New Functionalized Ionic Liquids Based on POSS for the Detection of Fe3+ Ion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization and Measurements
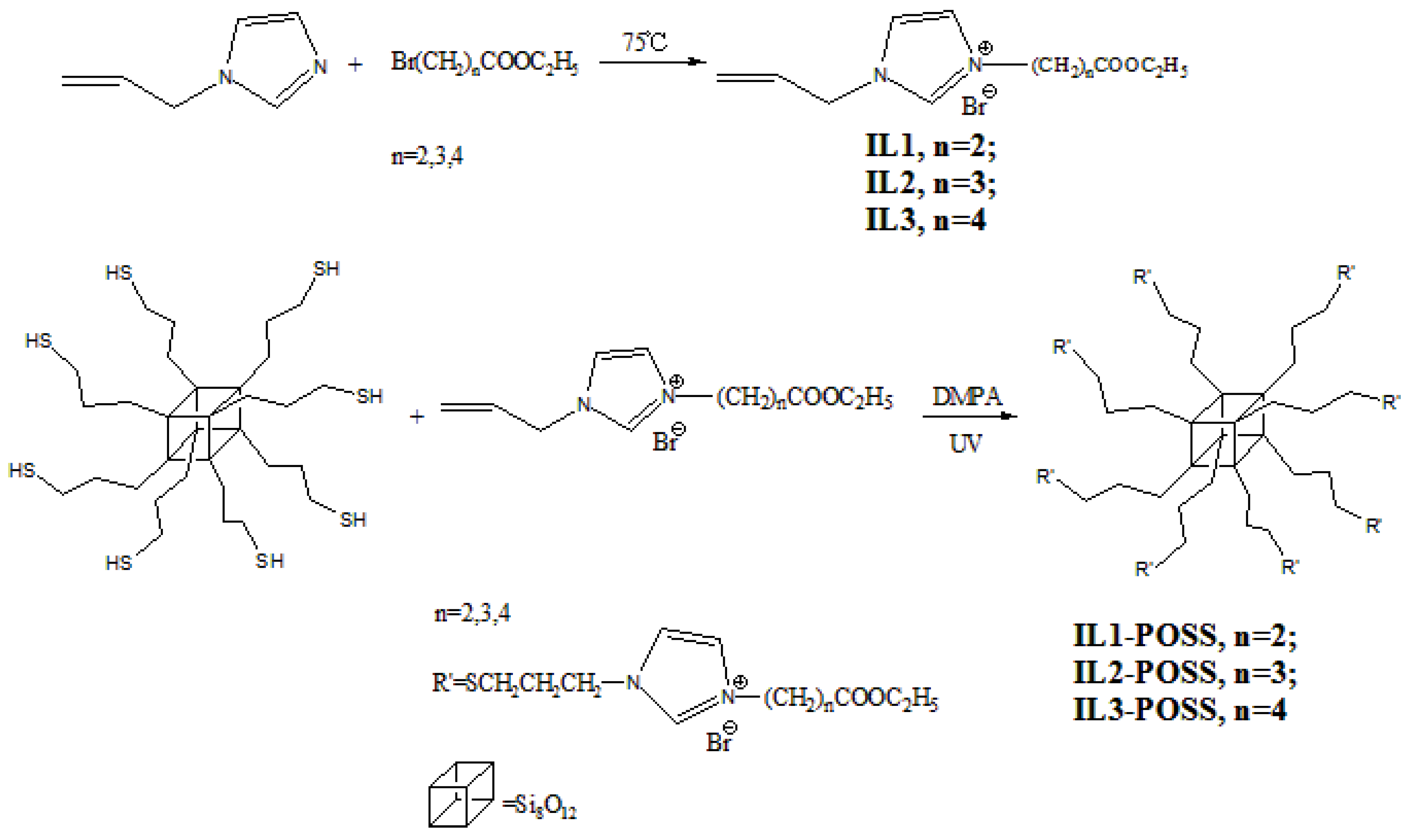
2.3. General Procedures to Synthesize ILs-POSS
3. Results
3.1. Synthesis and Characterization
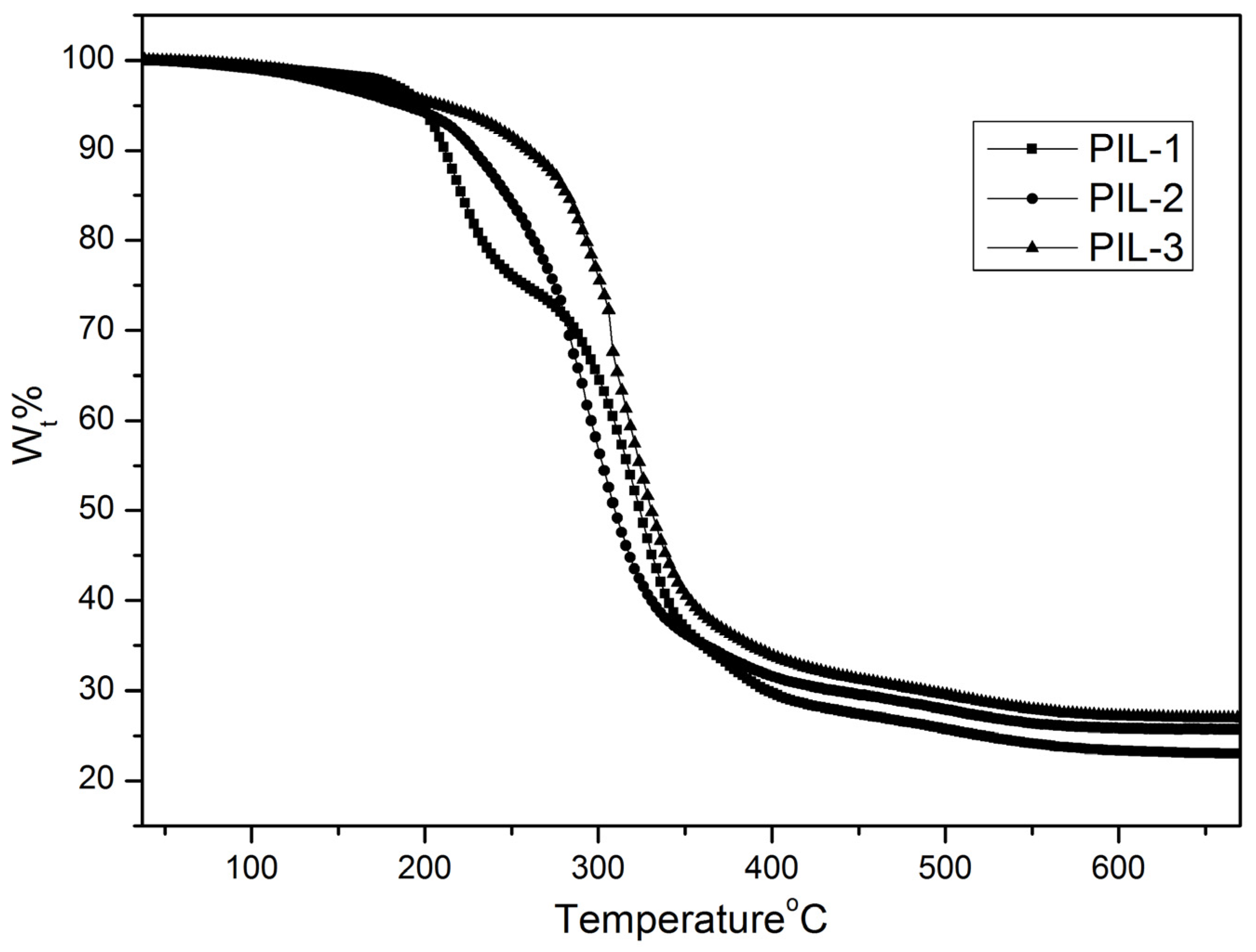
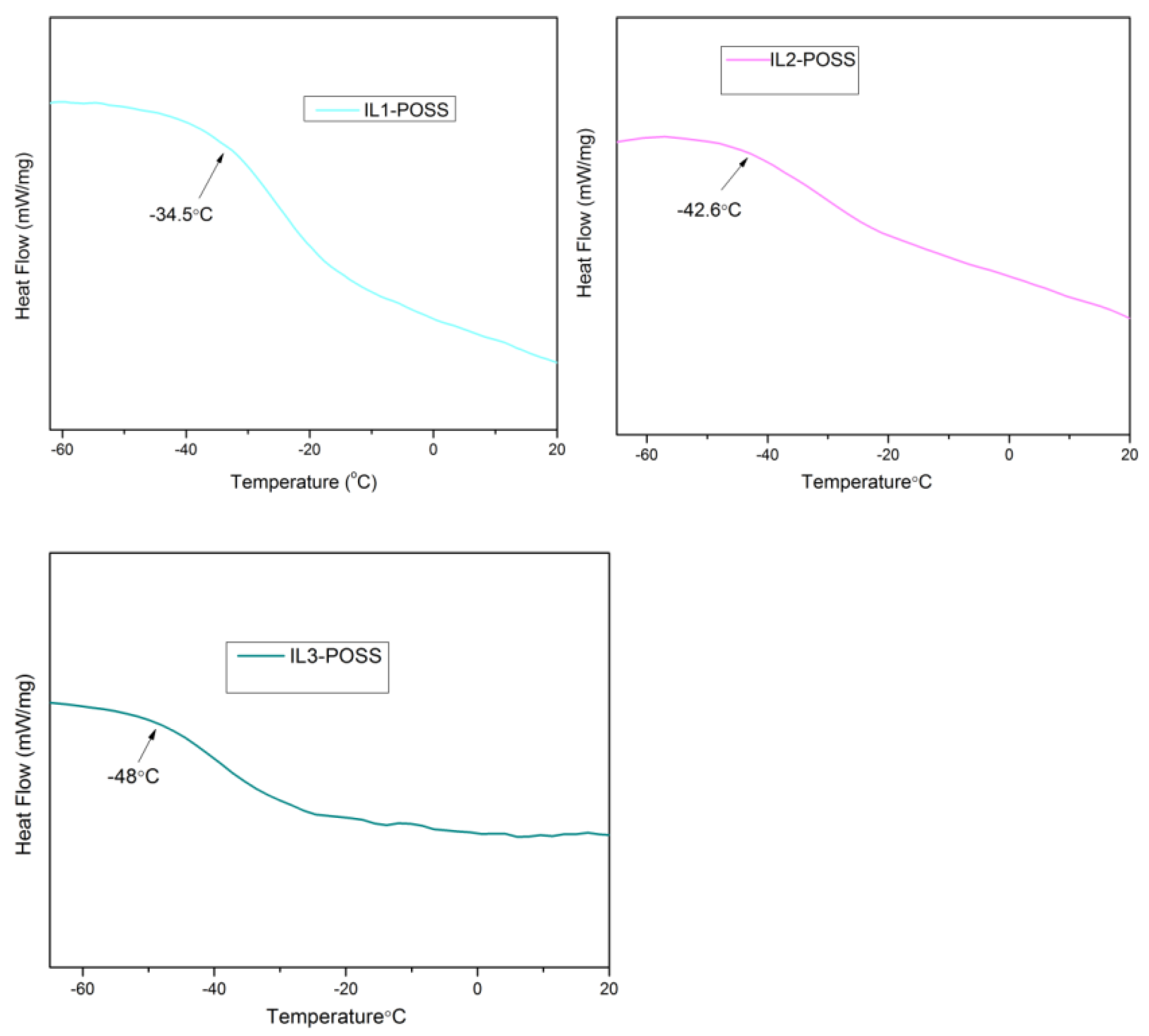
3.2. Thermal Properties
3.3. Self- Assembly Behaviors
3.4. Optical Properties
4. Discussion
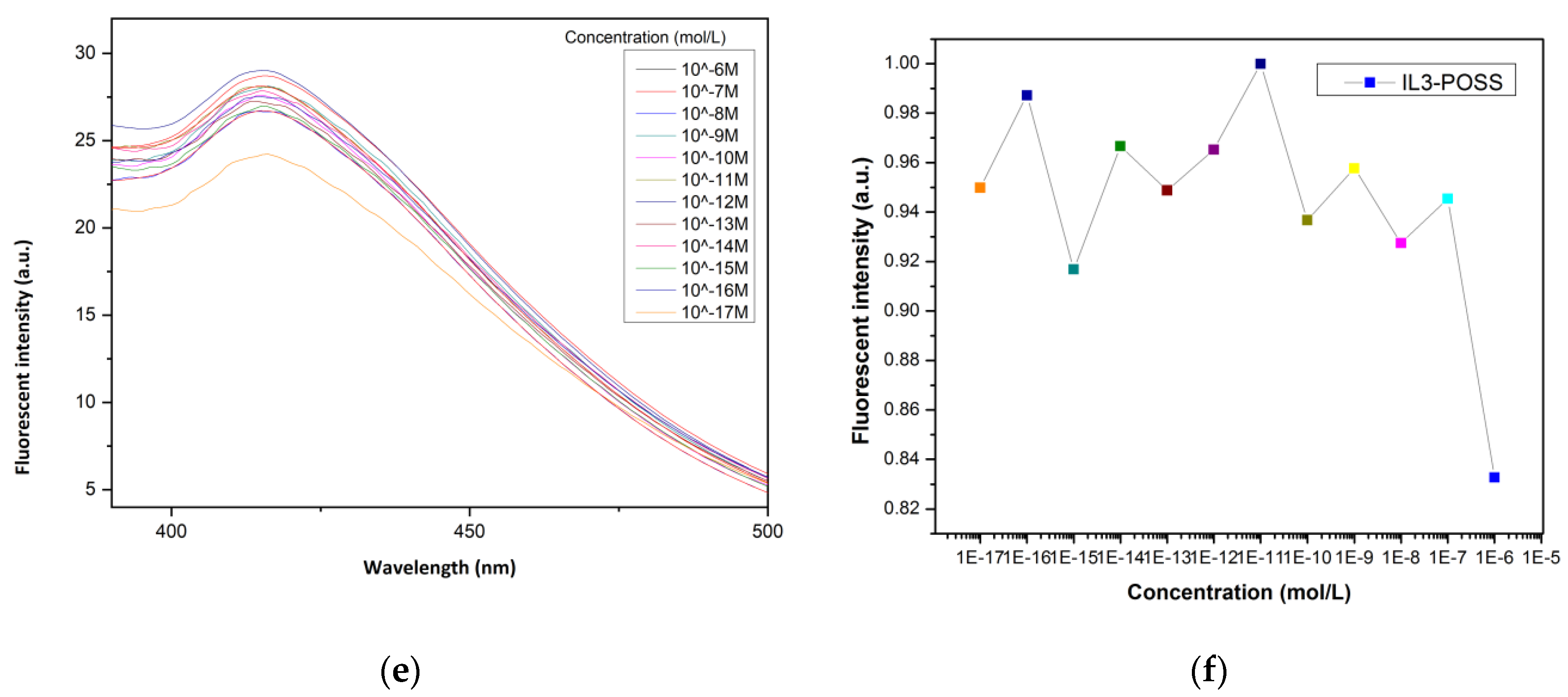
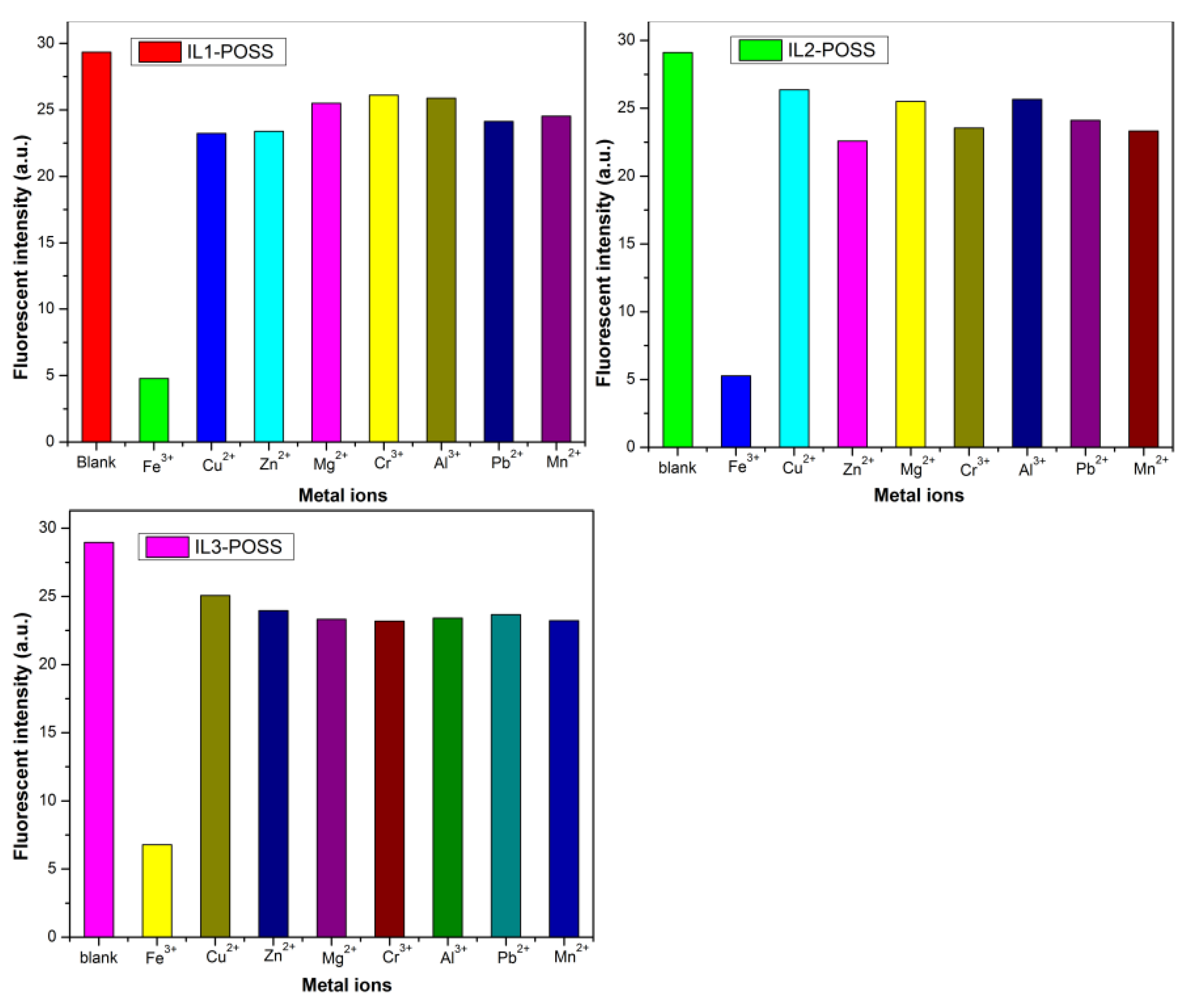
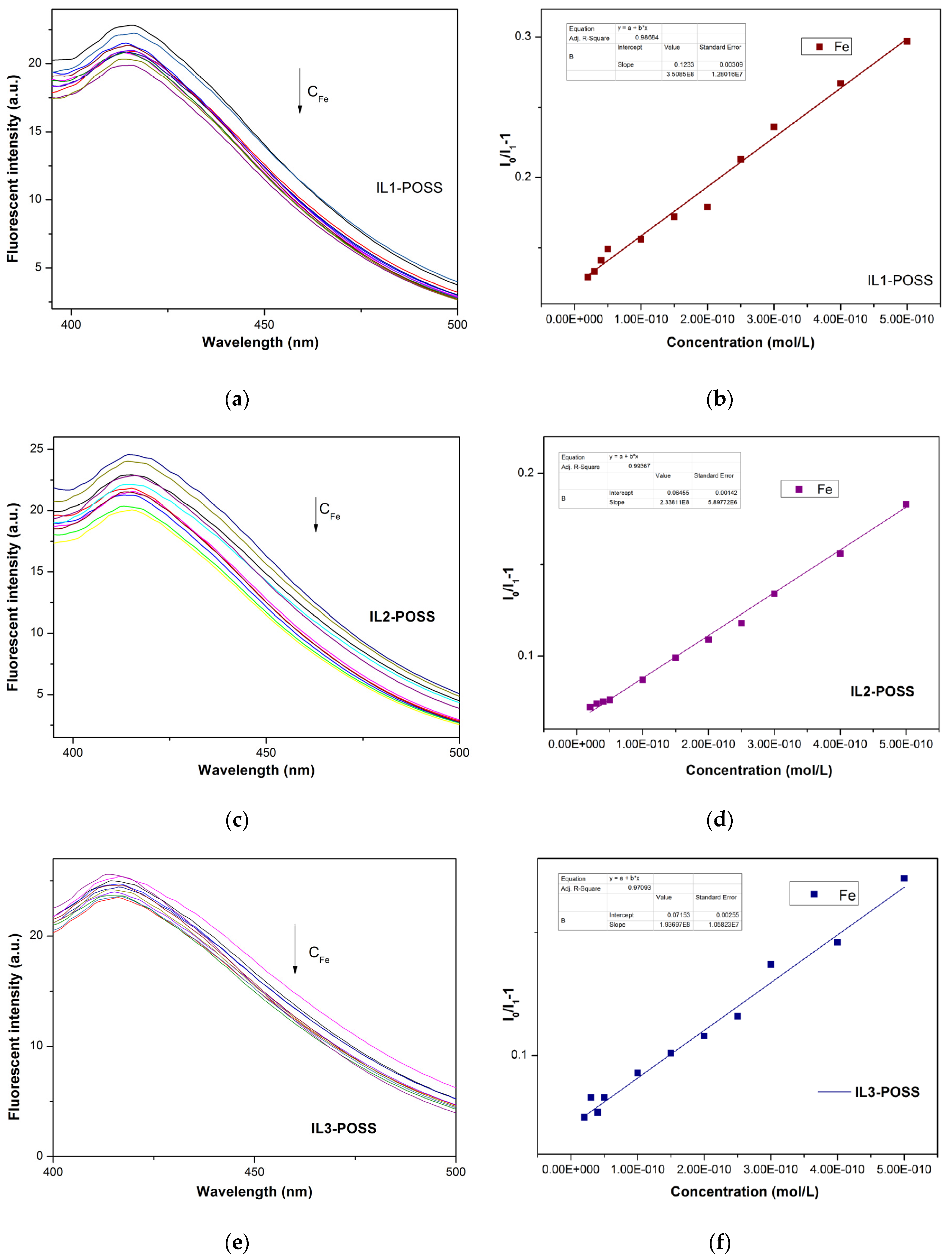
4.1. ILs-POSS in the Detection of Fe3+
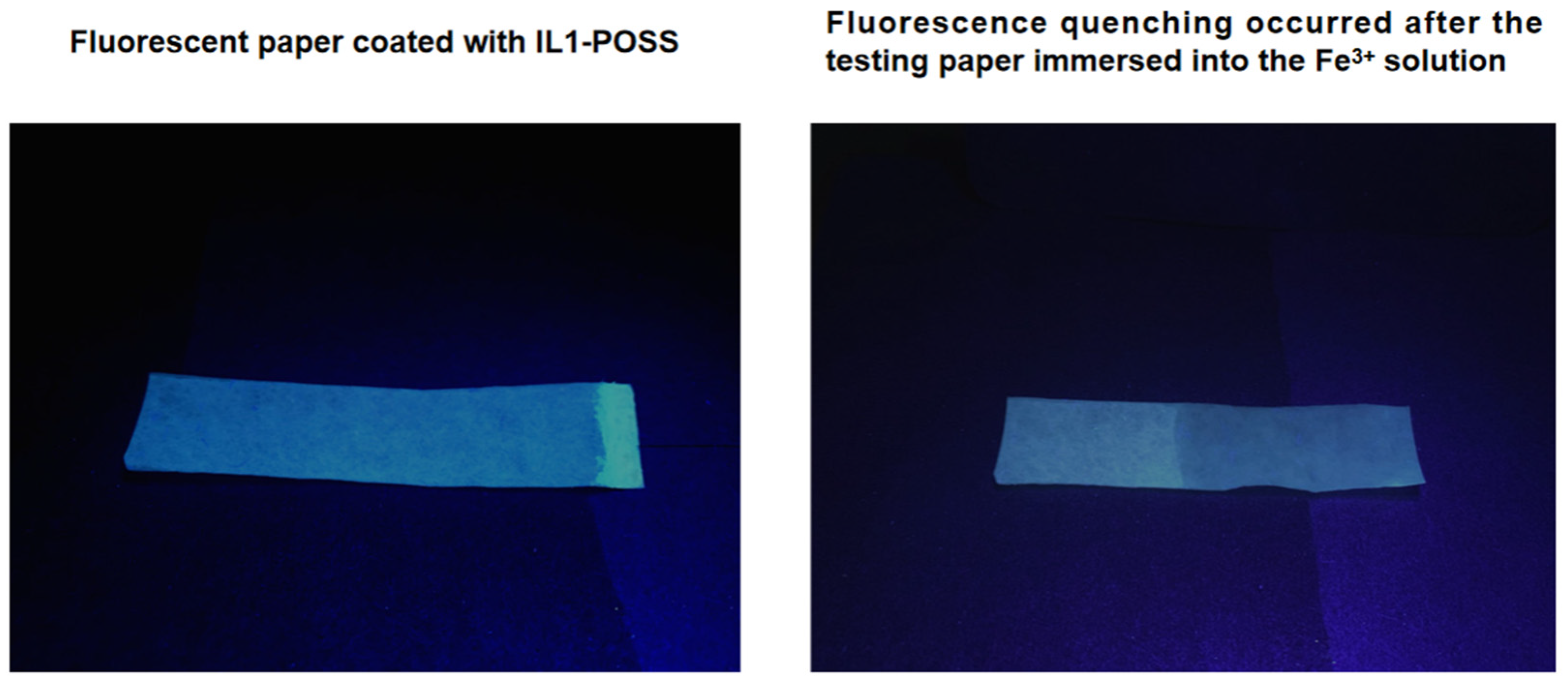
4.2. Efficient Fluorescent Paper for Detecting Fe3+
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbott, A.P.; Barron, J.C.; Ryder, K.S.; Wilson, D. Eutectic-based ionic liquids with metal-containing anions and cations. Chemistry 2007, 13, 6495–6501. [Google Scholar] [CrossRef] [PubMed]
- Bakerb, H.Z.; Gary, A.B. Ionic liquids and deep eutectic solvents for biodiesel synthesis: A review. J. Chem. Technol. Biotechnol. 2013, 88, 3–12. [Google Scholar] [CrossRef]
