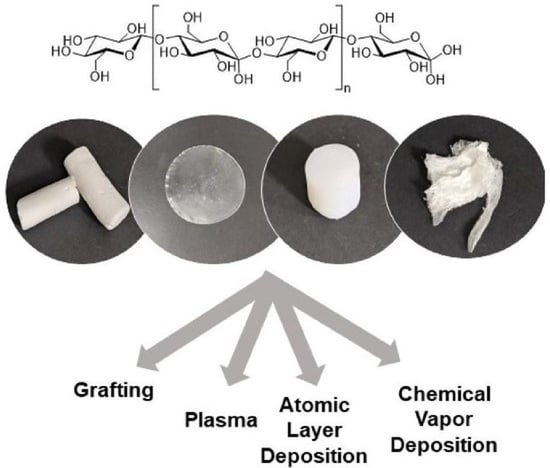
Production and Surface Modification of Cellulose Bioproducts
Abstract
:1. Introduction
2. Surface Modification
2.1. Plasma
2.2. Surface Grafting
2.3. Chemical Vapor Deposition
2.4. Atomic Layer Deposition
3. Surface Modification of Cellulose and Cellulose Bioproducts
3.1. Surface Modification of Textile Materials
3.2. Surface Modification of Cellulose Bioproducts
3.2.1. Porous Cellulose Products
3.2.2. Cellulose-Based Hydrogels
3.2.3. Aerocellulose Monoliths
3.2.4. Cellulose Beads
3.2.5. Films and Membranes
3.3. Surface Modification of Cellulose Nanomaterials
3.3.1. Cellulose Nanofibrils (CNFs)
3.3.2. Cellulose Nanocrystals (CNCs)
3.3.3. Bacterial Nanocellulose (BC)
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent strategies in preparation of cellulose nanocrystals and cellulose nanofibrils derived from raw cellulose materials. Int. J. Polym. Sci. 2018, 7923068. [Google Scholar] [CrossRef]
- Shende, P.; Pathan, N. Potential of carbohydrate-conjugated graphene assemblies in biomedical applications. Carbohydr. Polym. 2021, 255, 117385. [Google Scholar] [CrossRef]
- Nobles, D.R.; Brown, R.M. Many paths up the mountain: Tracking the evolution of cellulose byosynthesis. In Cellulose: Molecular and Structural Biology; Brown, R.M., Saxena, I.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 1–16. [Google Scholar]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Chang, X.X.; Mubarak, N.M.; Mazari, S.A.; Jatoi, A.S.; Ahmad, A.; Khalid, M.; Walvekar, R.; Abdullah, E.; Karri, R.R.; Siddiqui, M.; et al. A review on the properties and applications of chitosan, cellulose and deep eutectic solvent in green chemistry. J. Ind. Eng. Chem. 2021, in press. [Google Scholar]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
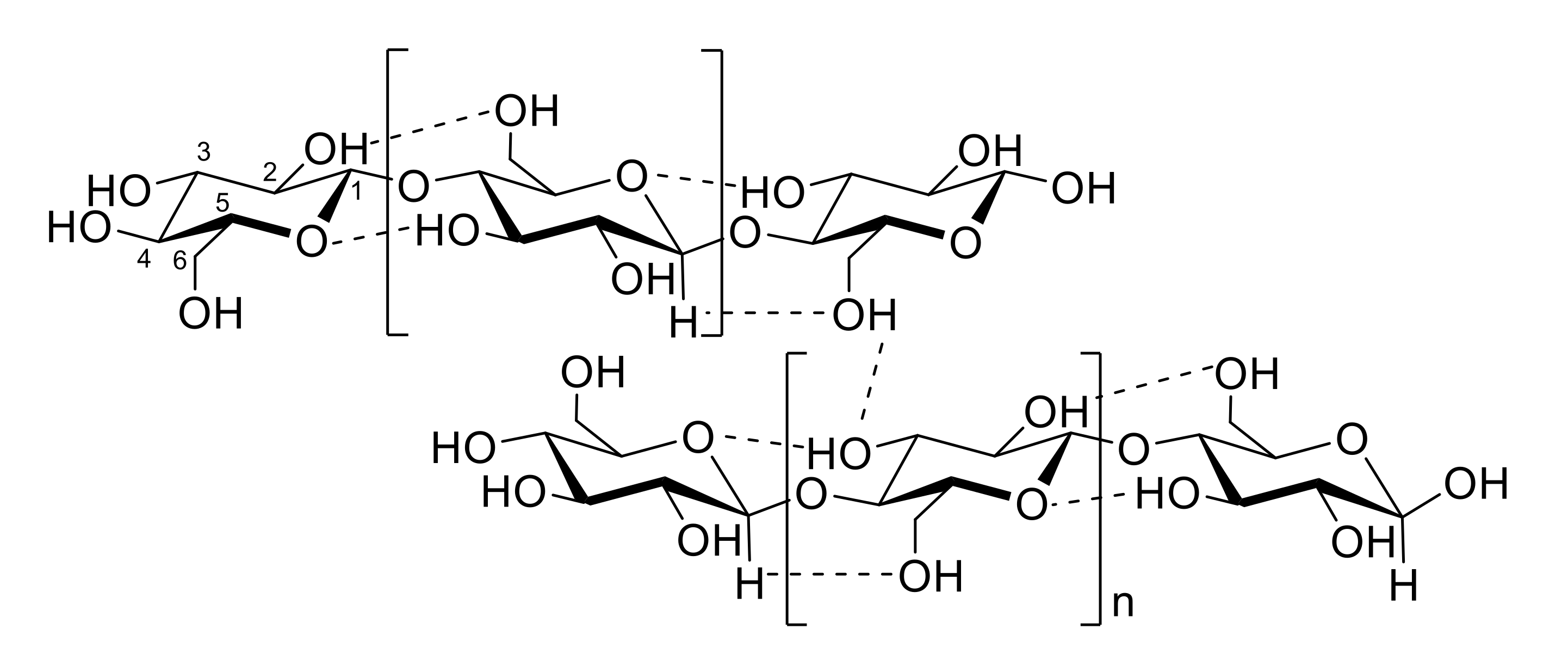
- Parthasarathi, R.; Bellesia, G.; Chundawat, S.P.S.; Dale, B.E.; Langan, P.; Gnanakaran, S. Insights into hydrogen bonding and stacking interactions in cellulose. J. Phys. Chem. A 2011, 115, 14191–14202. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Himmel, M.E. The maize primary cell wall microfibril: A new model derived from direct visualization. J. Agric. Food Chem. 2006, 54, 597–606. [Google Scholar] [CrossRef]
