Antibacterial Synergism of Electrospun Nanofiber Mats Functioned with Silver Nanoparticles and Pulsed Electromagnetic Waves
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
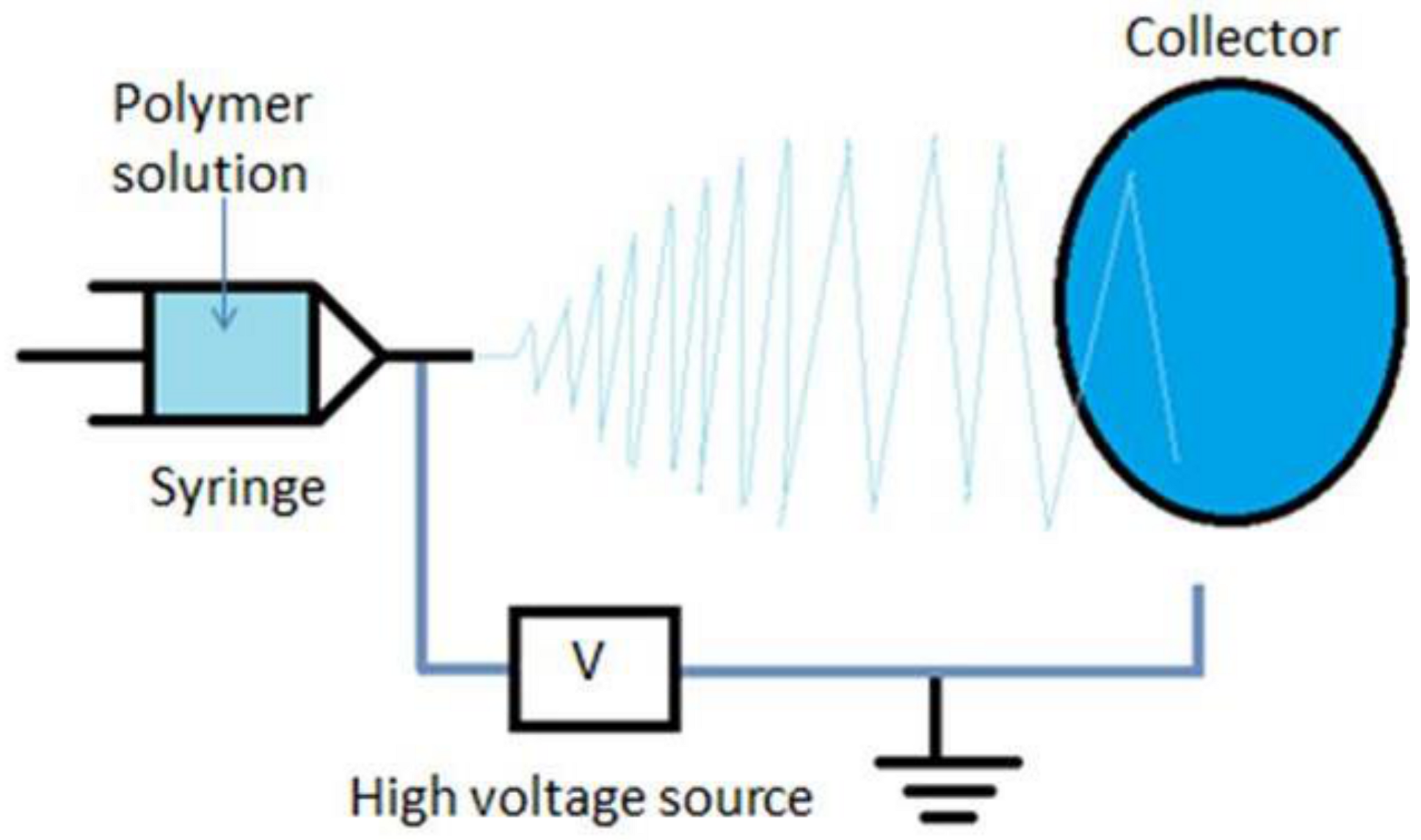
2.2. Electrospinning Process
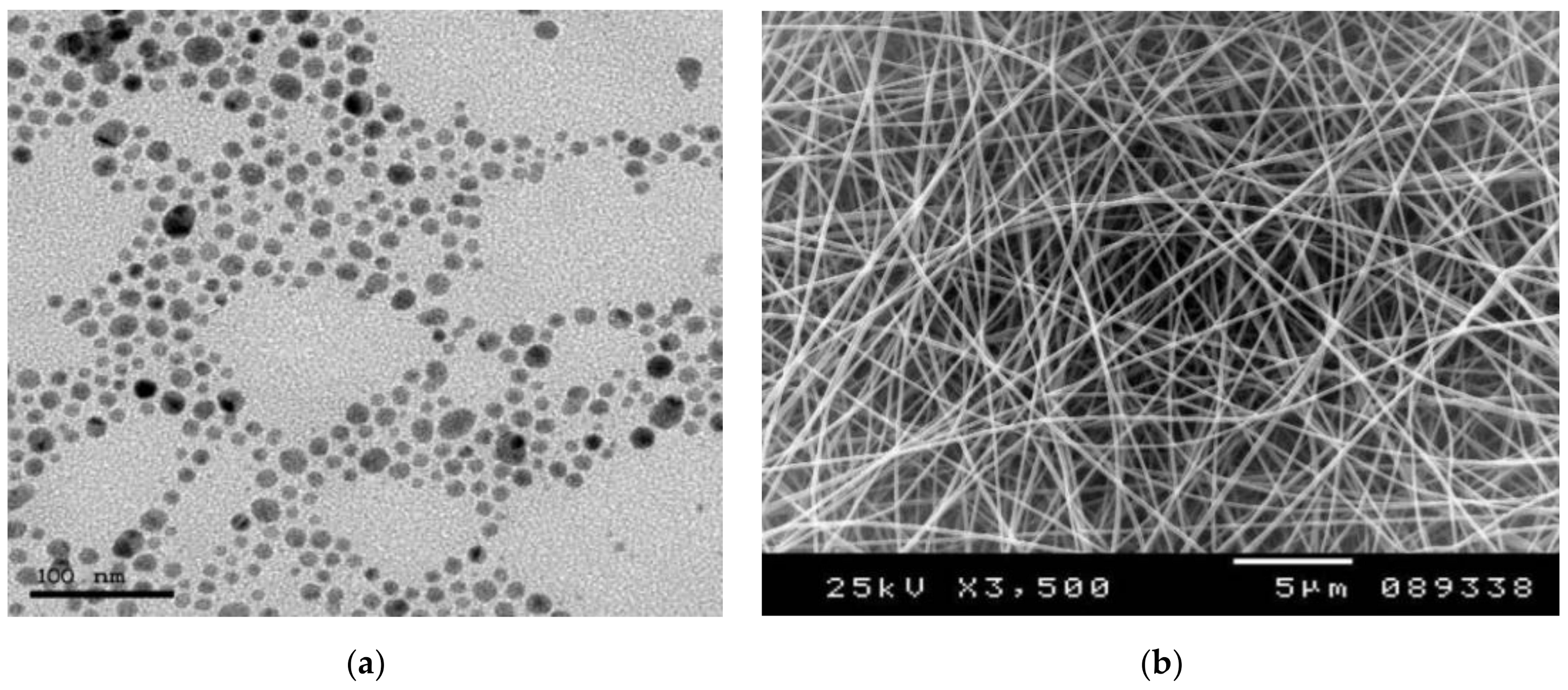
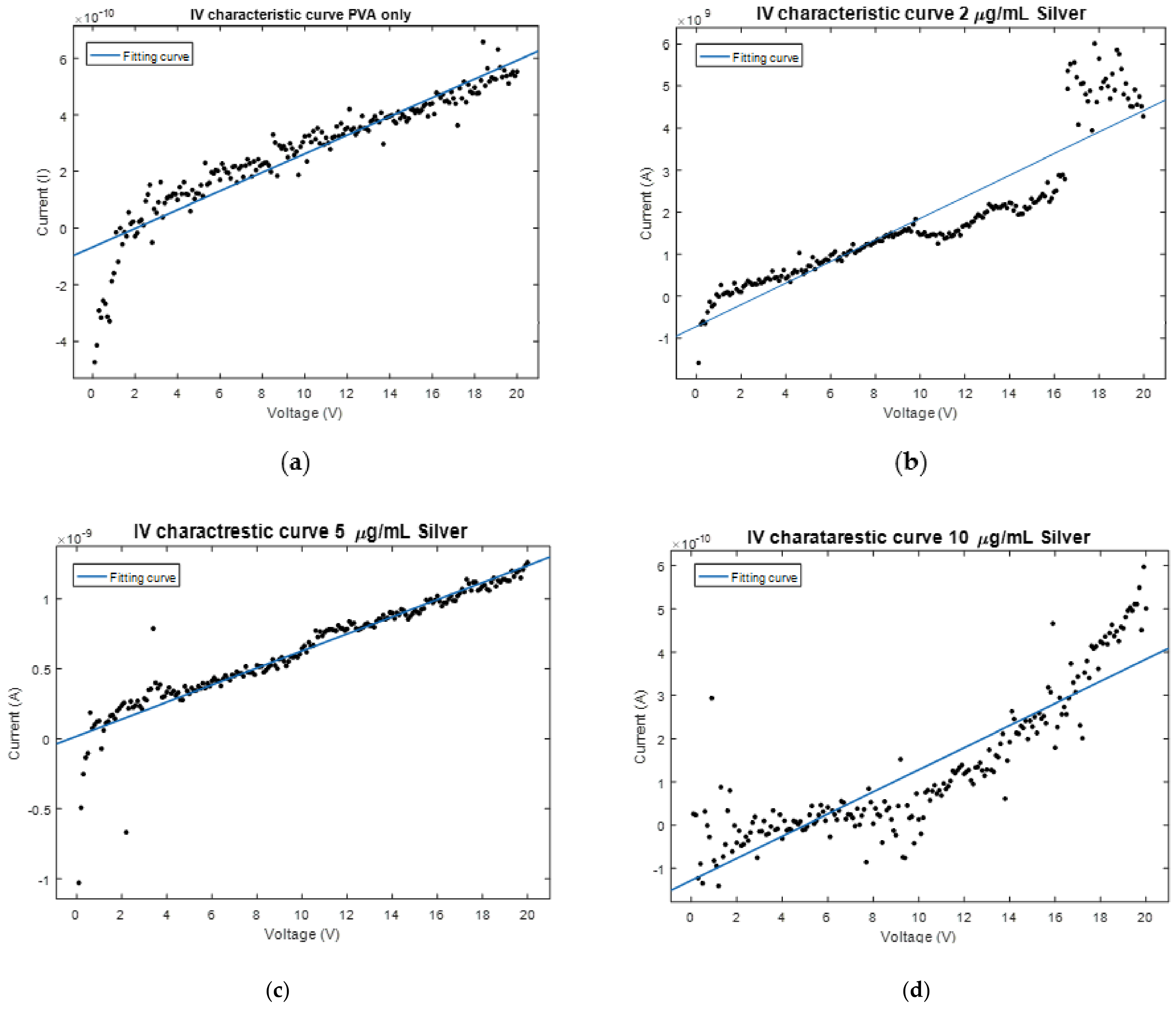
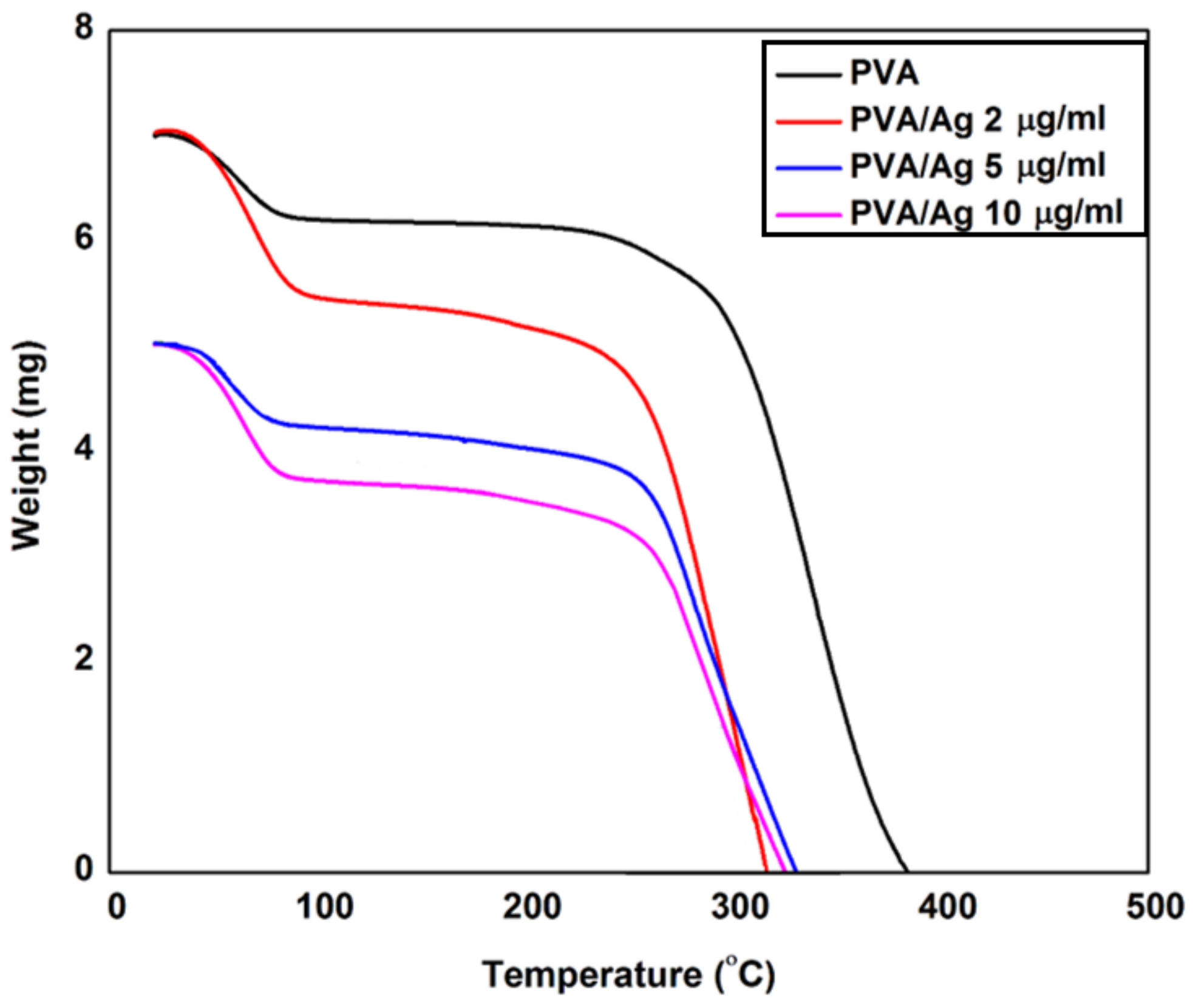
2.3. Nanocomposite Characterizations
2.4. Pulsed Electromagnetic Waves
2.5. Bacterial Strain and Replication
2.6. Bacterial Availability and Antibacterial Test
2.7. Bacterial Cytotoxicity Test
2.8. Statistical Analysis
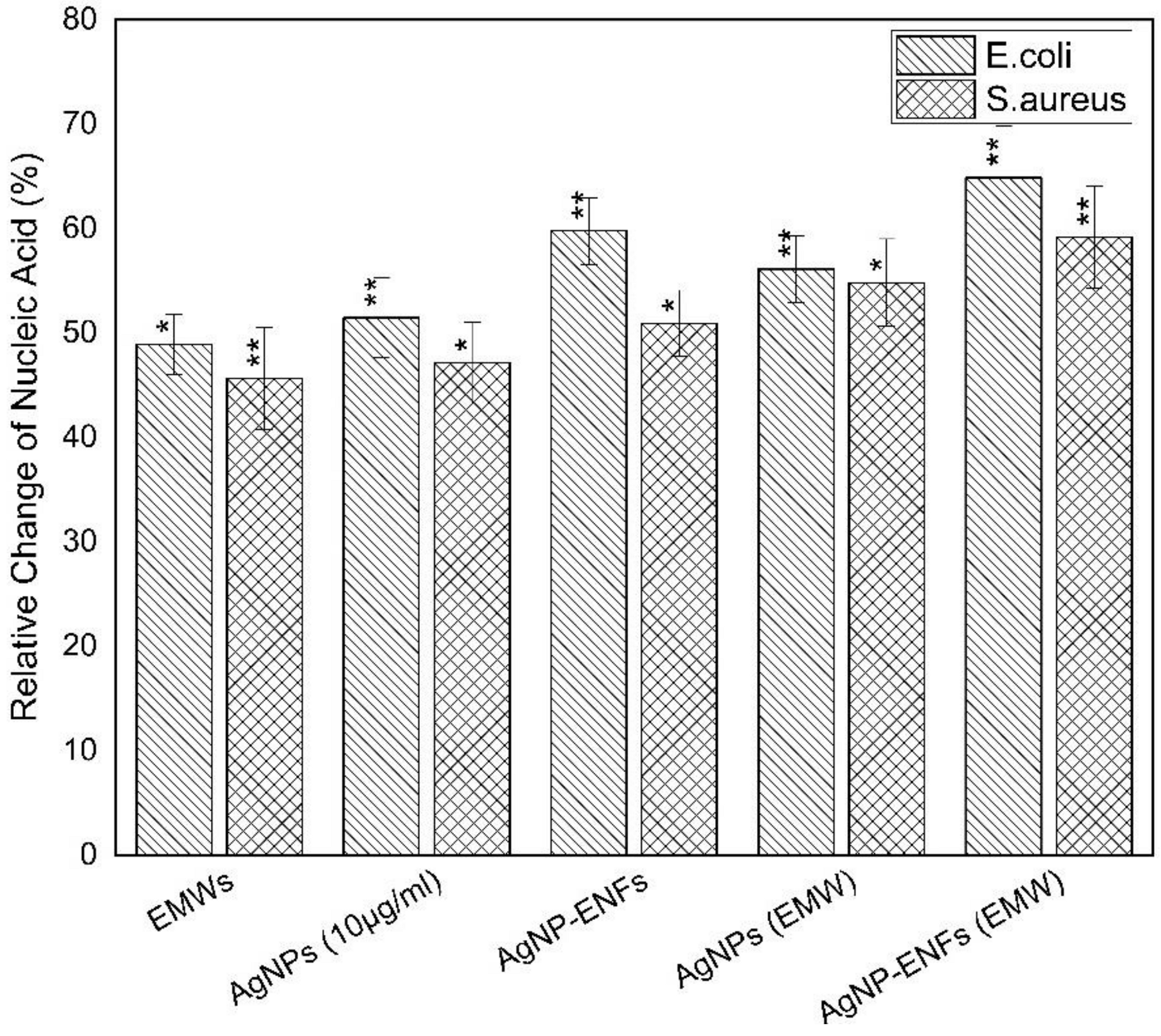
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hong, K.H.; Park, J.L.; Sul, I.H.; Youk, J.H.; Kang, T.J. Preparation of antimicrobial poly(vinyl alcohol) nanofibers containing silver nanoparticles. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2468–2474. [Google Scholar] [CrossRef]
- El-Khatib, A.M.; Khalil, A.M.; El-Kaliuoby, M.I.; Elkhatib, M. The combined effects of multisized silver nanoparticles and pulsed magnetic field on K. pneumoniae. Bioinspired Biomim. Nanobiomater. 2019, 8, 154–160. [Google Scholar] [CrossRef]
- Espinosa-Cristóbal, L.F.; Martínez-Castañon, G.; Martínez-Martínez, R.; Loyola-Rodriguez, J.P.; Patiño-Marín, N.; Reyes-Macías, J.; Ruiz, F. Antibacterial effect of silver nanoparticles against Streptococcus mutans. Mater. Lett. 2009, 63, 2603–2606. [Google Scholar] [CrossRef]
