Improving the Tensile and Tear Properties of Thermoplastic Starch/Dolomite Biocomposite Film through Sonication Process
Abstract
1. Introduction
2. Materials and Chemicals
2.1. Preparation of Dolomite (DOL(P)) and Ultrasonicated Dolomite (DOL(U)
2.2. Plasticization of Starch to Form Thermoplastic Starch (TPS)
2.3. Preparation of TPS-DOL Biocomposites
3. Characterization and Mechanical Testing
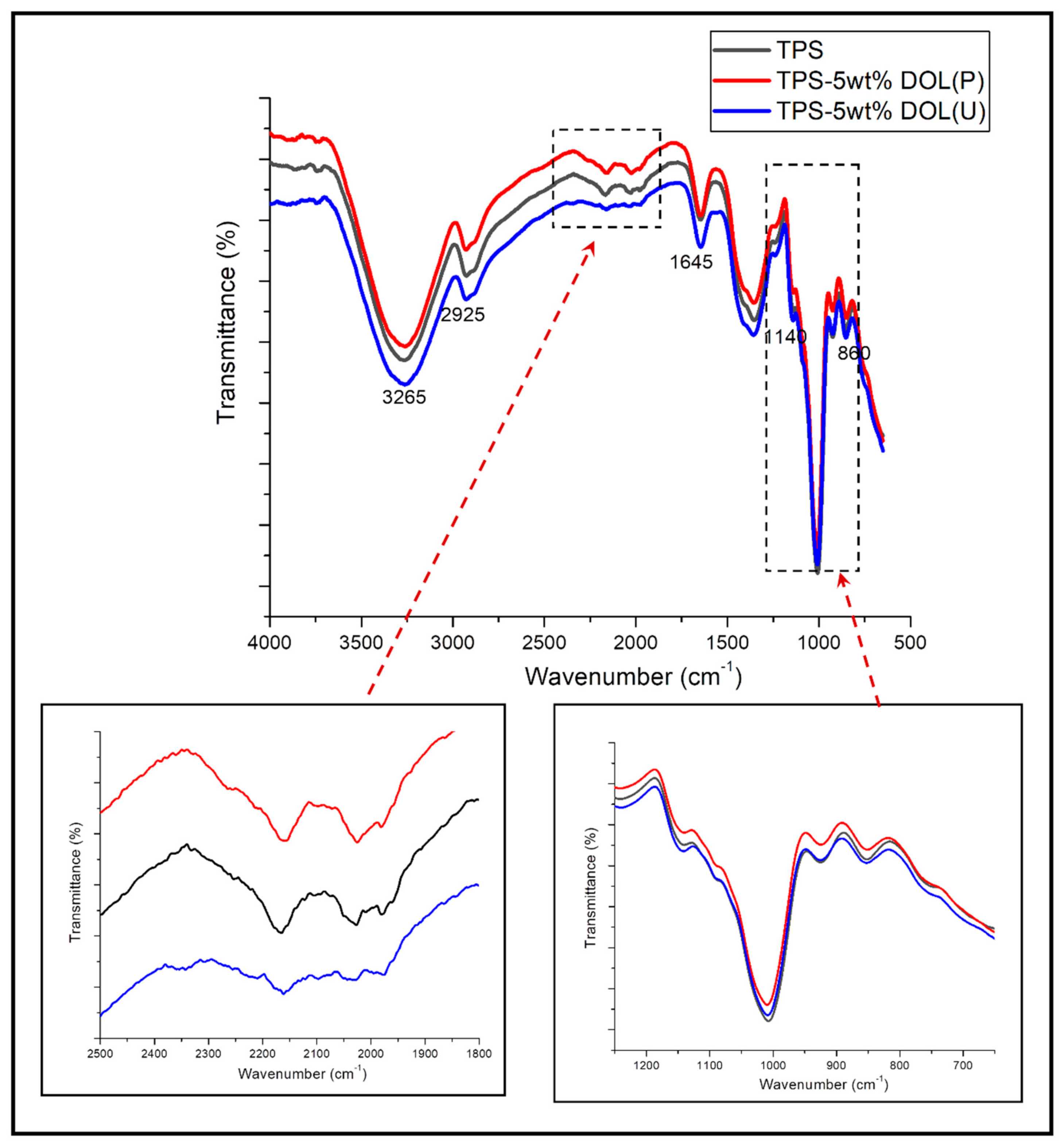
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
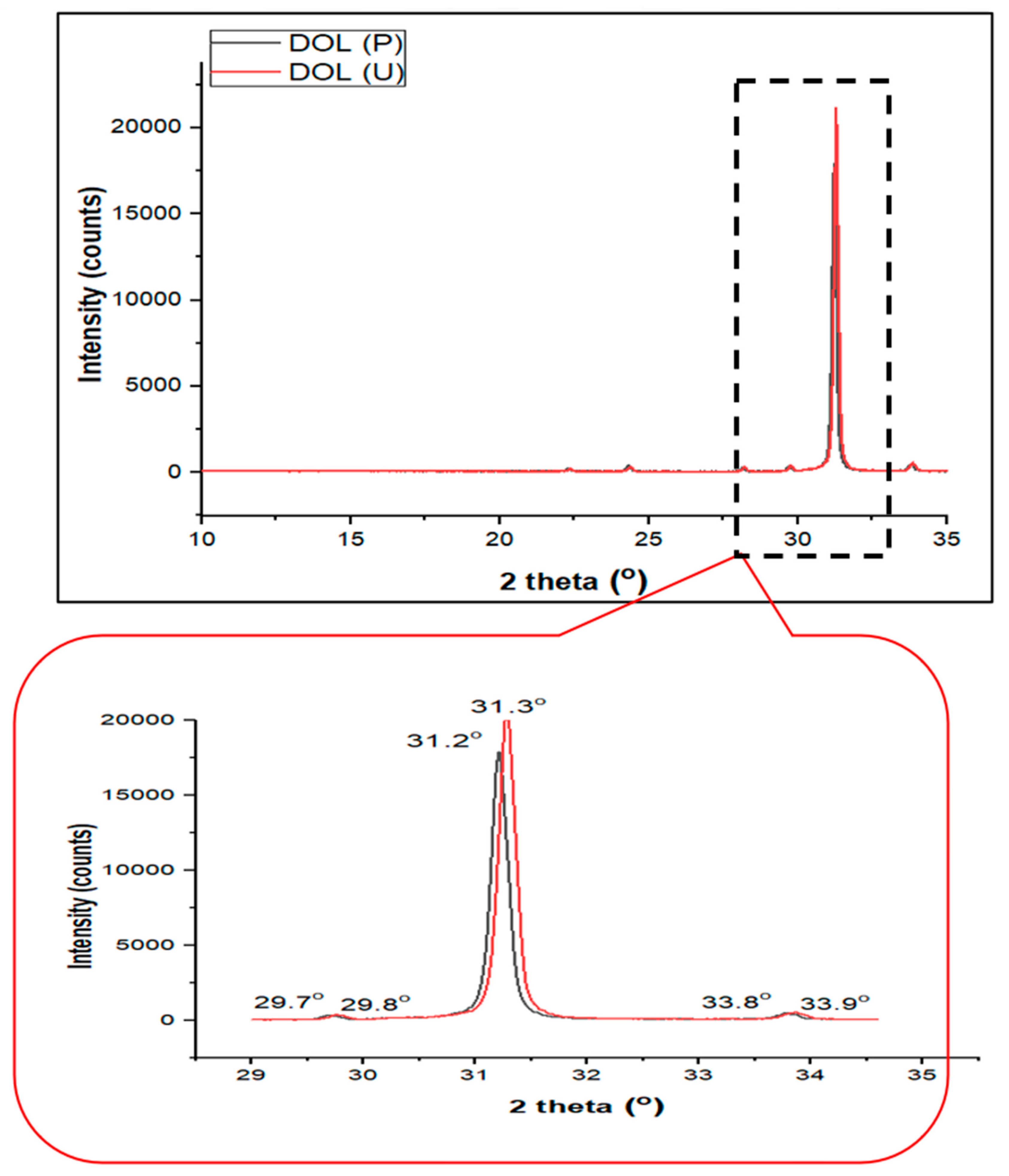
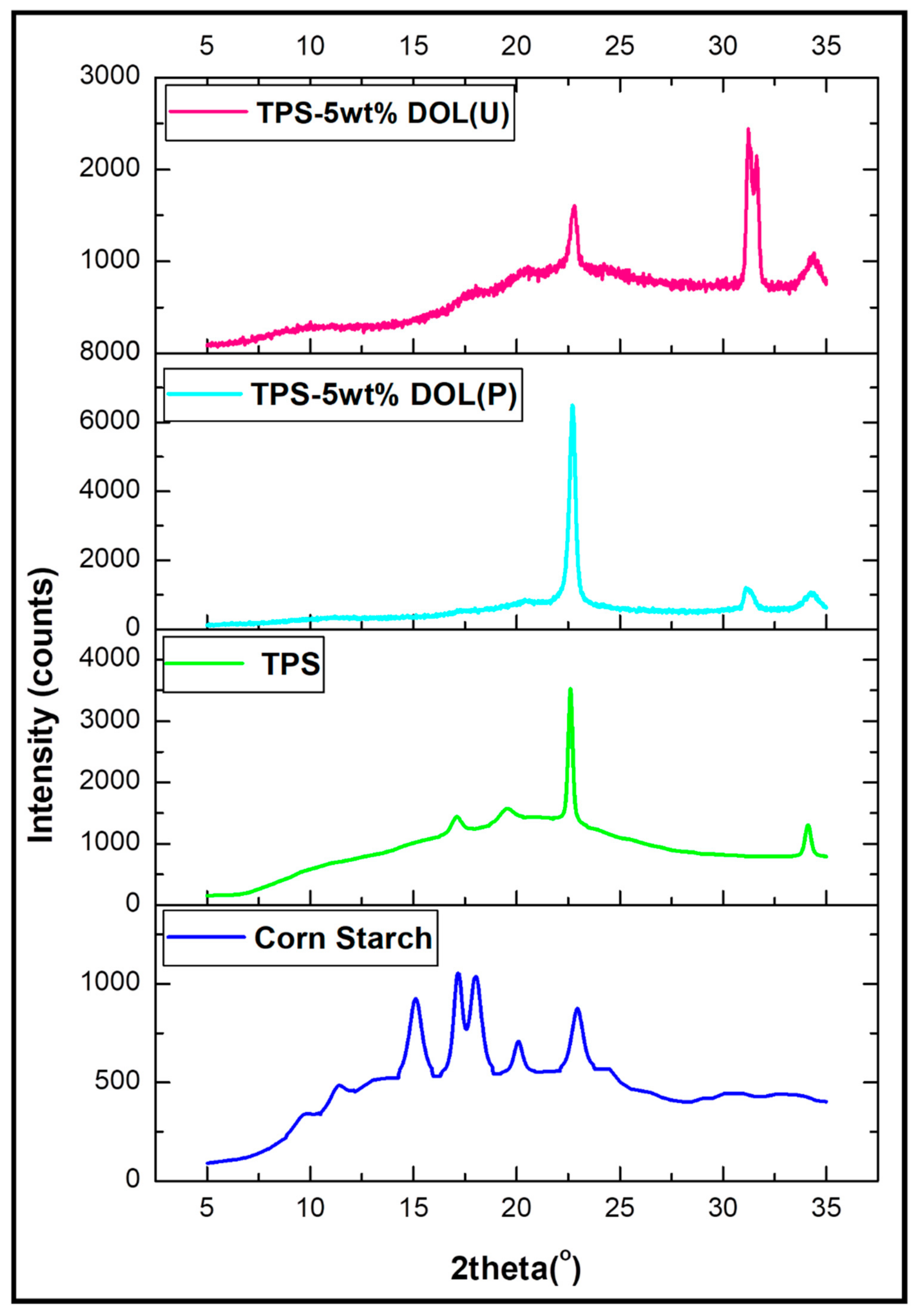
3.2. X-ray Diffraction (XRD)
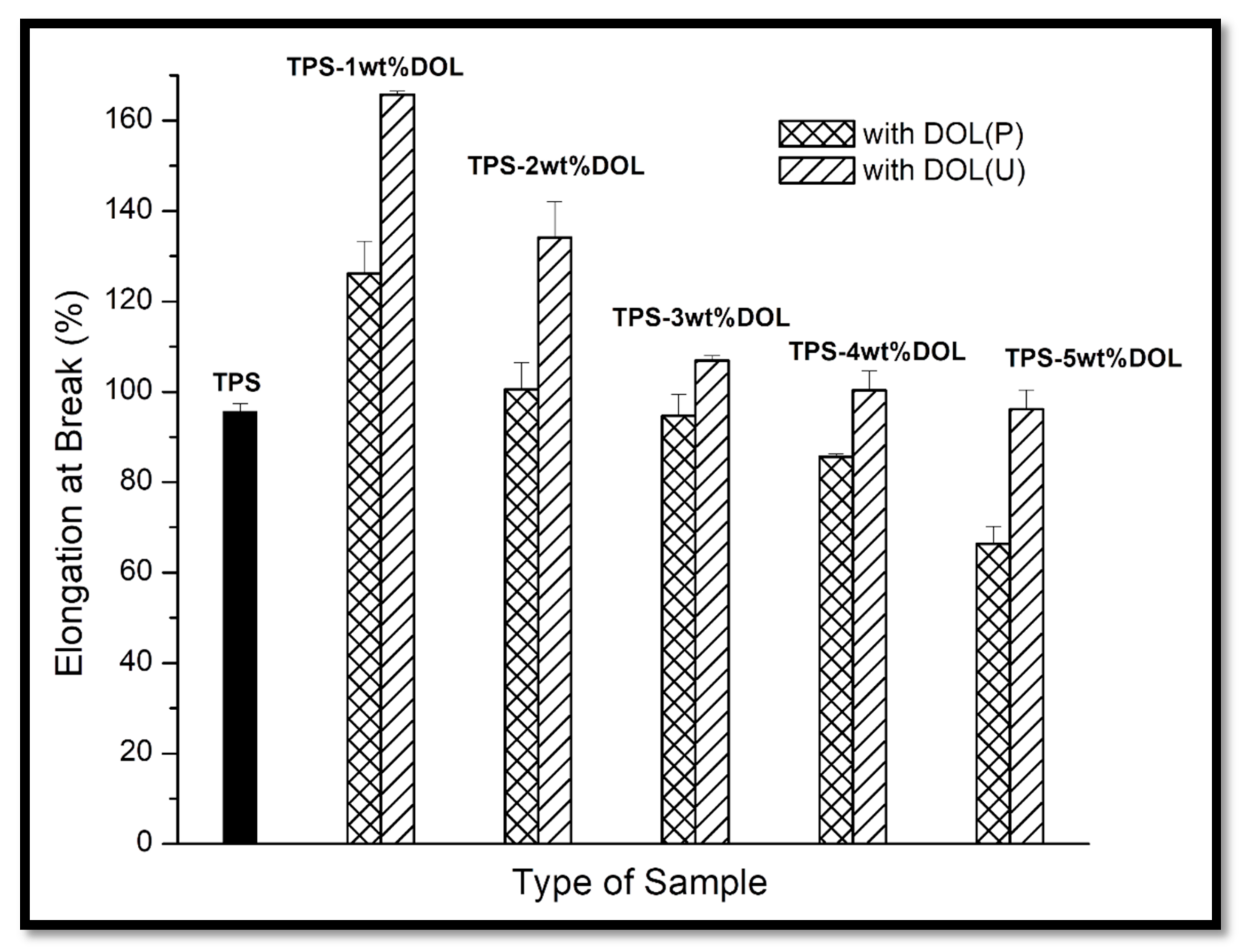
3.3. Tensile Test
3.4. Tear Test
4. Results and Discussion
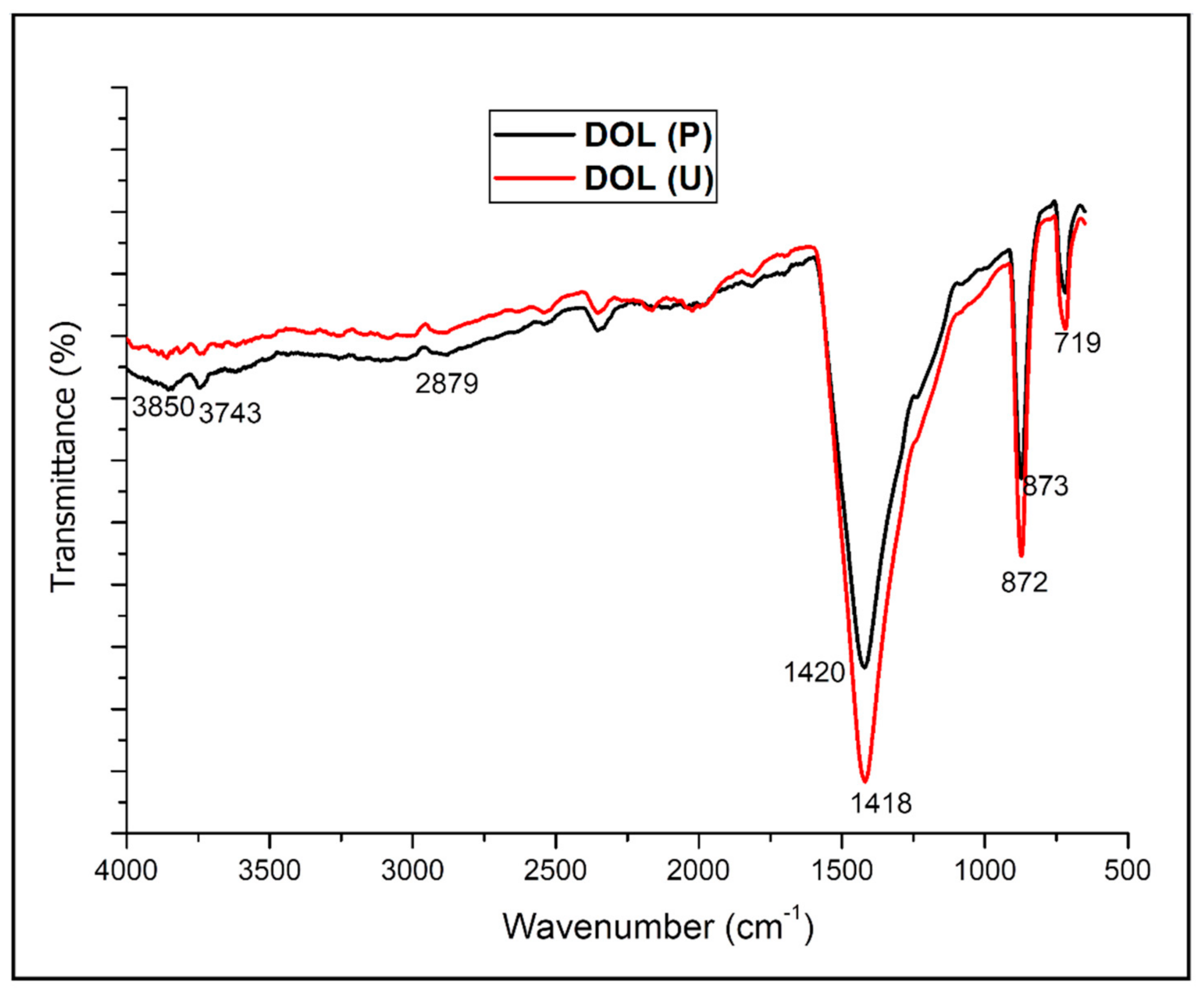
4.1. Functional Group Analysis of the Dolomite Filler (DOL(P) and DOL(U))
4.2. Functional Groups Analysis on TPS Film and TPS-DOL Biocomposite Films
4.3. Crystallinity Analysis of the Dolomite Filler (DOL(P) and DOL(U))
4.4. Crystallinity Analysis of the Corn Starch, TPS Film, and TPS-DOL Biocomposite Films
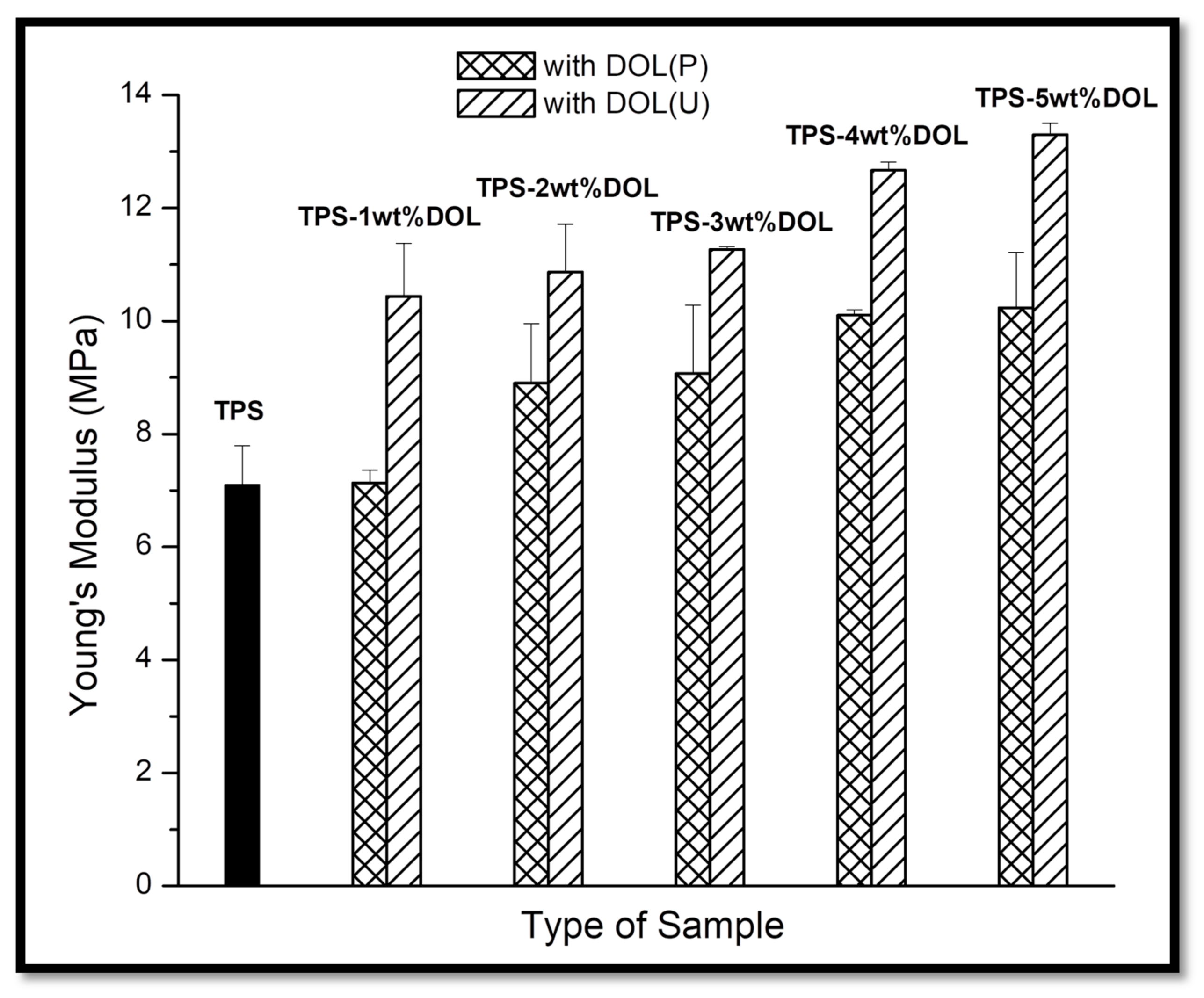
4.5. Mechanical Properties Evaluation through Tensile Test
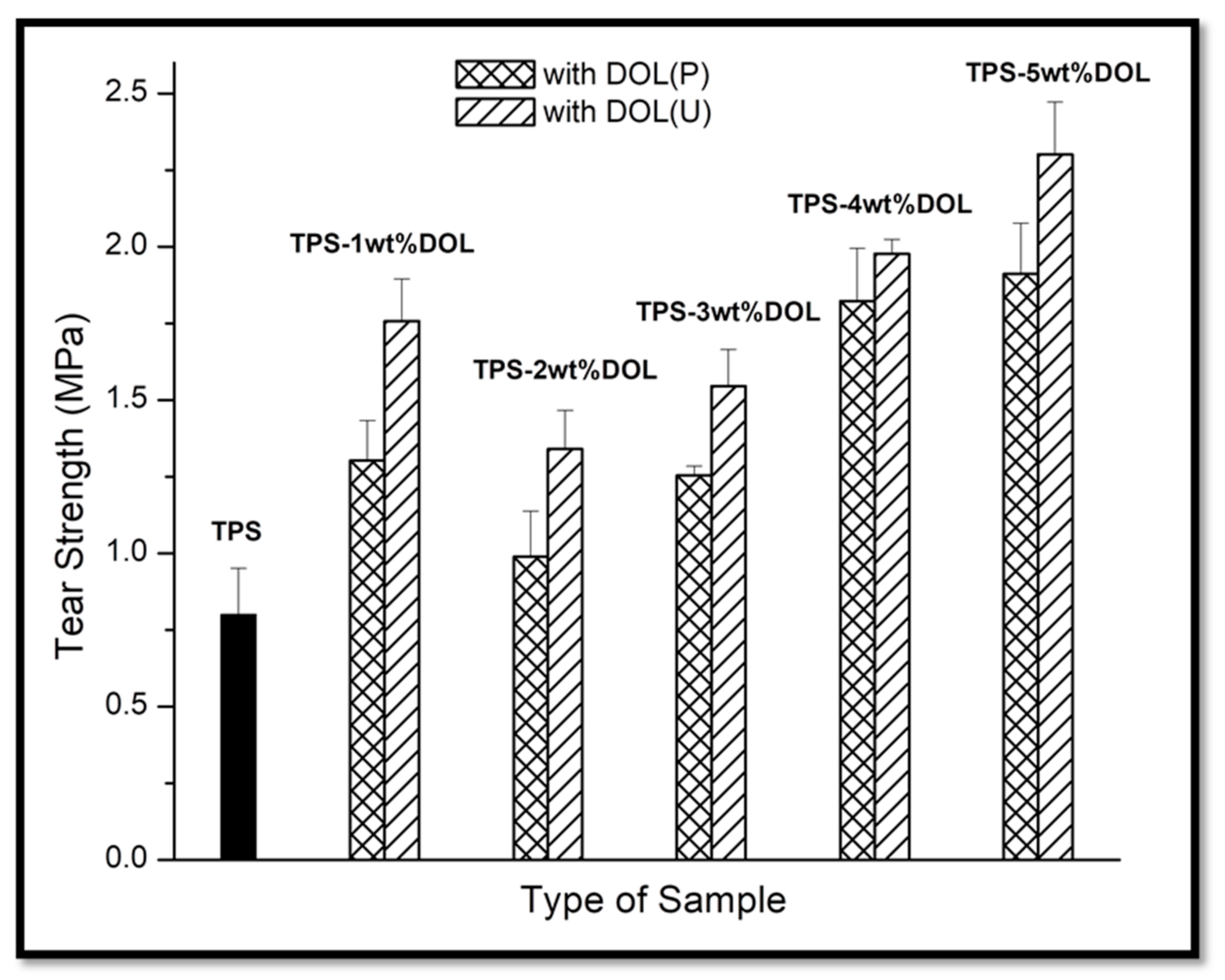
4.6. Mechanical Properties Evaluation through Tear Test
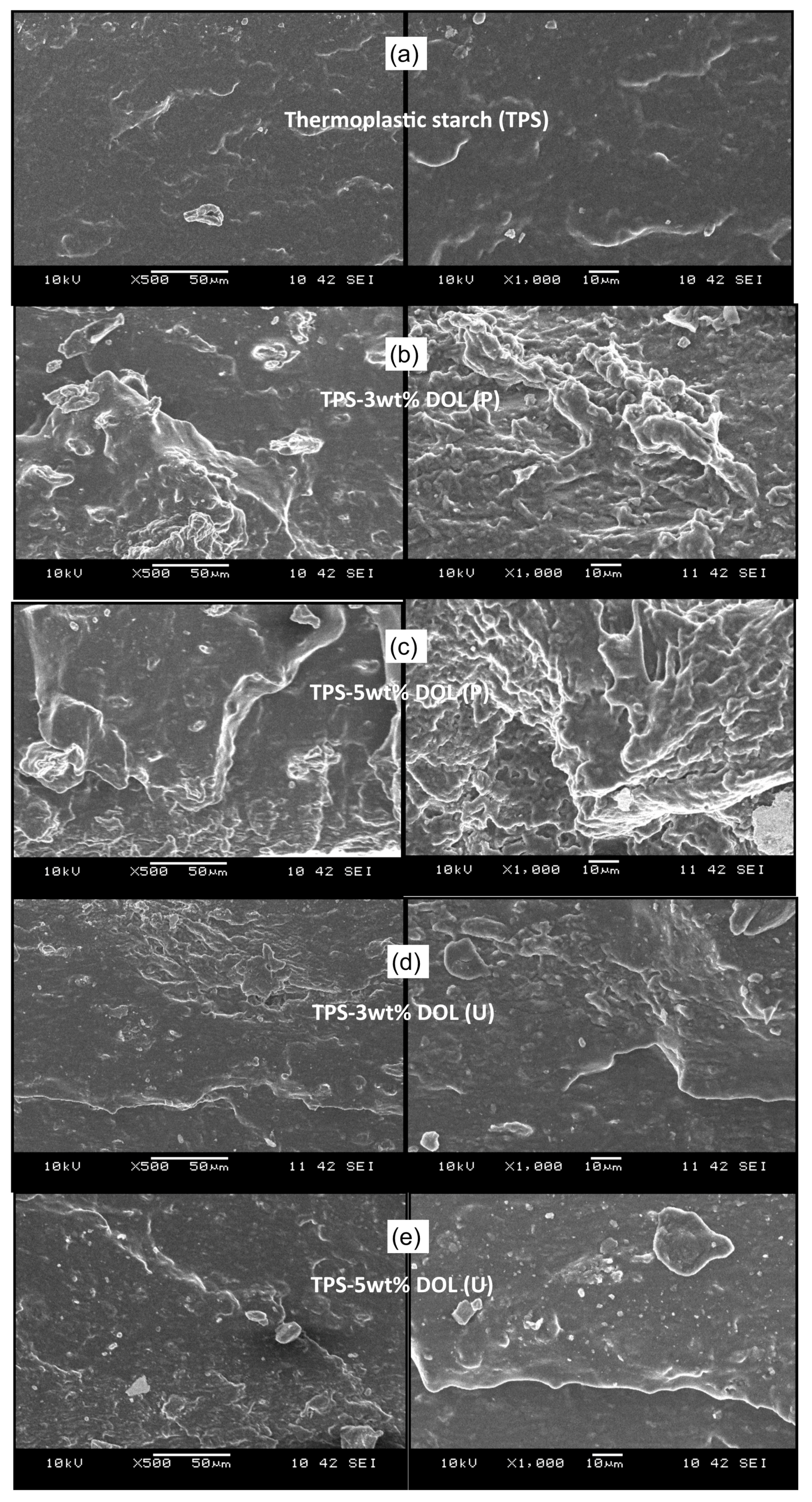
