Synthesis and Application of Arylaminophosphazene as a Flame Retardant and Catalyst for the Polymerization of Benzoxazines
Abstract
1. Introduction
2. Materials and Methods
2.1. Starting Materials
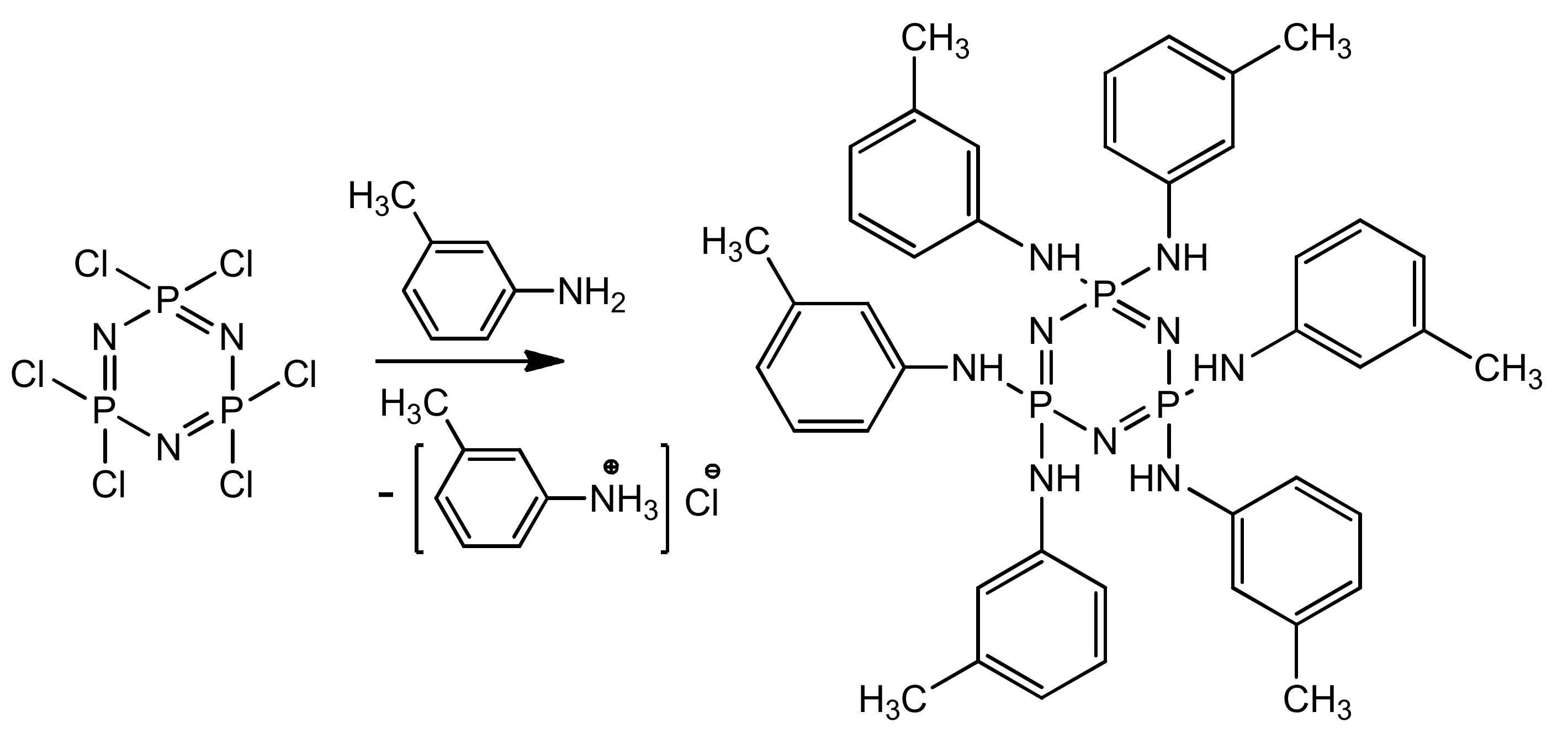
2.2. Synthesis of Hexakis-(3-methylphenylamino)cyclotriphosphazene (PN-mt)
2.3. Preparation of Benzoxazine Monomer Based on Bisphenol A, M-Toluidine and Paraphormaldehyde (BA-mt)
2.4. Composition Preparation
2.5. Measurements
3. Results and Discussion
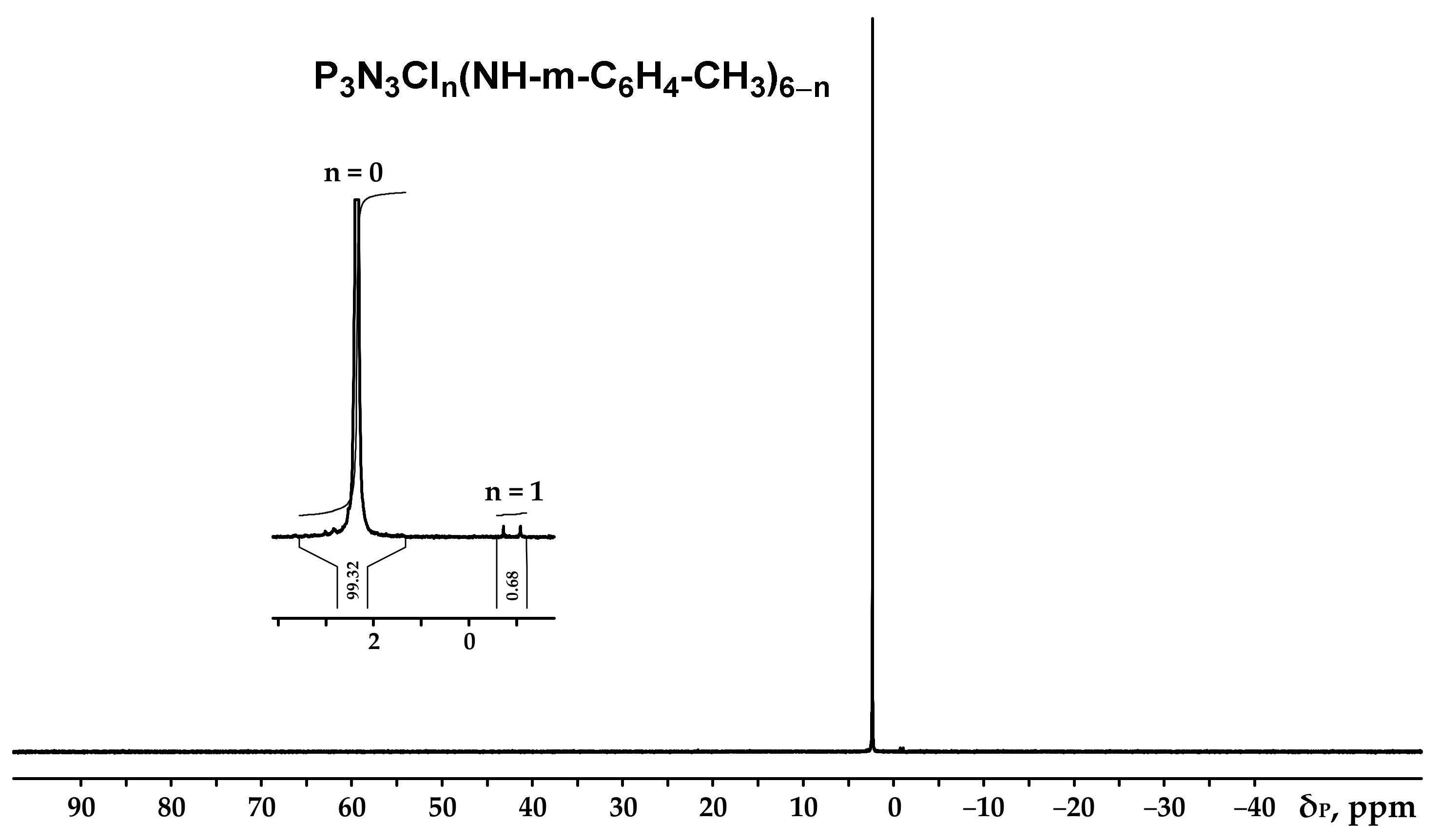
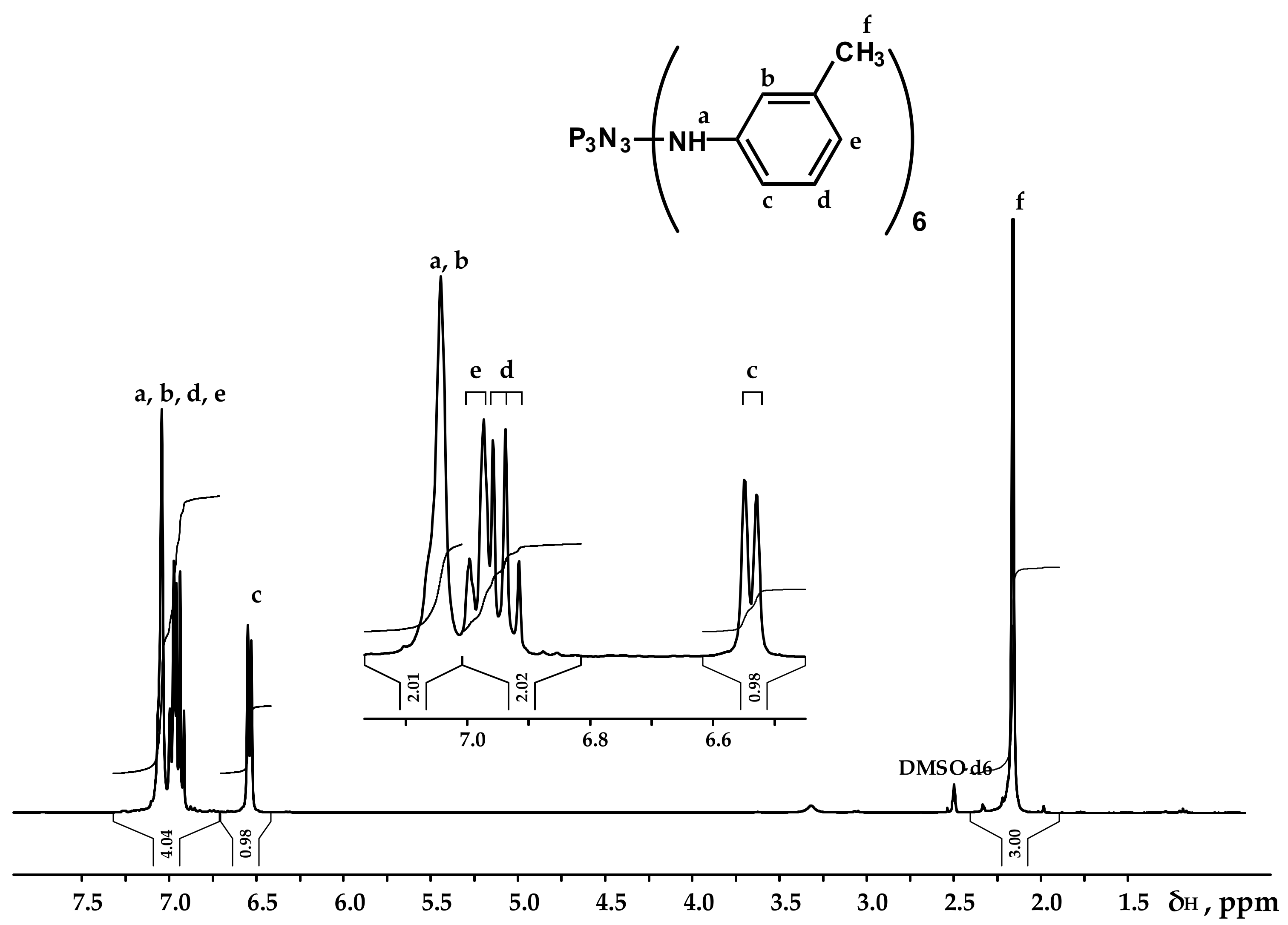
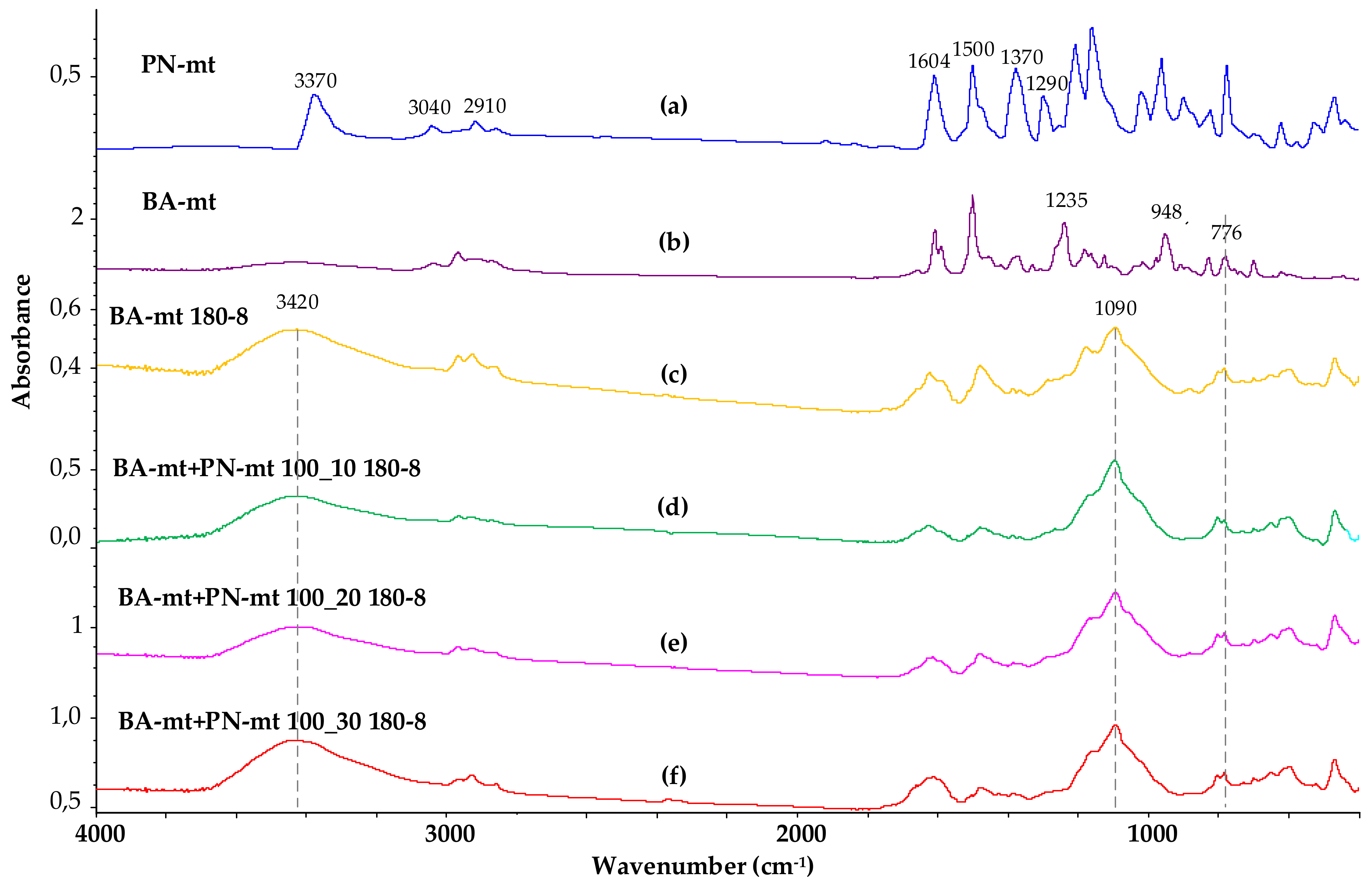
3.1. Synthesis and Characteristics of Hexakis-(3-methylphenylamino)cyclotriphosphazene (PN-mt)
3.2. Synthesis and Characteristics of BA-mt
3.3. Polymerization of BA-mt in Presence of PN-mt
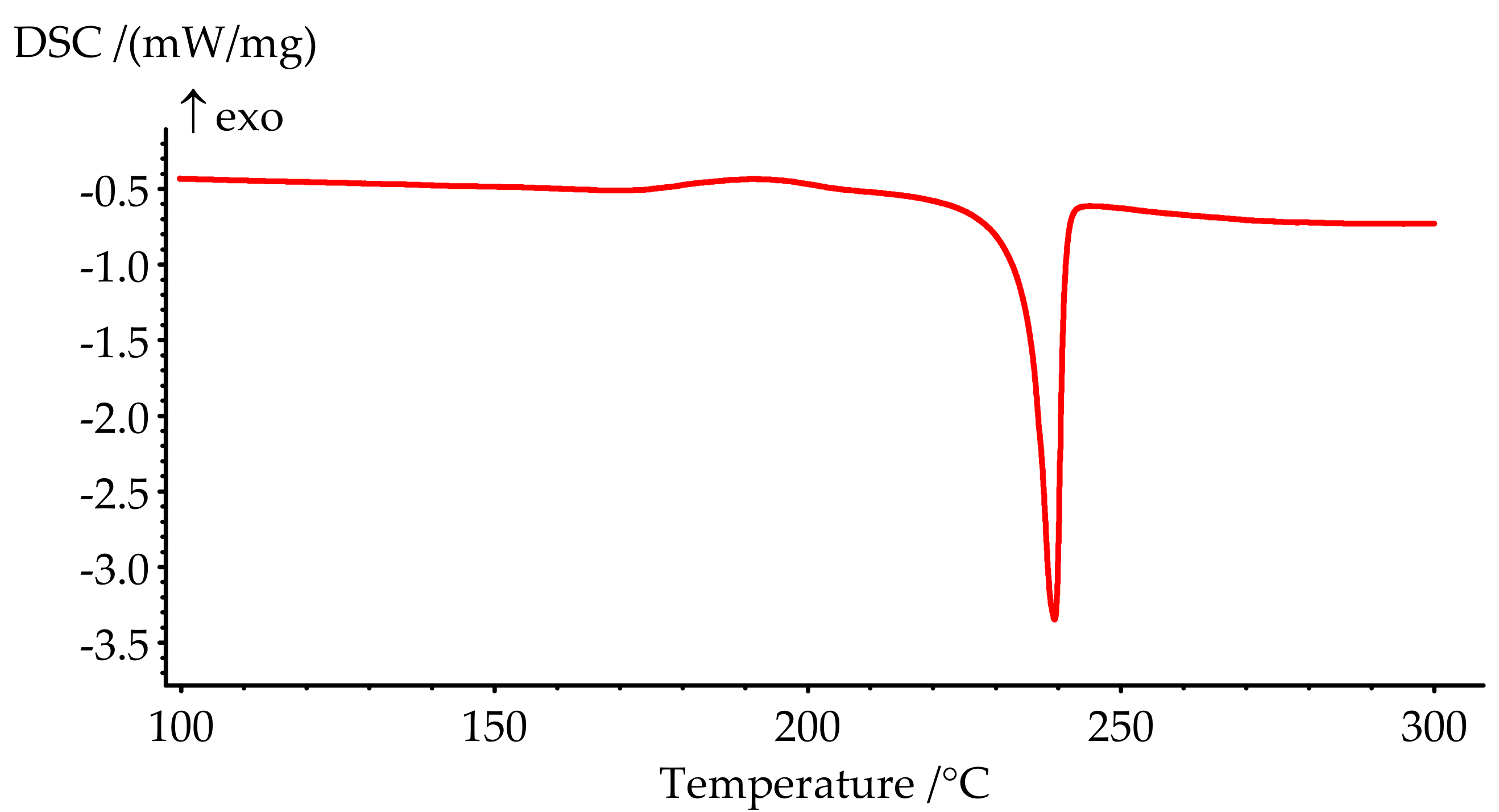
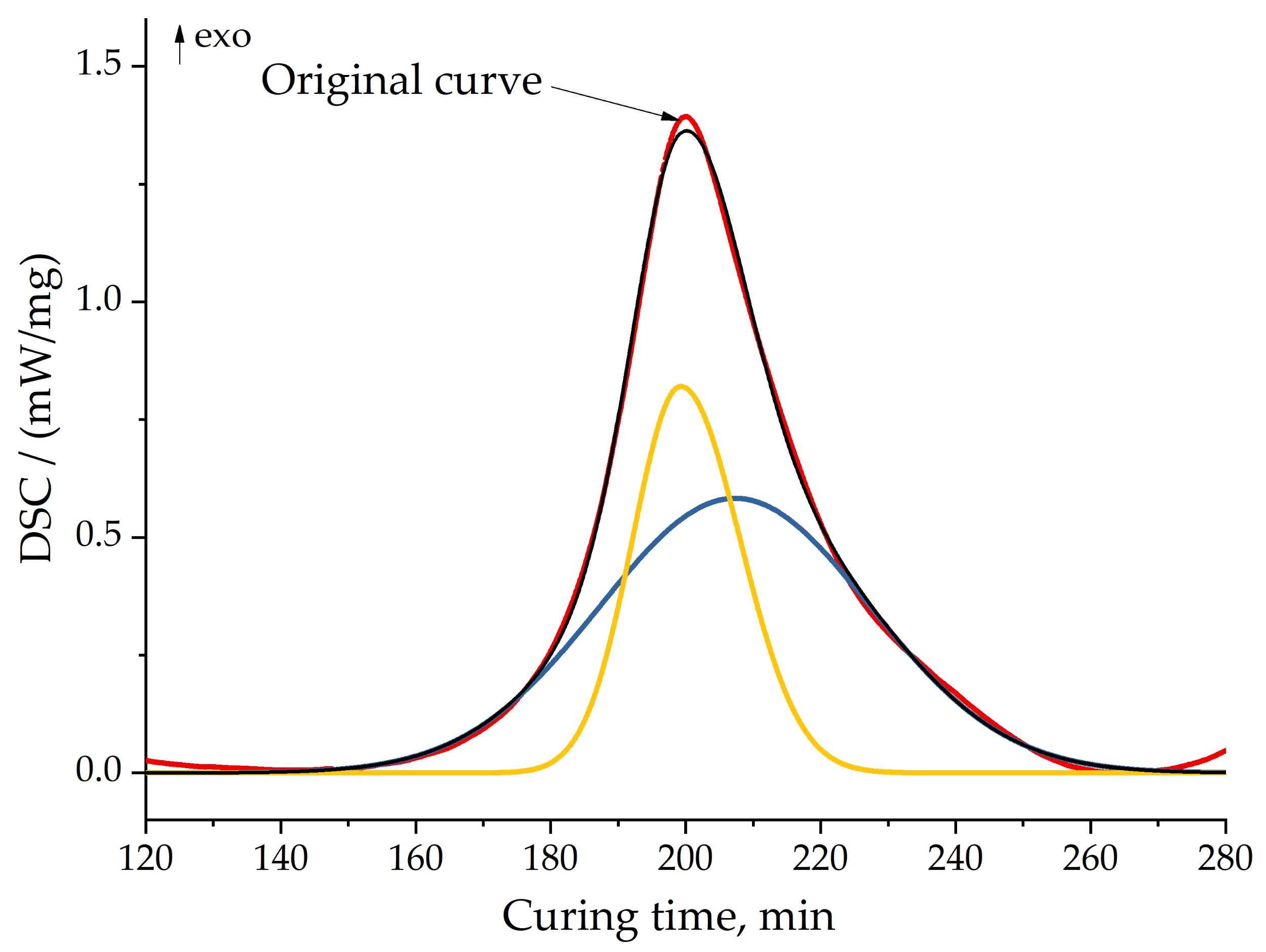
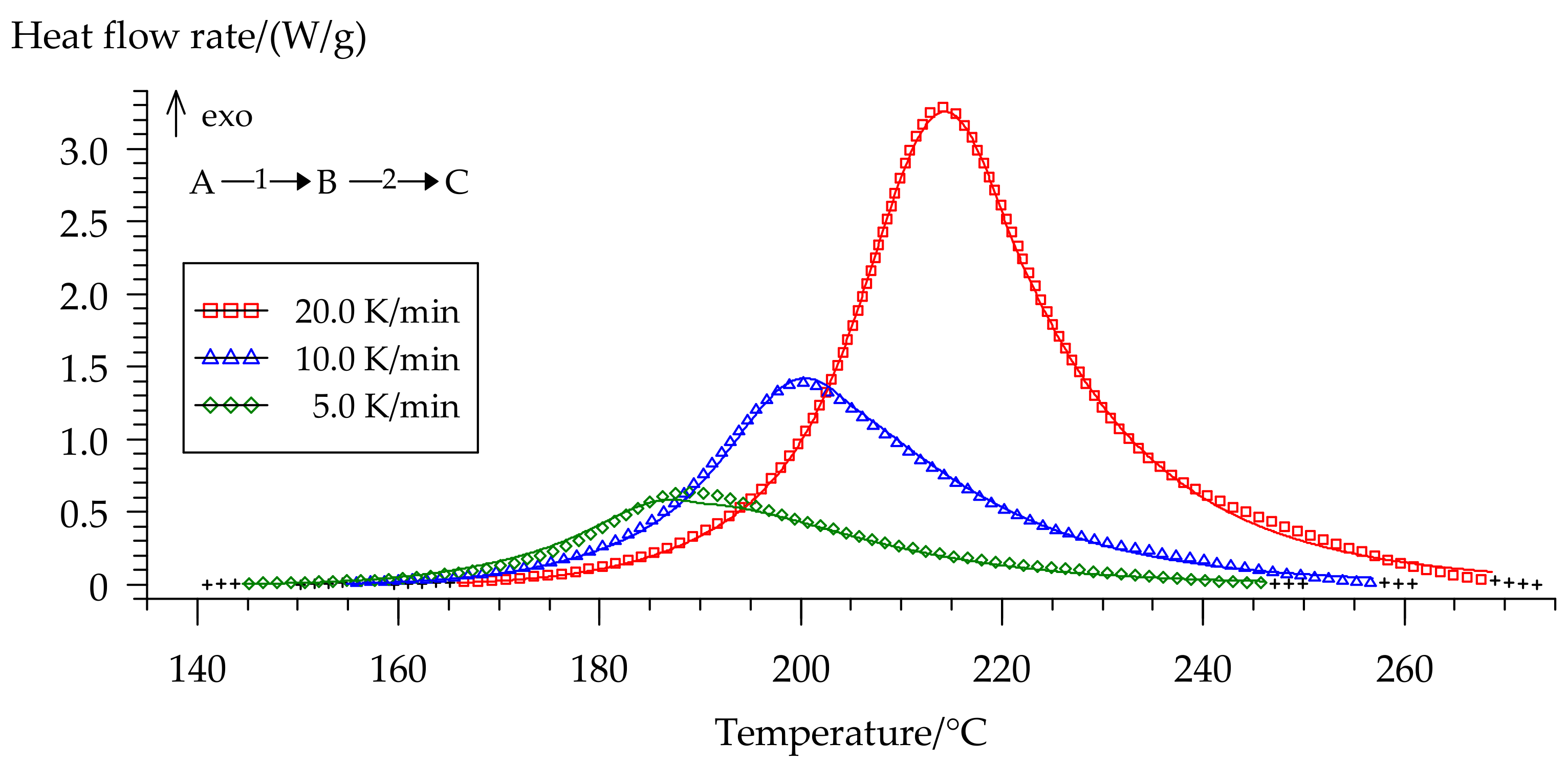
3.4. Curing Kinetics
3.5. Flammability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, C.; Yu, L.; Liu, H.; Chen, L. Development of Self-Reinforced Polymer Composites. Prog. Polym. Sci. 2012, 37, 767–780. [Google Scholar]
- Blanco, I. The Use of Composite Materials in 3D Printing. J. Compos. Sci. 2020, 4, 42. [Google Scholar]
- Singh, D.K.; Vaidya, A.; Thomas, V.; Theodore, M.; Kore, S.; Vaidya, U. Finite Element Modeling of the Fiber-Matrix Interface in Polymer Composites. J. Compos. Sci. 2020, 4, 58. [Google Scholar]