- Benedetto, A.; Ballone, P. Room-Temperature Ionic Liquids and Biomembranes: Setting the Stage for Applications in Pharmacology, Biomedicine, and Bionanotechnology. Langmuir 2018, 34, 9579–9597. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rodriguez, J.; Valls, A.; Arias Abrodo, P.; Gutierrez Alvarez, M.D.; Gonzalez-Alvarez, J.; Altava, B.; Luis, S.V. Polymeric Ionic Liquids Derived from L-Valine for the Preparation of Highly Selective Silica-Supported Stationary Phases in Gas Chromatography. Polymers 2020, 12, 2348. [Google Scholar] [CrossRef] [PubMed]
- Illia, V.; Kapitanov, A.J.; Yevgen, K.; Marcel, S.; Lourdes, P.; Nicholas, G.A.K.; Klaus, K.N. Gathergood Synthesis, self-assembly, bacterial and fungal toxicity, and preliminary biodegradation studies of a series of L-phenylalanine-derived surface-active ionic liquids. Green. Chem. 2019, 21, 1777–1794. [Google Scholar] [CrossRef]
- Ishii, T.; Enoki, T.; Mizumo, T.; Ohshita, J.; Kaneko, Y. Preparation of imidazolium-type ionic liquids containing silsesquioxane frameworks and their thermal and ion-conductive properties. RSC Adv. 2015, 5, 15226–15232. [Google Scholar] [CrossRef]