4.7. Morphology of Tensile Fractured Surface of the TPS Film and TPS-DOL Biocomposites
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASTM D | American Society for Testing and Materials D Standard |
| DOL (P) | Pristine dolomite |
| DOL (U) | Sonicated dolomite |
| FTIR | Fourier Transform Infrared Spectroscopy |
| ISO | International Organization for Standardization |
| mm | Micronmeter |
| MPa | Mega Pascal |
| PE | Polyethylene |
| PP | Polypropylene |
| TPS | Thermoplastic starch |
| TPS-Xwt%DOL(P) | TPS biocomposite containing X wt% pristine dolomite |
| TPS-Xwt%DOL(U) | TPS biocomposite containing X wt% sonicated dolomite |
| XRD | X-ray Diffraction |
| μm | Micrometer |
References
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, P.; Gopi, S.; Sreekala, M.S.; Thomas, S. UV resistant transparent bionanocomposite films based on potato starch/cellulose for sustainable packaging. Starch 2018, 70, 1700139. [Google Scholar] [CrossRef]
- Osman, A.F.; Ashafee, A.M.T.; Adnan, S.A.; Alakrach, A. Influence of Hybrid Cellulose/Bentonite Fillers on Structure, Ambient and Low Temperature Tensile Properties of Thermoplastic Starch Composites. Polym. Eng. Sci. 2020, 60, 810–822. [Google Scholar] [CrossRef]