- Holbery, J.; Houston, D. Natural-Fiber-Reinforced Polymer Composites in Automotive Applications. JOM 2006, 58, 80–86. [Google Scholar]
- Elanchezhian, C.; Ramnath, B.V.; Ramakrishnan, G.; Raghavendra, K.S.; Muralidharan, M.; Kishore, V. Review on Metal Matrix Composites for Marine Applications. Mater. Today Proc. 2018, 5, 1211–1218. [Google Scholar]
- Eliasson, J.; Sandström, R. Applications of Aluminium Matrix Composites. Key Eng. Mater. 1995, 104–107, 3–36. [Google Scholar]
- Kilinc, F.S. Handbook of Fire Resistant Textiles; Textile Institute (Manchester, UK), Ed.; Woodhead Publishing series in textiles; Woodhead Pub: Oxford, UK; Philadelphia, PA, USA, 2013; ISBN 978-0-85709-123-9. [Google Scholar]
- Levchik, S.V.; Weil, E.D. A Review of Recent Progress in Phosphorus-Based Flame Retardants. J. Fire Sci. 2006, 24, 345–364. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Hamerton, I. Recent Developments in the Chemistry of Halogen-Free Flame Retardant Polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Leu, T.-S.; Wang, C.-S. Synergistic Effect of a Phosphorus-Nitrogen Flame Retardant on Engineering Plastics. J. Appl. Polym. Sci. 2004, 92, 410–417. [Google Scholar] [CrossRef]
- Sarychev, I.A.; Sirotin, I.S.; Borisov, R.S.; Mu, J.; Sokolskaya, I.B.; Bilichenko, J.V.; Filatov, S.N.; Kireev, V.V. Synthesis of Resorcinol-Based Phosphazene-Containing Epoxy Oligomers. Polymers 2019, 11, 614. [Google Scholar] [CrossRef]