- Parviainen, H.; Parviainen, A.; Virtanen, T.; Kilpeläinen, I.; Ahvenainen, P.; Serimaa, R.; Grönqvist, S.; Maloney, T.; Maunu, S.L. Dissolution enthalpies of cellulose in ionic liquids. Carbohydr. Polym. 2014, 113, 67–76. [Google Scholar] [CrossRef]
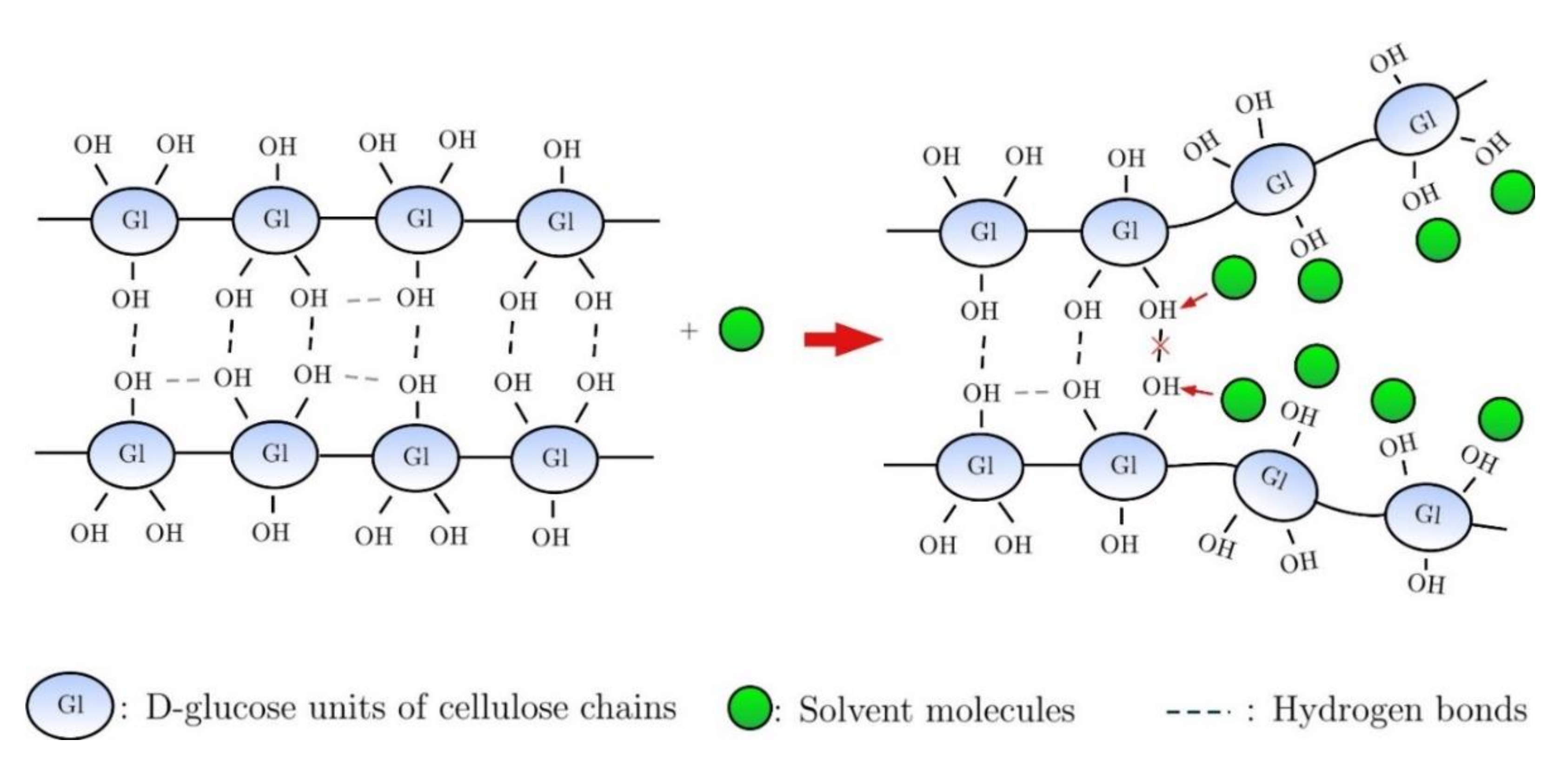
- Medronho, B.; Lindman, B. Competing forces during cellulose dissolution: From solvents to mechanisms. Curr. Opin. Colloid Interface Sci. 2014, 19, 32–40. [Google Scholar] [CrossRef]
- Atalla, R.H.; Vanderhart, D.L. Native cellulose: A composite of two distinct crystalline forms. Science 1984, 223, 283–285. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Acharya, S.; Hu, Y.; Abidi, N. Mild condition dissolution of high molecular weight cotton cellulose in 1-butyl-3-methylimidazolium acetate/N,N-dimethylacetamide solvent system. J. Appl. Polym. Sci. 2016, 135, 45928–45935. [Google Scholar] [CrossRef]
- Zhang, L.; Ruan, D.; Gao, S. Dissolution and regeneration of cellulose in NaOH/thiourea aqueous solution. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1521–1529. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K.R. Chemistry: Ionic liquids—Solvents of the future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Zhang, Y.; Jiang, K.; Wang, J.; Zhang, S. Why only ionic liquids with unsaturated heterocyclic cations can dissolve cellulose: A simulation study. ACS Sustain. Chem. Eng. 2017, 5, 3417–3428. [Google Scholar] [CrossRef]
- Dissanayake, N.; Thalangamaarachchige, V.D.; Thakurathi, M.; Knight, M.; Quitevis, E.L.; Abidi, N. Dissolution of cotton cellulose in 1:1 mixtures of 1-butyl-3-methylimidazolium methylphosphonate and 1-alkylimidazole co-solvents. Carbohydr. Polym. 2019, 221, 63–72. [Google Scholar] [CrossRef]
- Andanson, J.-M.; Bordes, E.; Devémy, J.; Leroux, F.; Padua, A.; Gomes, M.C. Understanding the role of co-solvents in the dissolution of cellulose in ionic liquids. Green Chem. 2014, 16, 2528–2538. [Google Scholar] [CrossRef] [Green Version]
- Velioğlu, S.; Yao, X.; Devémy, J.; Ahunbay, M.G.; Tantekin-Ersolmaz, S.B.; Dequidt, A.; Gomes, M.F.C.; Pádua, A.A.H. Solvation of a cellulose microfibril in imidazolium acetate ionic liquids: Effect of a cosolvent. J. Phys. Chem. B 2014, 118, 14860–14869. [Google Scholar] [CrossRef]