- Klueh, U.; Wagner, V.; Kelly, S.; Johnson, A.; Bryers, J.D. Efficacy of silver-coated fabric to prevent bacterial colonization and subsequent device-based biofilm formation. J. Biomed. Mater. Res. 2000, 53, 621–631. [Google Scholar] [CrossRef]
- Chen, X.; Schluesener, H. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Liu, J.; Hurt, R.H. Ion Release Kinetics and Particle Persistence in Aqueous Nano-Silver Colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Chairuangkitti, P.; Lawanprasert, S.; Roytrakul, S.; Aueviriyavit, S.; Phummiratch, D.; Kulthong, K.; Chanvorachote, P.; Maniratanachote, R. Silver nanoparticles induce toxicity in A549 cells via ROS-dependent and ROS-independent pathways. Toxicol. In Vitro 2013, 27, 330–338. [Google Scholar] [CrossRef]
- Panáček, A.; Kvitek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Zbořil, R. Silver Colloid Nanoparticles: Synthesis, Characterization, and Their Antibacterial Activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef]
- Li, P.; Li, J.; Wu, C.; Wu, Q.; Li, J. Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnology 2005, 16, 1912–1917. [Google Scholar] [CrossRef]
- Hong, K.H. Preparation and properties of electrospun poly(vinyl alcohol)/silver fiber web as wound dressings. Polym. Eng. Sci. 2006, 47, 43–49. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Bowlin, G.L.; Mansfield, K.; Layman, J.; Simpson, D.G.; Sanders, E.H.; Wnek, G. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J. Control. Release 2002, 81, 57–64. [Google Scholar] [CrossRef]
