QbD Enabled Azacitidine Loaded Liposomal Nanoformulation and Its In Vitro Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
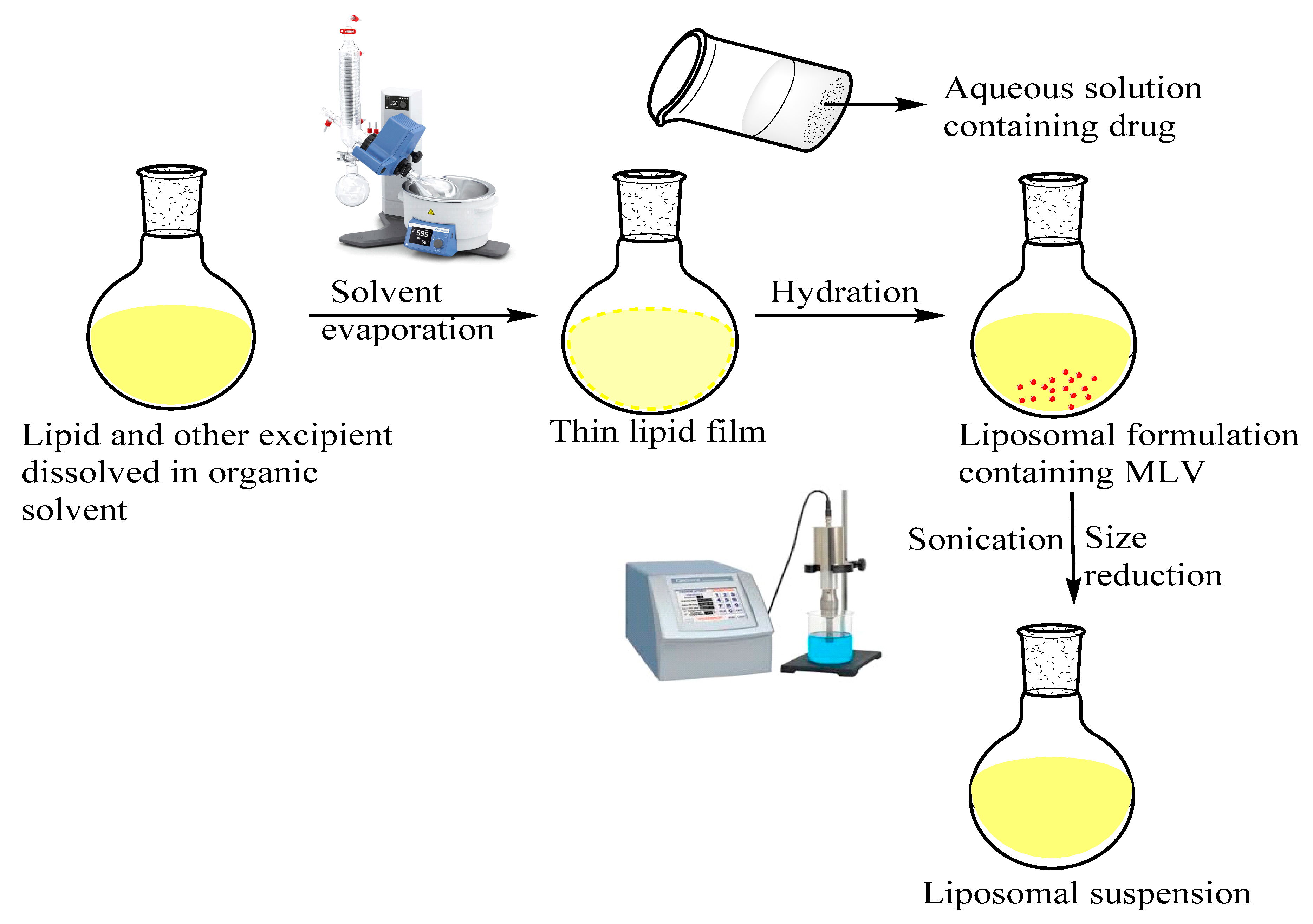
2.2. Preparation Method of AZA Loaded Liposomes
2.3. Box Behnken Design and Optimization Condition (BBD)
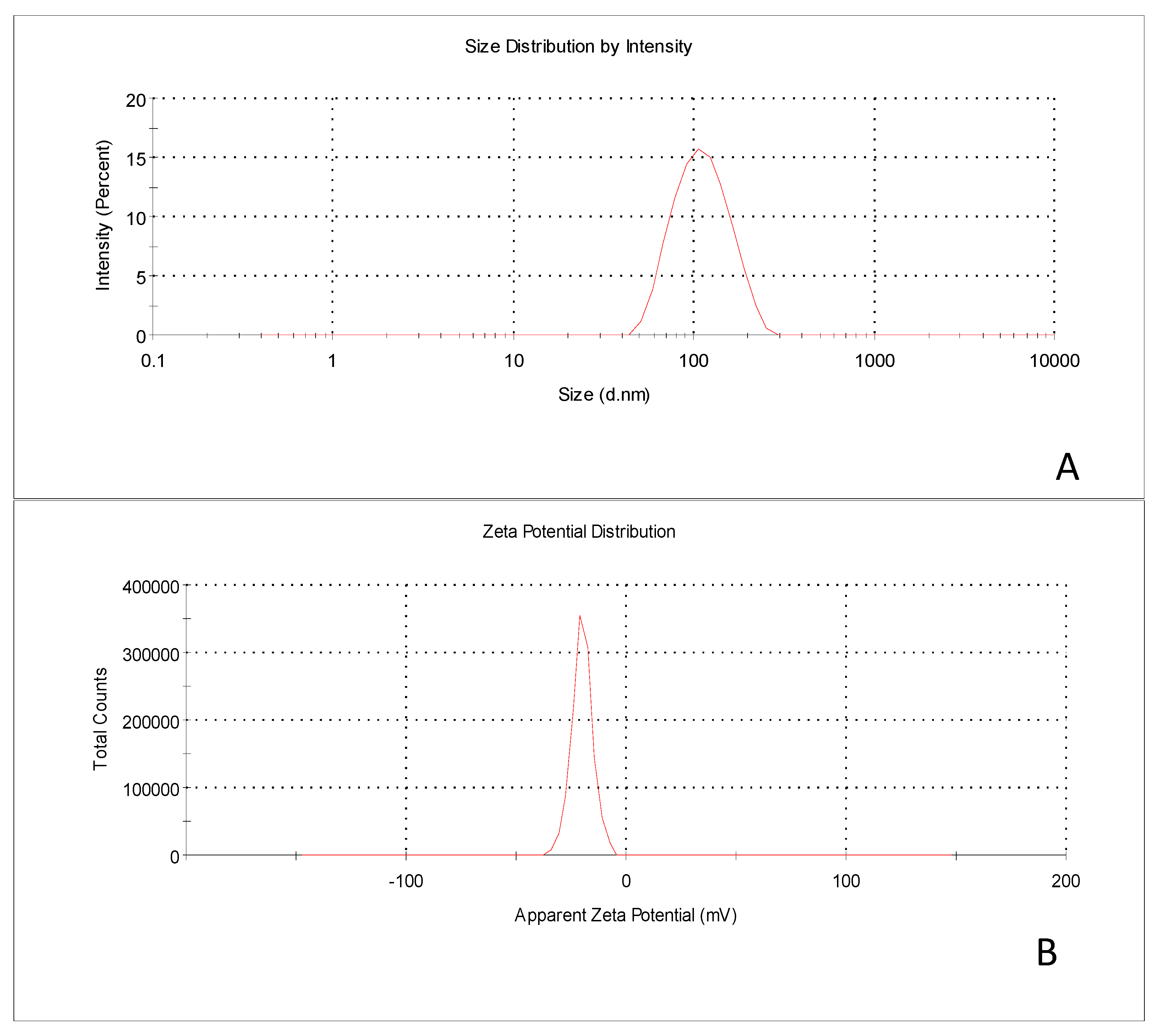
2.4. Particle Size, PDI and Zeta Potential
2.5. Drug Entrapment and Drug Loading Efficiency
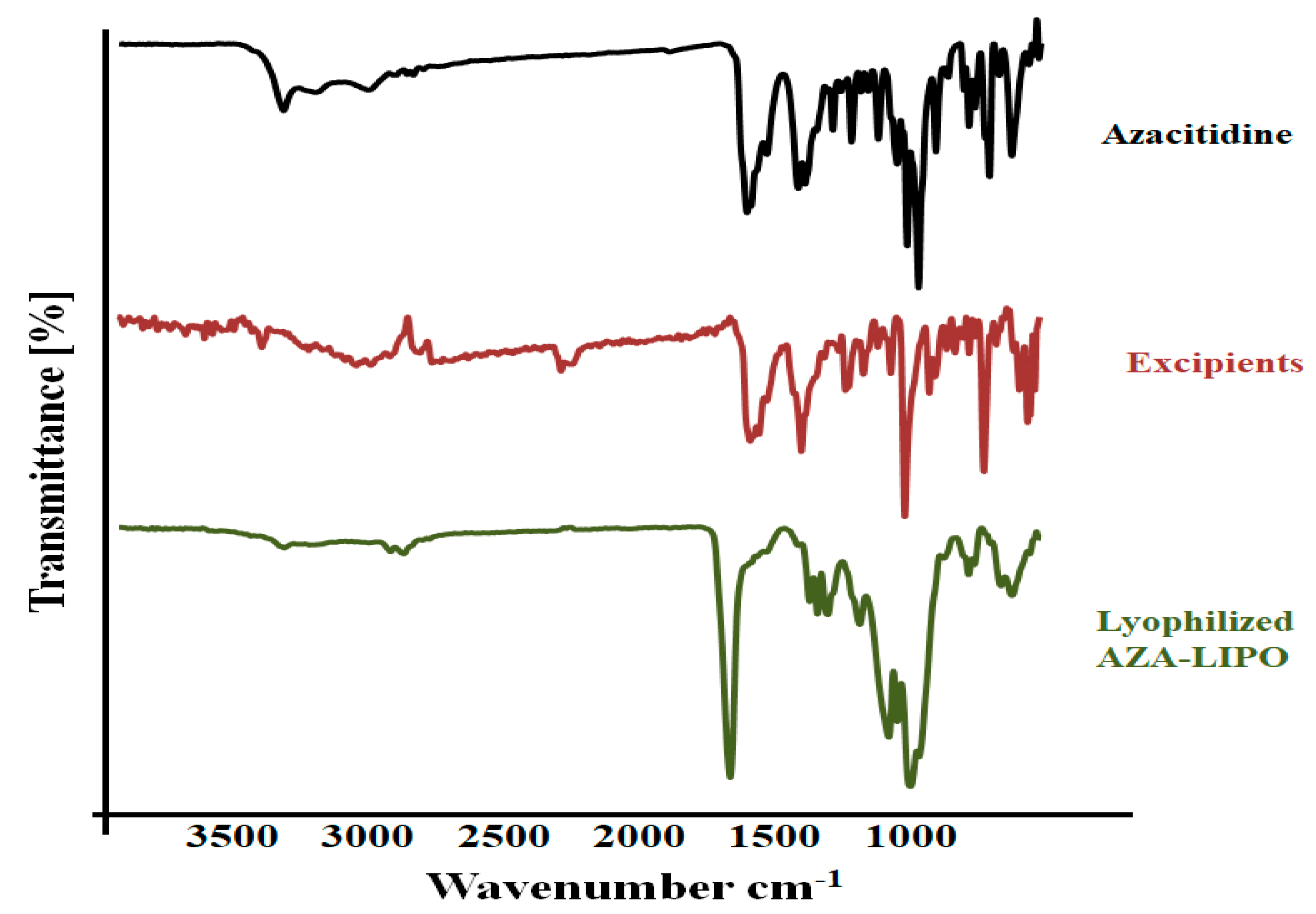
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.7. Differential Scanning Calorimetry (DSC)
2.8. X-Ray Diffraction Study
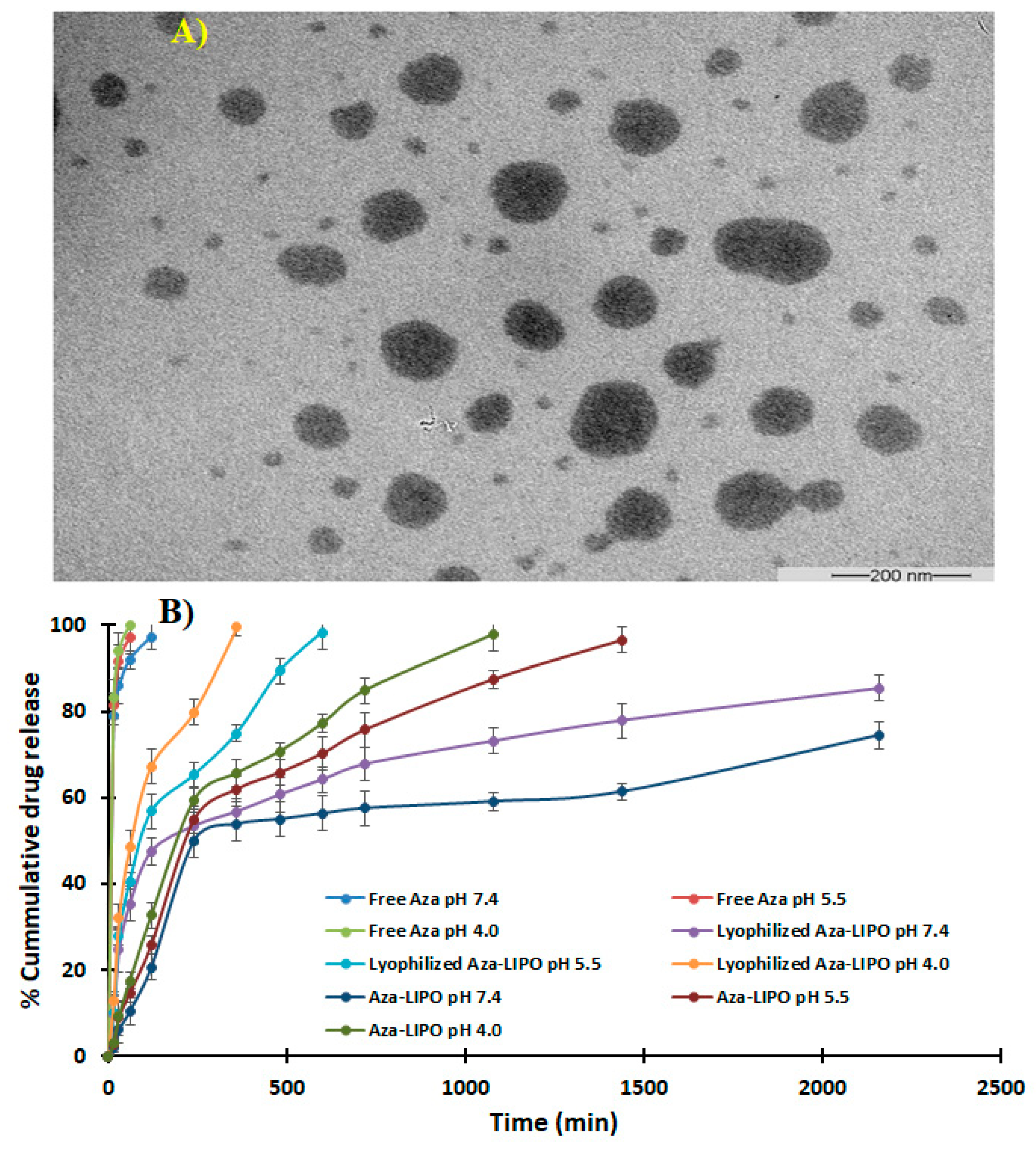
2.9. Transmission Electron Microscopy (TEM)
2.10. In-Vitro Drug Release Study
2.11. Hemolytic Toxicity
2.12. Cytotoxicity Assay
2.13. Confocal Microscopy
2.14. Determination of Bax, Bcl-2 and Caspase-3 Proteins Expression
2.15. Statistical Analysis
3. Result and Discussion
3.1. Preparation of AZA-LIPO
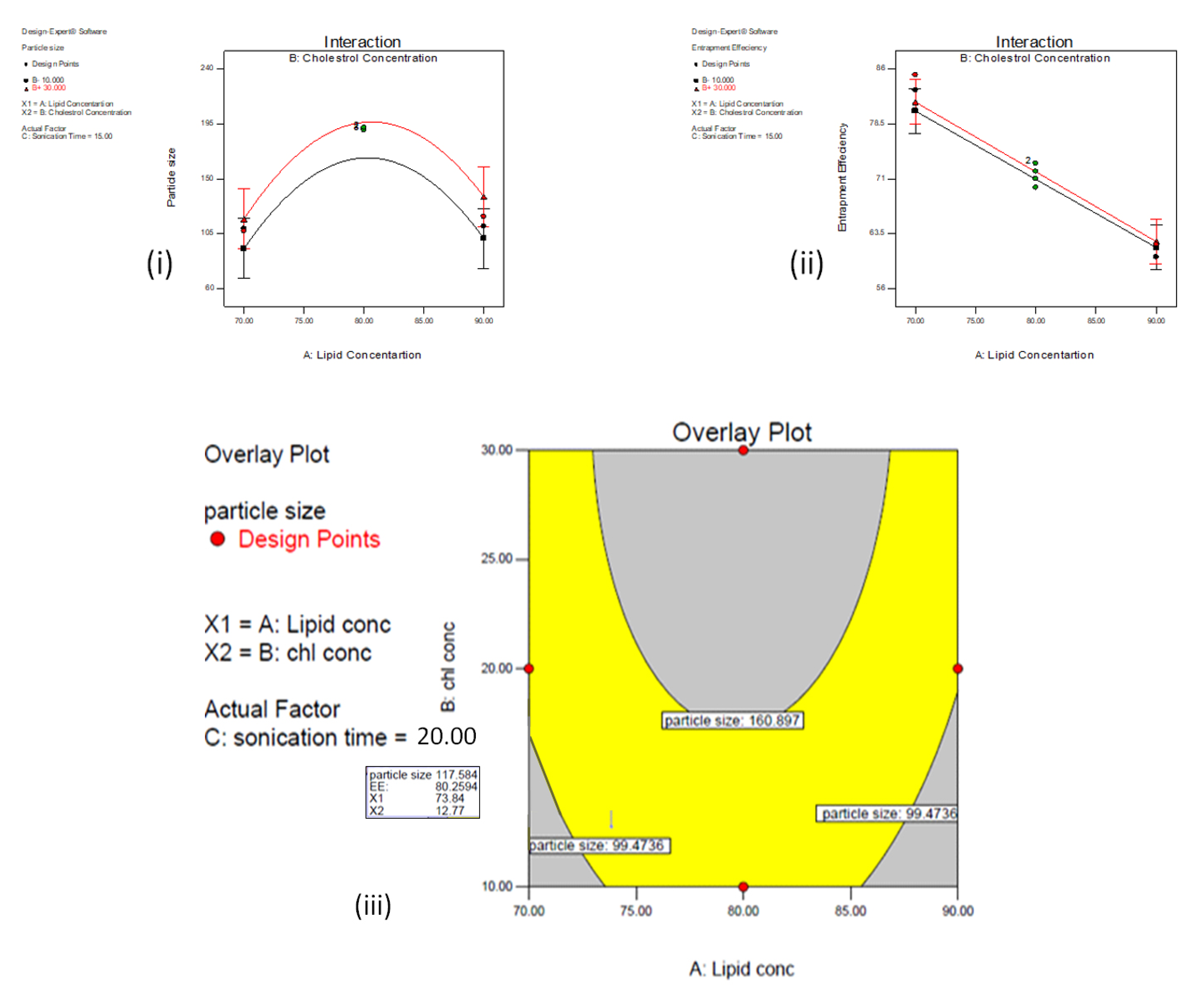
3.2. Optimization of AZA-LIPO
3.3. Particle Size
3.4. Entrapment Efficiency
3.5. Data Optimization and Model Validation
3.6. FTIR
3.7. DSC
3.8. X-Ray Diffraction Study
3.9. TEM
3.10. In Vitro Drug Release Study
3.11. Hemolytic Toxicity
3.12. Cytotoxicity Assay
3.13. Confocal Microscopy
3.14. Effect of the AZA LPO on the Expression of Bax, Bcl-2 and Caspase-3 Proteins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neupane, Y.R.; Srivastava, M.; Ahmad, N.; Kumar, N.; Bhatnagar, A.; Kohli, K. Lipid based nanocarrier system for the potential oral delivery of decitabine: Formulation design, characterization, ex vivo, and in vivo assessment. Int. J. Pharm. 2014, 477, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, K.; Handa, M.; Shukla, R. Azacitidine Loaded PLGA Nanoparticles and their Dual Release Mechanism. Curr. Nanomed. 2020, 10, 280–289. [Google Scholar] [CrossRef]
- Gore, S.D.; Jones, C.; Kirkpatrick, P. Decitabine. Nat. Rev. Drug Discov. 2006, 5, 891–892. [Google Scholar] [CrossRef] [PubMed]
- Gkionis, L.; Campbell, R.A.; Aojula, H.; Harris, L.K.; Tirella, A. Manufacturing drug co-loaded liposomal formulations targeting breast cancer: Influence of preparative method on liposomes characteristics and in vitro toxicity. Int. J. Pharm. 2020, 590, 119926. [Google Scholar] [CrossRef] [PubMed]