- Dissanayake, N.; Thalangamaarachchige, V.D.; Troxell, S.; Quitevis, E.L.; Abidi, N. Substituent effects on cellulose dissolution in imidazolium-based ionic liquids. Cellulose 2018, 25, 6887–6900. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Liu, X.; Zhang, S. Towards a molecular understanding of cellulose dissolution in ionic liquids: Anion/cation effect, synergistic mechanism and physicochemical aspects. Chem. Sci. 2018, 9, 4027–4043. [Google Scholar] [CrossRef] [Green Version]
- Medronho, B.; Lindman, B. Brief overview on cellulose dissolution/regeneration interactions and mechanisms. Adv. Colloid Interface Sci. 2014, 222, 502–508. [Google Scholar] [CrossRef]
- Liyanage, S.; Abidi, N. Molecular weight and organization of cellulose at different stages of cotton fiber development. Text. Res. J. 2019, 89, 726–738. [Google Scholar] [CrossRef]
- Abidi, N.; Manike, M. X-ray diffraction and FTIR investigations of cellulose deposition during cotton fiber development. Text. Res. J. 2017, 88, 719–730. [Google Scholar] [CrossRef]
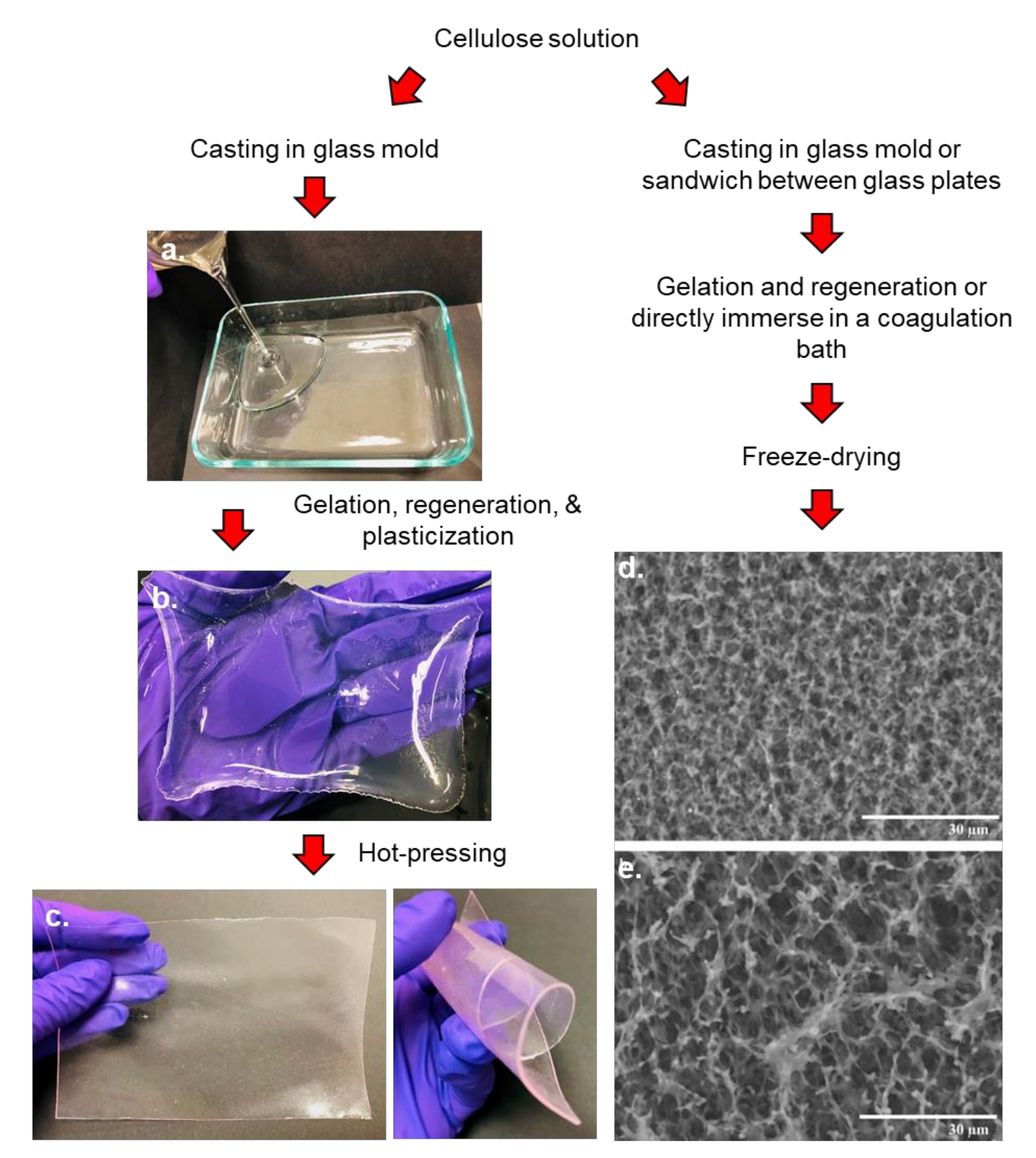
- Acharya, S.; Hu, Y.; Moussa, H.; Abidi, N. Preparation and characterization of transparent cellulose films using an improved cellulose dissolution process. J. Appl. Polym. Sci. 2017, 134, 44871. [Google Scholar] [CrossRef]
- Kosan, B.; Michels, C.; Meister, F. Dissolution and forming of cellulose with ionic liquids. Cellulose 2008, 15, 59–66. [Google Scholar] [CrossRef]
- Hauru, L.; Hummel, M.; Michud, A.; Sixta, H. Dry jet-wet spinning of strong cellulose filaments from ionic liquid solution. Cellulose 2014, 21, 4471–4481. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.G.; Zhang, Z.N.; Wu, J.; Zhang, J.; He, J.S. Regenerated-cellulose/multiwalled- carbon-nanotube composite fibers with enhanced mechanical properties prepared with the ionic liquid 1-allyl-3-methylimidazolium chloride. Adv. Mater. 2007, 19, 698–704. [Google Scholar] [CrossRef]
- Frenot, A.; Henriksson, M.W.; Walkenström, P. Electrospinning of cellulose-based nanofibers. J. Appl. Polym. Sci. 2007, 103, 1473–1482. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Electrospinning of ultrafine cellulose acetate fibers: Studies of a new solvent system and deacetylation of ultrafine cellulose acetate fibers. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 5–11. [Google Scholar] [CrossRef]
