Bacteria Adhesion of Textiles Influenced by Wettability and Pore Characteristics of Fibrous Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film and Electrospun Web Preparation
2.3. Surface Modification
2.4. Characterization of Materials
2.5. Quantification of Surface-Adhered Bacteria
3. Results and Discussion
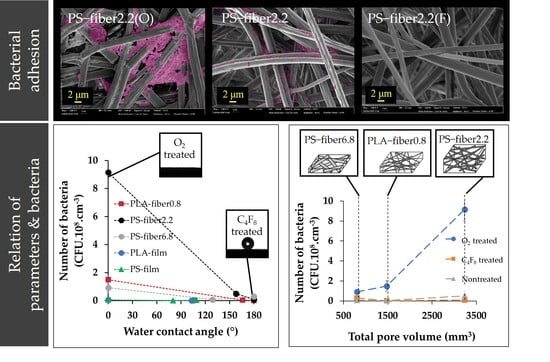
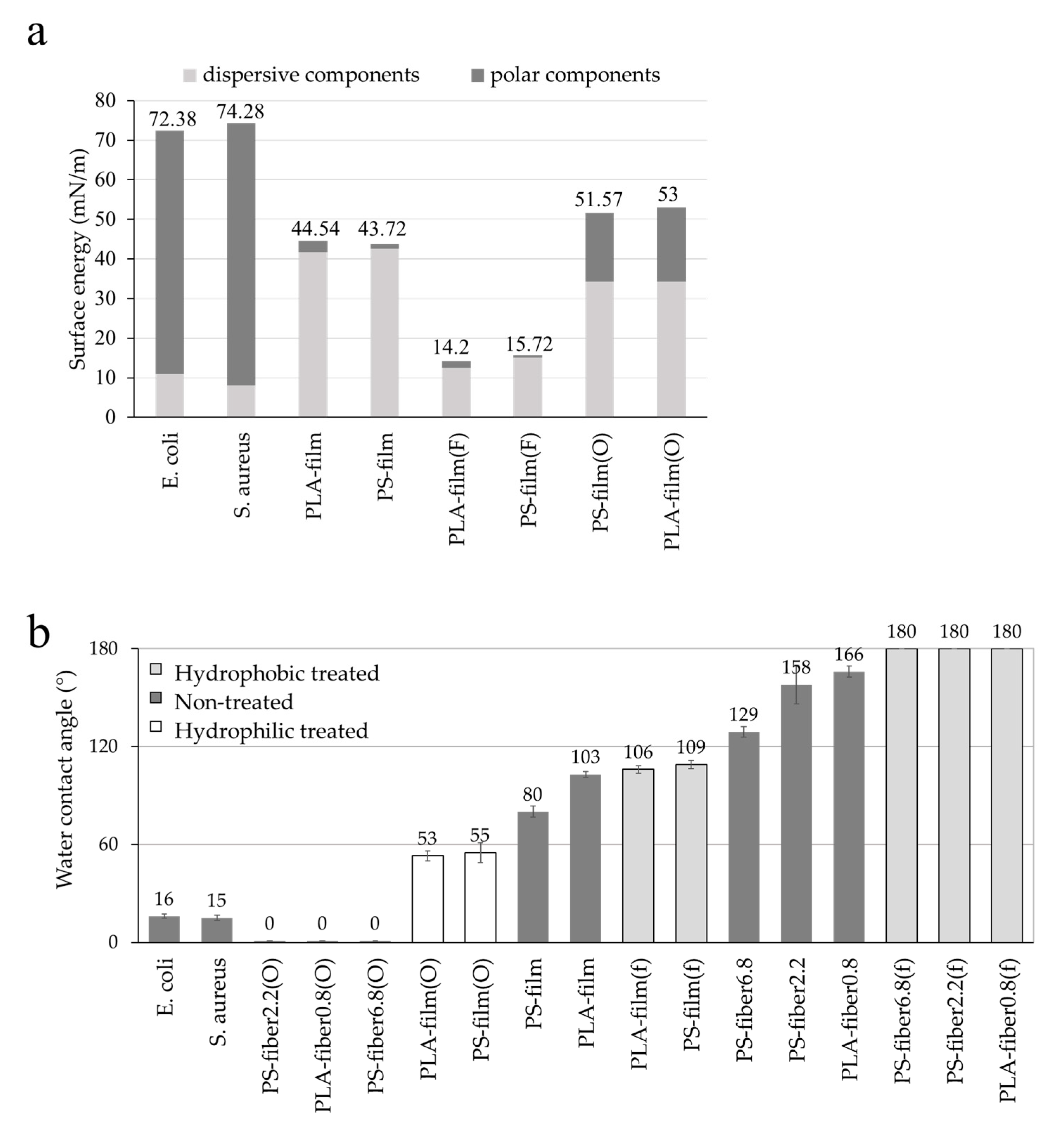
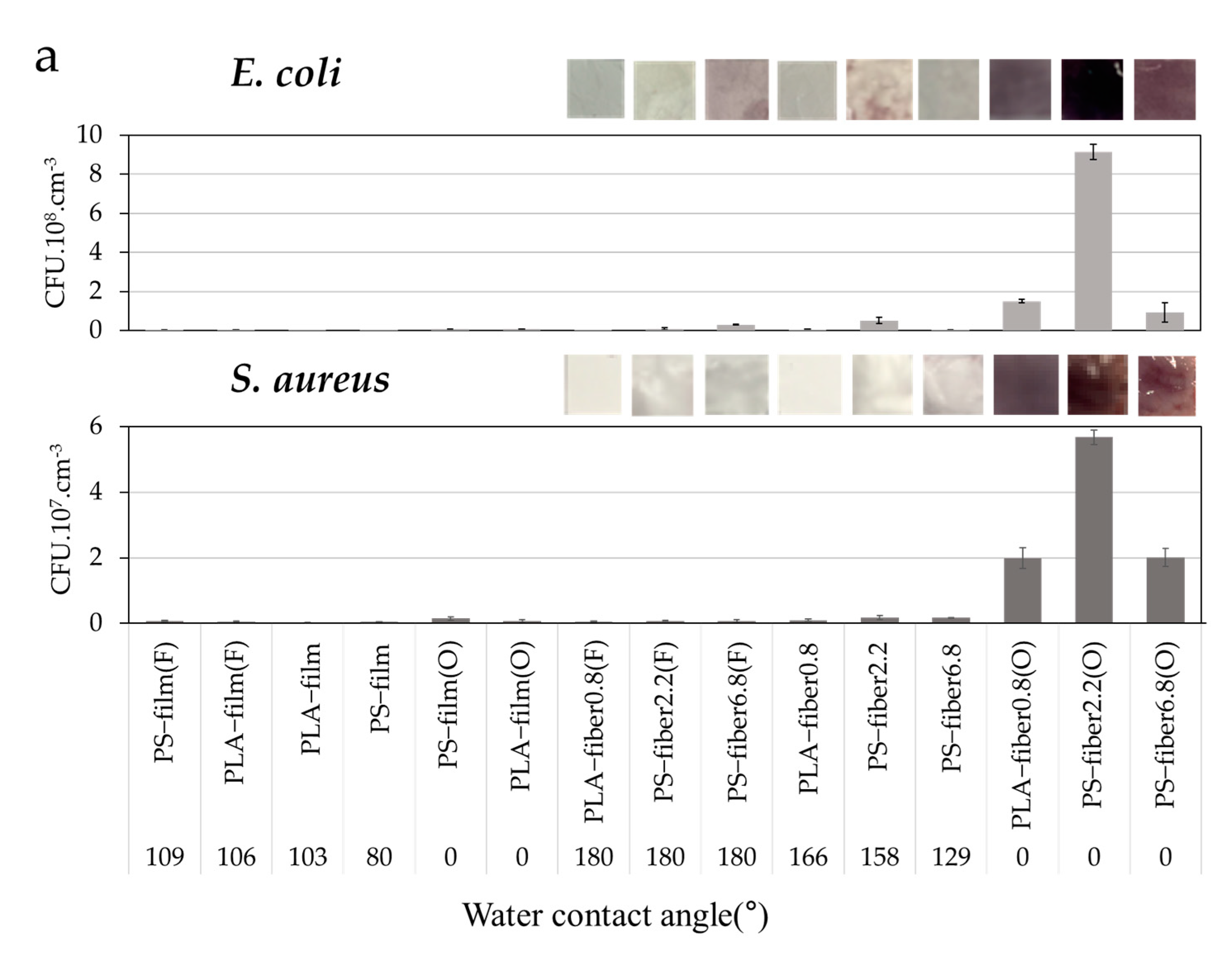
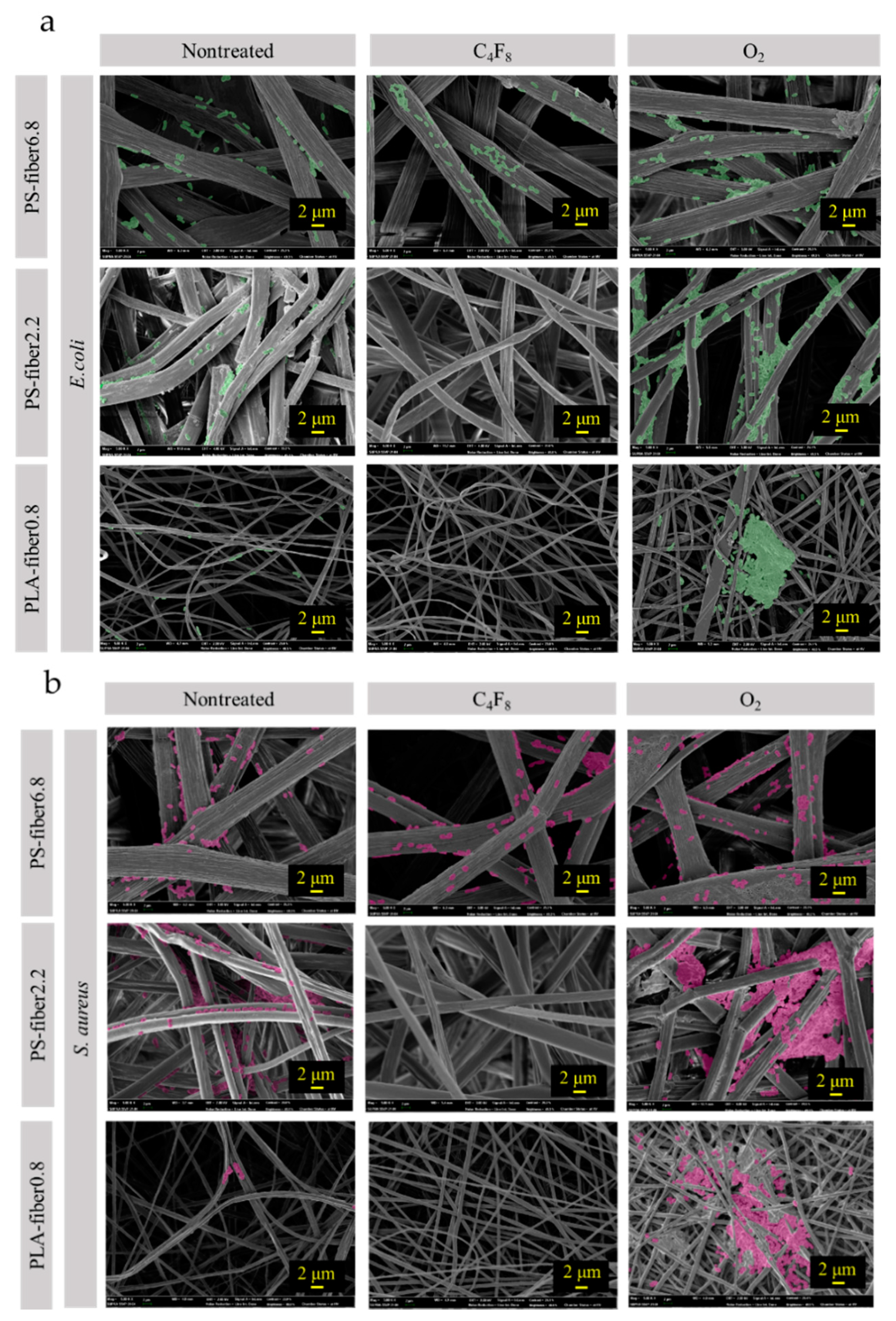
3.1. Effect of Wettability on Cell Adhesion
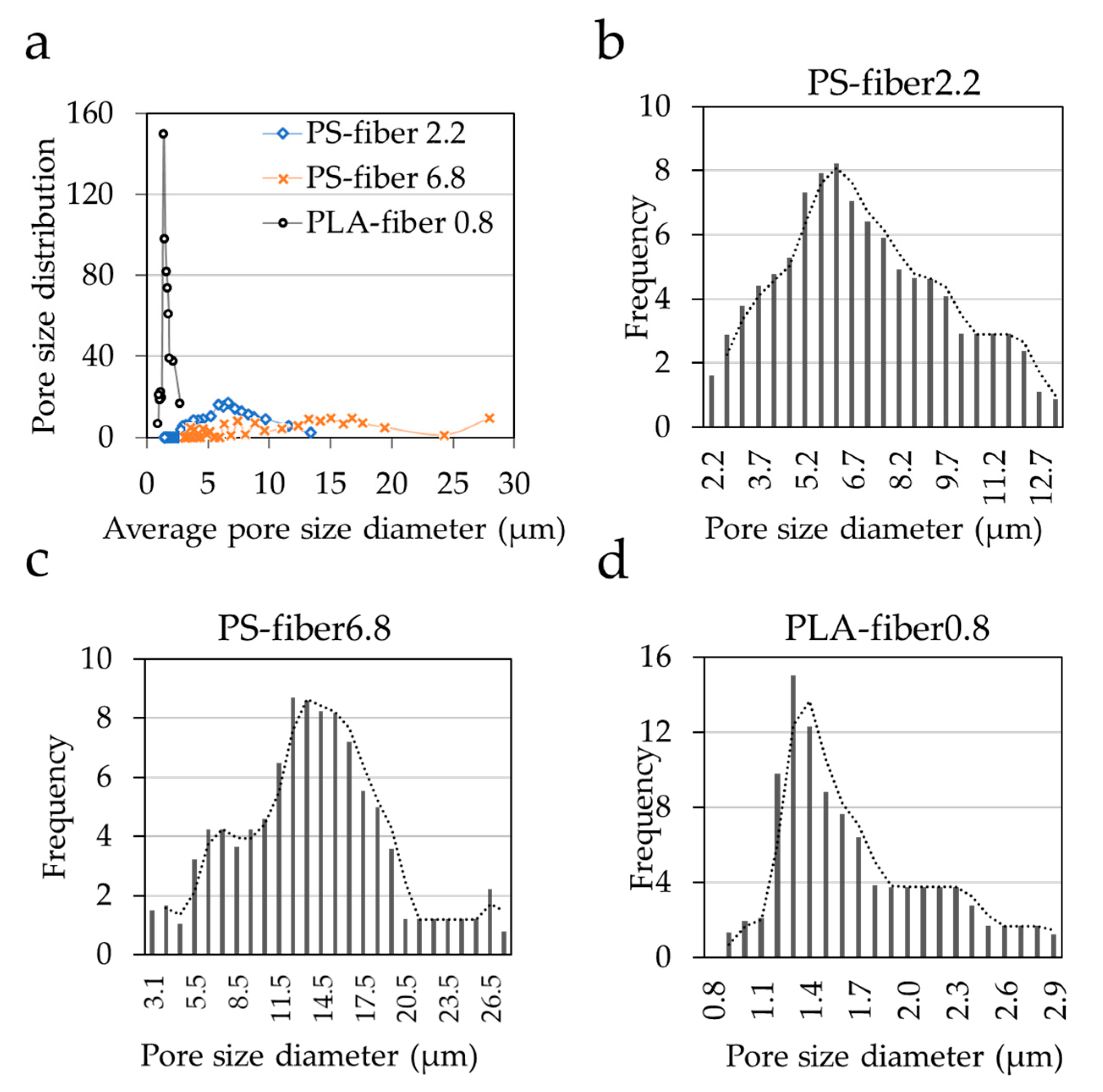
3.2. Effect of Morphology and Pore Characteristics on Bacteria Adhesion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Landry, K.S.; Morey, J.M.; Bharat, B.; Haney, N.M.; Panesar, S.S. Biofilms—Impacts on human health and its relevance to space travel. Microorganisms 2020, 8, 998. [Google Scholar] [CrossRef] [PubMed]
- Karim, N.; Afroj, S.; Lloyd, K.; Oaten, L.C.; Andreeva, D.V.; Carr, C.; Farmery, A.D.; Kim, I.D.; Novoselov, K.S. Sustainable personal protective clothing for healthcare applications: A Rreview. ACS Nano 2020, 14, 12313–12340. [Google Scholar] [CrossRef] [PubMed]
- Eid, B.M.; Ibrahim, N.A. Recent developments in sustainable finishing of cellulosic textiles employing biotechnology. J. Clean. Prod. 2020, 284, 124701. [Google Scholar] [CrossRef]
- Galante, A.J.; Haghanifar, S.; Romanowski, E.G.; Shanks, R.M.Q.; Leu, P.W. Superhemophobic and antivirofouling coating for mechanically durable and wash-stable medical textiles. ACS Appl. Mater. Interfaces 2020, 12, 22120–22128. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.K.; Pham, V.T.H.; Truong, V.K.; Sbarski, I.; Wang, J.; Balčytis, A.; Juodkazis, S.; Mainwaring, D.E.; Crawford, R.J.; Ivanova, E.P. Role of topological scale in the differential fouling of Pseudomonas aeruginosa and Staphylococcus aureus bacterial cells on wrinkled gold-coated polystyrene surfaces. Nanoscale 2018, 10, 5089–5096. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.K.; Lapovok, R.; Estrin, Y.S.; Rundell, S.; Wang, J.Y.; Fluke, C.J.; Crawford, R.J.; Ivanova, E.P. The influence of nano-scale surface roughness on bacterial adhesion to ultrafine-grained titanium. Biomaterials 2010, 31, 3674–3683. [Google Scholar] [CrossRef]
- Song, L.; Sun, L.; Zhao, J.; Wang, X.; Yin, J.; Luan, S.; Ming, W. Synergistic superhydrophobic and photodynamic cotton textiles with remarkable antibacterial activities. ACS Appl. Bio Mater. 2019, 2, 2756–2765. [Google Scholar] [CrossRef]
- Rodriguez-Palacios, A.; Cominelli, F.; Basson, A.R.; Pizarro, T.T.; Ilic, S. Textile masks and surface covers—A spray simulation method and a “universal droplet reduction model” against respiratory pandemics. Front. Med. 2020, 7, 260. [Google Scholar] [CrossRef]
- Elgadir, M.A.; Uddin, M.S.; Ferdosh, S.; Adam, A.; Chowdhury, A.J.K.; Sarker, M.Z.I. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J. Food Drug Anal. 2015, 23, 619–629. [Google Scholar] [CrossRef]
- Gokce, Y.; Aktas, Z.; Capar, G.; Kutlu, E.; Anis, P. Improved antibacterial property of cotton fabrics coated with waste sericin/silver nanocomposite. Mater. Chem. Phys. 2020, 254, 123508. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Liang, X.; Vorstius, J.; Keatch, R.; Corner, G.; Nabi, G.; Davidson, F.; Gadd, G.M.; Zhao, Q. Enhanced antibacterial and antiadhesive activities of silver-PTFE nanocomposite coating for urinary catheters. ACS Biomater. Sci. Eng. 2019, 5, 2804–2814. [Google Scholar] [CrossRef] [PubMed]
- Gadkari, R.R.; Ali, S.W.; Joshi, M.; Rajendran, S.; Das, A.; Alagirusamy, R. Leveraging antibacterial efficacy of silver loaded chitosan nanoparticles on layer-by-layer self-assembled coated cotton fabric. Int. J. Biol. Macromol. 2020, 162, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Swar, S.; Máková, V.; Horáková, J.; Kejzlar, P.; Parma, P.; Stibor, I. A comparative study between chemically modified and copper nanoparticle immobilized Nylon 6 films to explore their efficiency in fighting against two types of pathogenic bacteria. Eur. Polym. J. 2020, 122, 109392. [Google Scholar] [CrossRef]
