Synthesis of Mono Ethylene Glycol (MEG)-Based Polyurethane and Effect of Chain Extender on Its Associated Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Aromatic Polyester Polyol
2.2. Preparation of the Polyurethane Elastomer Using Glycol-Based Polyol
2.3. Examination of Properties of Polyester Polyols
2.3.1. Viscosity
2.3.2. Acid Number
2.3.3. Hydroxyl Value
- VAN (mg KOH/g sample) is the previously calculated acid value of the sample.
- Volblank (L) is the volume of potassium hydroxide solution used in the blank titration.
- Vol (L) denotes the volume of KOH solution used in the sample titration.
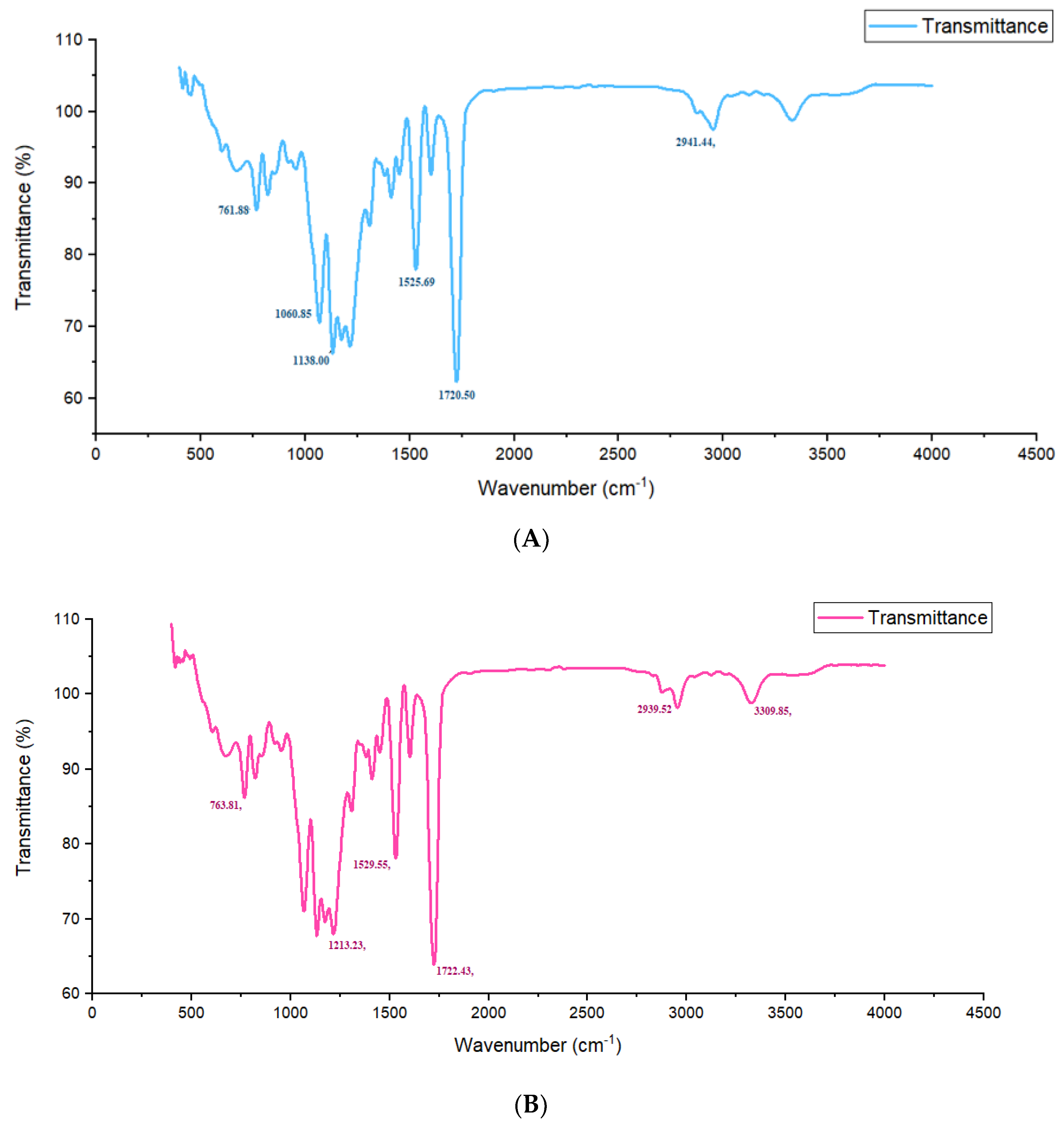
2.4. FTIR Analysis
3. Results and Discussion
3.1. Formation of Aromatic Polyester Polyol
3.2. Formation of Polyurethane Elastomers
3.3. Physical Properties of Aromatic Polyester Polyol
3.4. Hardness of Polyurethane Molecules
3.5. DIN Abrasion Wear Resistance
3.6. Density
3.7. Fourier-Transform Infrared Spectroscopy (FTIR) of Polyurethane
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Omrani, I.; Farhadian, A.; Babanejad, N.; Shendi, H.K.; Ahmadi, A.; Nabid, M.R. Synthesis of novel high primary hydroxyl functionality polyol from sunflower oil using thiol-yne reaction and their application in polyurethane coating. Eur. Polym. J. 2016, 82, 220–231. [Google Scholar] [CrossRef]
- Akbarian, M.; Olya, M.E.; Ataeefard, M.; Mahdavian, M. The influence of nanosilver on thermal and antibacterial properties of a 2 K waterborne polyurethane coating. Prog. Org. Coat. 2012, 75, 344–348. [Google Scholar] [CrossRef]
- Eyvazzadeh Kalajahi, A.; Rezaei, M.; Abbasi, F.; Mir Mohamad Sadeghi, G. The effect of chain extender type on the physical, mechanical, and shape memory properties of poly(ε-caprolactone)-based polyurethane-ureas. Polym. -Plast. Technol. Eng. 2017, 56, 1977–1985. [Google Scholar] [CrossRef]
- Priester, R.D.; Moore, D.R.; Turner, R.B. Polyurethane Polymer Toughness. Polym. Toughening 2020, 54, 293–338. [Google Scholar]
- Marossy, C.K.K. Influence of chain extender on soft and hard segment of polyurethane elastomers. Mater. Sci. Eng. 2018, 43, 98–107. [Google Scholar]
- Al-Attabi, N.Y.; Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Evans, M.; Gunatillake, P.; Malherbe, F.; Yu, A. Preparation and characterization of highly conductive polyurethane composites containing graphene and gold nanoparticles. J. Mater. Sci. 2017, 52, 11774–11784. [Google Scholar] [CrossRef]
- Ali, E.S.; Zubir, S.A. The mechanical properties of medium density rigid polyurethane biofoam. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2016; Volume 39, p. 01009. [Google Scholar]
- Ansari, M.A.; Somdee, P.; Marossy, K. Synthesis of cross-linked polyurethane elastomers with the inclusion of polar-aromatic moieties (BA, PNBA and 3, 5-DNBA): Electrical and thermo-mechanical properties analysis. J. Polym. Res. 2021, 28, 1–11. [Google Scholar] [CrossRef]