- Greiner, A.; Wendroff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 38, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Preparation of Antimicrobial Ultrafine Cellulose Acetate Fibers with Silver Nanoparticles. Macromol. Rapid Commun. 2004, 25, 1632–1637. [Google Scholar] [CrossRef]
- Fussell, G.; Thomas, J.; Scanlon, J.L.; Lowman, A.; Marcolongo, M. The effect of protein-free versus protein-containing medium on the mechanical properties and uptake of ions of PVA/PVP hydrogels. J. Biomater. Sci. Polym. Ed. 2005, 16, 489–503. [Google Scholar] [CrossRef]
- Porel, S.; Singh, S.; Harsha, S.S.; Rao, D.N.; Radhakrishnan, T.P. Nanoparticle-Embedded Polymer: In Situ Synthesis, Free-Standing Films with Highly Monodisperse Silver Nanoparticles and Optical Limiting. Chem. Mater. 2005, 17, 9–12. [Google Scholar] [CrossRef]
- Shao, C.; Kim, H.-Y.; Gong, J.; Ding, B.; Lee, D.-R.; Park, S.-J. Fiber mats of poly(vinyl alcohol)/silica composite via electrospinning. Mater. Lett. 2003, 57, 1579–1584. [Google Scholar] [CrossRef]
- Pazos-Ortiz, E.; Roque-Ruiz, J.H.; Hinojos-Márquez, E.A.; López-Esparza, J.; Donohué-Cornejo, A.; González, J.C.C.; Espinosa-Cristóbal, L.F.; Reyes-López, S.Y. Dose-Dependent Antimicrobial Activity of Silver Nanoparticles on Polycaprolactone Fibers against Gram-Positive and Gram-Negative Bacteria. J. Nanomater. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- El-Kaliuoby, M.I.; El-Khatib, A.M.; Khalil, A.M. Does engineering of nanoshapes have antibacterial synergy with magnetic signal exposure? Surf. Innov. 2019, 7, 260–267. [Google Scholar] [CrossRef]
