Plonka, A.B.; Monje, A.; Taut, A.D.; Lanis, A.; Kang, B.; Giannobile, W.V. Tissue Engineering for Bone Regeneration and Osseointegration in the Oral Cavity. Dent. Mater. 2015, 31, 317–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Jun, Y.; Qin, J.; Lee, S.H. Electrospinning versus Microfluidic Spinning of Functional Fibers for Biomedical Applications. Biomaterials 2017, 10, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Zelkó, R.; Lamprou, D.A.; Sebe, I. Recent Development of Electrospinning for Drug Delivery. Pharmaceutics 2020, 12, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mwiiri, F.K.; Brandner, J.M.; Daniels, R. Erratum: Electrospun Bioactive Wound Dressing Containing Colloidal Dispersions of Birch Bark Dry Extract (Pharmaceutics, (2020) 12, 770, 10.3390/Pharmaceutics12080770). Pharmaceutics 2020, 12, 991. [Google Scholar] [CrossRef]
- Biranje, S.; Madiwale, P.; Adicarekar, A.R.V. Preparation and Characterization of Chitosan/Pva Polymeric Film for Its Potential Application As Wound Dressing Material. Indian J. Sci. Res. 2017, 14, 250–256. [Google Scholar]
- Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Electrospun Chitosan/Polyvinyl Alcohol Nanofibre Mats for Wound Healing. Int. Wound J. 2014, 11, 215–222. [Google Scholar] [CrossRef]
- Song, J.; Kim, M.; Lee, H. Recent Advances on Nanofiber Fabrications: Unconventional State-of-the-Art Spinning Techniques. Polymers 2020, 12, 1386. [Google Scholar] [CrossRef]
- Baghaban-Eslaminejad, M.; Oryan, A.; Kamali, A.; Moshiri, A. The Role of Nanomedicine, Nanotechnology, and Nanostructures on Oral Bone Healing, Modeling, and Remodeling. In Nanostructures for Oral Medicine; Chapter 25; Elsevier: Amsterdam, The Netherlands, 2017; pp. 777–832. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. PVA/CA Based Electrospun Nanofibers: Influence of Processing Parameters in the Fiber Diameter. IOP Conf. Ser. Mater. Sci. Eng. 2019, 634, 012040. [Google Scholar] [CrossRef]
- Jiang, T.; James, R.; Kumbar, S.G.; Laurencin, C.T. Chitosan as a Biomaterial: Structure, Properties, and Applications in Tissue Engineering and Drug Delivery. In Natural And Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 91–113. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the Art and New Perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yang, E. Green Electrospinning and Crosslinking of Polyvinyl Alcohol/Citric Acid. J. Nano Res. 2015, 32, 32–42. [Google Scholar] [CrossRef]