- Husseinsyah, S.; Zailuddin, N.L.I.; Osman, A.F.; Lili, C.; Al-rashdi, A.A.; Alakrach, A. Methyl methacrylate (MMA) treatment of empty fruit bunch (EFB) to improve the properties of regenerated cellulose biocomposite films. Polymers 2020, 12, 2618. [Google Scholar] [CrossRef]
- Syed Adam, S.N.S.; Osman, A.F.; Shamsudin, R. Tensile Properties, Biodegradability and Bioactivity of Thermoplastic Starch (TPS)/Bioglass Composites for Bone Tissue Engineering. Sains Malays. 2018, 47, 1303–1310. [Google Scholar] [CrossRef]
- Ismail, I.; Osman, A.F.; Ping, T.L. Effects of ultrasonication process on crystallinity and tear strength of thermoplastic starch/cellulose biocomposites. IOP Conf. Ser. Mater. Sci. Eng. 2019, 701, 012045. [Google Scholar] [CrossRef]
- Ni, L.; Mao, Y.; Liu, Y.; Cai, P.; Jiang, X.; Gao, X.; Cheng, X.; Chen, J. Synergistic reinforcement of waterborne polyurethane films using Palygorskite and dolomite as micro/nano-fillers. J. Polym. Res. 2020, 27, 23. [Google Scholar] [CrossRef]
- Osman, A.F.; Kalo, H.; Hassan, M.S.; Hong, T.W.; Azmi, F. Pre-dispersing of montmorillonite nanofiller: Impact on morphology and performance of melt compounded ethyl vinyl acetate nanocomposites. J. Appl. Polym. Sci. 2016, 133, 1–15. [Google Scholar] [CrossRef]