- Sirotin, I.S.; Sarychev, I.A.; Vorobyeva, V.V.; Kuzmich, A.A.; Bornosuz, N.V.; Onuchin, D.V.; Gorbunova, I.Y.; Kireev, V.V. Synthesis of Phosphazene-Containing, Bisphenol A-Based Benzoxazines and Properties of Corresponding Polybenzoxazines. Polymers 2020, 12, 1225. [Google Scholar] [CrossRef]
- Kireev, V.V.; Bilichenko, Y.V.; Borisov, R.S.; Mu, J.; Kuznetsov, D.A.; Eroshenko, A.V.; Filatov, S.N.; Sirotin, I.S. Synthesis of Bisphenol A Based Phosphazene-Containing Epoxy Resin with Reduced Viscosity. Polymers 2019, 11, 1914. [Google Scholar] [CrossRef]
- Chistyakov, E.M.; Terekhov, I.V.; Shapagin, A.V.; Filatov, S.N.; Chuev, V.P. Curing of Epoxy Resin DER-331 by Hexakis(4-Acetamidophenoxy)Cyclotriphosphazene and Properties of the Prepared Composition. Polymers 2019, 11, 1191. [Google Scholar] [CrossRef]
- Terekhov, I.V.; Filatov, S.N.; Chistyakov, E.M.; Borisov, R.S.; Kireev, V.V. Synthesis of Oligomeric Epoxycyclotriphosphazenes and Their Properties as Reactive Flame-Retardants for Epoxy Resins. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 544–554. [Google Scholar] [CrossRef]
- Tan, Z.-W.; Wu, X.; Zhang, M.; Qiu, J.-J.; Liu, C.-M. Synthesis and Properties of Main-Chain Oligomeric Benzoxazine Precursor Containing Cyclotriphosphazene Units. High Perform. Polym. 2014, 26, 906–913. [Google Scholar] [CrossRef]
- Yang, G.; Wu, W.-H.; Wang, Y.-H.; Jiao, Y.-H.; Lu, L.-Y.; Qu, H.-Q.; Qin, X.-Y. Synthesis of a Novel Phosphazene-Based Flame Retardant with Active Amine Groups and Its Application in Reducing the Fire Hazard of Epoxy Resin. J. Hazard. Mater. 2019, 366, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, S.-Z.; Tian, D.