- Truong, Y.B.; Choi, J.; Mardel, J.; Gao, Y.; Maisch, S.; Musameh, M.; Kyratzis, I.L. Functional Cross-Linked Electrospun Polyvinyl Alcohol Membranes and Their Potential Applications. Macromol. Mater. Eng. 2017, 302, 1700024. [Google Scholar] [CrossRef] [Green Version]
- Soares, R.M.D.; Siqueira, N.M.; Prabhakaram, M.P.; Ramakrishna, S. Electrospinning and Electrospray of Bio-Based and Natural Polymers for Biomaterials Development. Mater. Sci. Eng. C 2018, 8, 969–982. [Google Scholar] [CrossRef]
- Toullec, C.; Le Bideau, J.; Geoffroy, V.; Halgand, B.; Buchtova, N.; Molina-peña, R.; Garcion, E.; Avril, S.; Sindji, L.; Dube, A.; et al. Curdlan–Chitosan Electrospun Fibers as Potential Scaffolds for Bone Regeneration. Polymers 2021, 13, 526. [Google Scholar] [CrossRef]
- Pangon, A.; Saesoo, S.; Saengkrit, N.; Ruktanonchai, U.; Intasanta, V. Multicarboxylic Acids as Environment-Friendly Solvents and in Situ Crosslinkers for Chitosan/PVA Nanofibers with Tunable Physicochemical Properties and Biocompatibility. Carbohydr. Polym. 2016, 138, 156–165. [Google Scholar] [CrossRef]
- Shin, S.-Y.; Park, H.-N.; Kim, K.-H.; Lee, M.-H.; Choi, Y.S.; Park, Y.-J.; Lee, Y.-M.; Ku, Y.; Rhyu, I.-C.; Han, S.-B.; et al. Biological Evaluation of Chitosan Nanofiber Membrane for Guided Bone Regeneration. J. Periodontol. 2005, 76, 1778–1784. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, D.; Chen, X.; Xu, Q.; Lu, F.; Nie, J. Electrospun Water-Soluble Carboxyethyl Chitosan/Poly(Vinyl Alcohol) Nanofibrous Membrane as Potential Wound Dressing for Skin Regeneration. Biomacromolecules 2008, 9, 349–354. [Google Scholar] [CrossRef]
- Niranjan, R.; Kaushik, M.; Prakash, J.; Venkataprasanna, K.S.; Christy, A.; Pannerselvam, B.; Venkatasubbu, G.D. Enhanced Wound Healing by PVA/Chitosan/Curcumin Patches: In Vitro and in Vivo Study. Colloids Surfaces B Biointerfaces 2019, 182, 68. [Google Scholar] [CrossRef]
- Kang, Y.O.; Yoon, I.S.; Lee, S.Y.; Kim, D.D.; Lee, S.J.; Park, W.H.; Hudson, S.M. Chitosan-Coated Poly(Vinyl Alcohol) Nanofibers for Wound Dressings. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 568–576. [Google Scholar] [CrossRef]
- Deneke, N.; Dohadwala, S.; Moore, Q.; Nave, F.; Thompson, A. Evaluating Alternative Crosslinking Agents in Poly(Vinyl Alcohol) Hydrogels Membranes. Pursue 2018, 1, 63–81. [Google Scholar]
- Schiffman, J.D.; Schauer, C.L. Cross-Linking Chitosan Nanofibers. Biomacromolecules 2007, 8, 594–601. [Google Scholar] [CrossRef]