- Hulleman, S.H.D.; Kalisvaart, M.G.; Jansen, F.H.P.; Feil, H.; Vliegenthart, J.F.G. Origins of B-type crystallinity in glycerol-plasticized, compression moulded potato starches. Carbohydr. Polym. 1999, 39, 51–360. [Google Scholar] [CrossRef]
- ASTM D882-18. Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM D638-14. Standard Test Method for Tensile Properties of Plastics; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM D624-00. Standard Test Method for Tear Strength of Conventional Vulcanized Rubber and Thermoplastic; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- Mohammed, M.A.A.; Salmiaton, A.; Wan Azlina, W.A.K.G.; Mohamad Amran, M.S.; Taufiq-Yap, Y.H. Preparation and Characterization of Malaysian Dolomites as a Tar Cracking Catalyst in Biomass Gasification Process. J. Energy 2013, 1–8. [Google Scholar] [CrossRef]
- Wilson, K.; Hardacre, C.; Lee, A.F.; Montero, J.M.; Shellard, L. The application of calcined natural dolomitic rock as a solid base catalyst in triglyceride transesterification for biodiesel synthesis. Green Chem. 2008, 10, 654–659. [Google Scholar] [CrossRef]
- Ji, J.; Ge, Y.; Balsam, W.; Damuth, J.E.; Chen, J. Rapid identification of dolomite using a Fourier Transform Infrared Spectrophotometer (FTIR): A fast method for identifying Heinrich events in IODP Site U1308. Mar. Geol. 2009, 258, 60–68. [Google Scholar] [CrossRef]