- Vakhshiteh, F.; Khabazian, E.; Atyabi, F.; Ostad, S.N.; Madjd, Z.; Dinarvand, R. Peptide-conjugated liposomes for targeted miR-34a delivery to suppress breast cancer and cancer stem-like population. J. Drug Deliv. Sci. Technol. 2020, 57, 101687. [Google Scholar] [CrossRef]
- Briot, T.; Roger, E.; Lautram, N.; Verger, A.; Clavreul, A.; Lagarce, F. Development and in vitro evaluations of new decitabine nanocarriers for the treatment of acute myeloid leukemia. Int. J. Nanomed. 2017, 12, 8427–8442. [Google Scholar] [CrossRef][Green Version]
- Stresemann, C.; Lyko, F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int. J. Cancer 2008, 123, 8–13. [Google Scholar] [CrossRef]
- Momparler, R.L. Pharmacology of 5-aza-2′-deoxycytidine (decitabine). Semin. Hematol. 2005, 42. [Google Scholar] [CrossRef]
- Hirnle, P. Liposomes for drug targeting in the lymphatic system. Hybridoma 1997, 16, 127–132. [Google Scholar] [CrossRef]
- Jain, A.; Jain, K.; Kesharwani, P.; Jain, N.K. Low density lipoproteins mediated nanoplatforms for cancer targeting. J. Nanopart. Res. 2013, 15, 1888. [Google Scholar] [CrossRef]
- Ağardan, N.B.M.; Değim, Z.; Yılmaz, Ş.; Altıntaş, L.; Topal, T. Tamoxifen/raloxifene loaded liposomes for oral treatment of breast cancer. J. Drug Deliv. Sci. Technol. 2020, 57, 101612. [Google Scholar] [CrossRef]
- Swami, R.; Kumar, Y.; Chaudhari, D.; Katiyar, S.S.; Kuche, K.; Katare, P.B.; Banerjee, S.K.; Jain, S. pH sensitive liposomes assisted specific and improved breast cancer therapy using co-delivery of SIRT1 shRNA and Docetaxel. Mater. Sci. Eng. C 2020, 111664. [Google Scholar] [CrossRef]
- Tang, B.; Peng, Y.; Yue, Q.; Pu, Y.; Li, R.; Zhao, Y.; Hai, L.; Guo, L.; Wu, Y. Design, preparation and evaluation of different branched biotin modified liposomes for targeting breast cancer. Eur. J. Med. Chem. 2020, 193, 112204. [Google Scholar] [CrossRef] [PubMed]