- El-Khatib, A.M.; Badawi, M.; Ghatass, Z.F.; Mohamed, M.M.; Elkhatib, M. Synthesize of Silver Nanoparticles by Arc Discharge Method Using Two Different Rotational Electrode Shapes. J. Clust. Sci. 2018, 29, 1169–1175. [Google Scholar] [CrossRef]
- El-Kaliuoby, M.I.; Khalil, A.M.; El-Khatib, A.M. Alterations of bacterial dielectric characteristics due to pulsed magnetic field exposure. Bioinspired Biomim. Nanobiomater. 2020, 9, 103–111. [Google Scholar] [CrossRef]
- Masood, S.S. Effect of Weak Magnetic Field on Bacterial Growth. Biophys. Rev. Lett. 2017, 12, 177–186. [Google Scholar] [CrossRef]
- Balabel, N.M.; El-Kaliuoby, M.I.; Khalil, A.M. Effect of square pulsed magnetic field exposure on growth kinetics of Dickeya solani. Arch. Phytopathol. Plant Prot. 2019, 52, 989–1004. [Google Scholar] [CrossRef]
- Tiwari, D.K.; Behari, J.; Sen, P. Time and dose-dependent antimicrobial potential of Ag nanoparticles synthesized by top-down approach. Curr. Sci. 2008, 95, 647–655. [Google Scholar]
- Shi, Q.; Vitchuli, N.; Nowak, J.; Noar, J.; Caldwell, J.M.; Breidt, F.; Bourham, M.; Mccord, M.; Zhang, X. One-step synthesis of silver nanoparticle-filled nylon 6 nanofibers and their antibacterial properties. J. Mater. Chem. 2011, 21, 10330–10335. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, H.S.; Ryu, D.S.; Choi, S.J.; Lee, D.S. Antibacterial Activity of Silver-nanoparticles Against Staphy-lococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011, 39, 77–85. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Zeng, H.-Y.; Ouyang, Y.-S.; Chen, Y.-B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef]