- Workman, J., Jr.; Weyer, L. Practical Guide to Interpretive Near-Infrared Spectroscopy; CRC press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Flores-Morales, A.; Jiménez-Estrada, M.; Mora-Escobedo, R. Determination of the structural changes by FT-IR, Raman, and CP/MAS 13C NMR spectroscopy on retrograded starch of maize tortillas. Carbohydr. Polym. 2012, 87, 61–68. [Google Scholar] [CrossRef]
- Hong, L.F.; Cheng, L.H.; Lee, C.Y.; Peh, K.K. Characterisation of physicochemical properties of propionylated corn starch and its application as stabilizer. Food Technol. Biotechnol. 2015, 53, 278–285. [Google Scholar] [CrossRef]
- Muller, P.; Kapin, E.; Fekete, E. Effects of preparation methods on the structure and mechanical properties of wet conditioned starch/montmorillonite nanocomposite films. Carbohydr. Polym. 2014, 113, 569–576. [Google Scholar] [CrossRef]
- Mendes, J.F.; Paschoalin, R.T.; Carmona, V.B.; Neto, A.R.S.; Marques, A.C.P.; Marconcini, J.M.; Oliveira, J.E. Biodegradable polymer blends based on corn starch and thermoplastic chitosan processed by extrusion. Carbohydr. Polym. 2016, 137, 452–458. [Google Scholar] [CrossRef]