- Prorokova, N.; Kumeeva, T.; Kholodkov, I. Formation of coatings based on titanium dioxide nanosolson polyester fibre materials. Eur. Coat. J. 2020, 10, 82. [Google Scholar] [CrossRef]
- Chen, C.C.; Wang, C.C. Crosslinking of cotton cellulose with succinic acid in the presence of titanium dioxide nano-catalyst under UV irradiation. J. Sol-Gel Sci. Technol. 2006, 40, 31–38. [Google Scholar] [CrossRef]
- Prorokova, N.P.; Kumeeva, T.Y.; Agafonov, A.V.; Ivanov, V.K. Modification of polyester fabrics with nanosized titanium dioxide to impart photoactivity. Inorg. Mater. Appl. Res. 2017, 8, 696–703. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, M.; He, Y.; Zhang, M.; Shen, R.; Zhang, Y.; Wang, M.; Wu, G. Fabrication and potential applications of highly durable superhydrophobic polyethylene terephthalate fabrics produced by in-situ zinc oxide (ZnO) nanowires deposition and polydimethylsiloxane (PDMS) packaging. Polymers 2020, 12, 2333. [Google Scholar] [CrossRef]
- Hatamie, A.; Khan, A.; Golabi, M.; Turner, A.P.F.; Beni, V.; Mak, W.C.; Sadollahkhani, A.; Alnoor, H.; Zargar, B.; Bano, S. Zinc oxide nanostructure-modified textile and its application to biosensing, photocatalysis, and as antibacterial material. Langmuir 2015, 31, 10913–10921. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, M.; Pang, L.; Yang, C.; Zhang, Y.; Hu, J.; Wu, G. Fabrication of highly durable polysiloxane-zinc oxide (ZnO) coated polyethylene terephthalate (PET) fabric with improved ultraviolet resistance, hydrophobicity, and thermal resistance. J. Colloid Interface Sci. 2019, 537, 91–100. [Google Scholar] [CrossRef]