- Isik, M.; Zulfiqar, S.; Edhaim, F.; Ruiperez, F.; Rothenberger, A.; Mecerreyes, D. Sustainable Poly(Ionic Liquids) for CO2 Capture Based on Deep Eutectic Monomers. ACS Sustain. Chem. Eng. 2016, 4, 7200–7208. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, J. Pseudo ionic liquids and their applications. Sci. Sin. Chim. 2016, 46, 1317–1329. [Google Scholar] [CrossRef][Green Version]
- Jiang, Y.; Liu, X.; Deng, D. Absorption of SO2 in Furoate Ionic Liquids/PEG200 Mixtures and Thermodynamic Analysis. J. Chem. Eng. Data 2018, 63, 259–268. [Google Scholar] [CrossRef]
- Marta, B.F.P.-P.; Łukasz, M.; Marek, T. How green are ionic liquids? A multicriteria decision analysis approach. Ecotoxicol. Environ. Safety 2019, 174, 455–458. [Google Scholar] [CrossRef]
- Kamio, E.; Yasui, T.; Iida, Y.; Gong, J.P.; Matsuyama, H. Inorganic/Organic Double-Network Gels Containing Ionic Liquids. Adv. Mater 2017, 29. [Google Scholar] [CrossRef]
- Kujawa, J.; Rynkowska, E.; Fatyeyeva, K.; Knozowska, K.; Wolan, A.; Dzieszkowski, K.; Li, G.; Kujawski, W. Preparation and Characterization of Cellulose Acetate Propionate Films Functionalized with Reactive Ionic Liquids. Polymers 2019, 11, 1217. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, J.; Hu, R.; Lv, C.; Zheng, J. A Highly Ionic Conductive, Healable, and Adhesive Polysiloxane-Supported Ionogel. Macromol. Rapid Commun. 