- Girones, J.; Lopez, J.P.; Mutje, P.; Carvalho, A.J.F.; Curvelo, A.A.S.; Vilaseca, F. Natural fiber-reinforced thermoplastic starch composites obtained by melt processing. Compos. Sci. Techno. 2012, 72, 858–863. [Google Scholar] [CrossRef]
- Nik Adik, N.N.A.; Lin, O.H.; Akil, H.M.; Sandu, A.V.; Villagracia, A.R.; Santos, G.N. Effects of stearic acid on tensile, morphological and thermal analysis of polypropylene (PP)/dolomite (Dol) composites. Mater. Plast 2016, 53, 61–64. [Google Scholar]
- Saidi, M.A.A.; Mazlan, F.S.; Hassan, A.; Rashid, R.A.; Rahmat, A.R. Flammability, thermal and mechanical properties of polybutylene terephthalate/dolomite composites. J. Phys. Sci. 2019, 30, 175–189. [Google Scholar] [CrossRef]
- Adesakin, A.O.; Ajayi, O.O.; Imosili, P.E.; Attahdaniel, B.E.; Olusunle, S.O.O. Characterization and Evaluation of Mechanical Properties of Dolomite as Filler in Polyester. Chem. Mater. Res. 2013, 3, 36–40. [Google Scholar]
- Osman, A.F.; Edwards, G.; Martin, D. Effects of Processing Method and Nanofiller Size on Mechanical Properties of Biomedical Thermoplastic Polyurethane (TPU) Nanocomposites. Adv. Mat. Res. 2014, 911, 115–119. [Google Scholar] [CrossRef]
- Li, Z.; Reimer, C.; Wang, T.; Mohanty, A.K.; Misra, M. Thermal and Mechanical Properties of the Biocomposites of Miscanthus Biocarbon and Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) (PHBV). Polymers 2020, 12, 1300. [Google Scholar] [CrossRef] [PubMed]