-T.; Qiu, J.-J.; Liu, C.-M. Well-Defined Organic–Inorganic Hybrid Benzoxazine Monomers Based on Cyclotriphosphazene: Synthesis, Properties of the Monomers and Polybenzoxazines. Polymer 2011, 52, 4235–4245. [Google Scholar] [CrossRef]
- Tan, Z.-W.; Wu, X.; Zhang, M.; Qiu, J.-J.; Liu, C.-M. Performances Improvement of Traditional Polybenzoxazines by Copolymerizing with Cyclotriphosphazene-Based Benzoxazine Monomers. Polym. Bull. 2015, 72, 1417–1431. [Google Scholar] [CrossRef]
- Amarnath, N.; Appavoo, D.; Lochab, B. Eco-Friendly Halogen-Free Flame Retardant Cardanol Polyphosphazene Polybenzoxazine Networks. ACS Sustain. Chem. Eng. 2018, 6, 389–402. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, Y.; Liu, S.-Z.; Guo, Y.-N.; Qiu, J.-J.; Liu, C.-M. Highly Branched Benzoxazine Monomer Based on Cyclotriphosphazene: Synthesis and Properties of the Monomer and Polybenzoxazines. Polymer 2011, 52, 1004–1012. [Google Scholar] [CrossRef]
- He, J.; Li, X.; Fu, Y.; Zhu, H.; Zhao, G.; Wang, Z. Curing Reaction Mechanism and Heat Resistance Properties of Hexa-(4-Carboxyl-Phenoxy)-Cyclotriphosphazene/Bisphenol A Aniline Benzoxazine Blends. J. Appl. Polym. Sci. 2018, 135, 46389. [Google Scholar] [CrossRef]
- Liu, X.; Gu, Y. Study on the Volumetric Expansion of Benzoxazine Curing with Different Catalysts. J. Appl. Polym. Sci. 2002, 84, 1107–1113. [Google Scholar] [CrossRef]
- Ishida, H.; Rodriguez, Y. Catalyzing the Curing Reaction of a New Benzoxazine-Based Phenolic Resin. J. Appl. Polym. Sci. 1995, 58, 1751–1760. [Google Scholar] [CrossRef]