- Badri, K.B.H.; Sien, W.C.; Shahrom, M.S.B.R.; Hao, L.C.; Baderuliksan, N.Y.; Norzali, N.R. FTIR spectroscopy analysis of the prepolymerization of palm-based polyurethane. Solid State Sci. Technol. 2010, 18, 1–8. [Google Scholar]
- Badri, K.H. Biobased polyurethane from palm kernel oil-based polyol. Polyurethane 2012, 39, 447–470. [Google Scholar]
- Bayat, Y.; Shandi, H.K.; Khanlari, T. Superior improvement in thermo-mechanical properties of polyurethane based on glycidyl azide polymer/polyethylene adipate. Polym. Bull. 2020, 34, 1–17. [Google Scholar] [CrossRef]
- Bilir, M.H.; Şakalar, N.; Acemioğlu, B.; Baran, E.; Alma, M.H. Sorption of remazol brilliant blue R onto polyurethane-type foam prepared from peanut shell. J. Appl. Polym. Sci. 2013, 127, 4340–4351. [Google Scholar] [CrossRef]
- Clemitson, I.R. Castable Polyurethane Elastomers; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Datta, J.; Kacprzyk, M. Thermal analysis and static strength of polyurethanes obtained from glycolysates. J. Therm. Anal. Calorim. 2008, 93, 753–757. [Google Scholar] [CrossRef]
- Elreedy, A.; Tawfik, A.; Enitan, A.; Kumari, S.; Bux, F. Pathways of 3-biofules (hydrogen, ethanol and methane) production from petrochemical industry wastewater via anaerobic packed bed baffled reactor inoculated with mixed culture bacteria. Energy Convers. Manag. 2016, 122, 119–130. [Google Scholar] [CrossRef]
- Aghabararpour, M.; Mohsenpour, M.; Motahari, S. Effect of crosslinker molecular structure on mechanical and thermal properties of resorcinol formaldehyde aerogel. Mater. Res. Express 2019, 6, 075059. [Google Scholar] [CrossRef]
- Favero, D.; Marcon, V.; Figueroa, C.A.; Gómez, C.M.; Cros, A.; Garro, N.; Sanchis, M.J.; Carsí, M.; Bianchi, O. Effect of chain extender on the morphology, thermal, viscoelastic, and dielectric behavior of soybean polyurethane. J. Appl. Polym. Sci. 2021, 138, 50709. [Google Scholar] [CrossRef]
- Pielichowska, K.; Michal, N.; Piotr, S.; Beata, M. Pielichowska, Kinga, Michał Nowak, Piotr Szatkowski, and Beata Macherzyńska. “The influence of chain extender on properties of polyurethane-based phase change materials modified with graphene.”. Appl. Energy 2016, 162, 1024–1033. [Google Scholar] [CrossRef]
- Gao, L.; Zheng, G.; Zhou, Y.; Hu, L.; Feng, G. Thermal performances and fire behaviors of rosin-based rigid polyurethane foam nanocomposites. J. Therm. Anal. Calorim. 2015, 119, 411–424. [Google Scholar] [CrossRef]
- Hai, T.A.P.; Tessman, M.; Neelakantan, N.; Samoylov, A.A.; Ito, Y.; Rajput, B.S.; Pourahmady, N.; Burkart, M.D. Renewable polyurethanes from sustainable biological precursors. Biomacromolecules 2021, 22, 1770–1794. [Google Scholar] [CrossRef]
- Harris, R.F.; Joseph, M.D.; Davidson, C.; Deporter, C.D.; Dais, V.A. Polyurethane elastomers based on molecular weight advanced poly (ethylene ether carbonate) diols. I. Comparison to commercial diols. J. Appl. Polym. Sci. 1990, 41, 487–507. [Google Scholar] [CrossRef]
