Biocompatible Lipid Polymer Cationic Nanoparticles for Antigen Presentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of NPs by Emulsion Polymerization Method
2.3. Determining Physical Properties of NPs by Dynamic Light-Scattering (DLS)
2.4. Determining Solids Content, PDDA, and DODAB Concentration in NPs
2.5. Determining NaCl Concentration Effect on NPs Dz
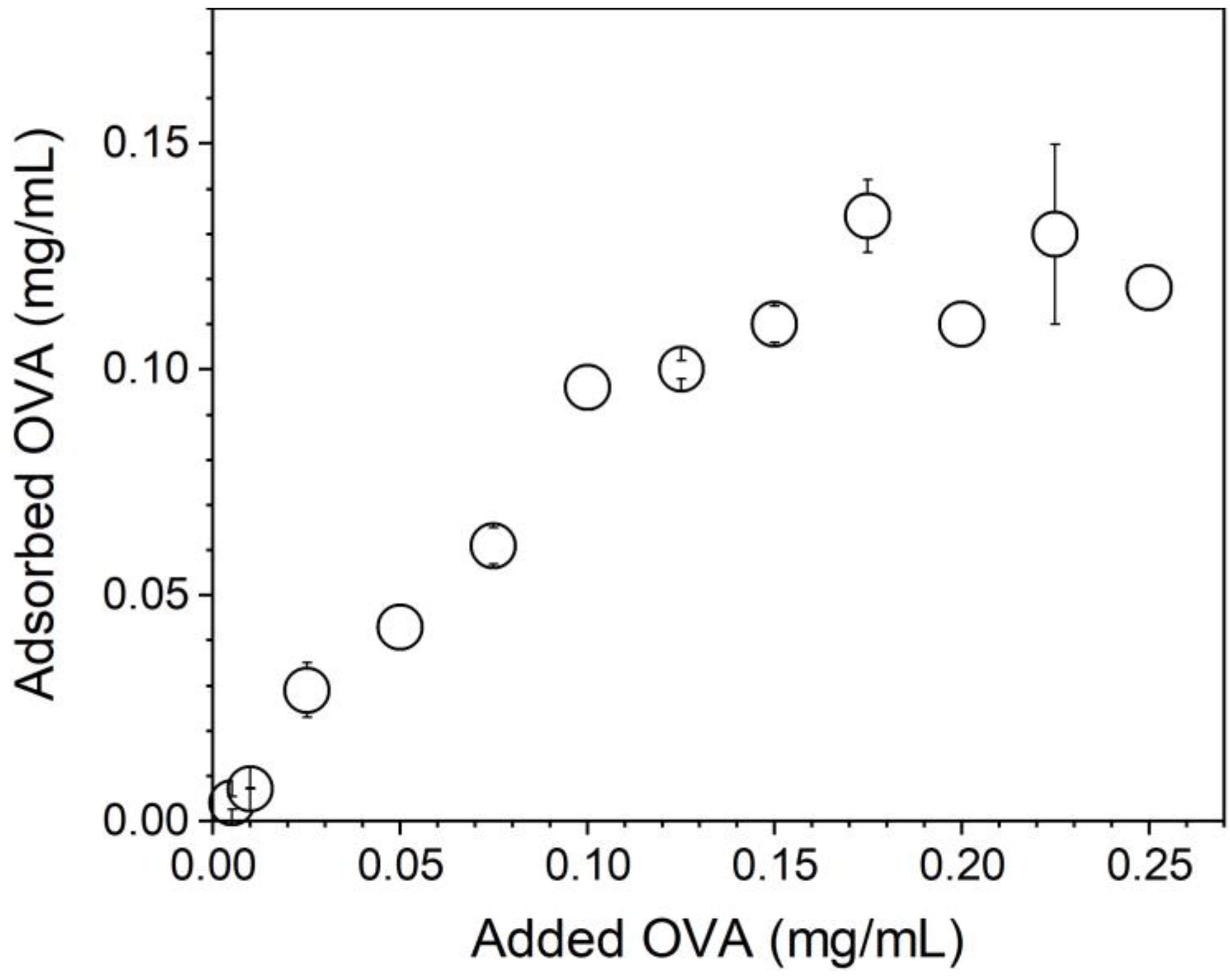
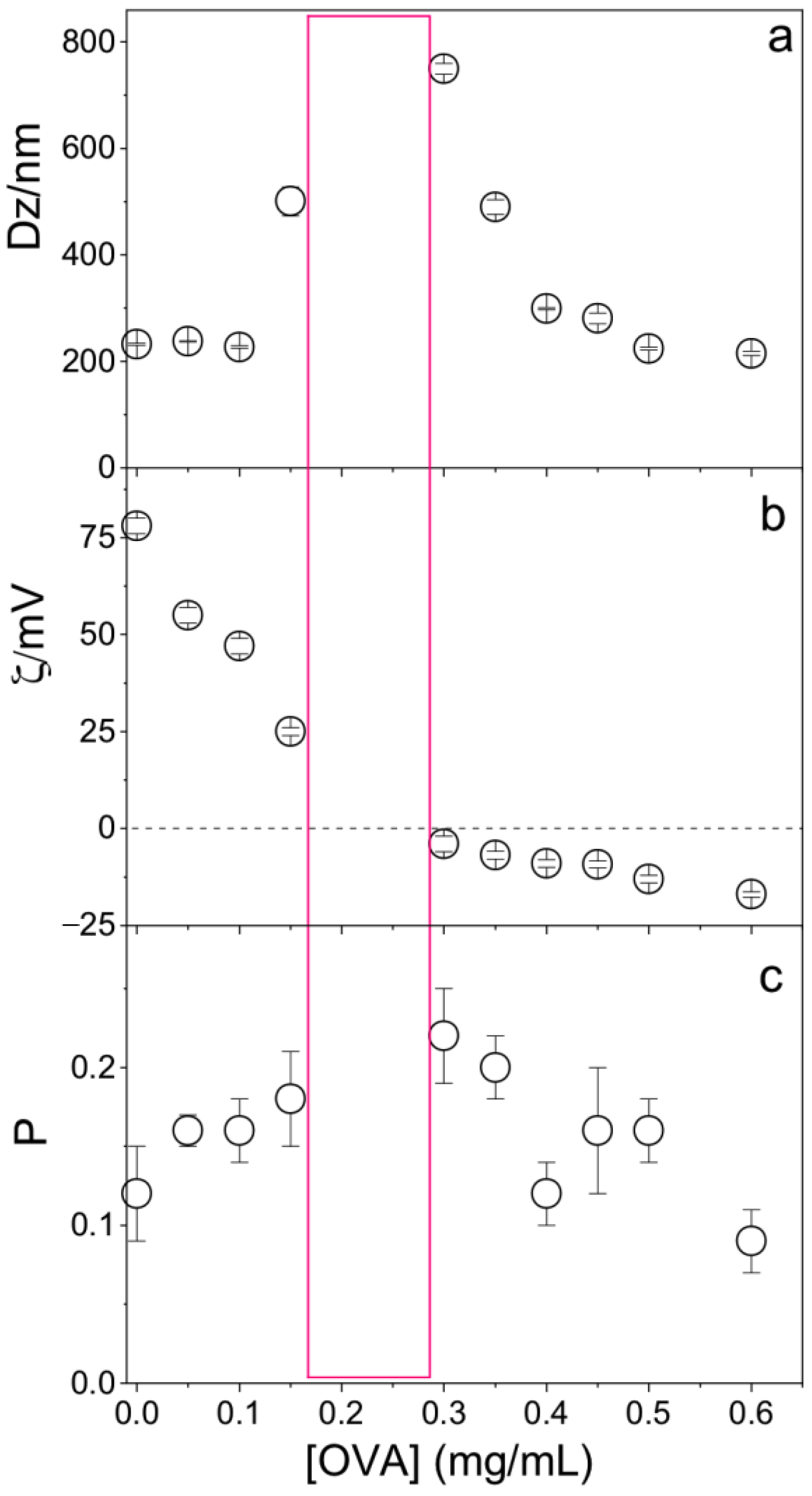
2.6. Evaluation of NPs/OVA Interaction
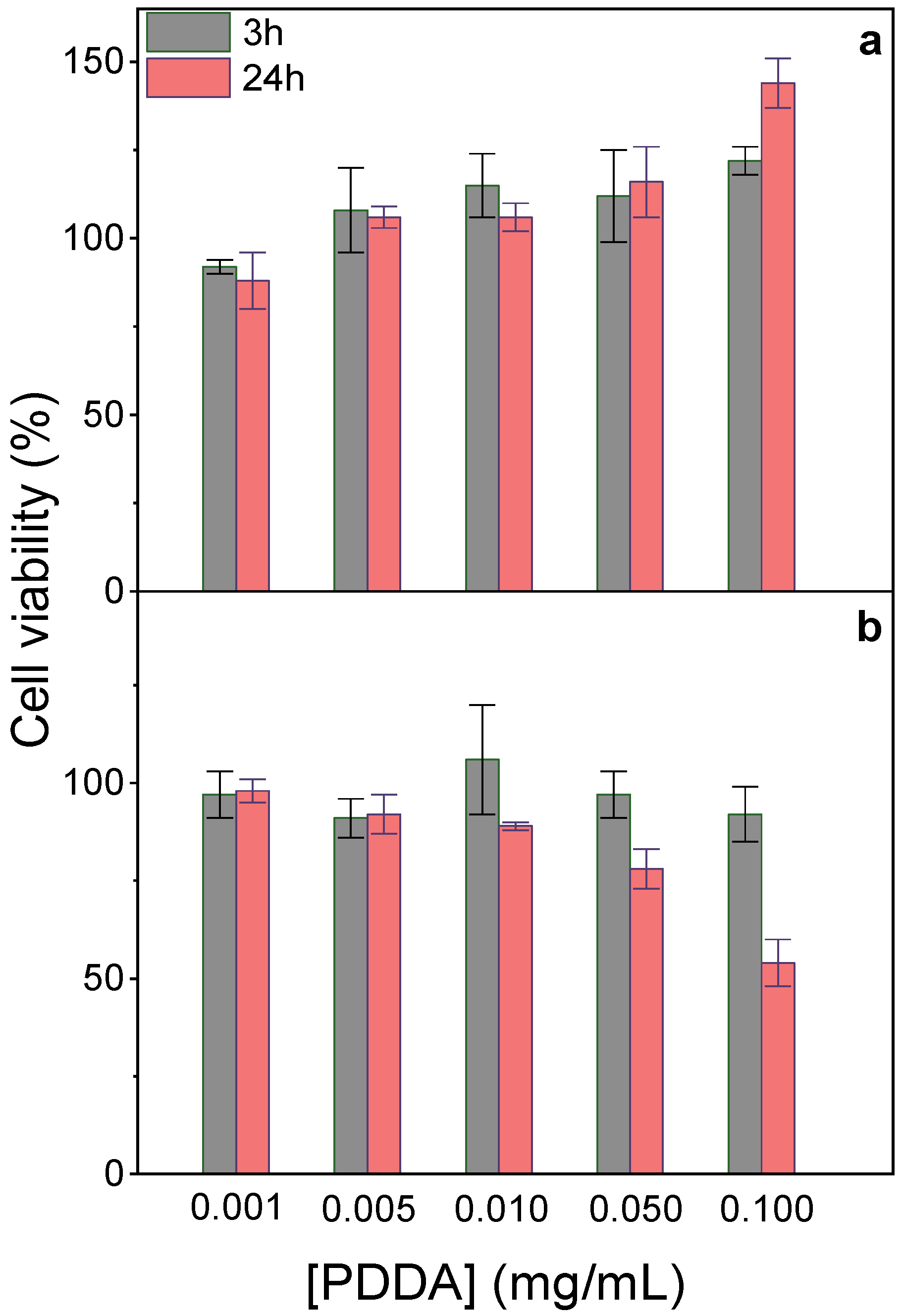
2.7. Determination of NPs Cytotoxicity by MTT Assay
2.8. Immunization Protocol
2.9. Determining OVA-Specific IgG1 and IgG2a
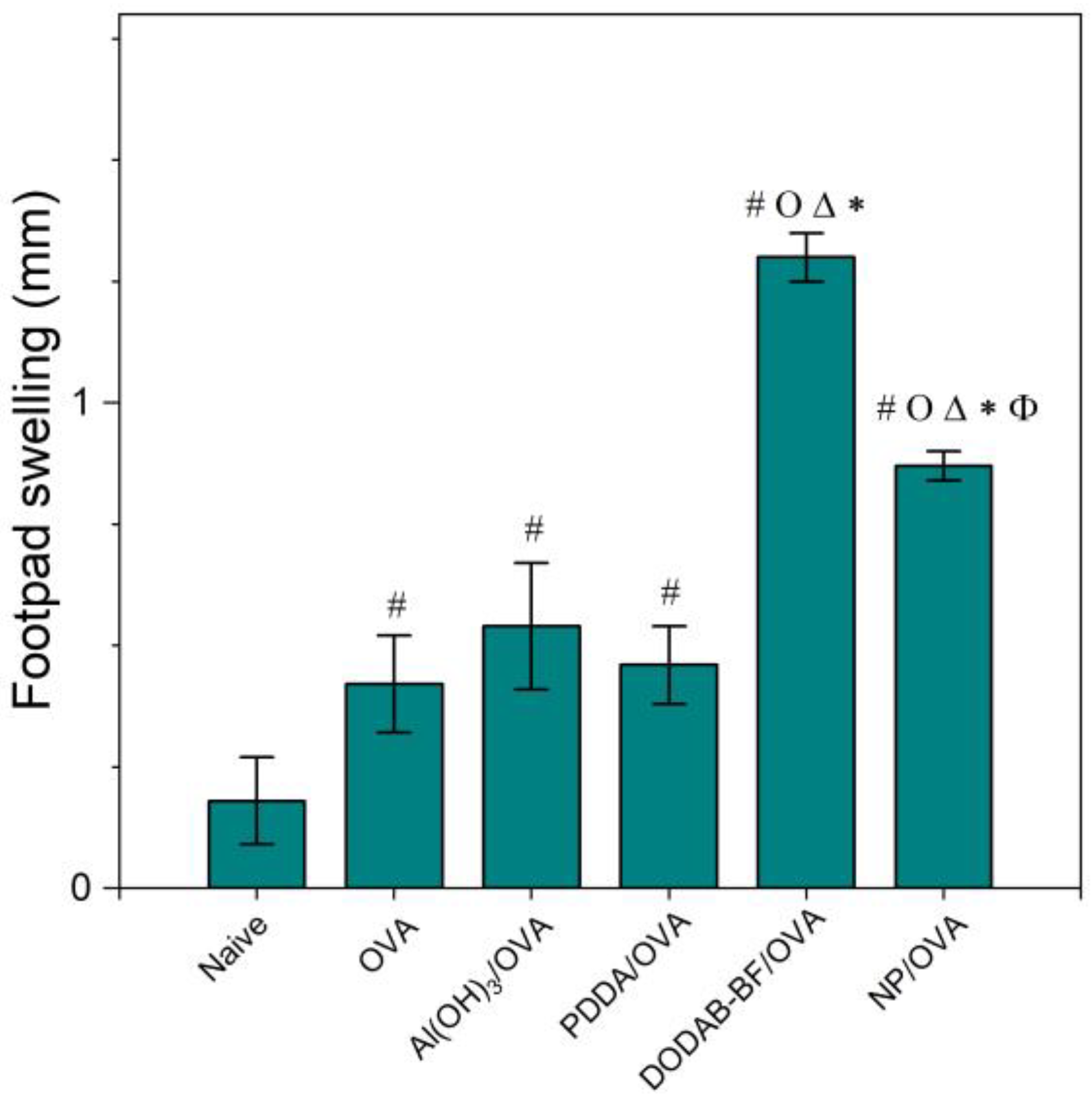
2.10. Determining DTH from Footpad Swelling
2.11. Culturing Spleen Cells for Cytokines Analysis
3. Results and Discussion
3.1. Synthesis of PMMA/DODAB/PDDA NPs
3.2. Properties and Cytotoxicity of PMMA/DODAB/PDDA NPs
3.3. OVA/NPs Interaction
3.4. PMMA/DODAB/PDDA NPs as Adjuvants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Date, T.; Nimbalkar, V.; Kamat, J.; Mittal, A.; Mahato, R.I.; Chitkara, D. Lipid-polymer hybrid nanocarriers for delivering cancer therapeutics. J. Control. Release 2018, 271, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Siewert, C.D.; Haas, H.; Cornet, V.; Nogueira, S.S.; Nawroth, T.; Uebbing, L.; Ziller, A.; Al-Gousous, J.; Radulescu, A.; Schroer, M.A.; et al. Hybrid biopolymer and lipid nanoparticles with improved transfection efficacy for mRNA. Cells 2020, 9, 2034. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, L.Y.; Pashirova, T.N.; Doktorovova, S.; Fernandes, A.R.; Sanchez-Lopez, E.; Silva, A.M.; Souto, S.B.; Souto, E.B. Cationic surfactants: Self-assembly, structure-activity correlation and their biological applications. Int. J. Mol. Sci. 2019, 20, 5534. [Google Scholar] [CrossRef] [PubMed]