- Espinosa, M.A.; Galià, M.; Cádiz, V. Novel Phosphorilated Flame Retardant Thermosets: Epoxy–Benzoxazine–Novolac Systems. Polymer 2004, 45, 6103–6109. [Google Scholar] [CrossRef]
- Dunkers, J.; Ishida, H. Reaction of Benzoxazine-Based Phenolic Resins with Strong and Weak Carboxylic Acids and Phenols as Catalysts. J. Polym. Sci. A Polym. Chem. 1999, 37, 1913–1921. [Google Scholar] [CrossRef]
- Andreu, R.; Reina, J.A.; Ronda, J.C. Carboxylic Acid-Containing Benzoxazines as Efficient Catalysts in the Thermal Polymerization of Benzoxazines. J. Polym. Sci. A Polym. Chem. 2008, 46, 6091–6101. [Google Scholar] [CrossRef]
- Gamal Mohamed, M.; Hsiao, C.-H.; Luo, F.; Dai, L.; Kuo, S.-W. Multifunctional Polybenzoxazine Nanocomposites Containing Photoresponsive Azobenzene Units, Catalytic Carboxylic Acid Groups, and Pyrene Units Capable of Dispersing Carbon Nanotubes. RSC Adv. 2015, 5, 45201–45212. [Google Scholar] [CrossRef]
- Kasapoglu, F.; Cianga, I.; Yagci, Y.; Takeichi, T. Photoinitiated Cationic Polymerization of Monofunctional Benzoxazine. J. Polym. Sci. A Polym. Chem. 2003, 41, 3320–3328. [Google Scholar] [CrossRef]
- Liu, C.; Shen, D.; Sebastián, R.M.; Marquet, J.; Schönfeld, R. Mechanistic Studies on Ring-Opening Polymerization of Benzoxazines: A Mechanistically Based Catalyst Design. Macromolecules 2011, 44, 4616–4622. [Google Scholar] [CrossRef]