- Cui, Z.; Zheng, Z.; Lin, L.; Si, J.; Wang, Q.; Peng, X.; Chen, W. Electrospinning and Crosslinking of Polyvinyl Alcohol/Chitosan Composite Nanofiber for Transdermal Drug Delivery. Adv. Polym. Technol. 2018, 37, 1917–1928. [Google Scholar] [CrossRef]
- Beauchamp, R.O.; Clair, M.B.; Fennell, T.R.; Clarke, D.O.; Morgan, K.T.; Kair, F.W. A Critical Review of the Toxicology of Glutaraldehyde. Gastroenterol. Nurs. 1993, 16, 42–43. [Google Scholar] [CrossRef]
- Gough, J.E.; Scotchford, C.A.; Downes, S. Cytotoxicity of Glutaraldehyde Crosslinked Collagen/Poly(Vinyl Alcohol) Films Is by the Mechanism of Apoptosis. J. Biomed. Mater. Res. 2002, 61, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Mudunkotuwa, I.A.; Grassian, V.H. Citric Acid Adsorption on TiO2 Nanoparticles in Aqueous Suspensions at Acidic and Circumneutral PH: Surface Coverage, Surface Speciation, and Its Impact on Nanoparticle-Nanoparticle Interactions. J. Am. Chem. Soc. 2010, 132, 14986–14994. [Google Scholar] [CrossRef] [PubMed]
- Sonker, A.K.; Teotia, A.K.; Kumar, A.; Nagarale, R.K.; Verma, V. Development of Polyvinyl Alcohol Based High Strength Biocompatible Composite Films. Macromol. Chem. Phys. 2017, 218, 130. [Google Scholar] [CrossRef]
- Zhuang, L.; Zhi, X.; Du, B.; Sichun, Y. Preparation of Elastic and Antibacterial Chitosan–Citric Membranes with High Oxygen Barrier Ability by in Situ Cross-Linking. ACS Omega 2020, 9, 1086–1097. [Google Scholar] [CrossRef]
- Riwu, Y.F.; Loi, F.H.P.; Kusumaatmaja, A.; Roto; Triyana, K. Effect of Chitosan Concentration and Heat Treatment on Electrospun PVA/Chitosan Nanofibers. AIP Conf. Proc. 2016, 1755, 58. [Google Scholar] [CrossRef] [Green Version]
- Park, J.C.; Ito, T.; Kim, K.O.; Kim, K.W.; Kim, B.S.; Khil, M.S.; Kim, H.Y.; Kim, I.S. Electrospun Poly(Vinyl Alcohol) Nanofibers: Effects of Degree of Hydrolysis and Enhanced Water Stability. Polym. J. 2010, 42, 273–276. [Google Scholar] [CrossRef] [Green Version]
- Lai, J.Y.; Luo, L.J. Chitosan-g-Poly(N-Isopropylacrylamide) Copolymers as Delivery Carriers for Intracameral Pilocarpine Administration. Eur. J. Pharm. Biopharm. 2017, 113, 140–148. [Google Scholar] [CrossRef]
- Hajdu, P.; Lampé, I.; Rácz, R.; Biri, S.; Csík, A.; Tóth, F.; Szalóki, M.; Hegedűs, V.; Dombrádi, Z.; Varga, I.; et al. Optimized Size and Distribution of Silver Nanoparticles on the Surface of Titanium Implant Regarding Cell Viability. Appl. Sci. 2020, 10, 7063. [Google Scholar] [CrossRef]