- Padovani, G.C.; Feitosa, V.P.; Sauro, S.; Tay, F.R.; Durán, G.; Paula, A.J.; Durán, N. Advances in dental materials through nanotechnology: Facts, perspectives and toxicological aspects. Trends Biotechnol. 2015, 33, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.; Lee, J. Recent nanotechnology approaches for prevention and treatment of biofilm-associated infections on medical devices. BioMed Res. Int. 2016, 2016, 1851242. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Astruc, D. Nanomaterials for removal of toxic elements from water. Coord. Chem. Rev. 2018, 356, 147–164. [Google Scholar] [CrossRef]
- de Melo Carrasco, L.D.; Sampaio, J.L.M.; Carmona-Ribeiro, A.M. Supramolecular cationic assemblies against multidrug-resistant microorganisms: Activity and mechanism of action. Int. J. Mol. Sci. 2015, 16, 6337–6352. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.M.A.; Kosaka, P.M.; Rosa, H.; Vieira, D.B.; Kawano, Y.; Petri, D.F.S.; Carmona-Ribeiro, A.M. Hybrid materials from intermolecular associations between cationic lipid and polymers. J. Phys. Chem. B 2008, 112, 9301–9310. [Google Scholar] [CrossRef]
- Lincopan, N.; Espindola, N.M.; Vaz, A.J.; Carmona-Ribeiro, A.M. Cationic supported lipid bilayers for antigen presentation. Int. J. Pharm. 2007, 340, 216–222. [Google Scholar] [CrossRef]
- Naves, A.F.; Palombo, R.R.; Carrasco, L.D.M.; Carmona-Ribeiro, A.M. Antimicrobial particles from emulsion polymerization of methyl methacrylate in the presence of quaternary ammonium surfactants. Langmuir 2013, 29, 9677–9684. [Google Scholar] [CrossRef] [PubMed]