- Liu, C.; Shen, D.; Sebastián, R.M.; Marquet, J.; Schönfeld, R. Catalyst Effects on the Ring-Opening Polymerization of 1,3-Benzoxazine and on the Polymer Structure. Polymer 2013, 54, 2873–2878. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Ishida, H. Cationic Ring-Opening Polymerization of Benzoxazines. Polymer 1999, 40, 4563–4570. [Google Scholar] [CrossRef]
- Mhlanga, P.; Hassan, W.A.W.; Hamerton, I.; Howlin, B.J. Using Combined Computational Techniques to Predict the Glass Transition Temperatures of Aromatic Polybenzoxazines. PLoS ONE 2013, 8, e53367. [Google Scholar] [CrossRef] [PubMed]
- Sirotin, I.S.; Bilichenko, Y.V.; Suraeva, O.V.; Solodukhin, A.N.; Kireev, V.V. Synthesis of Oligomeric Chlorophosphazenes in the Presence of ZnCl2. Polym. Sci. Ser. B 2013, 55, 63–68. [Google Scholar] [CrossRef]
- Riddick, J.A.; Bunger, W.B.; Sakano, T.; Weissberger, A. Organic Solvents: Physical Properties and Methods of Purification. In Techniques of Chemistry, 4th ed.; Wiley: New York, NY, USA, 1986; ISBN 978-0-471-08467-9. [Google Scholar]
- Aizawa, T.; Hirai, Y.; Numata, S. Method for Producing Benzoxazine Resin. U.S. Patent 7,041,772, 9 May 2006. [Google Scholar]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee Recommendations for Performing Kinetic Computations on Thermal Analysis Data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- 14:00-17:00 ISO 11357-5:1999. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/02/71/27143.html (accessed on 14 January 2021).
- 14:00-17:00 ISO 11357-2:1999. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/02/55/25545.html (accessed on 14 January 2021).