- Zengeni, B.T. Bingham Yield Stress and Bingham Plastic Viscosity of Homogeneous Non-Newtonian Slurries. Master’s Thesis, Faculty of Engineering, Cape Town, South Africa, October 2016. [Google Scholar]
- Bi, S.; Wang, P.; Hu, S.; Li, S.; Pang, J.; Zhou, Z.; Sun, G.; Huang, L.; Cheng, X.; Xing, S.; et al. Construction of Physical-Crosslink Chitosan/PVA Double-Network Hydrogel with Surface Mineralization for Bone Repair. Carbohydr. Polym. 2019, 224, 10034. [Google Scholar] [CrossRef]
- Szewczyk, P.K.; Ura, D.P.; Metwally, S.; Knapczyk-Korczak, J.; Gajek, M.; Marzec, M.M.; Bernasik, A.; Stachewicz, U. Roughness and Fiber Fraction Dominated Wetting of Electrospun Fiber-Based Porous Meshes. Polymers 2018, 11, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sargazi, G.; Afzali, D.; Mostafavi, A.; Shadman, A.; Rezaee, B.; Zarrintaj, P.; Saeb, M.R.; Ramakrishna, S.; Mozafari, M. Chitosan/Polyvinyl Alcohol Nanofibrous Membranes: Towards Green Super-Adsorbents for Toxic Gases. Heliyon 2019, 5, e01527. [Google Scholar] [CrossRef] [Green Version]
- Blout, E.R.; Karplus, R. The Infrared Spectrum of Polyvinyl Alcohol. J. Am. Chem. Soc. 1948, 70, 862–864. [Google Scholar] [CrossRef]
- Pervez, M.N.; Stylios, G.K. Investigating the Synthesis and Characterization of a Novel “Green” H2O2-Assisted, Water-Soluble Chitosan/Polyvinyl Alcohol Nanofiber for Environmental End Uses. Nanomaterials 2018, 8, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Yamaguchi, K.; Nagaishi, T.; Murai, M.; Kim, M.; Wei, K.; Zhang, K.Q.; Kim, I.S. Enhancement of Mechanical Properties of Polymeric Nanofibers by Controlling Crystallization Behavior Using a Simple Freezing/Thawing Process. RSC Adv. 2017, 7, 43994–44000. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.C.; Yuan, Q.; Ahmad, Z.; Huang, J.; Li, J.S.; Chang, M.W. Controlled Morphing of Microbubbles to Beaded Nanofibers via Electrically Forced Thin Film Stretching. Polymers 2017, 9, 265. [Google Scholar] [CrossRef] [Green Version]
- Satpathy, A.; Pal, A.; Sengupta, S.; Das, A.; Hasan, M.M.; Ratha, I.; Barui, A.; Bodhak, S. Bioactive Nano-Hydroxyapatite Doped Electrospun PVA-Chitosan Composite Nanofibers for Bone Tissue Engineering Applications. J. Indian Inst. Sci. 2019, 99, 289–302. [Google Scholar] [CrossRef]
- Molnar, K.; Jozsa, B.; Barczikai, D.; Krisch, E.; Puskas, J.E.; Jedlovszky-hajdu, A. Plasma Treatment as an Effective Tool for Crosslinking of Electrospun Fi Bers. J. Mol. Liq. 2020, 303, 112628. [Google Scholar] [CrossRef]