- Feuser, P.E.; Cordeiro, A.P.; de Bem Silveira, G.; Borges Corrêa, M.E.A.; Lock Silveira, P.C.; Sayer, C.; de Araújo, P.H.H.; Machado-de-Ávila, R.A.; Dal Bó, A.G. Co-encapsulation of sodium diethyldithiocarbamate (DETC) and zinc phthalocyanine (ZnPc) in liposomes promotes increases phototoxic activity against (MDA-MB 231) human breast cancer cells. Colloids Surf. B Biointerfaces 2021, 197, 111434. [Google Scholar] [CrossRef]
- Ahmed, K.S.; Hussein, S.A.; Ali, A.H.; Korma, S.A.; Lipeng, Q.; Jinghua, C. Liposome: Composition, characterisation, preparation, and recent innovation in clinical applications. J. Drug Target. 2019, 27, 742–761. [Google Scholar] [CrossRef]
- Xu, X.; Khan, M.A.; Burgess, D.J. A quality by design (QbD) case study on liposomes containing hydrophilic API: I. Formulation, processing design and risk assessment. Int. J. Pharm. 2011, 419, 52–59. [Google Scholar] [CrossRef]
- Sylvester, B.; Porfire, A.; Muntean, D.M.; Vlase, L.; Lupuţ, L.; Licarete, E.; Sesarman, A.; Alupei, M.C.; Banciu, M.; Achim, M.; et al. Optimization of prednisolone-loaded long-circulating liposomes via application of Quality by Design (QbD) approach. J. Liposome Res. 2018, 28, 49–61. [Google Scholar] [CrossRef]
- Shi, J.; Ma, F.; Wang, X.; Wang, F.; Liao, H. Formulation of liposomes gels of paeonol for transdermal drug delivery by Box-Behnken statistical design. J. Liposome Res. 2012, 22, 270–278. [Google Scholar] [CrossRef]
- Rane, S.; Prabhakar, B. Optimization of paclitaxel containing pH-sensitive liposomes by 3 factor, 3 level box-behnken design. Indian J. Pharm. Sci. 2013, 75, 420–426. [Google Scholar] [CrossRef]
- Patel, R.P.; Patel, H.; Baria, A.H. Formulation and Evaluation of Liposomes of Ketoconazole. Int. J. Drug Deliv. Technol. 2009, 1, 16–23. [Google Scholar] [CrossRef]
- Muppidi, K.; Pumerantz, A.S.; Wang, J.; Betageri, G. Development and Stability Studies of Novel Liposomal Vancomycin Formulations. ISRN Pharm. 2012, 2012, 636743. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, E.; Georgopoulos, A.; Kagkadis, K.A.; Demetzos, C. Preparation and characterization of lyophilized liposomes with incorporated quercetin. J. Liposome Res. 2006, 16, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.H.; Jobanputra, A. Enhanced Ungual Permeation of Terbinafine HCl Delivered Through Liposome-Loaded Nail Lacquer Formulation Optimized by QbD Approach. AAPS PharmSciTech 2018, 19, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Gupta, G.; Singodia, D.; Shukla, R.; Verma, A.K.; Dwivedi, P.; Kansal, S.; Mishra, P.R. Emerging trend in nano-engineered polyelectrolyte-based surrogate carriers for delivery of bioactives. Expert Opin. Drug Deliv. 