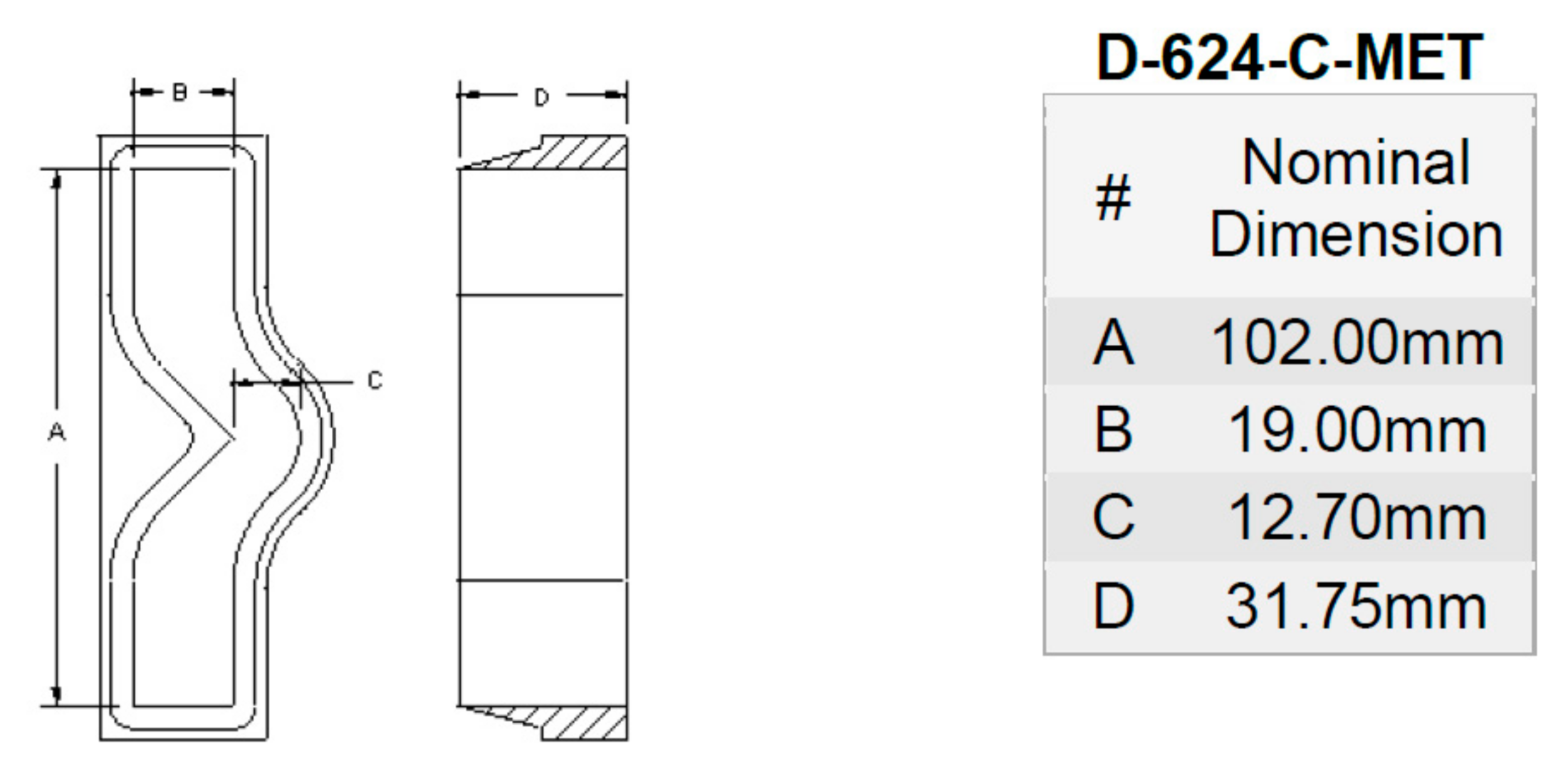
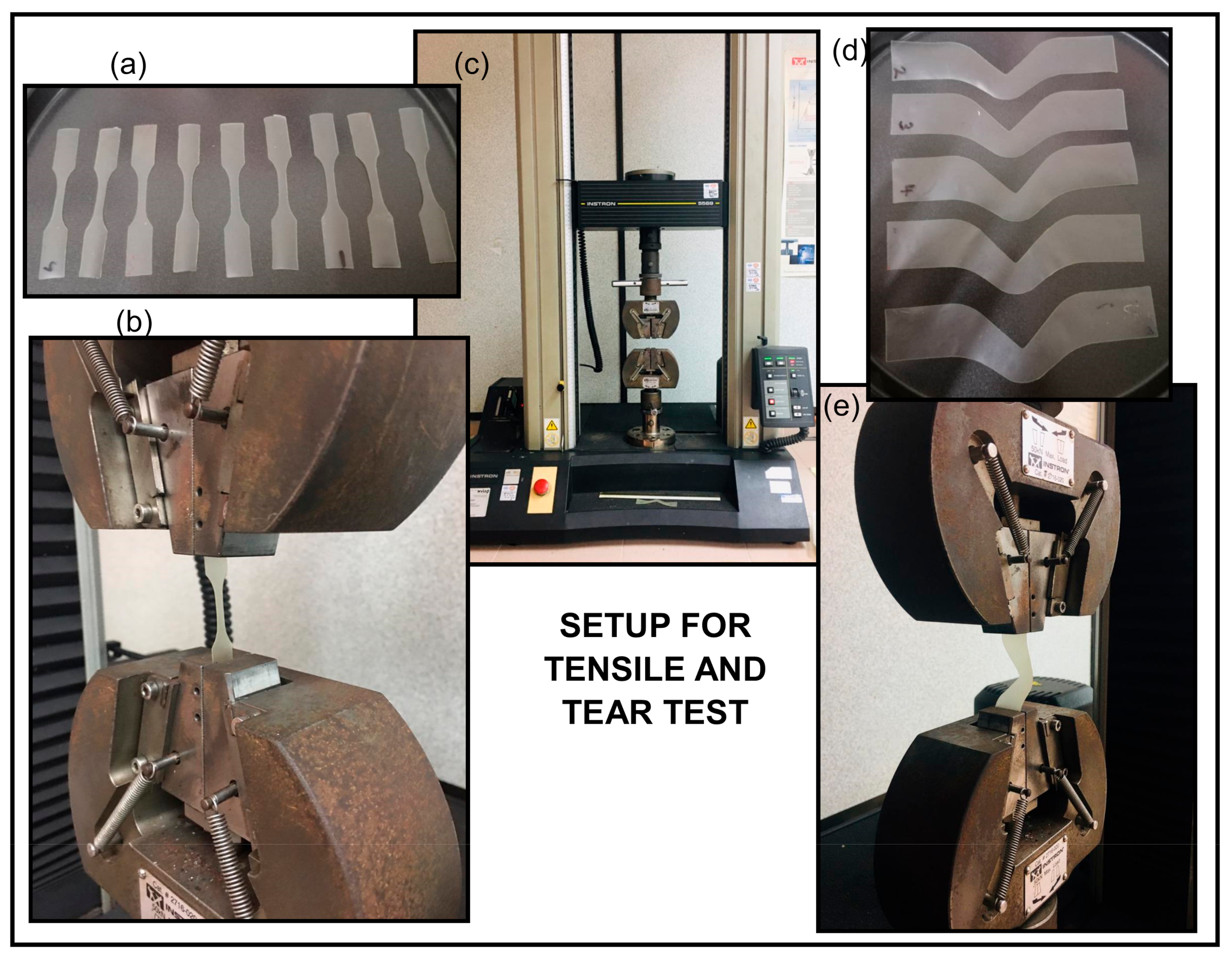

| TPS (wt%) | Dolomite Filler without Sonication Process (wt%) | Dolomite Filler Subjected to 120 min Sonication Process (wt%) | Acronym of Biocomposite Film |
|---|---|---|---|
| 100 | - | - | TPS |
| 99 | 1 | - | TPS-1wt%DOL(P) |
| 99 | - | 1 | TPS-1wt%DOL(U) |
| 98 | 2 | - | TPS-2wt%DOL(P) |
| 98 | - | 2 | TPS-2wt%DOL(U) |
| 97 | 3 | - | TPS-3wt%DOL(P) |
| 97 | - | 3 | TPS-3wt%DOL(U) |
| 96 | 4 | - | TPS-4wt%DOL(P) |
| 96 | - | 4 | TPS-4wt%DOL(U) |
| 95 | 5 | - | TPS-5wt%DOL(P) |
| 95 | - | 5 | TPS-5wt%DOL(U) |
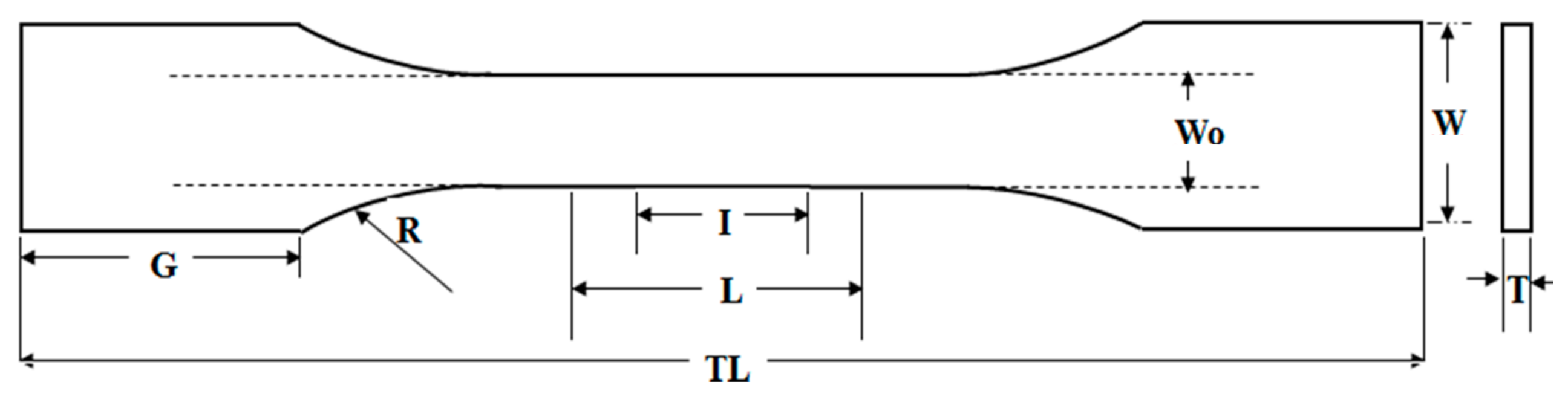
| Symbol | Description | Dimension (mm) |
|---|---|---|
| I | Gauge length | 7.62 |
| L | Length of narrow selection | 9.53 |
| Wo | Width of narrow selection | 3.18 |
| G | Grip length | 19.05 |
| W | Total width | 9.53 |
| TL | Total length | 63.50 |
| R | Radius of curvature | 12.70 |
| Samples | 2θ (°) | Percent (%) | |
|---|---|---|---|
| Crystallinity | Amorphous | ||
| DOL(P) | 22.4, 24.4, 28.2, 29.7, 31.2, 33.8 | 76.57 | 23.43 |
| DOL(U) | 22.4, 24.3, 28.1, 29.8, 31.3, 33.9 | 77.10 | 22.90 |
| Corn starch powder | 11.4, 15.1, 17.1,18, 20, 22.9 | 58.03 | 41.97 |