- Heiran, R.; Ghaderian, A.; Reghunadhan, A.; Sedaghati, F.; Thomas, S.; Haghighi, A.H. Glycolysis: An efficient route for recycling of end of life polyurethane foams. J. Polym. Res. 2021, 28, 1–19. [Google Scholar] [CrossRef]
- Hoang, C.N.; Pham, C.T.; Dang, T.M.; Hoang, D.; Lee, P.C.; Kang, S.J.; Kim, J. Novel oligo-ester-ether-diol prepared by waste poly(ethylene terephthalate) glycolysis and its use in preparing thermally stable and flame retardant polyurethane foam. Polymers 2019, 11, 236. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.; Shin, J. Modification of waterborne polyurethane by forming latex interpenetrating polymer networks with acrylate rubber. Colloid Polym. Sci. 2002, 280, 716–724. [Google Scholar] [CrossRef]
- Liu, L.; Ma, X.; Lv, R.; Wang, Z. Synthesis and characterization of a novel modified expandable graphite and its application in rigid polyurethane foams. Proceedings of the 2017 6th International Conference on Energy, Environment and Sustainable Development (ICEESD 2017), 11-12 March 2017, Zhuhai, China, 2017; 1027–1033. [Google Scholar]
- Korodi, T.; Marcu, N.; Tirnaveanu, A. Polyurethane microcellular elastomers: 2. Effect of chain extender on the mechanical properties. Polymer 1984, 25, 1211–1213. [Google Scholar] [CrossRef]
- Lin, F.; Lin, H.; Ke, J.; Liu, J.; Bai, X.; Chen, D. Preparation of reactive and additive flame retardant with different oxidation state of phosphorus on the thermal and flammability of thermoplastic polyurethane. Compos. Mater. 2019, 3, 43. [Google Scholar]
- Lingier, S.; Spiesschaert, Y.; Dhanis, B.; De Wildeman, S.; Du Prez, F.E. Rigid polyurethanes, polyesters, and polycarbonates from renewable ketal monomers. Macromolecules 2017, 50, 5346–5352. [Google Scholar] [CrossRef]
- Madkour, T.M.; Azzam, R.A. Non-Gaussian behavior of self-assembled thermoplastic polyurethane elastomers synthesized using two-step polymerization and investigated using constant-strain stress relaxation and molecular modeling techniques. Eur. Polym. J. 2013, 49, 439–451. [Google Scholar] [CrossRef]
- Prisacariu, C.; Scortanu, E. Influence of the type of chain extender and urethane group content on the mechanical properties of polyurethane elastomers with flexible hard segments. High Perform. Polym. 2011, 23, 308–313. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, G.; Wei, Z.; Sang, L.; Qi, M. Hemocompatibility evaluation of polyurethane film with surface-grafted poly (ethylene glycol) and carboxymethyl-chitosan. J. Appl. Polym. Sci. 2013, 127, 308–315. [Google Scholar] [CrossRef]
- Mohd Tahir, S.; Wan Salleh, W.N.; Nor Hadid, N.S.; Enderus, N.F.; Ismail, N.A. Synthesis of waste cooking oil-based polyol using sugarcane bagasse activated carbon and transesterification reaction for rigid polyurethane foam. Mater. Sci. Forum 2018, 1946, 020010. [Google Scholar]
- Napiórkowski, J.; Ligier, K. Investigation of wear resistance of polyurethanes in abrasive soil mass. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; Volume 1946, p. 020010. [Google Scholar]