2019, 40, e1800776. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Abedin, R.; Hung, F.R. On the Performance of Confined Deep Eutectic Solvents and Ionic Liquids for Separations of Carbon Dioxide from Methane: Molecular Dynamics Simulations. Langmuir 2019, 35, 3658–3671. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Ionic Liquids and Deep Eutectic Solvents in Natural Products Research: Mixtures of Solids as Extraction Solvents. J. Nat. Prod. 2013, 76, 12. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Naderahmadian, A.; Eftekhari-Sis, B. Silver and copper nanoparticles stabilized on ionic liquids-functionalized polyhedral oligomeric silsesquioxane (POSS): Highly active and recyclable hybrid catalysts. Polyhedron 2019, 171, 228–236. [Google Scholar] [CrossRef]
- Campanella, A.; Dohler, D.; Binder, W.H. Self-Healing in Supramolecular Polymers. Macromol. Rapid Commun. 2018, 39, e1700739. [Google Scholar] [CrossRef]
- Tanner, E.E.L.; Curreri, A.M.; Balkaran, J.P.R.; Selig-Wober, N.C.; Yang, A.B.; Kendig, C.; Fluhr, M.P.; Kim, N.; Mitragotri, S. Design Principles of Ionic Liquids for Transdermal Drug Delivery. Adv. Mater. 2019, 31, e1901103. [Google Scholar] [CrossRef]