- Sanches, L.M.; Petri, D.F.S.; de Melo Carrasco, L.D.; Carmona-Ribeiro, A.M. The antimicrobial activity of free and immobilized poly (diallyldimethylammonium) chloride in nanoparticles of poly (methylmethacrylate). J. Nanobiotechnol. 2015, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.T.; Galvão, C.N.; Betancourt, Y.P.; Mathiazzi, B.I.; Carmona-Ribeiro, A.M. Microbicidal dispersions and coatings from hybrid nanoparticles of poly (methyl methacrylate), poly (diallyl dimethyl ammonium) chloride, lipids, and surfactants. Int. J. Mol. Sci. 2019, 20, 6150. [Google Scholar] [CrossRef] [PubMed]
- Galvão, C.N.; Sanches, L.M.; Mathiazzi, B.I.; Ribeiro, R.T.; Petri, D.F.S.; Carmona-Ribeiro, A.M. Antimicrobial coatings from hybrid nanoparticles of biocompatible and antimicrobial polymers. Int. J. Mol. Sci. 2018, 19, 2965. [Google Scholar] [CrossRef] [PubMed]
- Mathiazzi, B.I.; Carmona-Ribeiro, A.M. Hybrid nanoparticles of poly (methyl methacrylate) and antimicrobial quaternary ammonium surfactants. Pharmaceutics 2020, 12, 340. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.D.; Mamizuka, E.M.; Carmona-Ribeiro, A.M. Antimicrobial particles from cationic lipid and polyelectrolytes. Langmuir 2010, 26, 12300–12306. [Google Scholar] [CrossRef] [PubMed]
- Lincopan, N.; Espindola, N.M.; Vaz, A.J.; da Costa, M.H.B.; Faquim-Mauro, E.; Carmona-Ribeiro, A.M. Novel immunoadjuvants based on cationic lipid: Preparation, characterization and activity in vivo. Vaccine 2009, 27, 5760–5771. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-Q.; Zhang, W.-J.; Lai, L.; Mei, P.; Wu, L.-M.; Wang, Y.-Q. Different cationic surfactants-modified silica nanoparticles for Pickering emulsions. J. Mol. Liq. 2019, 291, 111341. [Google Scholar] [CrossRef]
- Lincopan, N.; Santana, M.R.; Faquim-Mauro, E.; da Costa, M.H.B.; Carmona-Ribeiro, A.M. Silica-based cationic bilayers as immunoadjuvants. BMC Biotechnol. 2009, 9, 5. [Google Scholar] [CrossRef]