- Quan, S.-L.; Kang, S.-G.; Chin, I.-J. Characterization of cellulose fibers electrospun using ionic liquid. Cellulose 2010, 17, 223–230. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, B.; Li, H.-S.; Li, Y.-Q.; Ou, S.-Y. Green composite films composed of nanocrystalline cellulose and a cellulose matrix regenerated from functionalized ionic liquid solution. Carbohydr. Polym. 2010, 84, 383–389. [Google Scholar] [CrossRef]
- Wang, Q.; Cai, J.; Zhang, L.; Xu, M.; Cheng, H.; Han, C.C.; Kuga, S.; Xiao, J.; Xiao, R. A bioplastic with high strength constructed from a cellulose hydrogel by changing the aggregated structure. J. Mater. Chem. A 2013, 1, 6678–6686. [Google Scholar] [CrossRef]
- Tan, C.; Fung, B.M.; Newman, J.K.; Vu, C. Organic aerogels with very high impact strength. Adv. Mater. 2001, 13, 644–646. [Google Scholar] [CrossRef]
- Parajuli, P.; Acharya, S.; Hu, Y.; Abidi, N. Cellulose-based monoliths with enhanced surface area and porosity. J. Appl. Polym. Sci. 2020, 137, 48975. [Google Scholar] [CrossRef]
- Hu, Y.; Li, S.; Jackson, T.; Moussa, H.; Abidi, N. Preparation, characterization, and cationic functionalization of cellulose-based aerogels for wastewater clarification. J. Mater. 2016, 3186589. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Song, Y.; Xu, F. Highly transparent and hazy cellulose nanopaper simultaneously with a self-cleaning superhydrophobic surface. ACS Sustain. Chem. Eng. 2018, 6, 5173–5181. [Google Scholar] [CrossRef]
- Lin, X.; Ma, W.; Chen, L.; Huang, L.; Wu, H.; Takahara, A. Self-healing cellulose nanocrystal-stabilized droplets for water collection under oil. Soft Matter 2018, 14, 9308–9311. [Google Scholar] [CrossRef]
- Rasouli, S.; Rezaei, N.; Hamedi, H.; Zendehboudi, S.; Duan, X. Superhydrophobic and superoleophilic membranes for oil-water separation application: A comprehensive review. Mater. Des. 2021, 204, 109599. [Google Scholar] [CrossRef]
- Zhan, Y.; Hao, X.; Wang, L.; Jiang, X.; Cheng, Y.; Wang, C.; Meng, Y.; Xia, H.; Chen, Z. Superhydrophobic and flexible silver nanowire-coated cellulose filter papers with sputter-deposited nickel nanoparticles for ultrahigh electromagnetic interference shielding. ACS Appl. Mater. Interfaces 2021, 13, 14623–14633. [Google Scholar] [CrossRef]