- Bai, H.; Zheng, Y.; Yang, R. Recyclable liquid-like POSS derivatives with designed structures and their potential for CO2 capture. Mater. Design 2016, 99, 145–154. [Google Scholar] [CrossRef]
- Chen, S.C.; Fu, X.Z.; Chung, T.-S. Fouling behaviors of polybenzimidazole (PBI)–polyhedral oligomeric silsesquioxane (POSS)/polyacrylonitrile (PAN) hollow fiber membranes for engineering osmosis processes. Desalination 2014, 335, 17–26. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Xu, J.; Liu, X.; Liu, K.; Tong, M.; Long, Z. Imidazolium-based ionic porous hybrid polymers with POSS-derived silanols for efficient heterogeneous catalytic CO2 conversion under mild conditions. Chem. Eng. J. 2020, 381. [Google Scholar] [CrossRef]
- Çağlar, Y.; Biyiklioglu, Z. Spectrophotometric determination of Hg(II) in water samples by dispersive liquid liquid microextraction with use ionic liquid after derivatization with a water soluble Fe(II) phthalocyanine. J. Incl. Phenom. Macrocycl. Chem. 2018, 90, 331–339. [Google Scholar] [CrossRef]
- Kacmaz, S.; Ertekin, K.; Gocmenturk, M.; Suslu, A.; Ergun, Y.; Celik, E. Selective sensing of Fe3+ at pico-molar level with ethyl cellulose based electrospun nanofibers. React. Funct. Polym. 2013, 73, 674–682. [Google Scholar] [CrossRef]
