First-Principle Study of Rh-Doped Nitrogen Vacancy Boron Nitride Monolayer for Scavenging and Detecting SF6 Decomposition Products
Abstract
:1. Introduction
2. Modeling of the Gas/Rh-VNBN System
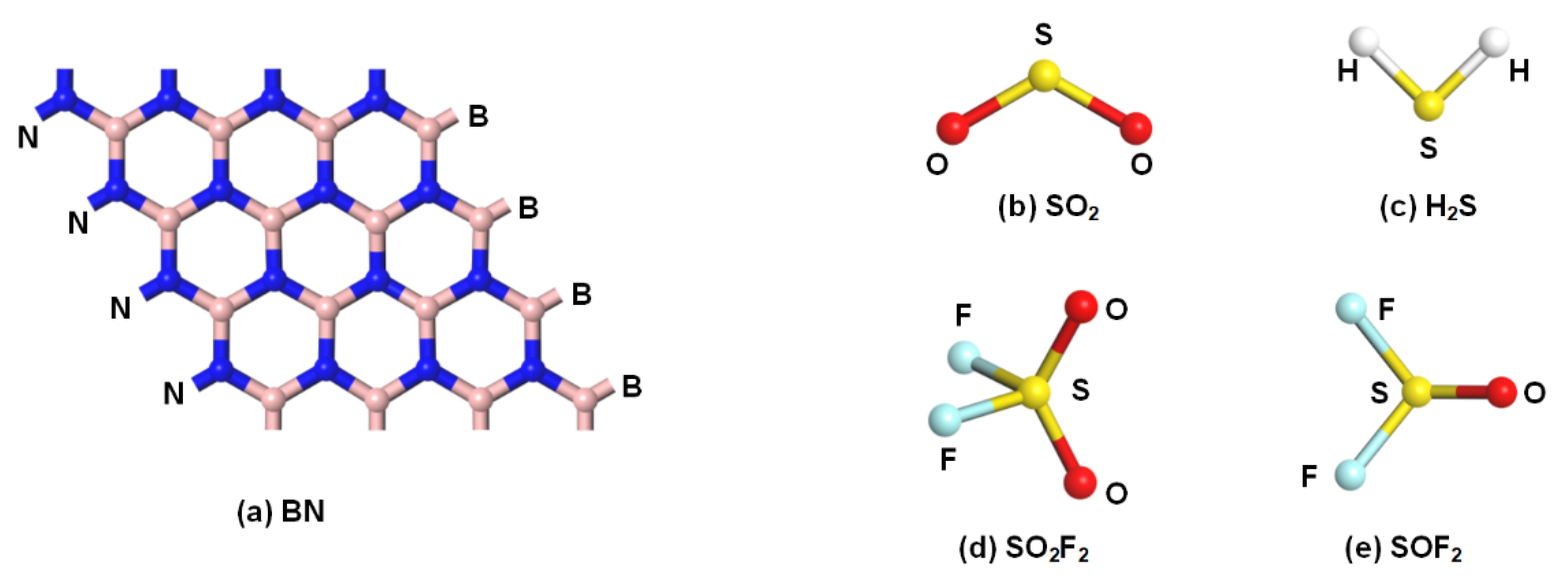
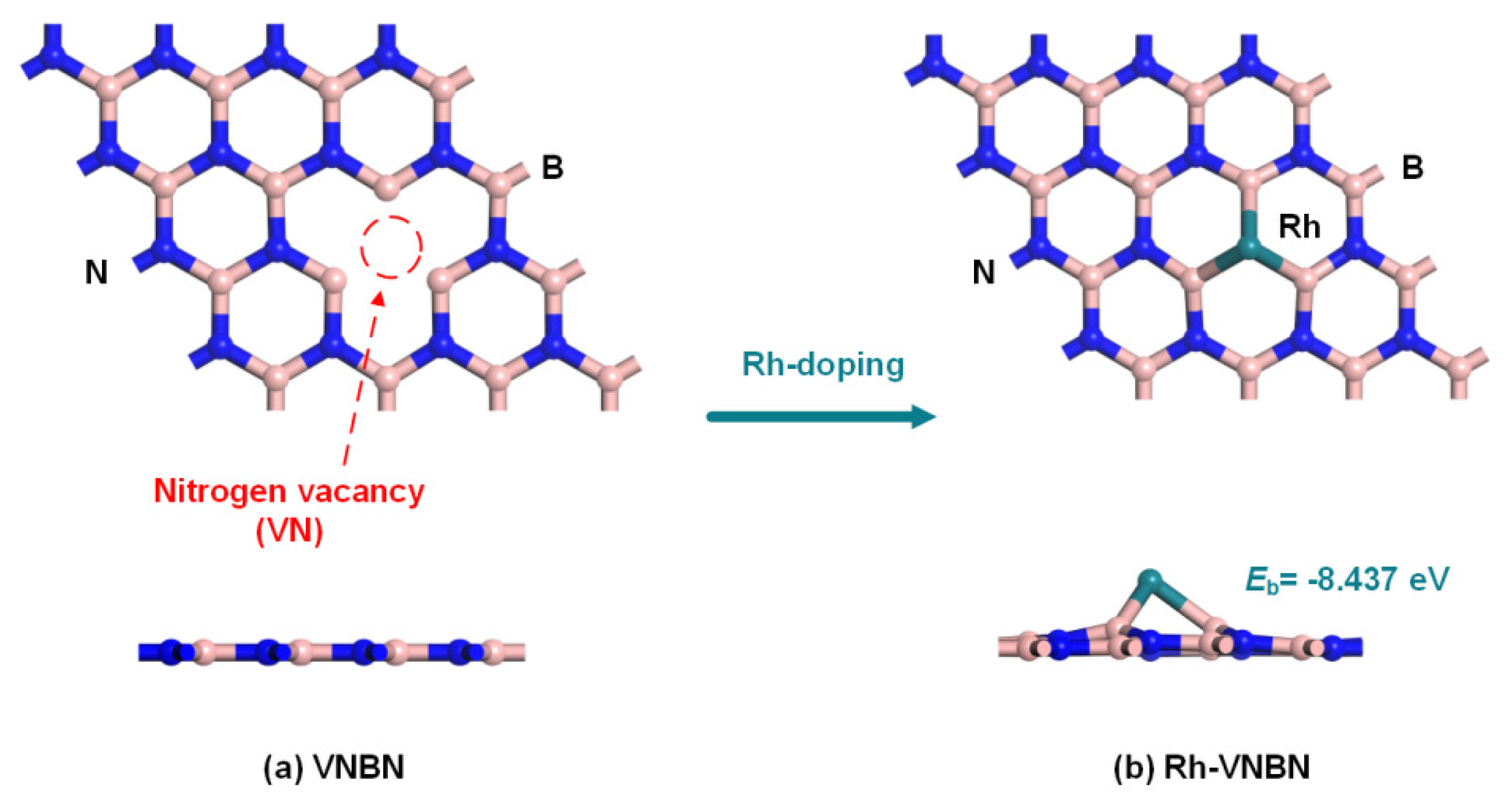
2.1. Configurations of Rh-VNBN and Gas Molecules