2010, 7, 993–1011. [Google Scholar] [CrossRef]
- Jain, P.; Tiwari, M.; Kumar, N.; Chonkar, A.; Rao, J.V.; Udupa, N. Oral administration of decitabine nanoparticles effectively suppresses Nmu-induced leukaemia in Sprague-Dawley rats and arrests K562 cells in S-phase. Int. J. Pharm. Clin. Res. 2016, 8, 1260–1268. [Google Scholar]
- Kesharwani, P.; Banerjee, S.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Parenterally administrable nano-micelles of 3,4-difluorobenzylidene curcumin for treating pancreatic cancer. Colloids Surf. B Biointerfaces 2015, 132, 138–145. [Google Scholar] [CrossRef]
- Wen, A.-H.; Choi, M.-K.; Kim, D.-D. Formulation of Liposome for topical delivery of arbutin. Arch. Pharm. Res. 2006, 29, 1187–1192. [Google Scholar] [CrossRef]
- Gorain, B.; Choudhury, H.; Pandey, M.; Kesharwani, P. Paclitaxel loaded vitamin E-TPGS nanoparticles for cancer therapy. Mater. Sci. Eng. C 2018. [Google Scholar] [CrossRef]
- Shukla, R.; Kumar, J.; Dwivedi, P.; Gatla, P.; Mishra, P.R. Microparticles of diethylcarbamazine citrate for the treatment of lymphatic filariasis. Asian J. Chem. 2013, 25, S302. [Google Scholar]
- Kesharwani, P.; Tekade, R.K.; Gajbhiye, V.; Jain, K.; Jain, N.K. Cancer targeting potential of some ligand-anchored poly(propylene imine) dendrimers: A comparison. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Gupta, J.; Shukla, P.; Dwivedi, P.; Tripathi, P.; Bhattacharya, S.M.; Mishra, P.R. Chitosan coated alginate micro particles for the oral delivery of antifilarial drugs and combinations for intervention in Brugia malayi induced lymphatic filariasis. RSC Adv. 2015, 5, 69047–69056. [Google Scholar] [CrossRef]