- Koubali, H.; El Louali, M.; Zahir, H.; Soufiani, S.; Mabrouki, M.; Latrache, H. Physicochemical characterization of glass and polyethylene surfaces treated with different surfactants and their effects on bacterial adhesion. Int. J. Adhes. Adhes. 2021, 104, 102754. [Google Scholar] [CrossRef]
- Lavanya, K.; Kalaimurugan, D.; Shivakumar, M.S.; Venkatesan, S. Gelatin stabilized silver nanoparticle provides higher antimicrobial efficiency as against chemically synthesized silver nanoparticle. J. Clust. Sci. 2020, 31, 265–275. [Google Scholar] [CrossRef]
- Marchioni, M.; Veronesi, G.; Worms, I.; Ling, W.L.; Gallon, T.; Leonard, D.; Gateau, C.; Chevallet, M.; Jouneau, P.H.; Carlini, L.; et al. Safer-by-design biocides made of tri-thiol bridged silver nanoparticle assemblies. Nanoscale Horiz. 2020, 5, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.C.; Fang, J.; Borca-Tasciuc, D.A.; Worobo, R.W.; Moraru, C.I. Effect of micro-and nanoscale topography on the adhesion of bacterial cells to solid surfaces. Appl. Environ. Microbiol. 2013, 79, 2703–2712. [Google Scholar] [CrossRef] [PubMed]
- Fiedot-Toboła, M.; Ciesielska, M.; Maliszewska, I.; Rac-Rumijowska, O.; Suchorska-Woźniak, P.; Teterycz, H.; Bryjak, M. Deposition of zinc oxide on different polymer textiles and their antibacterial properties. Materials 2018, 11, 707. [Google Scholar] [CrossRef]
- BinAhmed, S.; Hasane, A.; Wang, Z.; Mansurov, A.; Castrillón, S.R.V. Bacterial adhesion to ultrafiltration membranes: Role of hydrophilicity, natural organic matter, and cell-surface macromolecules. Environ. Sci. Technol. 2018, 52, 162–172. [Google Scholar] [CrossRef]
- Oh, J.K.; Yegin, Y.; Yang, F.; Zhang, M.; Li, J.; Huang, S.; Verkhoturov, S.V.; Schweikert, E.A.; Perez-Lewis, K.; Scholar, E.A.; et al. The influence of surface chemistry on the kinetics and thermodynamics of bacterial adhesion. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef]