- Pérez-Betancourt, Y.; Távora, B.C.L.F.; Colombini, M.; Faquim-Mauro, E.L.; Carmona-Ribeiro, A.M. Simple nanoparticles from the assembly of cationic polymer and antigen as immunoadjuvants. Vaccines 2020, 8, 105. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; Pérez-Betancourt, Y. Cationic nanostructures for vaccines design. Biomimetics 2020, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-W.; Na, W.; Kim, H.-O.; Yeom, M.; Park, G.; Kang, A.; Chun, H.; Park, C.; Oh, S.; Le, V.P.; et al. Cationic poly(amino acid) vaccine adjuvant for promoting both cell-mediated and humoral immunity against influenza virus. Adv. Healthc. Mater. 2019, 8, 1800953. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Y.; Chen, Z.; Li, W.; Liu, Y.; Wang, L.; Liu, Y.; Wu, X.; Ji, Y.; Zhao, Y.; et al. Surface-engineered gold nanorods: Promising DNA vaccine adjuvant for HIV-1 treatment. Nano Lett. 2012, 12, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Nawwab Al-Deen, F.M.; Selomulya, C.; Kong, Y.Y.; Xiang, S.D.; Ma, C.; Coppel, R.L.; Plebanski, M. Design of magnetic polyplexes taken up efficiently by dendritic cell for enhanced DNA vaccine delivery. Gene Ther. 2014, 21, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.L.; Howard, G.P.; Coatsworth, H.; Dinglasan, R.R.; Mao, H.-Q.; Plebanski, M. Biodegradable PLGA-b-PEG nanoparticles induce T helper 2 (Th2) immune responses and sustained antibody titers via TLR9 stimulation. Vaccines 2020, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.M.; Chew, C.H.; Ng, S.C.; Loh, S.E. Polymerization of methyl methacrylate in ternary systems: Emulsion and microemulsion. Langmuir 1993, 9, 2799–2803. [Google Scholar] [CrossRef]