- Bunjes, H.; Unruh, T. Characterization of lipid nanoparticles by differential scanning calorimetry, X-ray and neutron scattering. Adv. Drug Deliv. Rev. 2007, 59, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Pardhi, V.P.; Verma, T.; Flora, S.J.S.; Chandasana, H.; Shukla, R. Nanocrystals: An Overview of Fabrication, Characterization and Therapeutic Applications in Drug Delivery. Curr. Pharm. Des. 2019, 24, 5129–5146. [Google Scholar] [CrossRef]
- Pabst, G.; Rappolt, M.; Amenitsch, H.; Laggner, P. Structural information from multilamellar liposomes at full hydration: Full q-range fitting with high quality X-ray data. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 2000, 62, 4000. [Google Scholar] [CrossRef]
- De Jonge, N.; Ross, F.M. Electron microscopy of specimens in liquid. Nat. Nanotechnol. 2011, 6, 695–704. [Google Scholar] [CrossRef]
- Kesharwani, P.; Tekade, R.K.; Jain, N.K. Generation dependent safety and efficacy of folic acid conjugated dendrimer based anticancer drug formulations. Pharm. Res. 2015, 32, 1438–1450. [Google Scholar] [CrossRef]
- Niu, G.; Cogburn, B.; Hughes, J. Preparation and characterization of doxorubicin liposomes. Methods Mol. Biol. 2010, 624, 211–219. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, N.; Li, A.; Zou, W.; Xu, W. Preparation and evaluation of N3-O-toluyl-fluorouracil-loaded liposomes. Int. J. Pharm. 2008, 353, 243–250. [Google Scholar] [CrossRef]
- Kesharwani, P.; Tekade, R.K.; Jain, N.K. Formulation development and in vitro-in vivo assessment of the fourth-generation PPI dendrimer as a cancer-targeting vector. Nanomedicine 2014, 9, 2291–2308. [Google Scholar] [CrossRef]
- Kesharwani, P.; Tekade, R.K.; Jain, N.K. Generation dependent cancer targeting potential of poly(propyleneimine) dendrimer. Biomaterials 2014, 35, 5539–5548. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Gupta, R.; Jain, S.K.; Singh, S.; Kesharwani, P. Synthesis, characterization and in vitro assessment of colloidal gold nanoparticles of Gemcitabine with natural polysaccharides for treatment of breast cancer. J. Drug Del. Sci. Technol. 2020, 56A, 101565. [Google Scholar] [CrossRef]
- Kesharwani, P.; Mishra, V.; Jain, N.K. Generation dependent hemolytic profile of folate engineered poly(propyleneimine) dendrimer. J. Drug Deliv. Sci. Technol. 2015, 28, 1–6. [Google Scholar] [CrossRef]