- Prisacariu, C. Polyurethane Elastomers: From Morphology to Mechanical Aspects; Springer Science & Business Media: Berlin, Germany, 2011. [Google Scholar]
- Somdee, P.; Lassu-Kuknyo, T.; Konya, C.; Szabó, T.; Marossy, K. Investigating the properties and structure of polyurethane elastomers with monoethylene glycol chain extender. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2020; Volume 986, pp. 18–23. [Google Scholar]
- Rogante, M.; Söllradl, S. Characterization of mono-ethylene-glycol based industrial polyurethanes samples by fast-neutron radiography and neutron tomography. In J. Phys. Conf. Ser.; 2016; Volume 746. [Google Scholar]
- Sheel, A.; Pant, D. Chemical depolymerization of polyurethane foams via glycolysis and hydrolysis. In Recycling of Polyurethane Foams; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 67–75. [Google Scholar]
- Si, H.; Liu, H.; Shang, S.; Song, J.; Liao, S.; Wang, D.; Song, Z. Preparation and properties of maleopimaric acid-based polyester polyol dispersion for two-component waterborne polyurethane coating. Prog. Org. Coat. 2016, 90, 309–316. [Google Scholar] [CrossRef]
- Yeganeh, H.; Hojati-Talemi, P. Preparation and properties of novel biodegradable polyurethane networks based on castor oil and poly(ethylene glycol). Polym. Degrad. Stab. 2007, 92, 480–489. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; De Lucas, A.; Gutiérrez, C.; Rodríguez, J.F. Sustainable polyurethanes: Chemical recycling to get it. In Environment, Energy and Climate Change I; Springer: Cham, Switzerland, 2014; pp. 229–260. [Google Scholar]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J. Recycling of polyurethanes from laboratory to industry, a journey towards the sustainability. Waste Manag. 2018, 76, 147–171. [Google Scholar] [CrossRef] [PubMed]
- Somdee, P.; Lassú-Kuknyó, T.; Kónya, C.; Szabó, T.; Marossy, K. Thermal analysis of polyurethane elastomers matrix with different chain extender contents for thermal conductive application. J. Therm. Anal. Calorim. 2019, 138, 1003–1010. [Google Scholar] [CrossRef] [Green Version]
- Storey, R.F.; Hoffman, D.C. Polyurethane networks based on poly(ethylene ether carbonate) diols. Polymer 1992, 33, 2807–2816. [Google Scholar] [CrossRef]
- Strankowski, M. Effect of variation of hard segment content and graphene-based nanofiller concentration on morphological, thermal, and mechanical properties of polyurethane nanocomposites. Int. J. Polym. Sci. 2018, 51, 765–775. [Google Scholar] [CrossRef]
- Burns, V.; Emma, N.; Lear, B.J. Controlled rapid formation of polyurethane at 700 K: Thermodynamic and kinetic consequences of extreme photothermal heating. J. Phys. Chem. C 2019, 23, 14774–14780. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Chmiel, E.; Lubczak, J. Use of a mixture of polyols based on metasilicic acid and recycled pla for synthesis of rigid polyurethane foams susceptible to biodegradation. Int. J. Mol. Sci. 2021, 22, 69. [Google Scholar] [CrossRef]