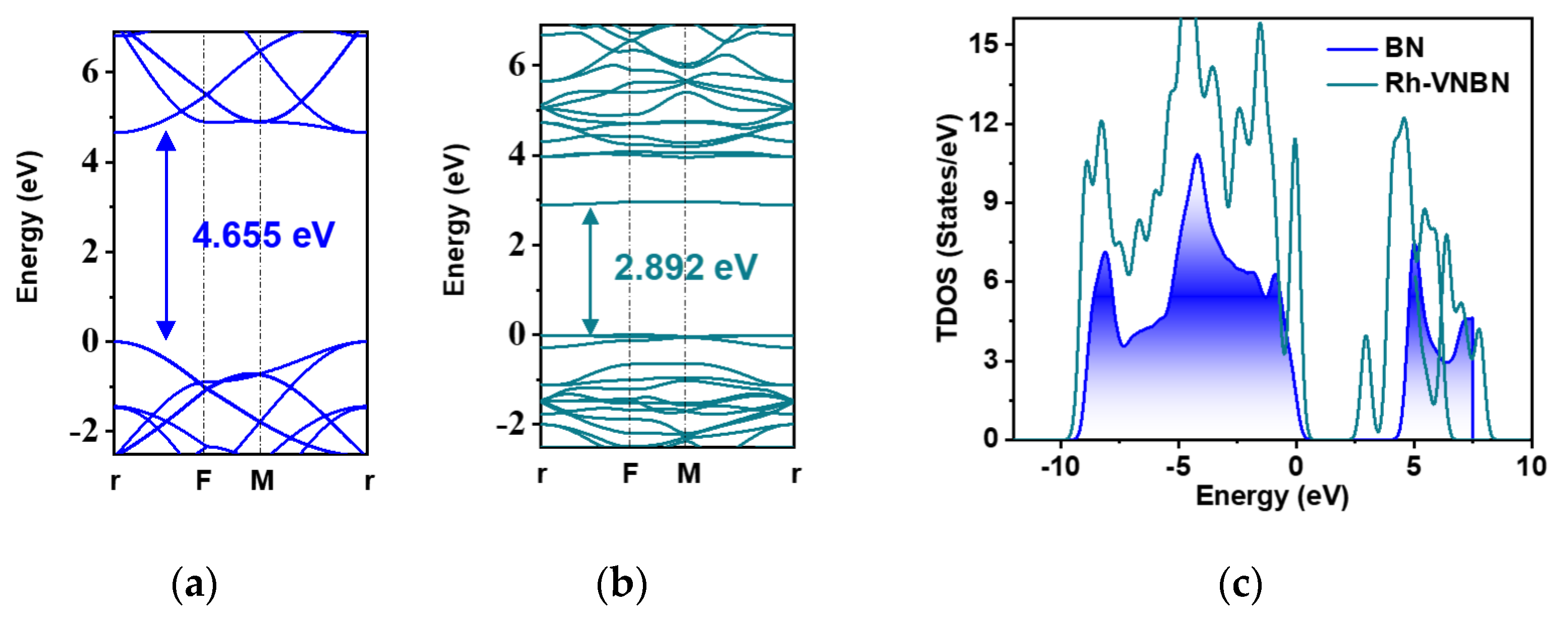
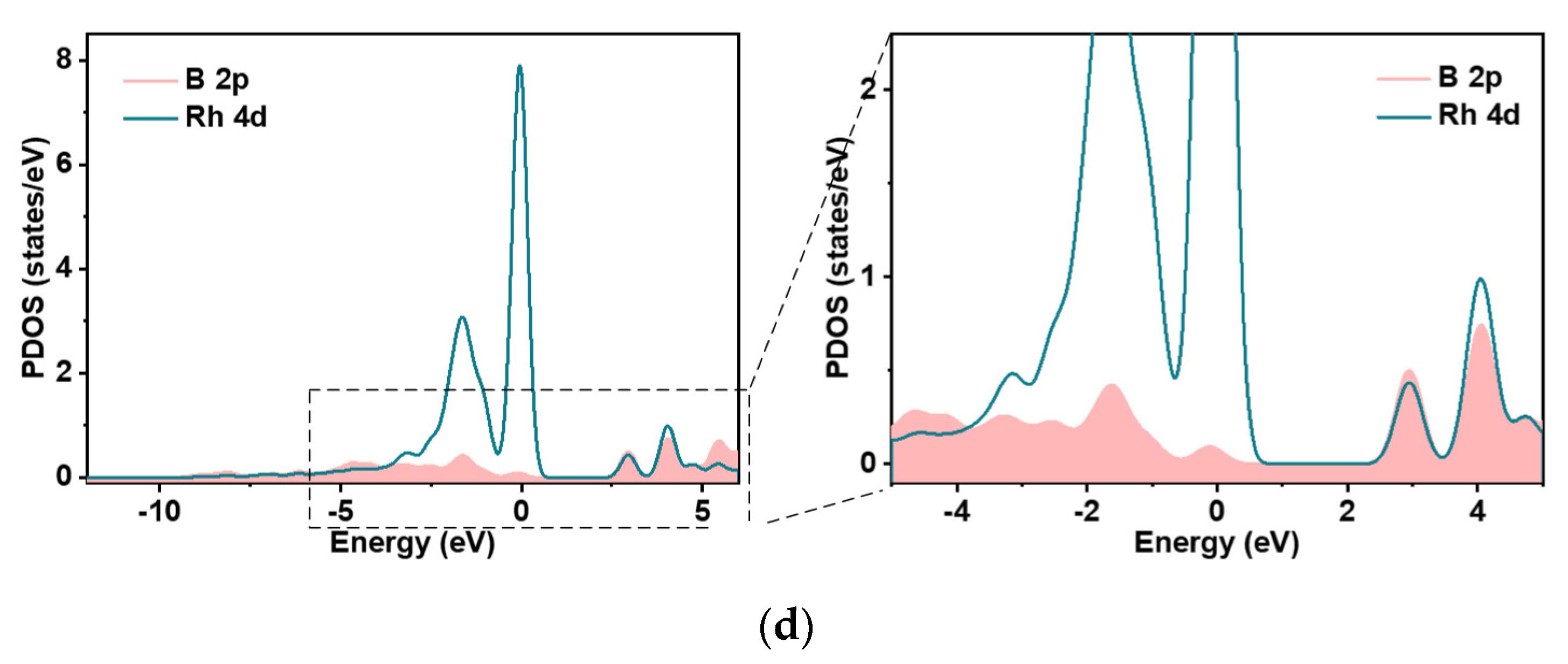
2.2. Electronic Properties of Rh-VNBN
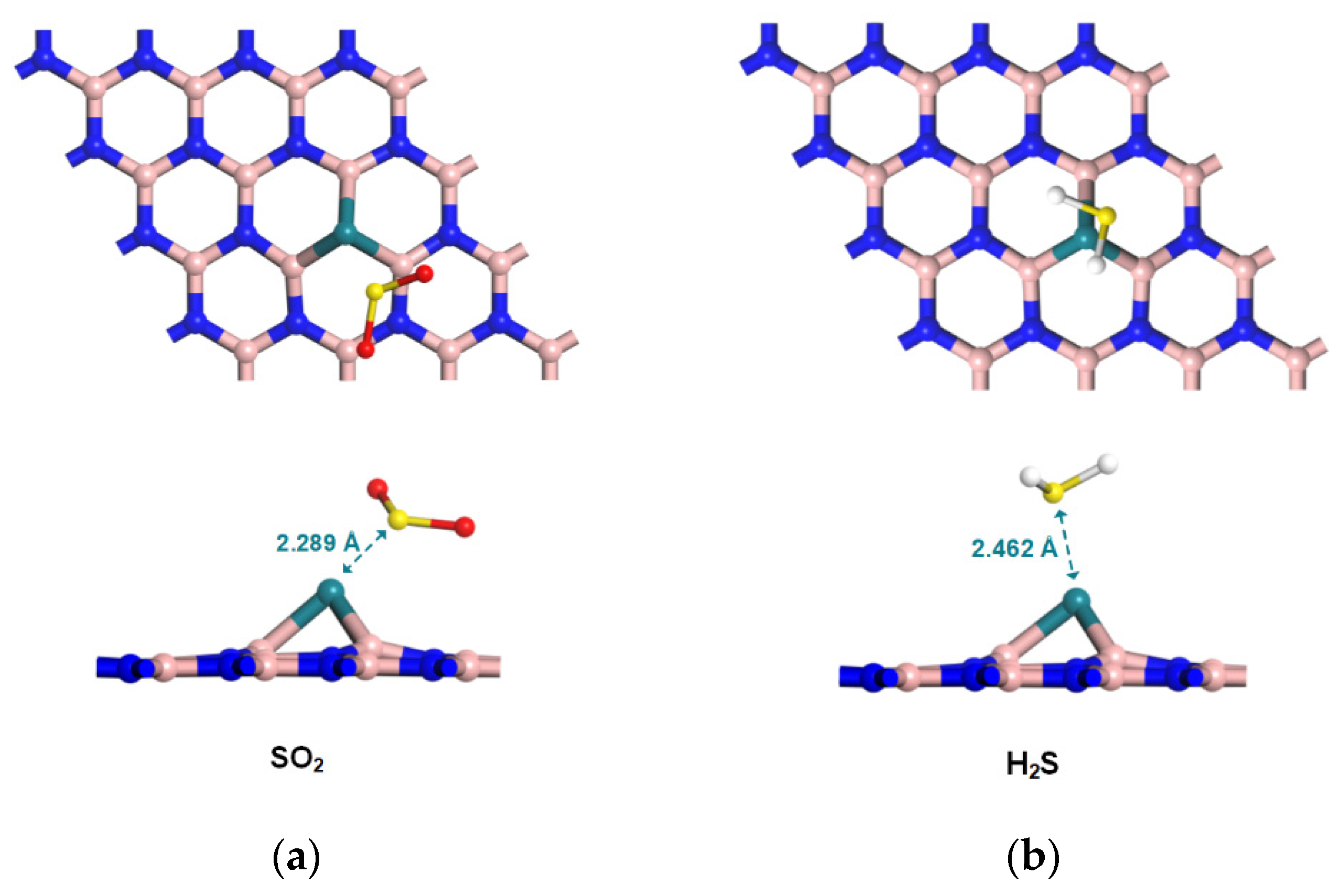
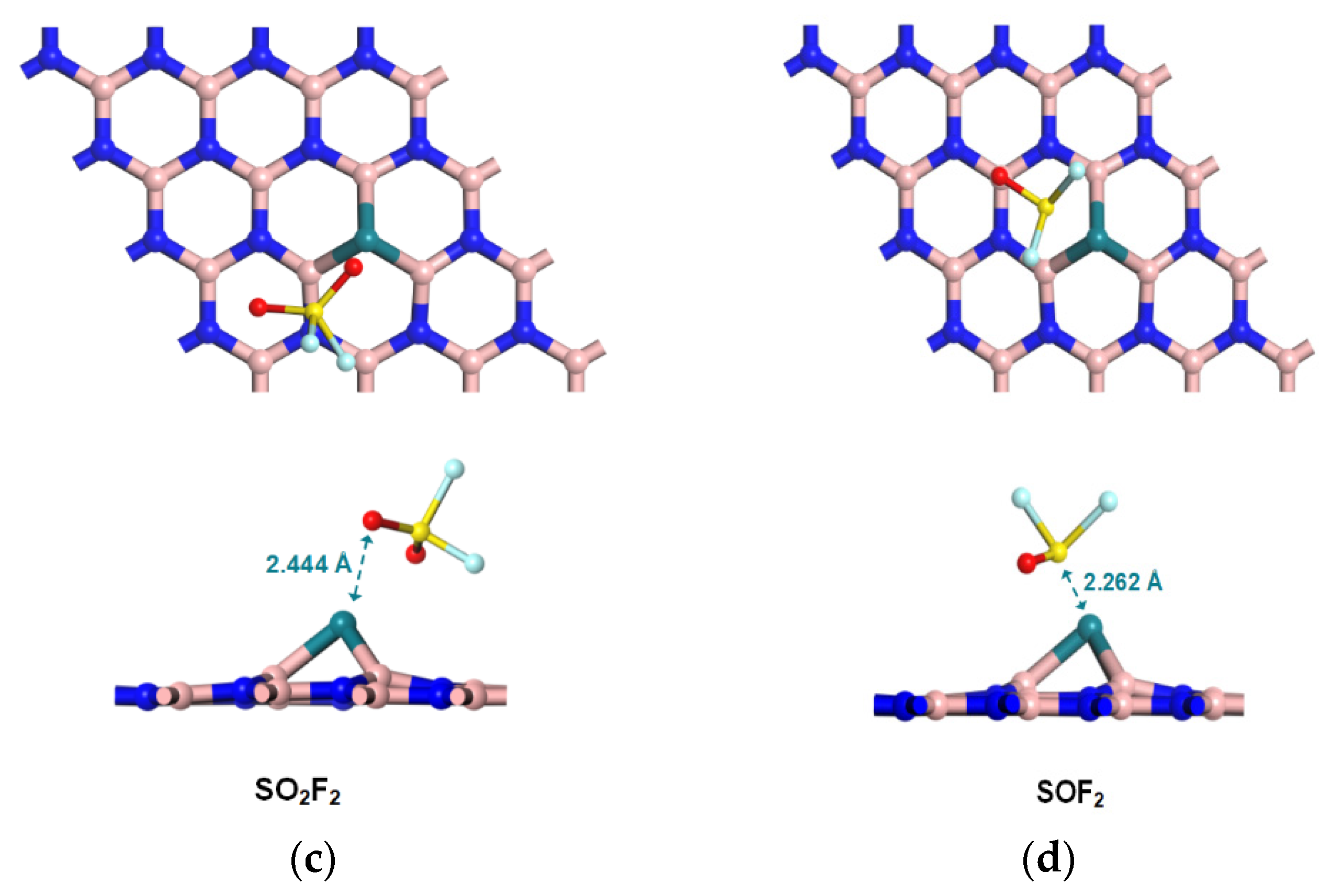
2.3. Configurations of Gas/Rh-VNBN Adsorption Systems
3. Results and Discussion
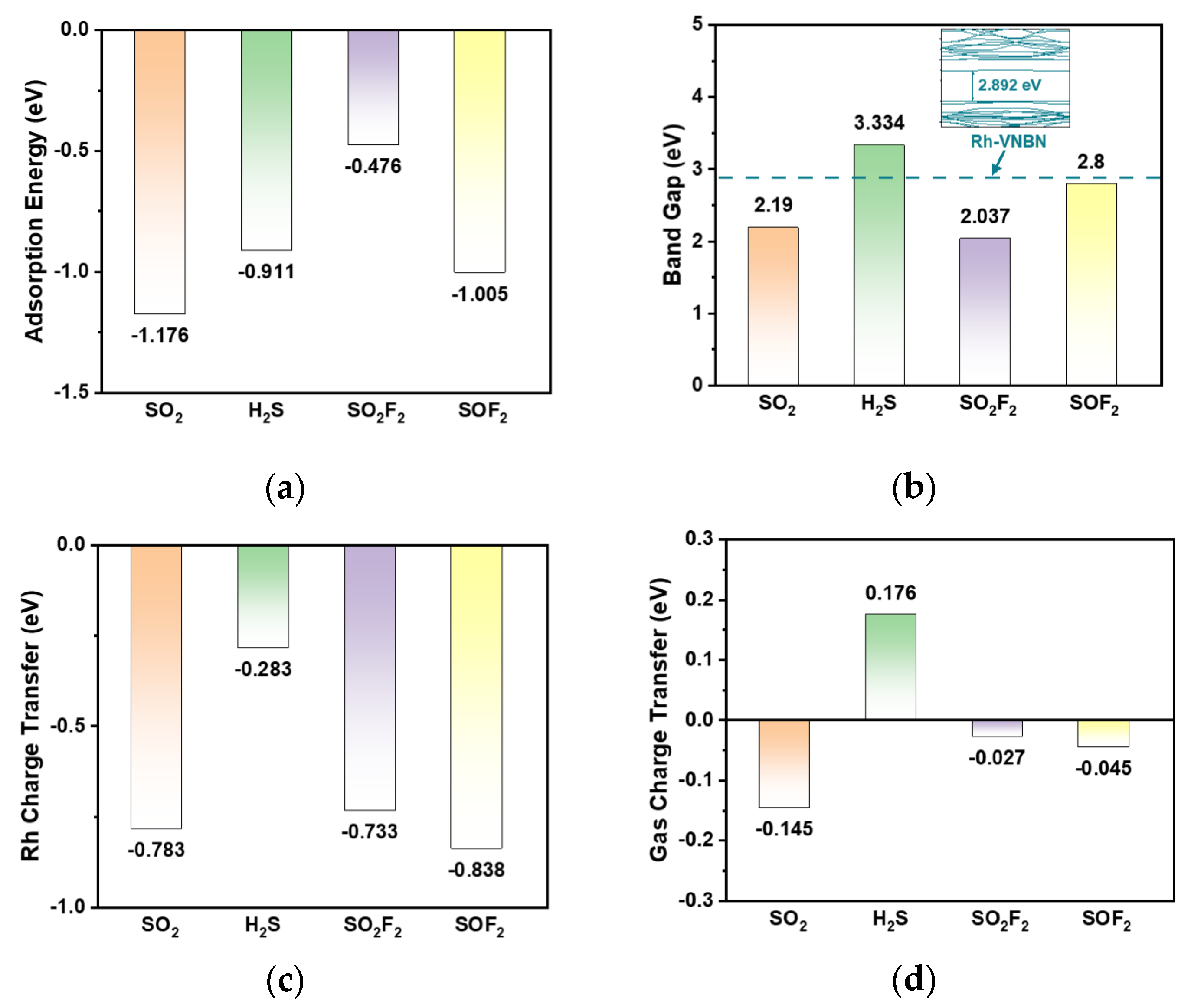
3.1. Adsorption and Sensing Properties of Gas/Rh-VNBN Adsorption System
3.2. Electronic Properties and Mechanisms of the Gas/Rh-VNBN Adsorption Systems
4. Conclusions
- Based on the analysis of the adsorption energy (Ead), the adsorption processes of SF6 decomposition products on Rh-VNBN are spontaneous and stable. In addition, the changes of the band gap (BG) suggest the sensing properties of Rh-VNBN for SO2, H2S, and SO2F2 are better than that for SOF2. Rh-VNBN exhibits outstanding adsorption and sensing capabilities for various SF6-decomposed gases;
- The electronic properties of gas/Rh-VNBN systems are studied, contributing to the understanding of the adsorption and sensing mechanisms. Additionally, the DOS further demonstrates the potential of Rh-VNBN for use in sensors and scavengers of SF6-decomposed products;
- From a long-term perspective, this computational study on gas/Rh-VNBN adsorption systems is important for future research on scavengers and sensors of SF6-decomposed gases, thereby ensuring the safe and stable operation of GIS equipment.