- Reddy, L.S.; Nisha, M.M.; Joice, M.; Shilpa, P.N. Antimicrobial activity of zinc oxide (ZnO) nanoparticle againstKlebsiella pneumoniae. Pharm. Biol. 2014, 52, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Villota, R. Comparative Study on Injury and Recovery of Staphylococcus aureus using Microwaves and Conventional Heating. J. Food Prot. 1988, 51, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Data sheet of Resistivity Measurements Using the Model 2450 Source eMeter SMU Instrument and a Four-Point Collinear Probe. Available online: https://www.academia.edu/34848316/ (accessed on 1 December 2020).
- Schiffman, J.D.; Wang, Y.; Giannelis, E.P.; Elimelech, M. Biocidal Activity of Plasma Modified Electrospun Polysulfone Mats Functionalized with Polyethyleneimine-Capped Silver Nanoparticles. Langmuir 2011, 27, 13159–13164. [Google Scholar] [CrossRef] [PubMed]
- De Faria, A.F.; Perreault, F.; Shaulsky, E.; Chavez, L.H.A.; Elimelech, M. Antimicrobial Electrospun Biopolymer Nanofiber Mats Functionalized with Graphene Oxide–Silver Nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 12751–12759. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.L.; Urban, D.A.; Rodriguez-Lorenzo, L.; Milosevic, A.; Crippa, F.; Spuch-Calvar, M.; Balog, S.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle administration method in cell culture alters particle-cell interaction. Sci. Rep. 2019, 9, 900. [Google Scholar] [CrossRef]