- Horner, A. Aircraft Materials Fire Test Handbook; Federal Aviation Administration (NTIS): Springfield, VA, USA, 2000. [Google Scholar]
- Stutz, H.; Mertes, J.; Neubecker, K. Kinetics of Thermoset Cure and Polymerization in the Glass Transition Region. J. Polym. Sci. A Polym. Chem. 1993, 31, 1879–1886. [Google Scholar] [CrossRef]
- Deng, Y.; Martin, G.C. Diffusion and Diffusion-Controlled Kinetics during Epoxy-Amine Cure. Macromolecules 1994, 27, 5147–5153. [Google Scholar] [CrossRef]
- Van Assche, G.; Van Hemelrijck, A.; Rahier, H.; Van Mele, B. Modulated Differential Scanning Calorimetry: Non-Isothermal Cure, Vitrification, and Devitrification of Thermosetting Systems. Thermochim. Acta 1996, 286, 209–224. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of Thermal Degradation of Char-Forming Plastics from Thermogravimetry. Application to a Phenolic Plastic. J. Polym. Sci. C Polym. Symp. 2007, 6, 183–195. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N.; Vyazovkin, S. Learning about Epoxy Cure Mechanisms from Isoconversional Analysis of DSC Data. Thermochim. Acta 2002, 388, 289–298. [Google Scholar] [CrossRef]
- Salla, J.M.; Ramis, X. Comparative Study of the Cure Kinetics of an Unsaturated Polyester Resin Using Different Procedures. Polym. Eng. Sci. 1996, 36, 835–851. [Google Scholar] [CrossRef]
- Tikhonov, N.A.; Arkhangelsky, I.V.; Belyaev, S.S.; Matveev, A.T. Carbonization of Polymeric Nonwoven Materials. Thermochim. Acta 2009, 486, 66–70. [Google Scholar] [CrossRef]

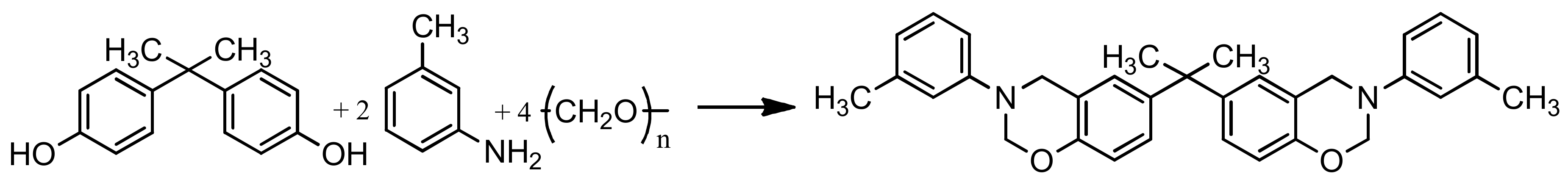


| Formulation Number | BA-mt 1, pbw | PN-mt 2, pbw |
|---|---|---|
| 1 | 100 | 0 |
| 2 | 100 | 10 |
| 3 | 100 | 20 |
| 4 | 100 | 30 |
| C, % | H, % | N, % | Cl, % | P, % | |
|---|---|---|---|---|---|
| Calculated | 65.36 | 6.27 | 16.33 | 0 | 12.04 |
| Found | 65.21 | 6.21 | 16.39 | 0 | 12.19 |
| Sample | Proton Chemical Shifts δH (ppm) | ||||
|---|---|---|---|---|---|
| Oxazine Ring | Amine | Diphenol | |||
| CH2N | CH2O | CH3– | CH3– | CH (Ar) | |
| BA-mt | 4.56 | 5.30 | 2.30 | 1.57 | 6.62–7.29 |
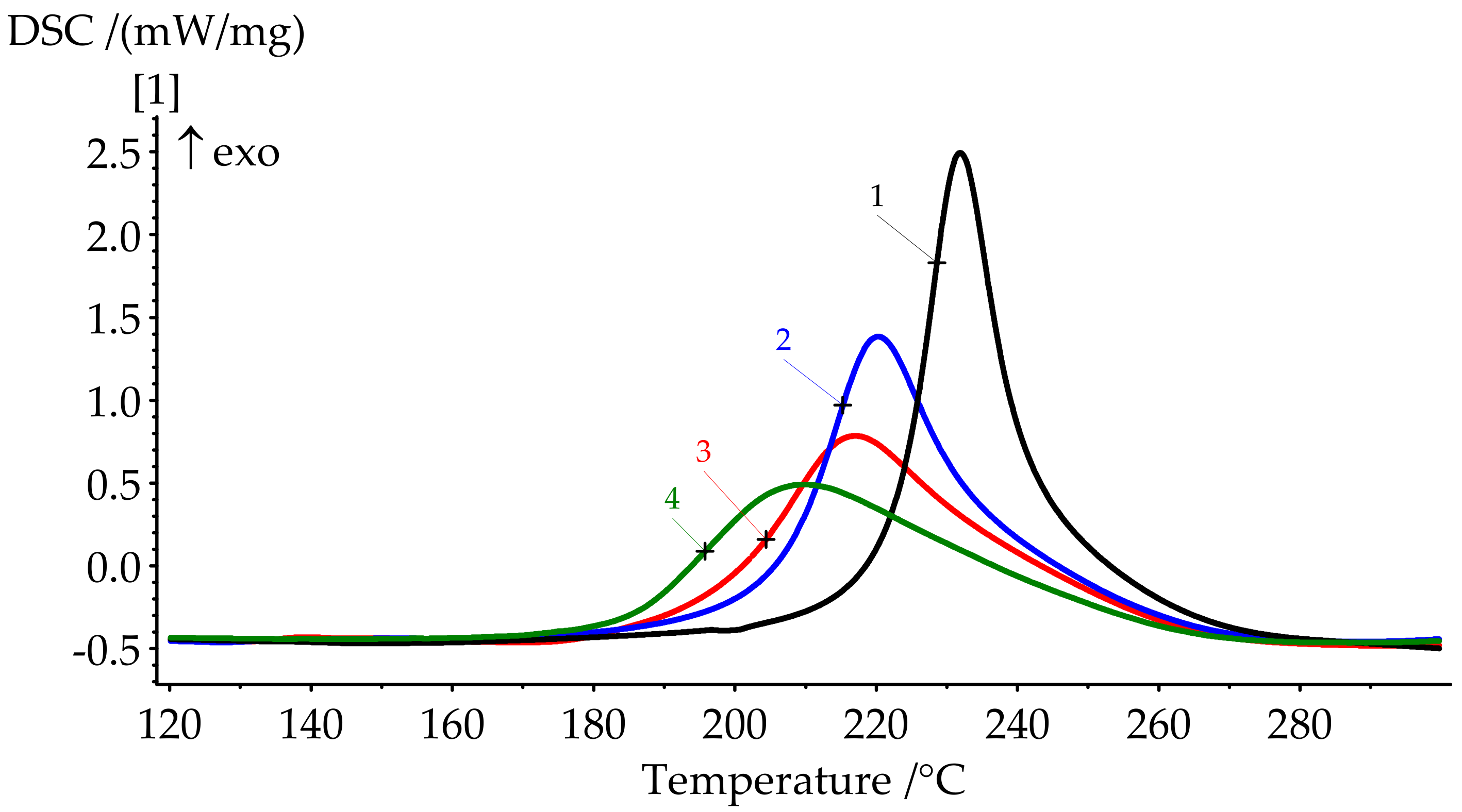
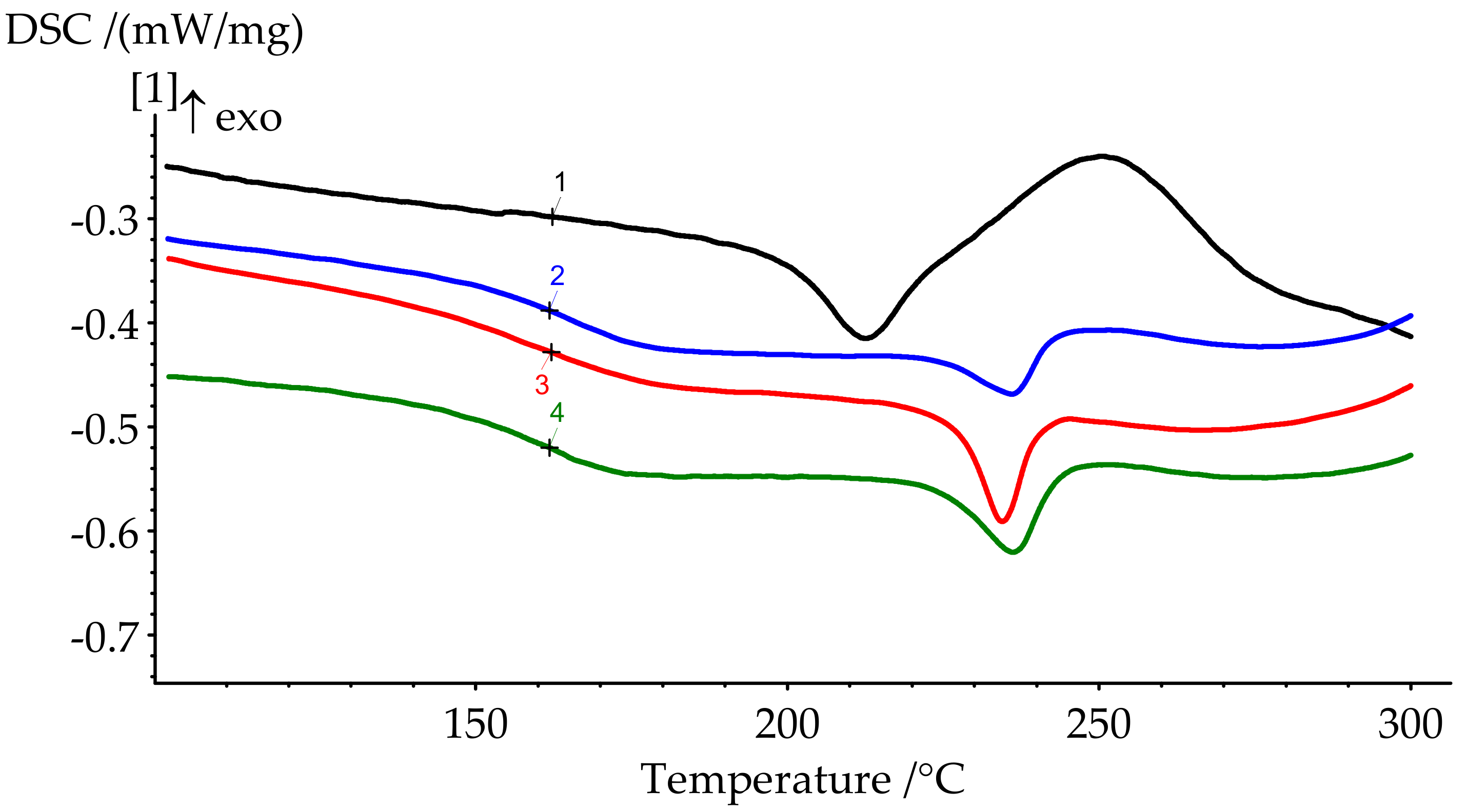
| Formulation № | Uncured Samples (Figure 6) | Cured Samples (Figure 7) | ||||
|---|---|---|---|---|---|---|
| Tonset, °C | Tpeak, °C | Tend, °C | ΔH, J/g | Tg(middle), °C | ΔHres, J/g | |
| 1 | 222.0 | 231.9 | 242.4 | 346.2 | 206.5 | 23.87 |