- Wei, D.W.; Wei, H.; Gauthier, A.C.; Song, J.; Jin, Y.; Xiao, H. Superhydrophobic modification of cellulose and cotton textiles: Methodologies and applications. J. Bioresour. Bioprod. 2020, 5, 1–15. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lee, H.-S.; Seo, J.; Kang, Y.-H.; Kim, W.; Kang, T.H.-K. State-of-the-art of cellulose nanocrystals and optimal method for their dispersion for construction-related applications. Appl. Sci. 2019, 9, 426. [Google Scholar] [CrossRef] [Green Version]
- Rauscher, H.; Perucca, M.; Buyle, G. (Eds.) Part I. Introduction to plasma technology for surface functionalization. In Plasma Technology for Hyperfunctional Surfaces. Food, Biomedical and Textile Applications; Wiley-VCH: Weinheim, Germany, 2010; pp. 3–32. [Google Scholar]
- Denes, F.; Manolache, S. Macromolecular plasma-chemistry: An emerging field of polymer science. Prog. Polym. Sci. 2004, 29, 815–885. [Google Scholar] [CrossRef]
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: A review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef] [Green Version]
- Morent, R.; De Geyter, N.; Desmet, T.; Dubruel, P.; Leys, C. Plasma surface modification of biodegradable polymers: A review. Plasma Process. Polym. 2011, 8, 171–190. [Google Scholar] [CrossRef]
- Abidi, N.; Hequet, E. Cotton fabric graft copolymerization using microwave plasma. I. Universal attenuated total reflectance-FTIR study. J. Appl. Polym. Sci. 2004, 93, 145–154. [Google Scholar] [CrossRef]
- Mather, R.R. Surface modification of textiles by plasma treatments. In Surface Modification of Textiles; Wei, Q., Ed.; Woodhead Publishing: Sawston, UK, 2009; pp. 296–317. [Google Scholar]
- Bhat, N.; Benjamin, Y. Surface resistivity behavior of plasma treated and plasma grafted cotton and polyester fabrics. Text. Res. J. 1999, 69, 38–42. [Google Scholar] [CrossRef]
- Ryu, G.H.; Yang, W.-S.; Roh, H.-W.; Lee, I.-S.; Kim, J.K.; Lee, G.H.; Lee, D.H.; Park, B.J.; Lee, M.S.; Park, J.-C. Plasma surface modification of poly (d,l-lactic-co-glycolic acid) (65/35) film for tissue engineering. Surf. Coatings Technol. 2005, 193, 60–64. [Google Scholar] [CrossRef]
- Lee, S.-D.; Hsiue, G.-H.; Kao, C.-Y.; Chang, P.C.-T. Artificial cornea: Surface modification of silicone rubber membrane by graft polymerization of pHEMA via glow discharge. Biomaterials 1996, 17, 587–595. [Google Scholar] [CrossRef]