- Patra, C.N.; Priya, R.; Swain, S.; Jena, G.K.; Panigrahi, K.C.; Ghose, D. Pharmaceutical significance of Eudragit: A review. Future J. Pharm. Sci. 2017, 3, 33–45. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; Midmore, B.R. Synthetic bilayer adsorption onto polystyrene microspheres. Langmuir 1992, 8, 801–806. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; de Moraes Lessa, M. Interactions between bilayer membranes and latex. Colloids Surf. A Physicochem. Eng. Asp. 1999, 153, 355–361. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M. Biomimetic nanomaterials from the assembly of polymers, lipids, and surfactants. In Surfactants and Detergents; Dutta, A.K., Ed.; IntechOpen: Rijeka, Croatia, 2019; pp. 1–16. ISBN 978-1-78984-661-4. [Google Scholar]
- Melo, L.D.; Palombo, R.R.; Petri, D.F.S.; Bruns, M.; Pereira, E.M.A.; Carmona-Ribeiro, A.M. Structure-activity relationship for quaternary ammonium compounds hybridized with poly(methyl methacrylate). ACS Appl. Mater. Interfaces 2011, 3, 1933–1939. [Google Scholar] [CrossRef]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Fifis, T.; Gamvrellis, A.; Crimeen-Irwin, B.; Pietersz, G.A.; Li, J.; Mottram, P.L.; McKenzie, I.F.C.; Plebanski, M. Size-dependent immunogenicity: Therapeutic and protective properties of nano-vaccines against tumors. J. Immunol. 2004, 173, 3148–3154. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, E.; Morrison, I. Particle Size Distribution from Analysis of Quasi-elastic Light Scattering Data. In Measurement of Suspended Particles by Quasi-Elastic Light Scattering; Dahneke, B., Ed.; Wiley-Interscience: New York, NY, USA, 1983; pp. 199–236. [Google Scholar]
- Schales, O.; Schales, S.S. A simple and accurate method for the determination of chloride in biological fluids. J. Biol. Chem 1941, 140, 879–882. [Google Scholar] [CrossRef]
- Walker, J.M. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol. Biol. 1994, 32, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Van der Lee, A.; Hamon, L.; Holl, Y.; Grohens, Y. Density profiles in thin PMMA supported films investigated by X-ray reflectometry. Langmuir 2001, 17, 7664–7669. [Google Scholar] [CrossRef]