- Do Nascimento, F.C.; de Aguiar, L.C.V.; Costa, L.A.T.; Fernandes, M.T.; Marassi, R.J.; de Gomes, A.S.; de Castro, J.A. Formulation and Characterization of Crosslinked Polyvinyl Alcohol (PVA) Membranes: Effects of the Crosslinking Agents. Polym. Bull. 2021, 78, 917–929. [Google Scholar] [CrossRef]
- López-córdoba, A.; Castro, G.R.; Goyanes, S. A Simple Green Route to Obtain Poly ( Vinyl Alcohol ) Electrospun Mats with Improved Water Stability for Use as Potential Carriers of Drugs. Mater. Sci. Eng. C 2016, 69, 726–732. [Google Scholar] [CrossRef]
- Hanaei, H.; Assadi, M.K.; Saidur, R. Highly Efficient Antireflective and Self-Cleaning Coatings That Incorporate Carbon Nanotubes (CNTs) into Solar Cells: A Review. Renew. Sustain. Energy Rev. 2016, 59, 620–635. [Google Scholar] [CrossRef]
- Wu, J.; Wang, L.; Dong, H.; Zhao, Y.; Jiang, L. Unidirectional Water-Penetration Composite Fibrous Film via Electrospinning. Soft Matter 2012, 8, 5996–5999. [Google Scholar] [CrossRef]
- Das, P.; Ojah, N.; Kandimalla, R.; Mohan, K.; Gogoi, D.; Dolui, S.K.; Choudhury, A.J. Surface Modification of Electrospun PVA/Chitosan Nanofibers by Dielectric Barrier Discharge Plasma at Atmospheric Pressure and Studies of Their Mechanical Properties and Biocompatibility. Int. J. Biol. Macromol. 2018, 114, 1026–1032. [Google Scholar] [CrossRef]

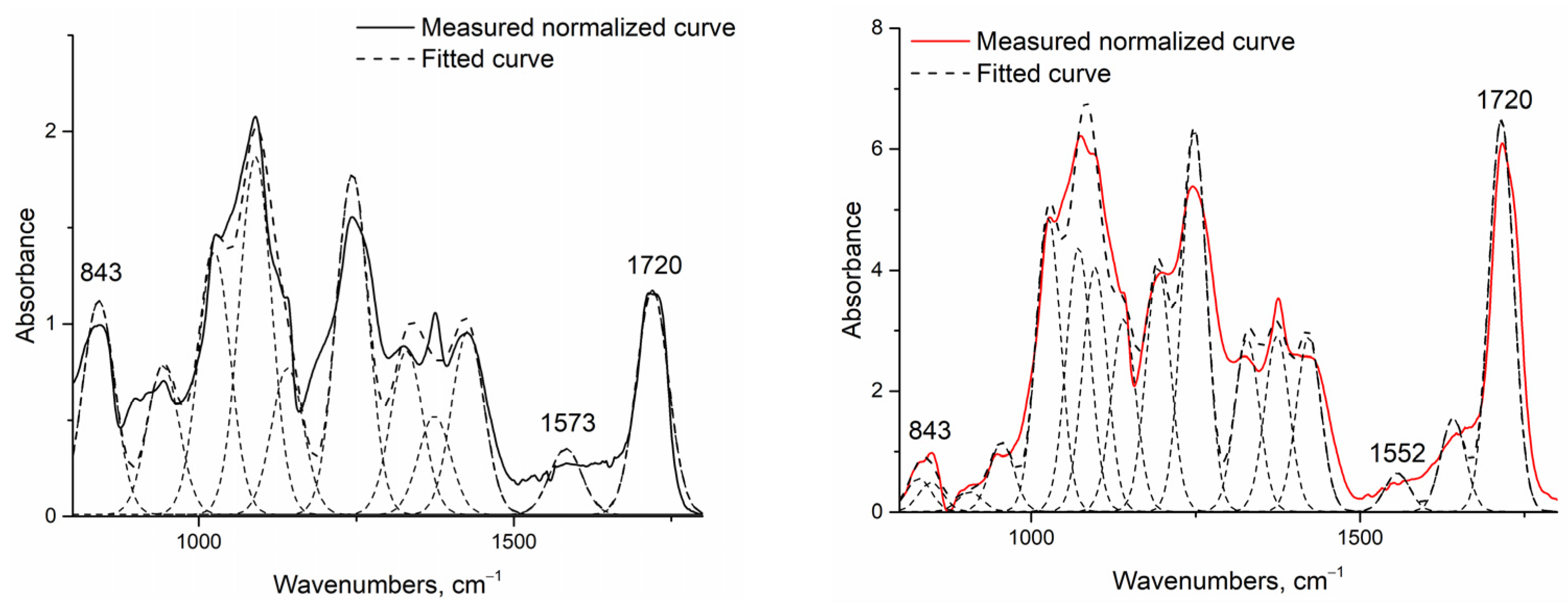




| Sample | PVA, g/100 g | Chitosan, g/100 g | Citric Acid, g/100 g | Sample Modification |
|---|---|---|---|---|
| F1 (PVA) | 10.0 | 0.0 | 0.0 | native |
| F1 * (PVA) | 10.0 | 0.0 | 6.1 | native |
| F2 (PVA) | 10.0 | 0.0 | 0.0 | heat-treated |
| F2 ** (PVA) | 10.0 | 0.0 | 6.1 | heat-treated |
| G1 | 7.8 | 0.8 | 1.8 | native |
| G2 | 7.8 | 0.8 | 1.8 | heat-treated |
| H1 | 6.5 | 1.3 | 2.9 | native |
| H2 | 6.5 | 1.3 | 2.9 | heat-treated |
| I1 | 4.8 | 1.9 | 4.2 | native |
| I2 | 4.8 | 1.9 | 4.2 | heat-treated |
| J1 | 4.2 | 2.1 | 4.7 | native |
| J2 | 4.2 | 2.1 | 4.7 | heat-treated |
| Sample Name | ηpl, mPas | St. dev |
|---|---|---|
| F1,2 (PVA) | 532 | 28 |
| F1,2 * | 490 | 25 |
| G1,2 | 905 | 51 |
| H1,2 | 1050 | 73 |
| I1,2 * | 1243 | 88 |
| J1,2 * | 1317 | 106 |
| Chitosan | 3561 | 209 |
| Samples | Full-Width at Half-Maximum Values of Distribution | St. dev. |
|---|---|---|
| F1, (PVA) | 0.1280 | 0.0084 |
| F2, (PVA) | 0.0989 | 0.0015 |
| G1 | 0.0829 | 0.0144 |
| G2 | 0.0937 | 0.0074 |
| H1 | 0.0815 | 0.0035 |
| H2 | 0.0919 | 0.0135 |
| I1 | 0.0844 | 0.0091 |
| I2 | 0.0753 | 0.0067 |
| J1 | 0.1737 | 0.0586 |
| J2 | 0.0673 | 0.0119 |