5. Computational Details
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cui, H.; Zhang, X.; Zhang, J.; Zhang, Y. Nanomaterials-based gas sensors of SF6 decomposed species for evaluating the operation status of high-voltage insulation devices. High Volt. 2019, 4, 242–258. [Google Scholar] [CrossRef]
- Liu, D.; Gui, Y.; Ji, C.; Tang, C.; Zhou, Q.; Li, J.; Zhang, X. Adsorption of SF6 decomposition components over Pd (1 1 1): A density functional theory study. Appl. Surf. Sci. 2019, 465, 172–179. [Google Scholar] [CrossRef]
- Qian, H.; Deng, J.; Xie, Z.; Pan, Z.; Zhang, J.; Zhou, H. Adsorption and Gas Sensing Properties of the Pt3-MoSe2 Monolayer to SOF2 and SO2F2. ACS Omega 2020, 5, 7722–7728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M. Adsorption Behavior of Ni-Doped ZnO Monolayer upon SF6 Decomposed Components and Effect of the Applied Electric Field. ACS Omega 2020, 5, 24118–24124. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bae, H.; Lee, S.; Shabbiri, K.; Hussain, T.; Lee, H. Highly sensitive and selective sensing properties of modified green phosphorene monolayers towards SF6 decomposition gases. Appl. Surf. Sci. 2020, 512, 145641. [Google Scholar] [CrossRef]
- Tang, J.; Liu, F.; Zhang, X.; Meng, Q.; Zhou, J. Partial discharge recognition through an analysis of SF6 decomposition products part 1: Decomposition characteristics of SF6 under four different partial discharges. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 29–36. [Google Scholar] [CrossRef]
- Tang, J.; Liu, F.; Zhang, X.; Meng, Q.; Zhou, J.; Tao, J. Partial discharge recognition through an analysis of SF6 decomposition products part 2: Feature extraction and decision tree-based pattern recognition. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 37–44. [Google Scholar] [CrossRef]
- Koch, H.; Goll, F.; Magier, T.; Juhre, K. Technical aspects of gas insulated transmission lines and application of new insulating gases. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1448–1453. [Google Scholar] [CrossRef]
- Hyrenbach, M.; Zache, S. Alternative insulation gas for medium-voltage switchgear. In Proceedings of the 2016 Petroleum and Chemical Industry Conference Europe (PCIC Europe), Berlin, Germany, 14–16 June 2016; pp. 1–9. [Google Scholar]
- Zhang, X.; Chen, Z.; Chen, D.; Cui, H.; Tang, J. Adsorption behaviour of SO2 and SOF2 gas on Rh-doped BNNT: A DFT study. Mol. Phys. 2020, 118, e1580394. [Google Scholar] [CrossRef]
- Wang, D.W.; Wang, X.H.; Yang, A.J.; Chu, J.F.; Lv, P.L.; Liu, Y.; Rong, M.Z. MoTe2: A Promising Candidate for SF6 Decomposition Gas Sensors with High Sensitivity and Selectivity. IEEE Electron Device Lett. 2018, 39, 292–295. [Google Scholar] [CrossRef]
- Sun, H.; Gui, Y.; Wei, H.; Long, Y.; Wang, Q.; Tang, C. DFT study of SF6 decomposed products on Pd–TiO2: Gas sensing mechanism study. Adsorption 2019, 25, 1643–1653. [Google Scholar] [CrossRef]
- Zhou, Q.; Ju, W.; Su, X.; Yong, Y.; Li, X. Adsorption behavior of SO2 on vacancy-defected graphene: A DFT study. J. Phys. Chem. Solids 2017, 109, 40–45. [Google Scholar] [CrossRef]
- Bhimanapati, G.R.; Lin, Z.; Meunier, V.; Jung, Y.; Cha, J.; Das, S.; Xiao, D.; Son, Y.; Strano, M.S.; Cooper, V.R.; et al. Recent Advances in Two-Dimensional Materials beyond Graphene. ACS Nano 2015, 9, 11509–11539. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Wu, X.; Hu, W. Experimental Sensing and Density Functional Theory Study of H2S and SOF2 Adsorption on Au-Modified Graphene. Adv. Sci. 2015, 2, 1500101. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Chang, H. Graphene and Graphene-like Two-Dimensional Materials in Photodetection: Mechanisms and Methodology. ACS Nano 2014, 8, 4133–4156. [Google Scholar] [CrossRef]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-Like Two-Dimensional Materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Jun, D.H.; Yang, J.H.; Zhou, Z.; Yang, S.S.; Cheng, M.M.-C. Carbon dioxide gas sensor using a graphene sheet. Sens. Actuators B Chem. 2011, 157, 310–313. [Google Scholar] [CrossRef]
- Wang, H.; Taychatanapat, T.; Hsu, A.; Watanabe, K.; Taniguchi, T.; Jarillo-Herrero, P.; Palacios, T. BN/Graphene/BN Transistors for RF Applications. IEEE Electron Device Lett. 2011, 32, 1209–1211. [Google Scholar] [CrossRef] [Green Version]