- Malagoli, D.; Lusvardi, M.; Gobba, F.; Ottaviani, E. 50 Hz magnetic fields activate mussel immunocyte p38 MAP kinase and induce HSP70 and 90. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 137, 75–79. [Google Scholar] [CrossRef]
- Gobba, F.; Malagoli, D.; Ottaviani, E. Effects of 50 Hz magnetic fields on fMLP-induced shape changes in invertebrate immunocytes: The role of calcium ion channels. Bioelectromagnetics 2003, 24, 277–282. [Google Scholar] [CrossRef]
- Sudarti, S.; Prihandono, T.; Yushardi, Y.; Ridlo, Z.R.; Kristinawati, A. Effective dose analysis of extremely low frequency (ELF) magnetic field exposure to growth of S. termophilus, L. lactis, L. acidophilus bacteria. IOP Conf. Ser. Mater. Sci. Eng. 2018, 432, 012010. [Google Scholar] [CrossRef]
- Ben Yakir-Blumkin, M.; Loboda, Y.; Schächter, L.; Finberg, J.P.M. Neuroprotective effect of weak static magnetic fields in primary neuronal cultures. Neuroscience 2014, 278, 313–326. [Google Scholar] [CrossRef]
- Oncul, S.; Cuce, E.M.; Aksu, B.; Garip, A.I. Effect of extremely low frequency electromagnetic fields on bacterial membrane. Int. J. Radiat. Biol. 2015, 92, 42–49. [Google Scholar] [CrossRef]
- Vanhauteghem, D.; Janssens, G.P.J.; Lauwaerts, A.; Sys, S.; Boyen, F.; Cox, E.; Meyer, E. Exposure to the Proton Scavenger Glycine under Alkaline Conditions Induces Escherichia coli Viability Loss. PLoS ONE 2013, 8, e60328. [Google Scholar] [CrossRef] [PubMed]