- Lin, R.; Li, A.; Zheng, T.; Lu, L.; Cao, Y. Hydrophobic and flexible cellulose aerogel as an efficient, green and reusable oil sorbent. RSC Adv. 2015, 5, 82027–82033. [Google Scholar] [CrossRef]
- Alanis, A.; Valdés, J.H.; Guadalupe, N.-V.M.; Lopez, R.; Mendoza, R.; Mathew, A.P.; de León, R.D.; Valencia, L. Plasma surface-modification of cellulose nanocrystals: A green alternative towards mechanical reinforcement of ABS. RSC Adv. 2019, 9, 17417–17424. [Google Scholar] [CrossRef] [Green Version]
- Rauscher, H.; Perucca, M.; Buyle, G. (Eds.) Part II. Hyperfunctional surfaces for textiles, food and biomedical applications plasma technology for hyperfunctional surfaces. In Plasma Technology for Hyperfunctional Surfaces. Food, Biomedical and Textile Applications; Wiley-VCH: Weinheim, Germany, 2010; pp. 33–65. [Google Scholar]
- Roy, D.; Semsarilar, M.; Guthrie, J.T.; Perrier, S. Cellulose modification by polymer grafting: A review. Chem. Soc. Rev. 2009, 38, 2046–2064. [Google Scholar] [CrossRef] [PubMed]
- Joubert, F.; Musa, O.M.; Hodgson, D.R.W.; Cameron, N.R. The preparation of graft copolymers of cellulose and cellulose derivatives using ATRP under homogeneous reaction conditions. Chem. Soc. Rev. 2014, 43, 7217–7235. [Google Scholar] [CrossRef] [Green Version]
- Abidi, N. Surface grafting of textiles. In Surface Modification of Textiles; Wei, Q., Ed.; Woodhead Publishing: Sawston, UK, 2009; pp. 91–107. [Google Scholar]
- Kumar, R.; Sharma, R.K.; Singh, A.P. Grafted cellulose: A bio-based polymer for durable applications. Polym. Bull. 2018, 75, 2213–2242. [Google Scholar] [CrossRef]
- Shipp, D. Reversible-deactivation radical polymerizations. Polym. Rev. 2011, 51, 99–103. [Google Scholar] [CrossRef]
- Glasing, J.; Champagne, P.; Cunningham, M. Graft modification of chitosan, cellulose and alginate using reversible deactivation radical polymerization (RDRP). Curr. Opin. Green Sustain. Chem. 2016, 2, 15–21. [Google Scholar] [CrossRef]
- Marić, M. History of nitroxide mediated polymerization in Canada. Can. J. Chem. Eng. 2021, 99, 832–852. [Google Scholar] [CrossRef]
- Littunen, K.; Hippi, U.; Johansson, L.-S.; Österberg, M.; Tammelin, T.; Laine, J.; Seppälä, J. Free radical graft copolymerization of nanofibrillated cellulose with acrylic monomers. Carbohydr. Polym. 2011, 84, 1039–1047. [Google Scholar] [CrossRef]