- Singh, A.; Vaishagya, K.; Verma, R.K.; Shukla, R. Temperature/pH-Triggered PNIPAM-Based Smart Nanogel System Loaded With Anastrozole Delivery for Application in Cancer Chemotherapy. AAPS PharmSciTech 2019, 20, 213. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Deepagan, V.G.; Divya Rani, V.V.; Menon, D.; Nair, S.V.; Jayakumar, R. Preparation, characterization, in vitro drug release and biological studies of curcumin loaded dextran sulphate-chitosan nanoparticles. Carbohydr. Polym. 2011, 84, 1158–1164. [Google Scholar] [CrossRef]
- Kesharwani, P.; Gajbhiye, V.; Tekade, R.K.; Jain, N.K. Evaluation of dendrimer safety and efficacy through cell line studies. Curr. Drug Targets 2011, 12, 1478–1497. [Google Scholar] [CrossRef]
- Li, S.Y.; Sun, R.; Wang, H.X.; Shen, S.; Liu, Y.; Du, X.J.; Zhu, Y.H.; Jun, W. Combination therapy with epigenetic-targeted and chemotherapeutic drugs delivered by nanoparticles to enhance the chemotherapy response and overcome resistance by breast cancer stem cells. J. Control. Release 2015, 205, 7–14. [Google Scholar] [CrossRef]
- Chawla, J.S.; Amiji, M.M. Cellular uptake and concentrations of tamoxifen upon administration in poly(E-caprolactone) nanoparticles. AAPS PharmSci 2003, 5, 28–34. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Husain, M.; Kotta, S.; Abdullah, S.T.; Fahmy, U.A.; Alfaleh, M.A.; Asfour, H.Z. Formulation design, statistical optimization, and in vitro evaluation of a naringenin nanoemulsion to enhance apoptotic activity in a549 lung cancer cells. Pharmaceuticals 2020, 13, 152. [Google Scholar] [CrossRef]
- Aldawsari, H.M.; Alhakamy, N.A.; Padder, R.; Husain, M.; Md, S. Preparation and characterization of chitosan coated plga nanoparticles of resveratrol: Improved stability, antioxidant and apoptotic activities in H1299 lung cancer cells. Coatings 2020, 10, 439. [Google Scholar] [CrossRef]
- Park, Y.I.; Kwon, S.-H.; Lee, G.; Motoyama, K.; Kim, M.W.; Lin, M.; Niidome, T.; Choi, J.H.; Lee, R. pH-sensitive multi-drug liposomes targeting folate receptor β for efficient treatment of non-small cell lung cancer. J. Control. Release 2021, 330, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Liang, Y.; Li, K.; Zhang, J.; Zhang, N.; Tian, B.; Han, J. Hyaluronic acid modified liposomes for targeted delivery of doxorubicin and paclitaxel to CD44 overexpressing tumor cells with improved dual-drugs synergistic effect. J. Drug Deliv. Sci. Technol. 2019, 53, 101179. [Google Scholar] [CrossRef]