- Fadel, M.A.; El-Gebaly, R.H.; Mohamed, S.A.; Abdelbacki, A.M. Biophysical control of the growth of Agrobacterium tumefaciens using extremely low frequency electromagnetic waves at resonance frequency. Biochem. Biophys. Res. Commun. 2017, 494, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.M.; Elkhatib, A.M.; Aboutaleb, W.M.; Abdelbacki, A.M.; Khalil, A.M.; Elkaliuoby, M.I. Control the Activity of Ralstonia Solanacearum Bacteria by Using Pulsed Electric Field. Jokull J. 2014, 64, 255–269. [Google Scholar]
- Oh, S.-J.; Kim, H.; Liu, Y.; Han, H.-K.; Kwon, K.; Chang, K.-H.; Park, K.; Kim, Y.; Shim, K.; An, S.S.A.; et al. Incompatibility of silver nanoparticles with lactate dehydrogenase leakage assay for cellular viability test is attributed to protein binding and reactive oxygen species generation. Toxicol. Lett. 2014, 225, 422–432. [Google Scholar] [CrossRef]
- Singh, M.; Mallick, A.K.; Banerjee, M.; Kumar, R. Loss of outer membrane integrity in Gram-negative bacteria by silver nanoparticles loaded with Camellia sinensis leaf phytochemicals: Plausible mechanism of bacterial cell disintegration. Bull. Mater. Sci. 2016, 39, 1871–1878. [Google Scholar] [CrossRef]
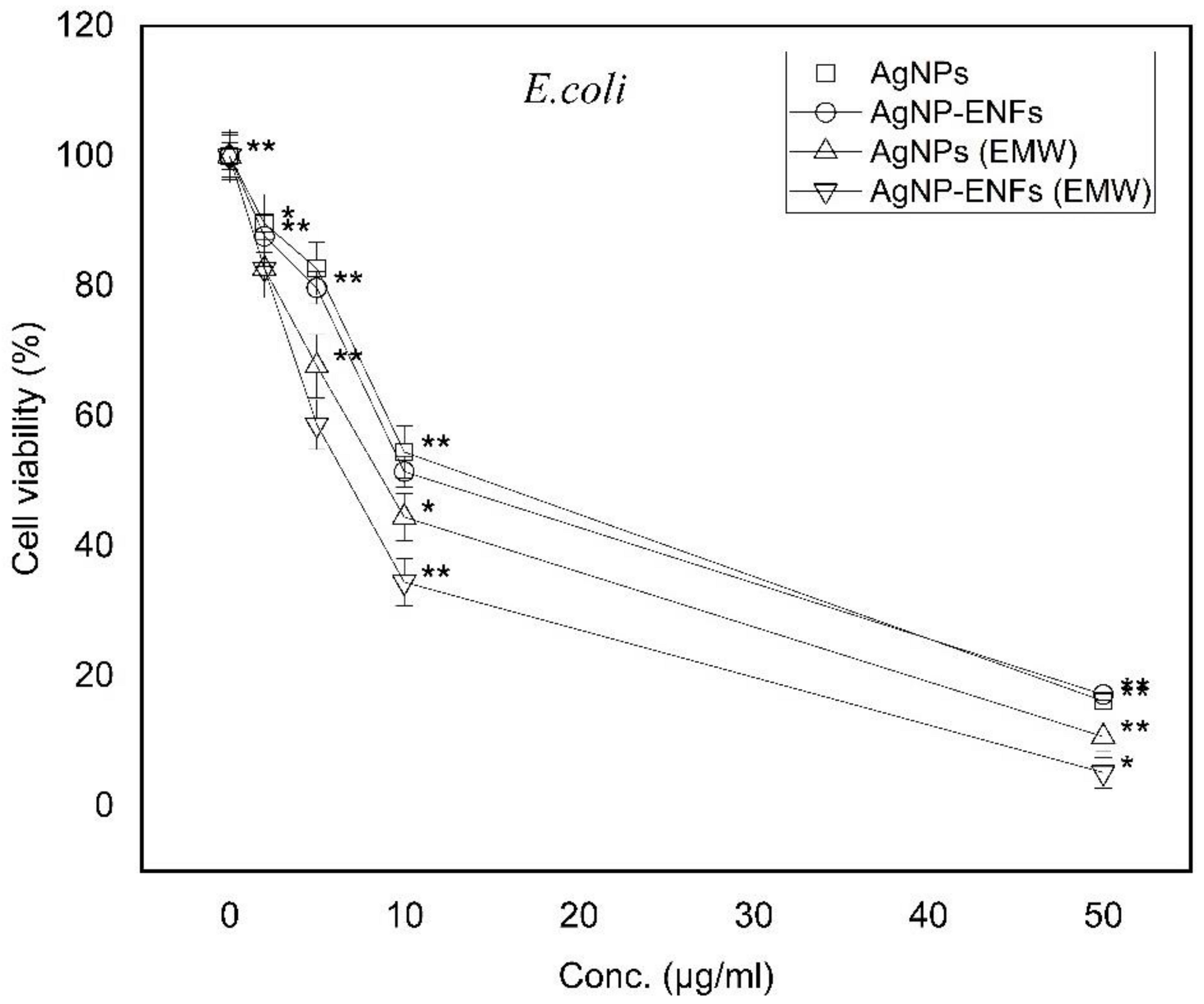
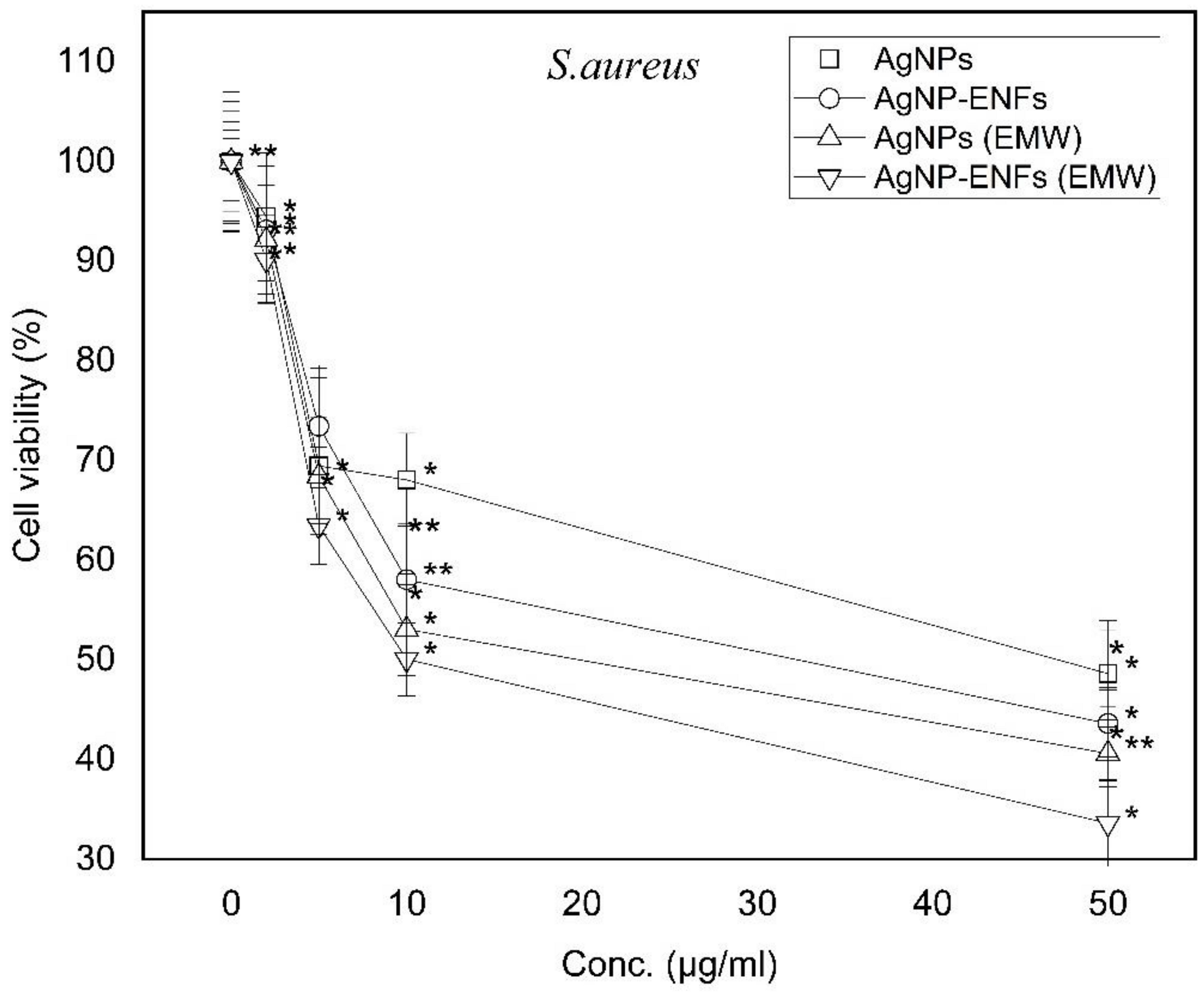
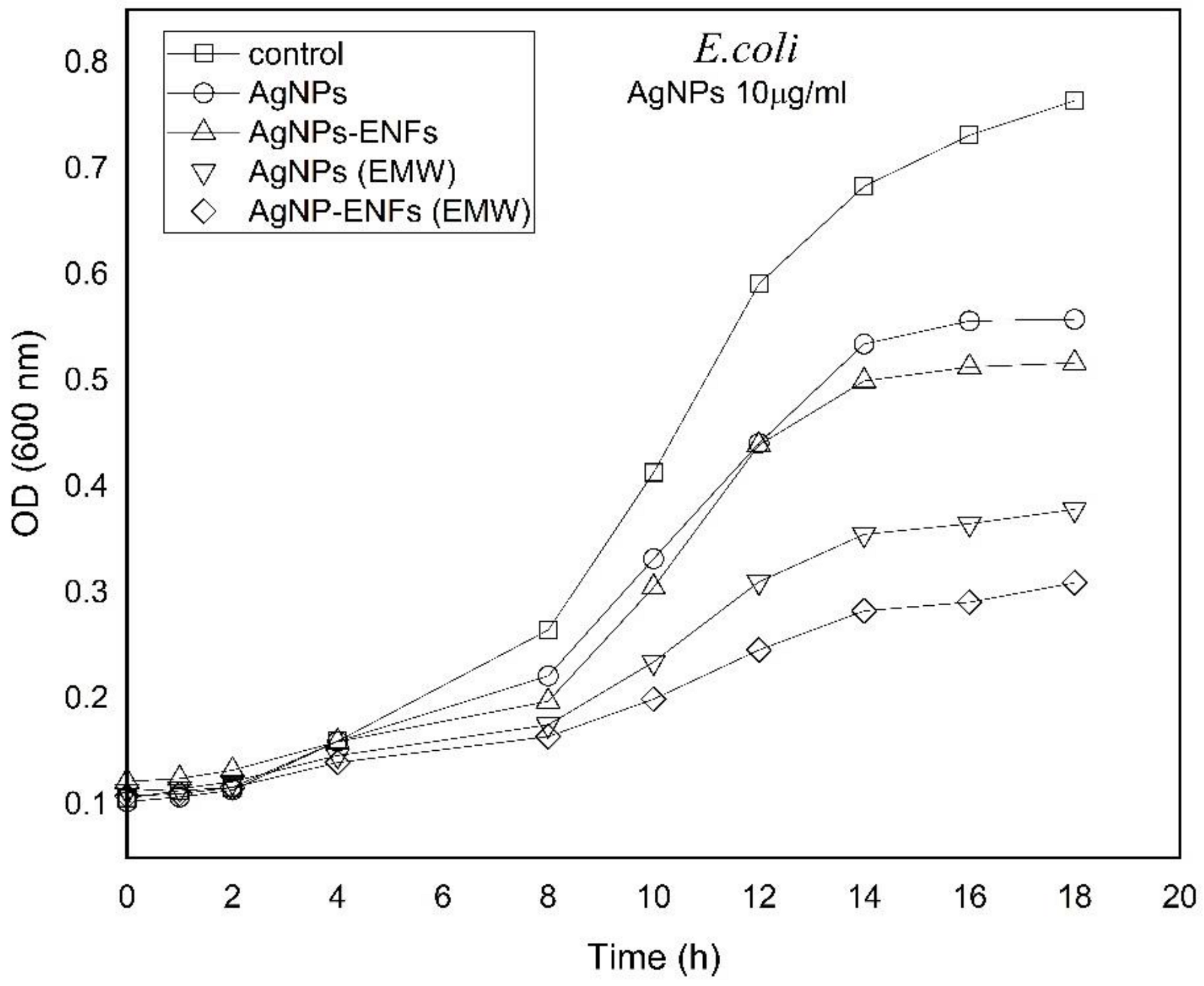
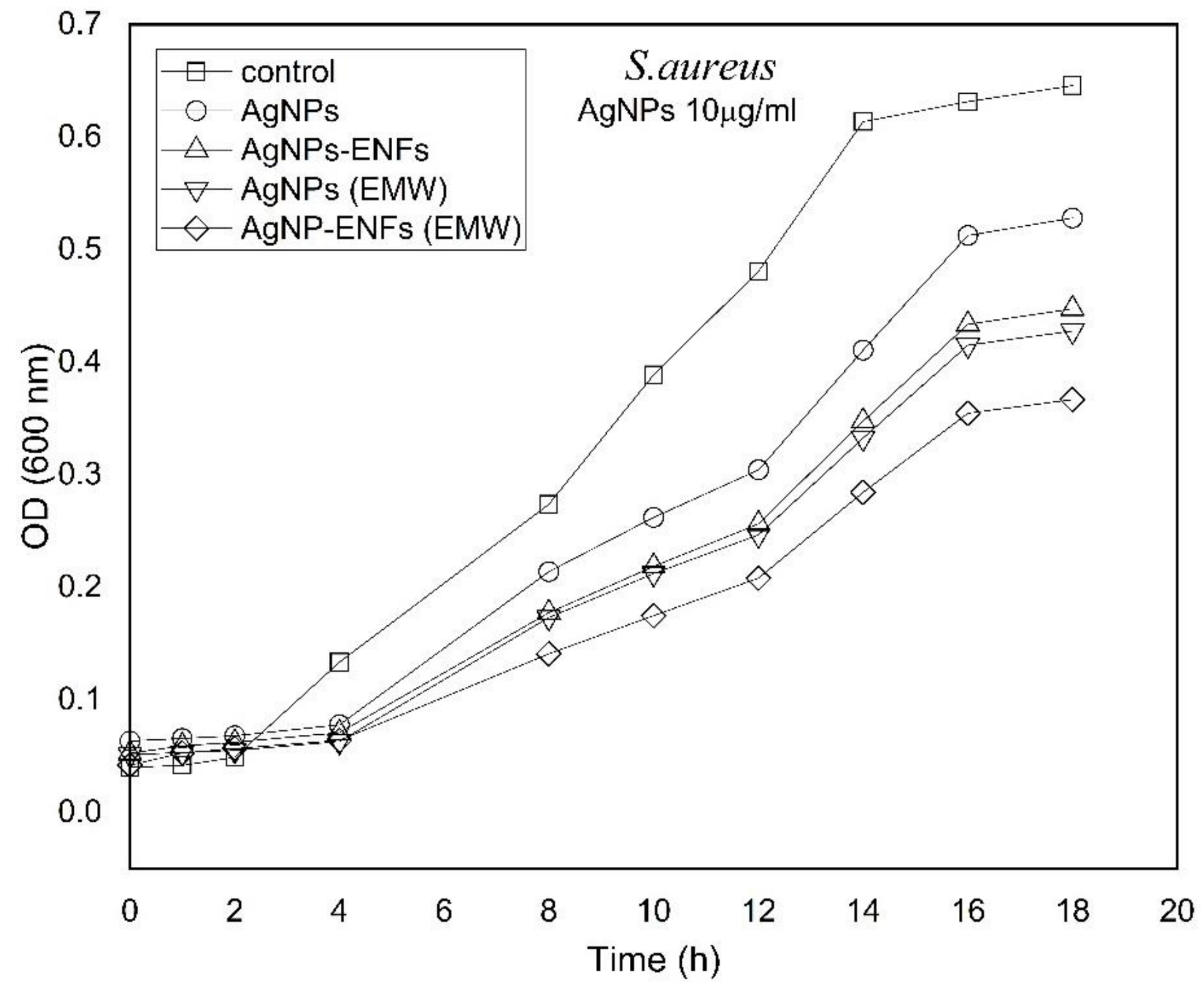
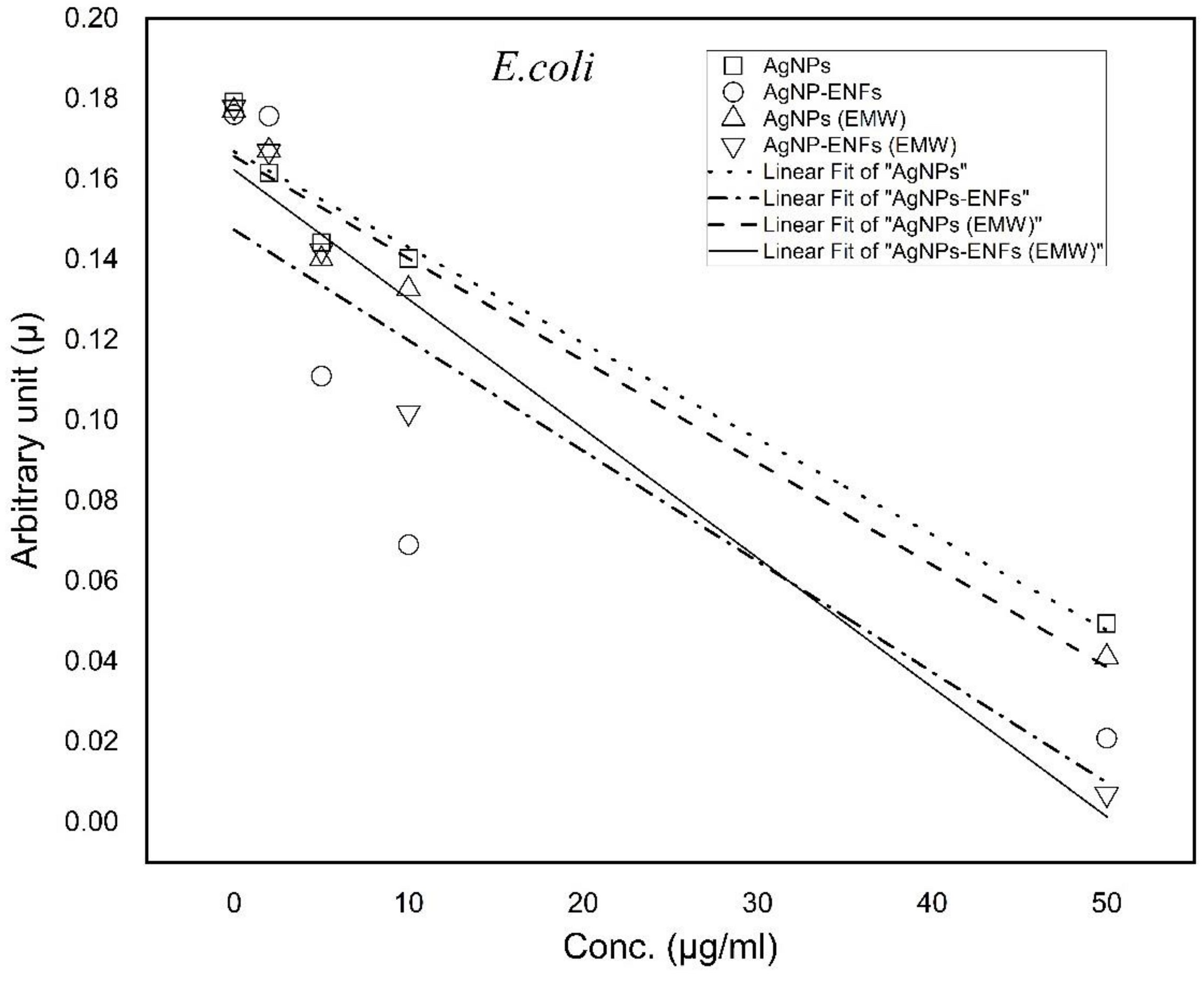
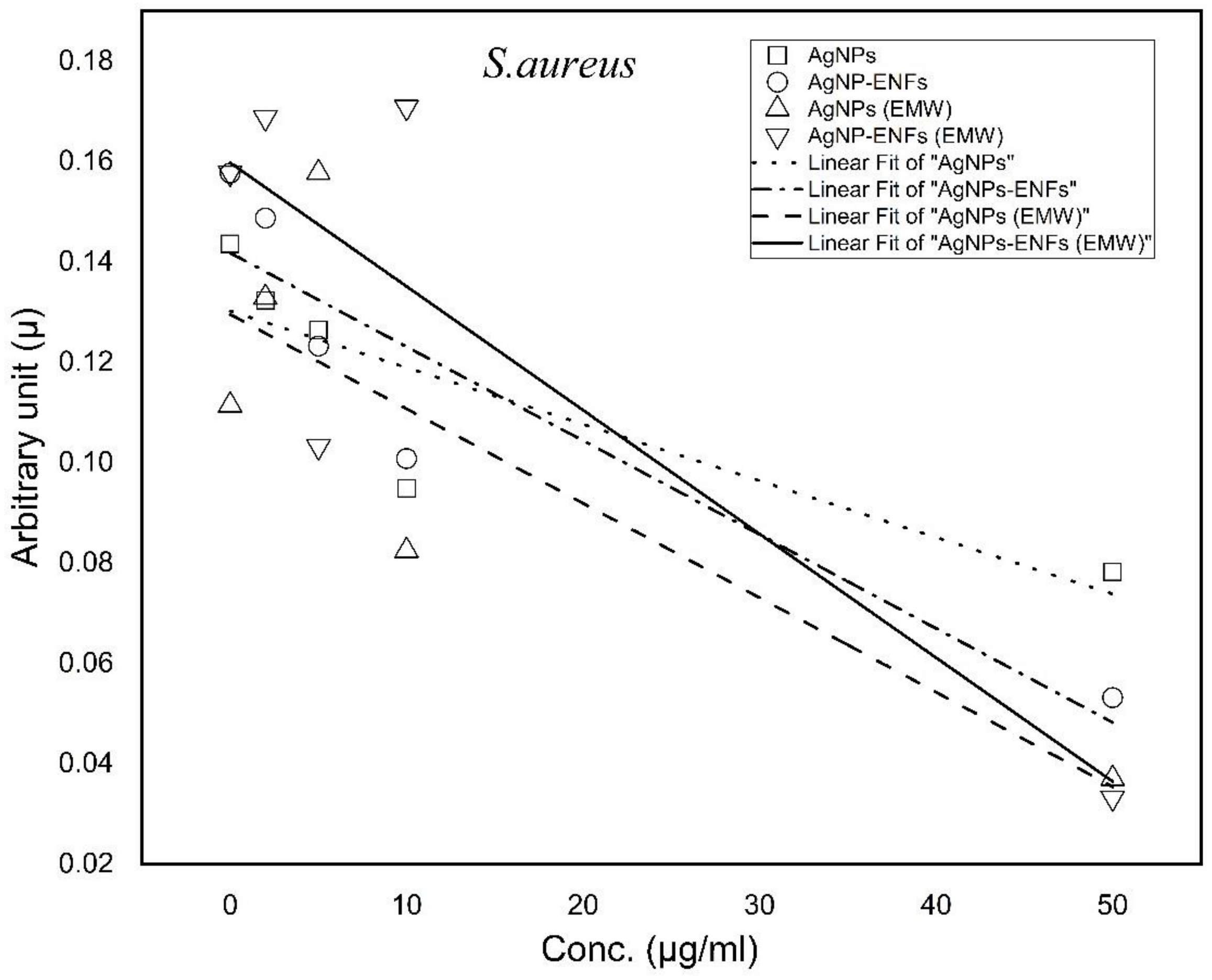
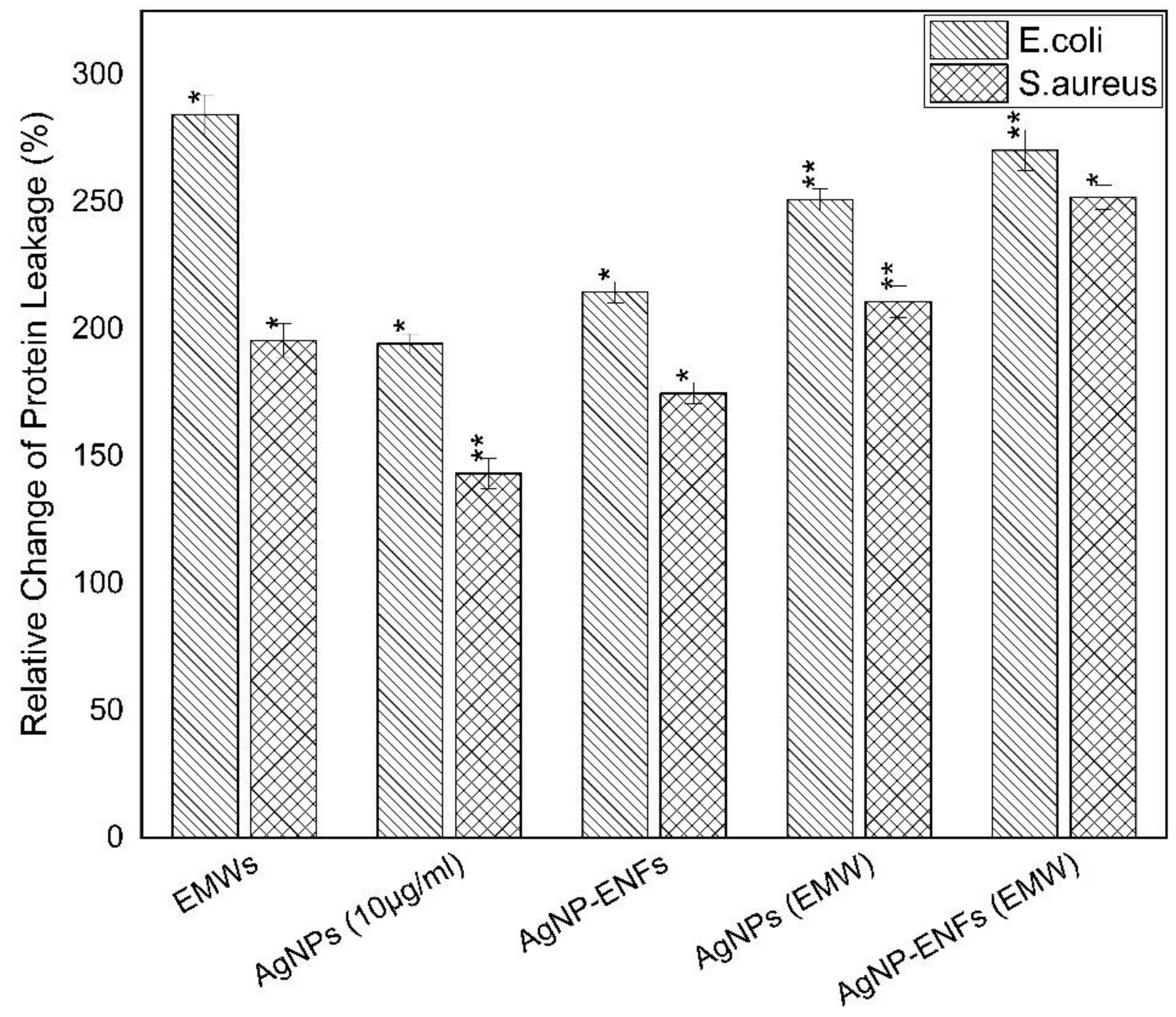
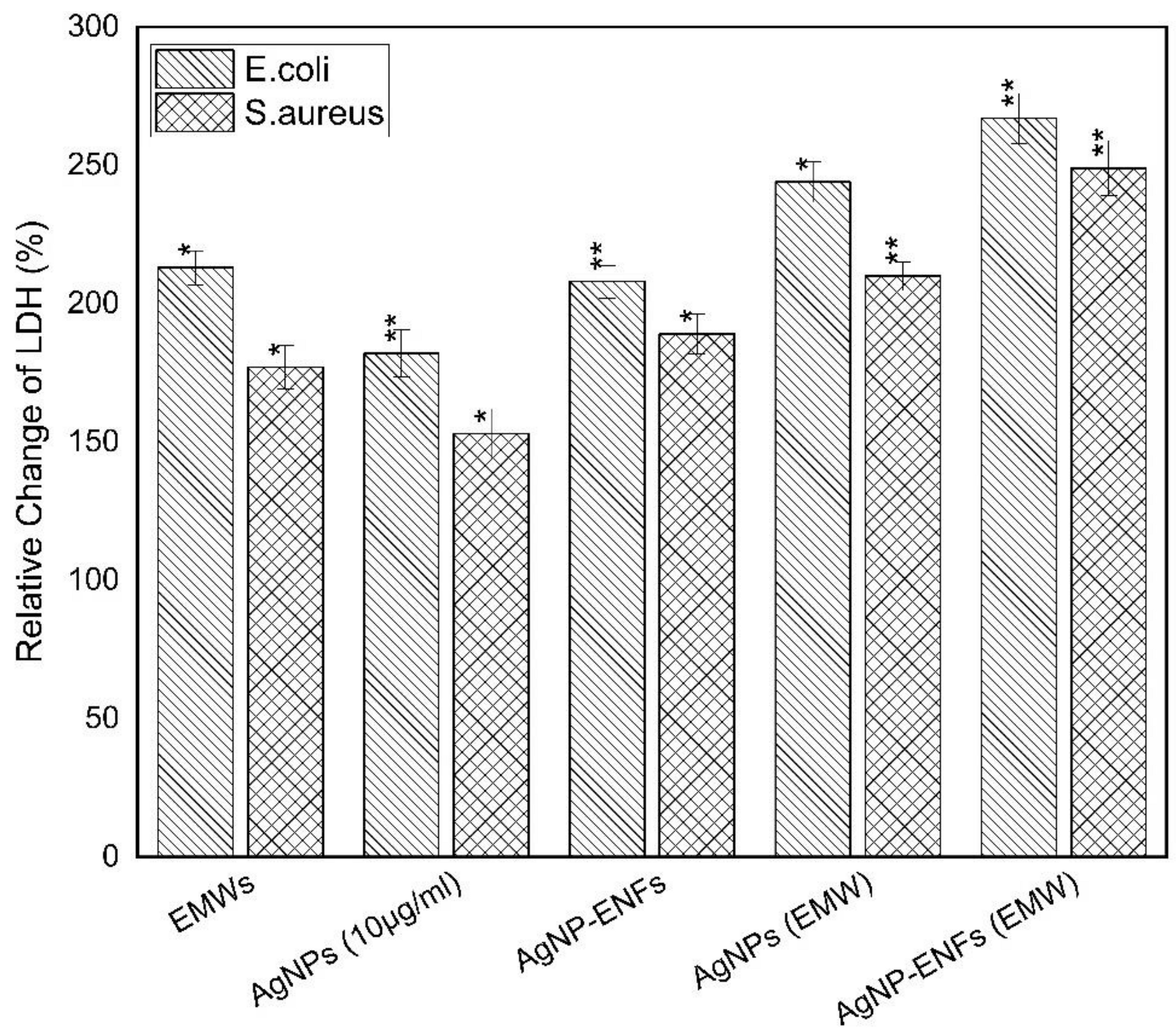