- Stuart, M.A.C. Adsorbed polymers in colloidal systems: From statics to dynamics. Polym. J. 1991, 23, 669–682. [Google Scholar] [CrossRef]
- Dubas, S.T.; Kittitheeranun, P.; Rangkupan, R.; Sanchavanakit, N.; Potiyaraj, P. Coating of polyelectrolyte multilayer thin films on nanofibrous scaffolds to improve cell adhesion. J. Appl. Polym. Sci. 2009, 114, 1574–1579. [Google Scholar] [CrossRef]
- Behera, S.S.; Das, U.; Kumar, A.; Bissoyi, A.; Singh, A.K. Chitosan/TiO2 composite membrane improves proliferation and survival of L929 fibroblast cells: Application in wound dressing and skin regeneration. Int. J. Biol. Macromol. 2017, 98, 329–340. [Google Scholar] [CrossRef]
- Carrasco, L.D.; Santos, H.C.; Sampaio, J.L.; Carmona-Ribeiro, A.M. Self-assembled antibiotic nanoparticles against intracellular bacteria. Drug Deliv. Lett. 2017, 7, 39–47. [Google Scholar]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Lindblad, E.B. Aluminium adjuvants—In retrospect and prospect. Vaccine 2004, 22, 3658–3668. [Google Scholar] [CrossRef] [PubMed]
- Rozenfeld, J.H.K.; Silva, S.R.; Raneia, P.A.; Faquim-Mauro, E.; Carmona-Ribeiro, A.M. Stable assemblies of cationic bilayer fragments and CpG oligonucleotide with enhanced immunoadjuvant activity in vivo. J. Control. Release 2012, 160, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M. Cationic nanostructures for vaccines. In Immune Response Activation; Duc, G.H.T., Ed.; IntechOpen: Rijeka, Croatia, 2014; pp. 1–45. ISBN 978-953-51-1374-4. [Google Scholar]
- Carmona-Ribeiro, A.M. Nanomaterials based on lipids for vaccine development. In Micro and Nano Technologies; Skwarczynski, M., Toth, I.B.T.-M., Eds.; Elsevier: Oxford, UK, 2017; pp. 241–257. ISBN 978-0-323-39981-4. [Google Scholar]