- Maikranz, E.; Spengler, C.; Thewes, N.; Thewes, A.; Nolle, F.; Jung, P.; Bischoff, M.; Santen, L.; Jacobs, K. Different binding mechanisms of Staphylococcus aureus to hydrophobic and hydrophilic surfaces. Nanoscale 2020, 12, 19267–19275. [Google Scholar] [CrossRef]
- Salerno, M.B.; Logan, B.E.; Velegol, D. Importance of molecular details in predicting bacterial adhesion to hydrophobic surfaces. Langmuir 2004, 20, 10625–10629. [Google Scholar] [CrossRef]
- Feng, G.; Cheng, Y.; Wang, S.Y.; Hsu, L.C.; Feliz, Y.; Borca-Tasciuc, D.A.; Worobo, R.W.; Moraru, C.I. Alumina surfaces with nanoscale topography reduce attachment and biofilm formation by Escherichia coli and Listeria spp. Biofouling 2014, 30, 1253–1268. [Google Scholar] [CrossRef]
- Bajpai, V.; Dey, A.; Ghosh, S.; Bajpai, S.; Jha, M.K. Quantification of bacterial adherence on different textile fabrics. Int. Biodeter. Biodegrad. 2011, 65, 1169–1174. [Google Scholar] [CrossRef]
- Cesare, F.D.; Mattia, E.D.; Zussman, E.; Macagnano, A. A study on the dependence of bacteria adhesion on the polymer nanofibre diameter. Environ. Sci. Nano 2019, 6, 778–797. [Google Scholar] [CrossRef]
- Alam, A.K.M.M.; Ewaldz, E.; Xiang, C.; Qu, W.; Bai, X. Tunable wettability of biodegradable multilayer sandwich-structured electrospun nanofibrous membranes. Polymers 2020, 12, 2092. [Google Scholar] [CrossRef] [PubMed]
- Varshney, S.; Sain, A.; Gupta, D.; Sharma, S. Factors affecting bacterial adhesion on selected textile fibres. Indian J. Microbiol. 2020, 1–7. [Google Scholar] [CrossRef]
- Schmidt-Emrich, S.; Stiefel, P.; Rupper, P.; Katzenmeier, H.; Amberg, C.; Maniura-Weber, K.; Ren, Q. Rapid assay to assess bacterial adhesion on textiles. Materials 2016, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; An, J.; Na, H.; Kim, J. Surface energy of filtration media influencing the filtration performance against solid particles, oily aerosol, and bacterial Aerosol. Polymers 2019, 11, 935. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.; Kim, S.; Kim, J. Facile functionalization via plasma-enhanced chemical vapor deposition for the effective filtration of oily aerosol. Polymers 2019, 11, 1490. [Google Scholar] [CrossRef]