| Treatment Condition | Arbitrary Constant (µ) E. coli | Arbitrary Constant (µ) S. aureus |
|---|---|---|
| Ag NPs | −(2.38 × 10−3 ± 2.31415 × 10−4) | −(1.13 × 10−3 ± 3.92243 × 10−4) |
| Ag NPs-ENFs | −(2.75 ×10−3 ± 9.99632 × 10−4) | −(1.87 × 10−3 ± 4.32734 × 10−4) |
| Ag NPs (EMWs) | −(2.54 × 10−3 ± 2.77668 × 10−4) | −(1.88 × 10−3 ± 7.06717 × 10−4) |
| Ag NPs-ENFs (EMWs) | −(3.22 × 10−3 ± 4.82432 × 10−4) | −(2.47 × 10−3 ± 8.13422 × 10−4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-kaliuoby, M.I.; Khalil, A.M.; El-Khatib, A.M.; Shehata, N. Antibacterial Synergism of Electrospun Nanofiber Mats Functioned with Silver Nanoparticles and Pulsed Electromagnetic Waves. Polymers 2021, 13, 277. https://doi.org/10.3390/polym13020277
El-kaliuoby MI, Khalil AM, El-Khatib AM, Shehata N. Antibacterial Synergism of Electrospun Nanofiber Mats Functioned with Silver Nanoparticles and Pulsed Electromagnetic Waves. Polymers. 2021; 13(2):277. https://doi.org/10.3390/polym13020277
Chicago/Turabian StyleEl-kaliuoby, Mai I., Alaa M. Khalil, Ahmed M. El-Khatib, and Nader Shehata. 2021. "Antibacterial Synergism of Electrospun Nanofiber Mats Functioned with Silver Nanoparticles and Pulsed Electromagnetic Waves" Polymers 13, no. 2: 277. https://doi.org/10.3390/polym13020277
APA StyleEl-kaliuoby, M. I., Khalil, A. M., El-Khatib, A. M., & Shehata, N. (2021). Antibacterial Synergism of Electrospun Nanofiber Mats Functioned with Silver Nanoparticles and Pulsed Electromagnetic Waves. Polymers, 13(2), 277. https://doi.org/10.3390/polym13020277