- Srivastava, S.; Thakur, N.; Singh, A.; Shukla, P.; Maikhuri, V.K.; Garg, N.; Prasad, A.; Pandey, R. Development of a fused imidazo[1,2-a]pyridine based fluorescent probe for Fe3+ and Hg2+ in aqueous media and HeLa cells. RSC Adv. 2019, 9, 29856–29863. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Santiago, A.R.P.; Nair, A.N.; Weller, J.M.; Sanad, M.F.; Valles-Rosales, D.J.; Chan, C.K.; Sreenivasan, S.; Noveron, J.C. Metal-Organic frameworks-derived multifunctional carbon encapsulated metallic nanocatalysts for catalytic peroxymonosulfate activation and electrochemical hydrogen generation. Mol. Catal. 2020, 498. [Google Scholar] [CrossRef]
- Fan, B.; Wei, J.; Ma, X.; Bu, X.; Xing, N.; Pan, Y.; Zheng, L.; Guan, W. Synthesis of Lanthanide-Based Room Temperature Ionic Liquids with Strong Luminescence and Selective Sensing of Fe(III) over Mixed Metal Ions. Ind. Eng. Chem. Res. 2016, 55, 2267–2271. [Google Scholar] [CrossRef]
- Yang, D.; Dai, C.; Hu, Y.; Liu, S.; Weng, L.; Luo, Z.; Cheng, Y.; Wang, L. A New Polymer-Based Fluorescent Chemosensor Incorporating Propane-1,3-Dione and 2,5-Diethynylbenzene Moieties for Detection of