| TPS | 17.2, 19.5, 22.6, 34.1 | 25.38 | 74.62 |
| TPS-5wt%DOL (P) | 20.4, 22.7, 31, 34.3 | 51.79 | 48.21 |
| TPS-5wt%DOL (U) | 17.4, 20.2, 22.8, 31.2, 31.6, 34.4 | 52.43 | 47.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, A.F.; Siah, L.; Alrashdi, A.A.; Ul-Hamid, A.; Ibrahim, I. Improving the Tensile and Tear Properties of Thermoplastic Starch/Dolomite Biocomposite Film through Sonication Process. Polymers 2021, 13, 274. https://doi.org/10.3390/polym13020274
Osman AF, Siah L, Alrashdi AA, Ul-Hamid A, Ibrahim I. Improving the Tensile and Tear Properties of Thermoplastic Starch/Dolomite Biocomposite Film through Sonication Process. Polymers. 2021; 13(2):274. https://doi.org/10.3390/polym13020274
Chicago/Turabian StyleOsman, Azlin Fazlina, Lilian Siah, Awad A. Alrashdi, Anwar Ul-Hamid, and Ismail Ibrahim. 2021. "Improving the Tensile and Tear Properties of Thermoplastic Starch/Dolomite Biocomposite Film through Sonication Process" Polymers 13, no. 2: 274. https://doi.org/10.3390/polym13020274
APA StyleOsman, A. F., Siah, L., Alrashdi, A. A., Ul-Hamid, A., & Ibrahim, I. (2021). Improving the Tensile and Tear Properties of Thermoplastic Starch/Dolomite Biocomposite Film through Sonication Process. Polymers, 13(2), 274. https://doi.org/10.3390/polym13020274