- Zaiyad, S.A.A.; Sapari, J.M.; Mohamed, A.H.; Hanifah, N.S.A.; Ghazali, S.A.I.S.M.; Dzulkifli, N.N. Synthesis of used frying oil-based polyol for rigid polyurethane foam. Grow. Creat. Innov. Solut. 2016, 108, 3456–3469. [Google Scholar]
- Vasil’eva, S.Y.; Khitrova, T.K.; Kolyamshin, O.A.; Kol’tsov, N.I. Synthesis and properties of polyurethanes based on oligourethane dimethacrylate and bismaleimides. Int. Polym. Sci. Technol. 2007, 34, 41–46. [Google Scholar] [CrossRef]

| Sr. No. | Raw Materials | Chemical Formula | Quantity in Grams | Parts by Weight |
|---|---|---|---|---|
| 1. | Adipic Acid | C₆H₁₀O₄ | 146 | 51.95 |
| 2. | Monoethylene Glycol | C2H6O2 | 115 | 40.92 |
| 3. | Terephthalic Acid | C8H6O4 | 20 | 7.11 |
| 4. | Tetra Isopropyl Titanate (TITP) Catalyst | C12H28O4Ti | - | 0.005 |
| Sr. No. | Material | Content |
|---|---|---|
| 1. | Aromatic polyester polyol | Table 3 |
| 2. | Pure monomeric MDI-based prepolymer | Table 3 |
| 3. | Chain extender (mEG) | Table 3 |
| 4. | DABCO EG catalyst | 1.77 parts by weight |
| 5. | Silicone DC 193 | 0.5 parts by weight |
| Sr. No. | Chain Extender (mEG) (g) | Pure Monomeric MDI-Based Prepolymer (Parts by Weight) (Prepolymer/Aromatic Polyester Polyol) |
|---|---|---|
| 1. | 4 | 50/100 |
| 2. | 6 | 54/100 |
| 3. | 8 | 58/100 |
| 4. | 10 | 62/100 |
| 5. | 12 | 66/100 |
| Sr. No. | Parameters | Value |
|---|---|---|
| 1. | Acid value | 0.55 mg KOH/g |
| 2. | Viscosity @ 35 °C | 3200 cps |
| 3. | Hydroxyl number | 60 mg KOH/g |
| Sr. No. | Concentration of Mono (Ethylene Glycol) | Hardness of Polyurethane (Shore A) |
|---|---|---|
| 1. | 4 | 42 |
| 2. | 6 | 44 |
| 3. | 8 | 52 |
| 4. | 10 | 54 |
| 5. | 12 | 56 |
| Sr. No. | Concentration of Mono (Ethylene Glycol) | Abrasion Resistance of Polyurethane (mm) |
|---|---|---|
| 1. | 4 | 90 |
| 2. | 6 | 148 |
| 3. | 8 | 203 |
| 4. | 10 | 265 |
| 5. | 12 | 345 |
| Sr. No. | Concentration of Mono Ethylene Glycol | Density of Polyurethane (g/cc) |
|---|---|---|
| 1. | 4 | 0.94 |
| 2. | 6 | 0.92 |
| 3. | 8 | 0.90 |
| 4. | 10 | 0.88 |
| 5. | 12 | 0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafiq, M.; Butt, M.T.Z.; Khan, S.M. Synthesis of Mono Ethylene Glycol (MEG)-Based Polyurethane and Effect of Chain Extender on Its Associated Properties. Polymers 2021, 13, 3436. https://doi.org/10.3390/polym13193436
Shafiq M, Butt MTZ, Khan SM. Synthesis of Mono Ethylene Glycol (MEG)-Based Polyurethane and Effect of Chain Extender on Its Associated Properties. Polymers. 2021; 13(19):3436. https://doi.org/10.3390/polym13193436
Chicago/Turabian StyleShafiq, Muhammad, Muhammad Taqi Zahid Butt, and Shahzad Maqsood Khan. 2021. "Synthesis of Mono Ethylene Glycol (MEG)-Based Polyurethane and Effect of Chain Extender on Its Associated Properties" Polymers 13, no. 19: 3436. https://doi.org/10.3390/polym13193436
APA StyleShafiq, M., Butt, M. T. Z., & Khan, S. M. (2021). Synthesis of Mono Ethylene Glycol (MEG)-Based Polyurethane and Effect of Chain Extender on Its Associated Properties. Polymers, 13(19), 3436. https://doi.org/10.3390/polym13193436