- Yu, Y.; Zu, C.; He, D.; Li, Y.; Chen, Q.; Chen, Q.; Wang, H.; Wang, R.; Chaurasiya, B.; Zaro, J.L.; et al. pH-dependent reversibly activatable cell-penetrating peptides improve the antitumor effect of artemisinin-loaded liposomes. J. Colloid Interface Sci. 2020, 586, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cai, F.; Shen, X.; Su, H. A high stable pH-temperature dual-sensitive liposome for tuning anticancer drug release. Synth. Syst. Biotechnol. 2020, 5, 103–110. [Google Scholar] [CrossRef]
- Naseri, M.H.; Mahdavi, M.; Davoodi, J.; Tackallou, H.S.; Goudarzvand, M.; Neishabouri, S.H. Up regulation of Bax and down regulation of Bcl2 during 3-NC mediated apoptosis in human cancer cells. Cancer Cell Int. 2015, 15, 55. [Google Scholar] [CrossRef]
- Sharifi, S.; Barar, J.; Hejazi, M.S.; Samadi, N. Doxorubicin changes Bax /Bcl-xL ratio, caspase-8 and 9 in breast cancer cells. Adv. Pharm. Bull. 2015, 5, 351–359. [Google Scholar] [CrossRef]





| Actual Coded Value | A | B | C |
|---|---|---|---|
| −1 | 70 | 10 | 10 |
| 0 | 80 | 20 | 15 |
| 1 | 90 | 30 | 20 |
| Y1 | Lipid Concentration (%w/v) | ||
| Y2 | Cholesterol concentration (%w/v) | ||
| Y3 | Sonication Time (min) | ||
| Dependent variables | Particle size (nm) and entrapment efficiency (%) | ||
| Run | Y1 | Y2 | Y3 | Particle Size (nm) ± SD | Entrapment Efficiency (%EE) ± SD |
|---|---|---|---|---|---|
| 1 | 80 | 20 | 15 | 190 ± 2.12 | 69.8 ± 2.24 |
| 2 | 80 | 10 | 20 | 106 ± 3.23 | 76.7 ± 1.31 |
| 3 | 80 | 10 | 10 | 220 ± 4.11 | 65.1 ± 3.22 |
| 4 | 80 | 20 | 15 | 190 ± 1.14 | 70.5 ± 2.03 |
| 5 | 70 | 10 | 15 | 109 ± 2.02 | 83.1 ± 0.52 |
| 6 | 80 | 30 | 20 | 202 ± 5.17 | 66.2 ± 2.14 |
| 7 | 70 | 20 | 20 | 116 ± 2.24 | 76.2 ± 1.02 |
| 8 | 80 | 20 | 15 | 190 ± 1.45 | 71.3 ± 4.01 |
| 9 | 90 | 10 | 15 | 111 ± 3.26 | 60.3 ± 3.36 |
| 10 | 90 | 20 | 10 | 175 ± 4.02 | 56.3 ± 2.13 |