- Song, K.; Lee, J.; Choi, S.O.; Kim, J. Interaction of surface energy components between solid and liquid on wettability, and its application to textile anti-wetting finish. Polymers 2019, 11, 498. [Google Scholar] [CrossRef]
- Hemmatian, T.; Kim, J. Quantification methods for textile-adhered bacteria: Extraction, colorimetric, and microscopic analysis. Polymers 2019, 11, 1666. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Wenzel, R.N. Surface roughness and contact angle. J. Phys. Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Yuan, Y.; Choi, S.O.; Kim, J. Analysis of contact area between water and irregular fibrous surface for prediction of wettability. RSC Adv. 2016, 6, 73313–73322. [Google Scholar] [CrossRef]
- Castellanos, T.; Ascencio, F.; Bashan, Y. Cell-surface hydrophobicity and cell-surface charge of Azospirillum spp. FEMS Microbiol. Ecol. 1997, 24, 159–172. [Google Scholar] [CrossRef]
- Zita, A.; Hermansson, M. Determination of bacterial cell surface hydrophobicity of single cells in cultures and in wastewater in situ. FEMS Microbiol. Lett. 1997, 152, 299–306. [Google Scholar] [CrossRef]
- Bruinsma, G.M.; van der Mei, H.C.; Busscher, H.J. Bacterial adhesion to surface hydrophilic and hydrophobic contact lenses. Biomaterials 2001, 22, 3217–3224. [Google Scholar] [CrossRef]
- Zita, A.; Hermansson, M. Effects of bacterial cell surface structures and hydrophobicity on attachment to activated sludge flocs. Appl. Environ. Microbiol. 1997, 63, 1168–1170. [Google Scholar] [CrossRef]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Role of adhesion forces in mechanosensitive channel gating in Staphylococcus aureus adhering to surfaces. NPJ Biofilms Microbiomes 2020, 6, 31. [Google Scholar] [CrossRef]