- Jain, N.; Bansal, T.; Durcan, C.; Yu, B. Graphene-Based Interconnects on Hexagonal Boron Nitride Substrate. IEEE Electron Device Lett. 2012, 33, 925–927. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Chi, Z.-T.; Liu, J.; Li, D.-H.; Sun, X.-J.; Yan, C.; Wang, Y.-C.; Li, H.; Wang, X.-D.; Xie, W.-F. Enhanced gas sensing performance based on p-NiS/n-In2O3 heterojunction nanocomposites. Sens. Actuators B Chem. 2020, 304, 127305. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Z.; Zhang, Y.; Li, H.-Y.; Gao, N.; Tian, Z.; Zhou, L.; Zhang, B.; Tang, J.; Zhang, J.; et al. MoS2 Nanosheets Sensitized with Quantum Dots for Room-Temperature Gas Sensors. Nano-Micro Lett. 2020, 12, 59. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Lin, S.; Gu, D.; Li, X. Two-Dimensional Nanomaterials for Gas Sensing Applications: The Role of Theoretical Calculations. Nanomaterials 2018, 8, 851. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zheng, K.; Huang, Y.; Yu, J.; Wu, H.; Chen, X.; Tao, L.-Q. An investigation of the positive effects of doping an Al atom on the adsorption of CO2 on BN nanosheets: A DFT study. Phys. Chem. Chem. Phys. 2020, 22, 9368–9374. [Google Scholar] [CrossRef] [PubMed]
- Bhati, V.S.; Kumar, M.; Banerjee, R. Gas sensing performance of 2D nanomaterials/metal oxide nanocomposites: A review. J. Mater. Chem. C 2021, 9, 8776–8808. [Google Scholar] [CrossRef]
- Mistry, K.; Ibrahim, K.H.; Novodchuk, I.; Ngo, H.T.; Imamura, G.; Sanderson, J.; Yavuz, M.; Yoshikawa, G.; Musselman, K.P. Nanomechanical Gas Sensing with Laser Treated 2D Nanomaterials. Adv. Mater. Technol. 2020, 5, 2000704. [Google Scholar] [CrossRef]
- Lin, S.; Ye, X.; Johnson, R.S.; Guo, H. First-Principles Investigations of Metal (Cu, Ag, Au, Pt, Rh, Pd, Fe, Co, and Ir) Doped Hexagonal Boron Nitride Nanosheets: Stability and Catalysis of CO Oxidation. J. Phys. Chem. C 2013, 117, 17319–17326. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, T.; Yang, L.; Liu, R.; Zhang, G.; Jiang, J.; Luo, Y.; Lian, P.; Tang, S. Graphene–boron nitride hybrid-supported single Mo atom electrocatalysts for efficient nitrogen reduction reaction. J. Mater. Chem. A 2019, 7, 15173–15180. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Yang, P.; Sun, X.; Wang, Z.G.; Zu, X.T.; Gao, F. First-principles study of the noble metal-doped BN layer. J. Appl. Phys. 2011, 109, 084308. [Google Scholar] [CrossRef]
- Wan, Q.; Chen, X.; Gui, Y. First-Principles Insight into a Ru-Doped SnS2 Monolayer as a Promising Biosensor for Exhale Gas Analysis. ACS Omega 2020, 5, 8919–8926. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Zhang, X.; Chen, D.; Tang, J. Adsorption of SF6 Decomposed Products on ZnO-Modified C3N: A Theoretical Study. Nanoscale Res. Lett. 2020, 15, 186. [Google Scholar] [CrossRef]
- He, X.; Gui, Y.; Liu, K.; Xu, L. Comparison of sensing and electronic properties of C2H2 on different transition metal oxide nanoparticles (Fe2O3, NiO, TiO2) modified BNNT (10, 0). Appl. Surf. Sci. 2020, 521, 146463. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Thomson, B.; Motayed, A.; Li, Q.; Rao, M.V. Functionalization of GaN Nanowire Sensors With Metal Oxides: An Experimental and DFT Investigation. IEEE Sens. J. 2020, 20, 7138–7147. [Google Scholar] [CrossRef]
- Xia, S.-Y.; Tao, L.-Q.; Jiang, T.; Sun, H.; Li, J. Rh-doped h-BN monolayer as a high sensitivity SF6 decomposed gases sensor: A DFT study. Appl. Surf. Sci. 2021, 536, 147965. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhong, L.; Wang, H.; Li, X. Structural Defects, Mechanical Behaviors, and Properties of Two-Dimensional Materials. Materials 2021, 14, 1192. [Google Scholar] [CrossRef]
- Lee, C.W.; Suh, J.M.; Jang, H.W. Chemical Sensors Based on Two-Dimensional (2D) Materials for Selective Detection of Ions and Molecules in Liquid. Front. Chem. 2019, 7, 708. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Hu, Z.; Probert, M.; Li, K.; Lv, D.; Yang, X.; Gu, L.; Mao, N.; Feng, Q.; Xie, L.; et al. Exploring atomic defects in molybdenum disulphide monolayers. Nat. Commun. 2015, 6, 6293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, N.; Gan, L.; Yang, R.; Wang, F.; Li, L.; Chen, Y.; Li, D.; Zhai, T. Nonlayered Two-Dimensional Defective Semiconductor γ-Ga 2 S 3 toward Broadband Photodetection. ACS Nano 2019, 13, 6297–6307. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Lin, F.; Suenaga, K.; Iijima, S. Fabrication of a Freestanding Boron Nitride Single Layer and Its Defect Assignments. Phys. Rev. Lett. 2009, 102, 195505. [Google Scholar] [CrossRef] [Green Version]