| Sample | dave. μm | St. dev. | Raave. μm | St. dev. | Ranot significantp > 0.1 |
|---|---|---|---|---|---|
| F1 | 0.230 | 0.005 | 0.353 | 0.033 | |
| F2 | 0.252 | 0.005 | 0.358 | 0.090 | vs. F1 |
| G1 | 0.175 | 0.020 | 0.283 | 0.043 | |
| G2 | 0.236 | 0.002 | 0.254 | 0.035 | vs. G1 |
| H1 | 0.150 | 0.040 | 0.230 | 0.018 | |
| H2 | 0.192 | 0.007 | 0.150 | 0.013 | |
| I1 | 0.116 | 0.030 | 0.221 | 0.040 | vs. H1 |
| I2 | 0.153 | 0.003 | 0.142 | 0.023 | vs. H2 |
| J1 | 0.101 | 0.050 | 0.179 | 0.016 | |
| J2 | 0.124 | 0.003 | 0.121 | 0.046 | vs. I2 |
| Samples | Contact Angle, ° | St. dev. | Not Significant p > 0.1 |
|---|---|---|---|
| F2, (PVA) | 25.4 | 2.6 | |
| G2 | 28.5 | 1.9 | vs. F2 |
| H2 | 42.6 | 4.39 | |
| I2 | 41.2 | 4.7 | vs. H2 |
| J2 | 51.8 | 4.3 |
| Samples/Peaks | 1552 to 1573 cm−1 Amide II Bond | St. dev. | 1720 to 1735 cm−1 Peak of Ester Bond | St. dev. |
|---|---|---|---|---|
| F1 (PVA) | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| F2 (PVA) | 0.0000 | 0.0000 | 0.0000 or co-peak * | 0.0000 |
| G1 | 0.1756 | 0.0487 | 0.8028 | 0.1834 |
| G2 | 0.1470 | 0.0258 | 1.4082 | 0.2887 |
| H1 | 0.1953 | 0.0275 | 1.1657 | 0.1802 |
| H2 | 0.3555 | 0.1016 | 6.2583 | 1.8627 |
| I1 | 0.6716 | 0.1211 | 2.9992 | 0.3213 |
| I2 | 1.5974 | 0.2282 | 6.2351 | 0.2024 |
| J1 | 0.7784 | 0.2724 | 2.2367 | 0.3972 |
| J2 | 2.4740 | 0.4948 | 17.0395 | 2.6624 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czibulya, Z.; Csík, A.; Tóth, F.; Pál, P.; Csarnovics, I.; Zelkó, R.; Hegedűs, C. The Effect of the PVA/Chitosan/Citric Acid Ratio on the Hydrophilicity of Electrospun Nanofiber Meshes. Polymers 2021, 13, 3557. https://doi.org/10.3390/polym13203557
Czibulya Z, Csík A, Tóth F, Pál P, Csarnovics I, Zelkó R, Hegedűs C. The Effect of the PVA/Chitosan/Citric Acid Ratio on the Hydrophilicity of Electrospun Nanofiber Meshes. Polymers. 2021; 13(20):3557. https://doi.org/10.3390/polym13203557
Chicago/Turabian StyleCzibulya, Zsuzsanna, Attila Csík, Ferenc Tóth, Petra Pál, István Csarnovics, Romána Zelkó, and Csaba Hegedűs. 2021. "The Effect of the PVA/Chitosan/Citric Acid Ratio on the Hydrophilicity of Electrospun Nanofiber Meshes" Polymers 13, no. 20: 3557. https://doi.org/10.3390/polym13203557
APA StyleCzibulya, Z., Csík, A., Tóth, F., Pál, P., Csarnovics, I., Zelkó, R., & Hegedűs, C. (2021). The Effect of the PVA/Chitosan/Citric Acid Ratio on the Hydrophilicity of Electrospun Nanofiber Meshes. Polymers, 13(20), 3557. https://doi.org/10.3390/polym13203557