- Thewes, N.; Loskill, P.; Jung, P.; Peisker, H.; Bischoff, M.; Herrmann, M.; Jacobs, K. Hydrophobic interaction governs unspecific adhesion of staphylococci: A single cell force spectroscopy study. Beilstein J. Nanotechnol. 2014, 5, 1501–1512. [Google Scholar] [CrossRef]
- Bendinger, B.; Rijnaarts, H.H.M.; Altendorf, K.; Zehnder, A.J.B. Physicochemical cell surface and adhesive properties of coryneform bacteria related to the presence and chain length of mycolic acids. Appl. Environ. Microbiol. 1993, 59, 3973–3977. [Google Scholar] [CrossRef]
- Das, T.; Sharma, P.K.; Krom, B.P.; van der Mei, H.C.; Busscher, H.J. Role of eDNA on the adhesion forces between Streptococcus mutans and substratum surfaces: Influence of ionic strength and substratum hydrophobicity. Langmuir 2011, 27, 10113–10118. [Google Scholar] [CrossRef] [PubMed]
- Hochbaum, A.I.; Aizenberg, J. Bacteria pattern spontaneously on periodic nanostructure arrays. Nano Lett. 2010, 10, 3717–3721. [Google Scholar] [CrossRef] [PubMed]
- Decker, J.T.; Sheats, J.T.; Brennan, A.B. Engineered antifouling microtopographies: Surface pattern effects on cell distribution. Langmuir 2014, 30, 15212–15218. [Google Scholar] [CrossRef] [PubMed]
- Erramilli, S.; Genzer, J. Influence of surface topography attributes on settlement and adhesion of natural and synthetic species. Soft Matter 2019, 15, 4045–4067. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun nanofibers: New concepts, materials, and applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef] [PubMed]
- Kargar, M.; Wang, J.; Nain, A.S.; Behkam, B. Controlling bacterial adhesion to surfaces using topographical cues: A study of the interaction of Pseudomonas aeruginosa with nanofiber-textured surfaces. Soft Matter 2012, 8, 10254–10259. [Google Scholar] [CrossRef]

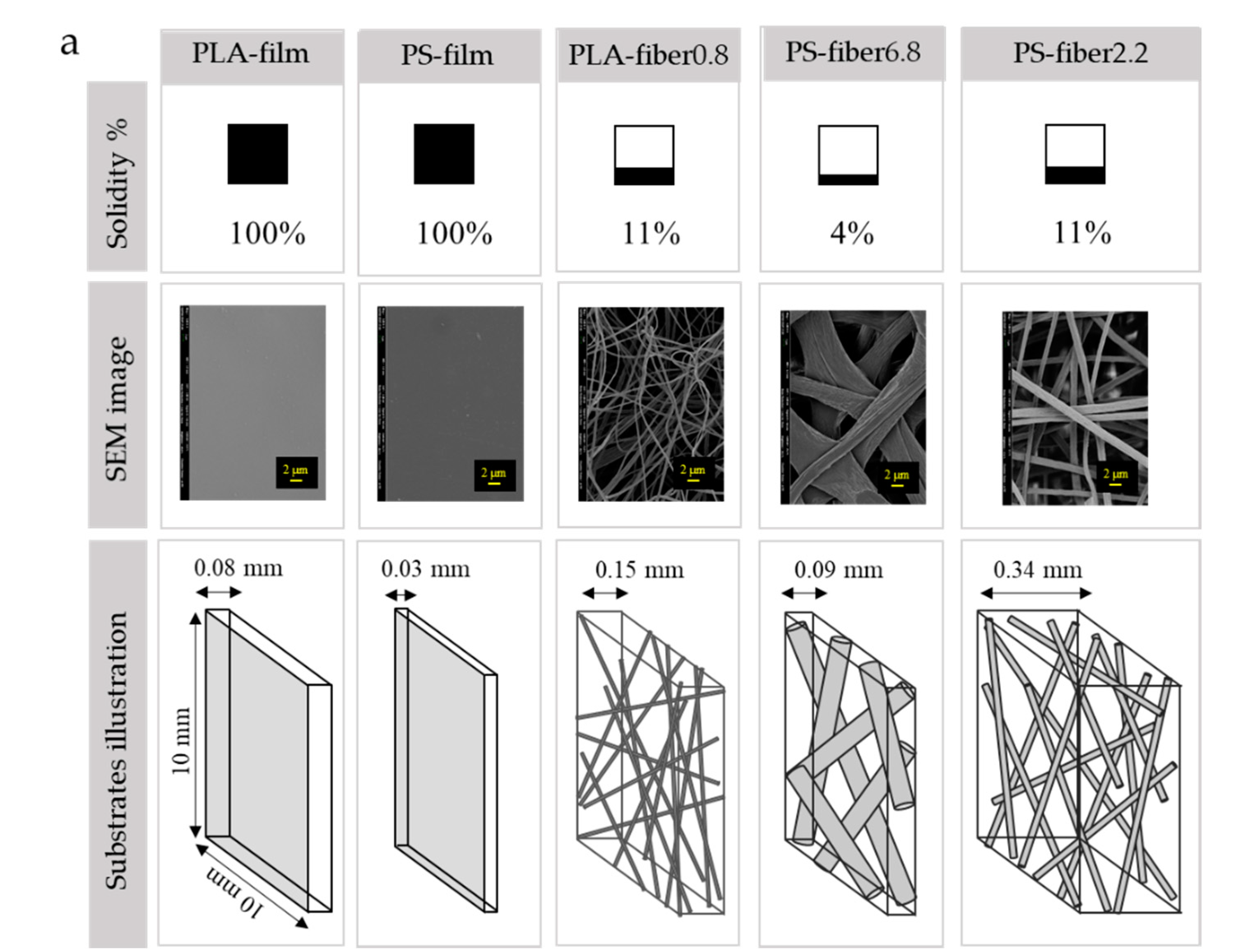

| Code | Description | Thickness (mm) | Basis Weight (g/m2) | Solidity (Unitless) | Porosity (%) | Mean Fiber Diameter (μm) | Volume (mm3) | Total Pore Volume (mm3) |