Copper(II) and Iron(III). Polymers 2017, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Qian, Y.; Jiao, Y.; Liu, J.; Xi, F.; Dong, X. Ionic liquid-capped graphene quantum dots as label-free fluorescent probe for direct detection of ferricyanide. Talanta 2017, 165, 429–435. [Google Scholar] [CrossRef]
- Wang, D.; Li, L.; Yang, W.; Zuo, Y.; Feng, S.; Liu, H. POSS-based luminescent porous polymers for carbon dioxide sorption and nitroaromatic explosives detection. RSC Adv. 2014, 4, 59877–59884. [Google Scholar] [CrossRef]
- Li, L.; Liu, H. Rapid Preparation of Silsesquioxane-Based Ionic Liquids. Chemistry 2016, 22, 4713–4716. [Google Scholar] [CrossRef]
- Liu, D.; Cheng, G.; Zhao, H.; Zeng, C.; Qu, D.; Xiao, L.; Tang, H.; Deng, Z.; Li, Y.; Su, B.-L. Self-assembly of polyhedral oligosilsesquioxane (POSS) into hierarchically ordered mesoporous carbons with uniform microporosity and nitrogen-doping for high performance supercapacitors. Nano Energy 2016, 22, 255–268. [Google Scholar] [CrossRef]
- Matějka, L.; Janata, M.; Pleštil, J.; Zhigunov, A.; Šlouf, M. Self-assembly of POSS-containing block copolymers: Fixing the hierarchical structure in networks. Polymer 2014, 55, 126–136. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, B.; Wen, L.; Liang, Q.; Zhang, L. Amphiphilic cholesteryl grafted sodium alginate derivative: Synthesis and self-assembly in aqueous solution. Carbohydr. Polym. 2007, 68, 218–225. [Google Scholar] [CrossRef]
- Liu, W.; Huang, X.; Xu, C.; Chen, C.; Yang, L.; Dou, W.; Chen, W.; Yang, H.; Liu, W. A Multi-responsive Regenerable Europium-Organic Framework Luminescent Sensor for Fe(3+), Cr(VI) Anions, and Picric Acid. Chemistry 2016, 22, 18769–18776. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Feng, S. New Functionalized Ionic Liquids Based on POSS for the Detection of Fe3+ Ion. Polymers 2021, 13, 196. https://doi.org/10.3390/polym13020196
Li W, Feng S. New Functionalized Ionic Liquids Based on POSS for the Detection of Fe3+ Ion. Polymers. 2021; 13(2):196. https://doi.org/10.3390/polym13020196
Chicago/Turabian StyleLi, Wensi, and Shengyu Feng. 2021. "New Functionalized Ionic Liquids Based on POSS for the Detection of Fe3+ Ion" Polymers 13, no. 2: 196. https://doi.org/10.3390/polym13020196
APA StyleLi, W., & Feng, S. (2021). New Functionalized Ionic Liquids Based on POSS for the Detection of Fe3+ Ion. Polymers, 13(2), 196. https://doi.org/10.3390/polym13020196