- Li, S.; Malmstadt, N. Deformation and poration of lipid bilayer membranes by cationic nanoparticles. Soft Matter 2013, 9, 4969–4976. [Google Scholar] [CrossRef]
- Lin, J.; Alexander-Katz, A. Cell membranes open “doors” for cationic nanoparticles/Biomolecules: Insights into uptake kinetics. ACS Nano 2013, 7, 10799–10808. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology, 9th ed.; Elsevier Ltd.: Philadelphia, PA, USA, 2018; ISBN 9780323479783. [Google Scholar]
- Kubo, T.; Morita, H.; Sugita, K.; Akdis, C.A. Introduction to mechanisms of allergic diseases. In Middleton’s Allergy Essentials; O’Hehir, R.E., Holgate, S.T., Sheikh, A.B.T.-M.A.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–27. ISBN 978-0-323-37579-5. [Google Scholar]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.M.; Avia, M.; Martín, V.; Sevilla, N. IL-10: A multifunctional cytokine in viral infections. J. Immunol. Res. 2017, 2017, 6104054. [Google Scholar] [CrossRef]
- Fritsche, G.; Dlaska, M.; Barton, H.; Theurl, I.; Garimorth, K.; Weiss, G. Nramp1 functionality increases inducible nitric oxide synthase transcription via stimulation of IFN regulatory factor 1 expression. J. Immunol. 2003, 171, 1994–1998. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Minami, Y. The IL-2/IL-2 receptor system: A current overview. Cell 1993, 73, 5–8. [Google Scholar] [CrossRef]
- Cote-Sierra, J.; Foucras, G.; Guo, L.; Chiodetti, L.; Young, H.A.; Hu-Li, J.; Zhu, J.; Paul, W.E. Interleukin 2 plays a central role in Th2 differentiation. Proc. Natl. Acad. Sci. USA 2004, 101, 3880–3885. [Google Scholar] [CrossRef]
- Zhu, J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 2015, 75, 14–24. [Google Scholar] [CrossRef]




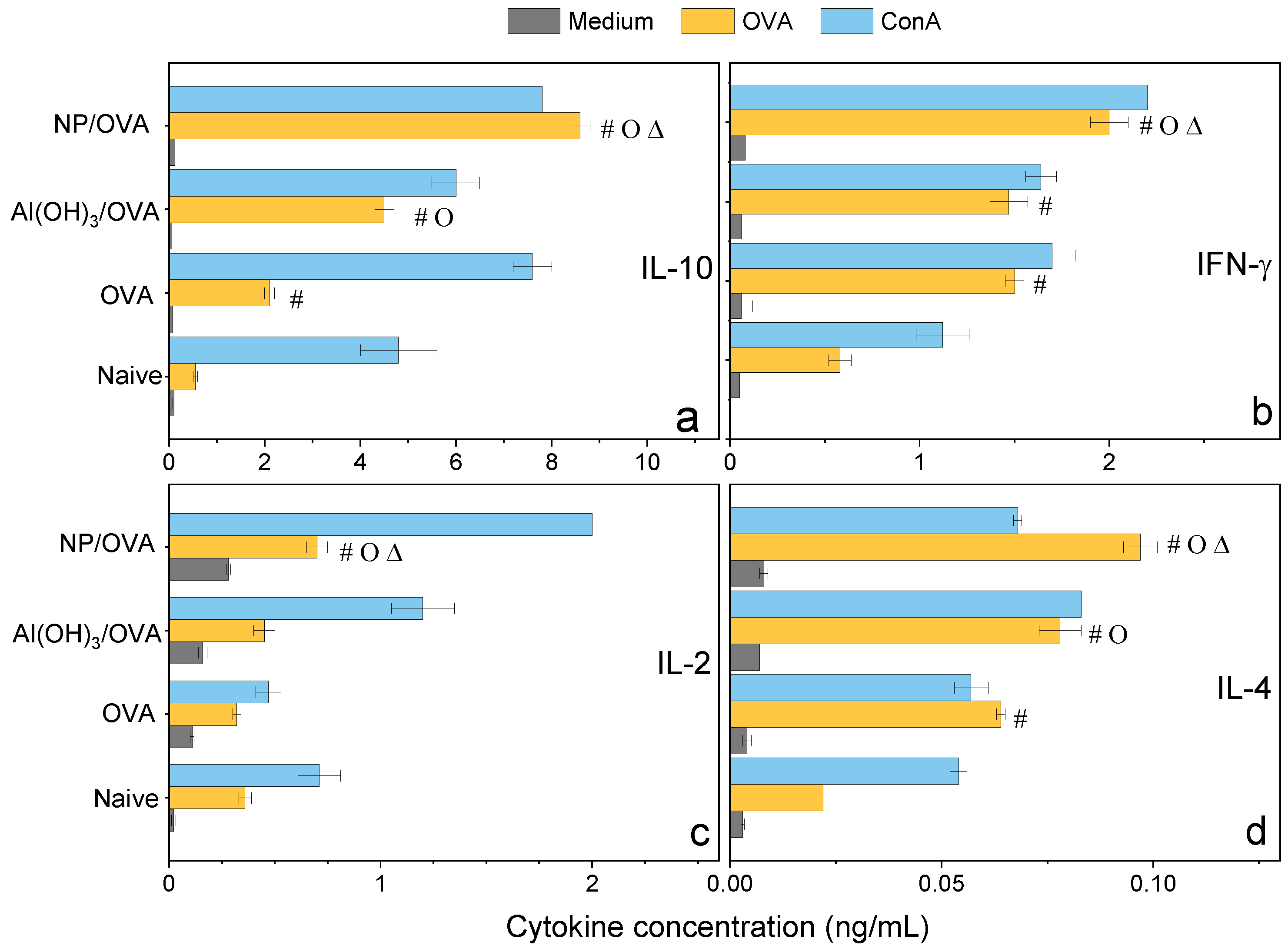
| [MMA]/M | Solids Content/mg mL−1 | Yield/% |
|---|---|---|
| 0.10 | 11.0 ± 1.0 | 100 |