| 11 | 90 | 30 | 15 | 119 ± 2.31 | 62.4 ± 5.24 |
| 12 | 70 | 20 | 10 | 168 ± 5.12 | 74.8 ± 1.18 |
| 13 | 80 | 20 | 15 | 190 ± 6.31 | 72.8 ± 0.53 |
| 14 | 90 | 20 | 20 | 104 ± 8.01 | 69.7 ± 4.12 |
| 15 | 70 | 30 | 15 | 107 ± 1.17 | 85.2 ± 0.51 |
| 16 | 80 | 20 | 15 | 192 ± 2.38 | 73.1 ± 2.18 |
| 17 | 80 | 30 | 10 | 235 ± 3.54 | 75.9 ± 1.27 |
| T1 | 70 | 30 | 13 | 115 ± 1.41 | 80.45 ± 2.46 |
| T2 | 70 | 20 | 10 | 124 ± 2.84 | 79 ± 1.13 |
| Response | Runs | |||
|---|---|---|---|---|
| T1 | T2 | Optimized Formulation | ||
| Particle size (nm) | Observed | 115 | 124 | 107 |
| Predicted | 130 | 145 | 110 | |
| Residuals | 7 | 8 | −4.82 | |
| EE (%) | Observed | 80.45 | 79 | 85.2 |
| Predicted | 75.2 | 72.8 | 81.32 | |
| Batch No. | Excipients Drug Ratio | Particle Size (nm) ± SD | PDI ± SD | %Entrapment Efficiency ± SD | %Drug Loading ± SD |
|---|---|---|---|---|---|
| B1 | 60:40:10 | 119 ± 5 | 0.07 ± 0.004 | 60.3% ± 2% | 5.48% ± 1% |
| B2 | 70:30:10 | 107 ± 3 | 0.035 ± 0.002 | 85.2% ± 1% | 5.82% ± 2% |
| B3 | 75:25:10 | 109 ± 6 | 0.082 ± 0.005 | 43.2% ± 3% | 3.92% ± 4% |
| B4 | 85:15:10 | 190 ± 8 | 0.163 ± 0.004 | 29% ± 4% | 2.64% ± 3% |
| B5 | 90:10:10 | 111 ± 2 | 0.049 ± 0.003 | 60% ± 1% | 5.45% ± 1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kesharwani, P.; Md, S.; Alhakamy, N.A.; Hosny, K.M.; Haque, A. QbD Enabled Azacitidine Loaded Liposomal Nanoformulation and Its In Vitro Evaluation. Polymers 2021, 13, 250. https://doi.org/10.3390/polym13020250
Kesharwani P, Md S, Alhakamy NA, Hosny KM, Haque A. QbD Enabled Azacitidine Loaded Liposomal Nanoformulation and Its In Vitro Evaluation. Polymers. 2021; 13(2):250. https://doi.org/10.3390/polym13020250
Chicago/Turabian StyleKesharwani, Prashant, Shadab Md, Nabil A. Alhakamy, Khaled M. Hosny, and Anzarul Haque. 2021. "QbD Enabled Azacitidine Loaded Liposomal Nanoformulation and Its In Vitro Evaluation" Polymers 13, no. 2: 250. https://doi.org/10.3390/polym13020250
APA StyleKesharwani, P., Md, S., Alhakamy, N. A., Hosny, K. M., & Haque, A. (2021). QbD Enabled Azacitidine Loaded Liposomal Nanoformulation and Its In Vitro Evaluation. Polymers, 13(2), 250. https://doi.org/10.3390/polym13020250