- Tang, J.; Lee, M.F.X.; Zhang, W.; Zhao, B.; Berry, R.M.; Tam, K.C. Dual responsive pickering emulsion stabilized by poly[2-(dimethylamino)ethyl methacrylate] grafted cellulose nanocrystals. Biomacromolecules 2014, 15, 3052–3060. [Google Scholar] [CrossRef]
- Abidi, N.; Hequet, E. Cotton fabric graft copolymerization using microwave plasma. II. Physical properties. J. Appl. Polym. Sci. 2005, 98, 896–902. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P.; Matyjaszewski, K. Modification of wood-based materials by atom transfer radical polymerization methods. Eur. Polym. J. 2019, 120, 109253. [Google Scholar] [CrossRef]
- Chmielarz, P. Cellulose-based graft copolymers prepared by simplified electrochemically mediated ATRP. Express Polym. Lett. 2017, 11, 140–151. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Jiang, F.; Fang, H.; Wang, Z. Synthesis and characterization of designed cellulose-graft-polyisoprene copolymers. Polym. Chem. 2014, 5, 3379–3388. [Google Scholar] [CrossRef]
- Moreira, G.; Fedeli, E.; Ziarelli, F.; Capitani, D.; Mannina, L.; Charles, L.; Viel, S.; Gigmes, D.; Lefay, C. Synthesis of polystyrene-grafted cellulose acetate copolymers via nitroxide-mediated polymerization. Polym. Chem. 2015, 6, 5244–5253. [Google Scholar] [CrossRef] [Green Version]
- Kodama, Y.; Barsbay, M.; Güven, O. Radiation-induced and RAFT-mediated grafting of poly(hydroxyethyl methacrylate) (PHEMA) from cellulose surfaces. Radiat. Phys. Chem. 2014, 94, 98–104. [Google Scholar] [CrossRef]
- Lönnberg, H.; Zhou, Q.; Brumer, H.; Teeri, T.T.; Malmström, E.; Hult, A. Grafting of cellulose fibers with poly(ε-caprolactone) and poly(l-lactic acid) via ring-opening polymerization. Biomacromolecules 2006, 7, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Wohlhauser, S.; Delepierre, G.; Labet, M.; Morandi, G.; Thielemans, W.; Weder, C.; Zoppe, J.O. Grafting polymers from cellulose nanocrystals: Synthesis, properties, and applications. Macromolecules 2018, 51, 6157–6189. [Google Scholar] [CrossRef] [Green Version]
- Gates, S.M. Surface chemistry in the chemical vapor deposition of electronic materials. Chem. Rev. 1996, 96, 1519–1532. [Google Scholar] [CrossRef]
- Coclite, A.M.; Howden, R.M.; Borrelli, D.C.; Petruczok, C.D.; Yang, R.; Yagüe, J.L.; Ugur, A.; Chen, N.; Lee, S.; Jo, W.J.; et al. 25th anniversary article: CVD polymers: A new paradigm for surface modification and device fabrication. Adv. Mater. 2013, 25, 5392–5423. [Google Scholar] [CrossRef]