| 0.20 | 14.0 ± 1.0 | 70 |
| 0.30 | 21.0 ± 1.6 | 70 |
| 0.40 | 30.0 ± 2.1 | 75 |
| 0.50 | 31.0 ± 1.6 | 62 |
| 0.56 | 36.0 ± 2.4 | 64 |
| Dz/nm | ζ/mV | P | G/µS | [PMMA]P | [DODAB]P | [PDDA]P |
|---|---|---|---|---|---|---|
| 225 ± 2 * | 70 ± 1* | 0.10 ± 0.02 * | 92 ± 3 * | |||
| 217 ± 1 | 73 ± 1 | 0.12 ± 0.01 | 4 ± 2 | 7.0 ± 0.7 | 0.32 ± 0.03 | 0.70 ± 0.02 |
| Assembly | Time/Days | A492nm (IgG1) | A492nm (IgG2a) | FS/mm |
|---|---|---|---|---|
| OVA | 14 | 0.40 (1/128) | 0.10(1/32) | 0.3 |
| 21 | 0.25 (1/128) | 0.10(1/32) | ||
| DODAB BF/OVA | 14 | 1.45 (1/128) | 0.70(1/32) | 1.3 |
| 21 | 0.80 (1/128) | 0.40(1/32) | ||
| PDDA/OVA | 14 | 1.20 (1/256) | 0.15(1/8) | 0.5 |
| 21 | 1.50 (1/256) | 0.50(1/8) | ||
| NPs/OVA | 14 | 1.90 (1/512) | 0.60(1/8) | 0.9 |
| 21 | 1.90 (1/512) | 0.50(1/8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Betancourt, Y.; Távora, B.d.C.L.F.; Faquim-Mauro, E.L.; Carmona-Ribeiro, A.M. Biocompatible Lipid Polymer Cationic Nanoparticles for Antigen Presentation. Polymers 2021, 13, 185. https://doi.org/10.3390/polym13020185
Pérez-Betancourt Y, Távora BdCLF, Faquim-Mauro EL, Carmona-Ribeiro AM. Biocompatible Lipid Polymer Cationic Nanoparticles for Antigen Presentation. Polymers. 2021; 13(2):185. https://doi.org/10.3390/polym13020185
Chicago/Turabian StylePérez-Betancourt, Yunys, Bianca de Carvalho Lins Fernandes Távora, Eliana L. Faquim-Mauro, and Ana Maria Carmona-Ribeiro. 2021. "Biocompatible Lipid Polymer Cationic Nanoparticles for Antigen Presentation" Polymers 13, no. 2: 185. https://doi.org/10.3390/polym13020185
APA StylePérez-Betancourt, Y., Távora, B. d. C. L. F., Faquim-Mauro, E. L., & Carmona-Ribeiro, A. M. (2021). Biocompatible Lipid Polymer Cationic Nanoparticles for Antigen Presentation. Polymers, 13(2), 185. https://doi.org/10.3390/polym13020185