| 2 | 205.9 | 220.3 | 238.2 | 268.6 | 164.9 | 3.18 |
| 3 | 195.8 | 217.0 | 247.4 | 260.9 | 162.3 | 0 |
| 4 | 184.8 | 209.9 | 255.7 | 252.0 | 160.1 | 0 |
| Parameter | Optimum Value |
|---|---|
| logA1 [s−1] | 7.3632 |
| E1 [kJ/mol] | 80.7604 |
| React. Ord. 1 | 0.6224 |
| Exponent a1 | 0.5311 |
| Log A2 [s−1] | 13.2443 |
| E2 [kJ/mol] | 137.0854 |
| React. Ord. 2 | 2.0102 |
| Exponent a2 | 0.0938 |
| Foll. React. 1 | 0.1963 |
| Correlation coefficient | 0.9993 |
| ΔH (5 °C/min), J/g | 278.7 |
| ΔH (10 °C/min), J/g | 268.6 |
| ΔH (20 °C/min), J/g | 257.5 |
| Formulation № | τ1, c | τ2, c | τ3, c | Στ, c | UL-94 |
|---|---|---|---|---|---|
| 1 | 16 | 19 | 14 | 49 | V-1 |
| 2 | 5 | 6 | 9 | 20 | V-0 |
| 3 | 3 | 5 | 7 | 15 | V-0 |
| 4 | 1 | 4 | 5 | 10 | V-0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bornosuz, N.V.; Gorbunova, I.Y.; Kireev, V.V.; Bilichenko, Y.V.; Chursova, L.V.; Svistunov, Y.S.; Onuchin, D.V.; Shutov, V.V.; Petrakova, V.V.; Kolenchenko, A.A.; et al. Synthesis and Application of Arylaminophosphazene as a Flame Retardant and Catalyst for the Polymerization of Benzoxazines. Polymers 2021, 13, 263. https://doi.org/10.3390/polym13020263
Bornosuz NV, Gorbunova IY, Kireev VV, Bilichenko YV, Chursova LV, Svistunov YS, Onuchin DV, Shutov VV, Petrakova VV, Kolenchenko AA, et al. Synthesis and Application of Arylaminophosphazene as a Flame Retardant and Catalyst for the Polymerization of Benzoxazines. Polymers. 2021; 13(2):263. https://doi.org/10.3390/polym13020263
Chicago/Turabian StyleBornosuz, Natalia V., Irina Yu. Gorbunova, Vyacheslav V. Kireev, Yulya V. Bilichenko, Larisa V. Chursova, Yuri S. Svistunov, Denis V. Onuchin, Vyacheslav V. Shutov, Viktoria V. Petrakova, Alexander A. Kolenchenko, and et al. 2021. "Synthesis and Application of Arylaminophosphazene as a Flame Retardant and Catalyst for the Polymerization of Benzoxazines" Polymers 13, no. 2: 263. https://doi.org/10.3390/polym13020263
APA StyleBornosuz, N. V., Gorbunova, I. Y., Kireev, V. V., Bilichenko, Y. V., Chursova, L. V., Svistunov, Y. S., Onuchin, D. V., Shutov, V. V., Petrakova, V. V., Kolenchenko, A. A., Nguyen, D. T., Pavlov, N. V., Orlov, A. V., Grebeneva, T. A., & Sirotin, I. S. (2021). Synthesis and Application of Arylaminophosphazene as a Flame Retardant and Catalyst for the Polymerization of Benzoxazines. Polymers, 13(2), 263. https://doi.org/10.3390/polym13020263