- Si, M.S.; Xue, D.S. Magnetic properties of vacancies in a graphitic boron nitride sheet by first-principles pseudopotential calculations. Phys. Rev. B 2007, 75, 193409. [Google Scholar] [CrossRef]
- Azevedo, S.; Kaschny, J.R.; de Castilho, C.M.C.; de Brito Mota, F. A theoretical investigation of defects in a boron nitride monolayer. Nanotechnology 2007, 18, 495707. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gui, Y.; Zhao, W.; Tang, C.; Dong, X. Palladium modified MoS2 monolayer for adsorption and scavenging of SF6 decomposition products: A DFT study. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 123, 114178. [Google Scholar] [CrossRef]
- Hou, W.; Mi, H.; Peng, R.; Peng, S.; Zeng, W.; Zhou, Q. First-Principle Insight into Ga-Doped MoS2 for Sensing SO2, SOF2 and SO2F2. Nanomaterials 2021, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Chen, W.; Lu, Y.; Tang, C.; Xu, L. Au Catalyst-Modified MoS2 Monolayer as a Highly Effective Adsorbent for SO2F2 Gas: A DFT Study. ACS Omega 2019, 4, 12204–12211. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Hong, Q.; Wu, T.; Cui, H. SOF 2 sensing by Rh-doped PtS2 monolayer for early diagnosis of partial discharge in the SF6 insulation device. Mol. Phys. 2021, 119, e1919774. [Google Scholar] [CrossRef]
- Zhu, H.; Cui, H.; He, D.; Cui, Z.; Wang, X. Rh-doped MoTe2 Monolayer as a Promising Candidate for Sensing and Scavenging SF6 Decomposed Species: A DFT Study. Nanoscale Res. Lett. 2020, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Zhou, Q.; Peng, S.; Xu, L.; Zeng, W. Adsorption behavior of Rh-doped MoS2 monolayer towards SO2, SOF2, SO2F2 based on DFT study. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 122, 114224. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Zhou, K.; Ma, W.; Zeng, Z.; Chen, R.; Xu, X.; Liu, B.; Li, H.; Li, H.; Li, L. Waste biomass-derived oxygen and nitrogen co-doped porous carbon/MgO composites as superior acetone adsorbent: Experimental and DFT study on the adsorption behavior. Chem. Eng. J. 2020, 387, 124173. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Li, P.; Pang, M.; Xue, Q. Flexible self-powered high-performance ammonia sensor based on Au-decorated MoSe2 nanoflowers driven by single layer MoS2-flake piezoelectric nanogenerator. Nano Energy 2019, 65, 103974. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Q.; Zeng, W. Competitive adsorption of SF6 decompositions on Ni-doped ZnO (100) surface: Computational and experimental study. Appl. Surf. Sci. 2019, 479, 185–197. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, L.; Zhang, M.; Ma, C. Effect of N-doping on NO2 adsorption and reduction over activated carbon: An experimental and computational study. Fuel 2019, 258, 116109. [Google Scholar] [CrossRef]
- Maximoff, S.N.; Ernzerhof, M.; Scuseria, G.E. Current-dependent extension of the Perdew–Burke–Ernzerhof exchange-correlation functional. J. Chem. Phys. 2004, 120, 2105–2109. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, G.; Zhang, X.; Tang, J. Rh-doped MoSe2 as a toxic gas scavenger: A first-principles study. Nanoscale Adv. 2019, 1, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Zhang, J.; Ali Mehmood, M. Interaction of CO and CH 4 Adsorption with Noble Metal (Rh, Pd, and Pt)-Decorated N 3 -CNTs: A First-Principles Study. ACS Omega 2018, 3, 16892–16898. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Xia, S.-Y. First-Principle Study of Rh-Doped Nitrogen Vacancy Boron Nitride Monolayer for Scavenging and Detecting SF6 Decomposition Products. Polymers 2021, 13, 3507. https://doi.org/10.3390/polym13203507
Shi Z, Xia S-Y. First-Principle Study of Rh-Doped Nitrogen Vacancy Boron Nitride Monolayer for Scavenging and Detecting SF6 Decomposition Products. Polymers. 2021; 13(20):3507. https://doi.org/10.3390/polym13203507
Chicago/Turabian StyleShi, Zhen, and Sheng-Yuan Xia. 2021. "First-Principle Study of Rh-Doped Nitrogen Vacancy Boron Nitride Monolayer for Scavenging and Detecting SF6 Decomposition Products" Polymers 13, no. 20: 3507. https://doi.org/10.3390/polym13203507
APA StyleShi, Z., & Xia, S.-Y. (2021). First-Principle Study of Rh-Doped Nitrogen Vacancy Boron Nitride Monolayer for Scavenging and Detecting SF6 Decomposition Products. Polymers, 13(20), 3507. https://doi.org/10.3390/polym13203507