|---|---|---|---|---|---|---|---|---|
| PLA-film | PLA film | 0.08 (±0.01) | 72.6 (±7.7) | 1.00 | 0 | N/A | 8 | 0 |
| PLA-film(O) | O2 plasma-treated | |||||||
| PLA-film(F) | C4F8 plasma-treated | |||||||
| PLA-fiber0.8 | electrospun PLA fiber | 0.15 (±0.02) | 19.6 (±4.0) | 0.11 | 89 | 0.8 (±0.1) | 15 | 1470 |
| PLA-fiber 0.8(O) | O2 plasma-treated | |||||||
| PLA-fiber 0.8(F) | C4F8 plasma-treated | |||||||
| PS-film | PS film | 0.03 (±0.01) | 33.4 (±2.1) | 1.00 | 0 | N/A | 13 | 0 |
| PS-film(O) | O2 plasma-treated | |||||||
| PS-film(F) | C4F8 plasma-treated | |||||||
| PS-fiber6.8 | Electrospun PS fiber | 0.09 (±0.00) | 11.0 (±1.4) | 0.11 | 89 | 6.8 (±1.9) | 9 | 801 |
| PS-fiber6.8(O) | O2 plasma-treated | |||||||
| PS-fiber6.8(F) | C4F8 plasma-treated | |||||||
| PS-fiber2.2 | Electrospun PS fiber | 0.34 (±0.05) | 17.0 (±5.5) | 0.04 | 95 | 2.2 (±0.3) | 34 | 3230 |
| PS-fiber2.2(O) | O2 plasma-treated | |||||||
| PS-fiber2.2(F) | C4F8 plasma-treated |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemmatian, T.; Lee, H.; Kim, J. Bacteria Adhesion of Textiles Influenced by Wettability and Pore Characteristics of Fibrous Substrates. Polymers 2021, 13, 223. https://doi.org/10.3390/polym13020223
Hemmatian T, Lee H, Kim J. Bacteria Adhesion of Textiles Influenced by Wettability and Pore Characteristics of Fibrous Substrates. Polymers. 2021; 13(2):223. https://doi.org/10.3390/polym13020223
Chicago/Turabian StyleHemmatian, Tahmineh, Halim Lee, and Jooyoun Kim. 2021. "Bacteria Adhesion of Textiles Influenced by Wettability and Pore Characteristics of Fibrous Substrates" Polymers 13, no. 2: 223. https://doi.org/10.3390/polym13020223
APA StyleHemmatian, T., Lee, H., & Kim, J. (2021). Bacteria Adhesion of Textiles Influenced by Wettability and Pore Characteristics of Fibrous Substrates. Polymers, 13(2), 223. https://doi.org/10.3390/polym13020223