- Chen, N.; Kim, D.H.; Kovacik, P.; Sojoudi, H.; Wang, M.; Gleason, K.K. polymer thin films and surface modification by chemical vapor deposition: Recent progress. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Nanda, D. Methods and fabrication techniques of superhydrophobic surfaces. In Superhydrophobic Polymer Coatings Fundamentals, Design, Fabrication, and Applications; Samal, S.K., Mohanty, S., Nayak, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 43–75. [Google Scholar]
- Yagüe, J.L.; Coclite, A.M.; Petruczok, C.; Gleason, K.K. Chemical vapor deposition for solvent-free polymerization at surfaces. Macromol. Chem. Phys. 2013, 214, 302–312. [Google Scholar] [CrossRef]
- Şakalak, H.; Yılmaz, K.; Gürsoy, M.; Karaman, M. Roll-to roll initiated chemical vapor deposition of super hydrophobic thin films on large-scale flexible substrates. Chem. Eng. Sci. 2020, 215, 115466. [Google Scholar] [CrossRef]
- Leal, S.; Cristelo, C.; Silvestre, S.; Fortunato, E.; Sousa, A.; Alves, A.; Correia, D.M.; Lanceros-Mendez, S.; Gama, M. Hydrophobic modification of bacterial cellulose using oxygen plasma treatment and chemical vapor deposition. Cellulose 2020, 27, 10733–10746. [Google Scholar] [CrossRef]
- Kettunen, M.; Silvennoinen, R.J.; Houbenov, N.; Nykänen, A.; Ruokolainen, J.; Sainio, J.; Pore, V.; Kemell, M.; Ankerfors, M.; Lindström, T.; et al. Photoswitchable superabsorbency based on nanocellulose aerogels. Adv. Funct. Mater. 2011, 21, 510–517. [Google Scholar] [CrossRef]
- Bashir, T.; Skrifvars, M.; Persson, N.-K. Production of highly conductive textile viscose yarns by chemical vapor deposition technique: A route to continuous process. Polym. Adv. Technol. 2011, 22, 2214–2221. [Google Scholar] [CrossRef]
- Leskela, M.; Niinisto, J.; Ritala, M. Atomic layer deposition. In Handbook of Thin Films, 4th ed.; Seshan, K., Schrepis, D., Eds.; Academic Press: Cambridge, MA, USA, 2002; pp. 103–159. [Google Scholar]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Ritala, M.; Niinistö, J. Industrial applications of atomic layer deposition. ECS Trans. 2019, 25, 641–652. [Google Scholar] [CrossRef]
- Hyde, G.K.; McCullen, S.D.; Jeon, S.; Stewart, S.M.; Jeon, H.; Loboa, E.G.; Parsons, G.N. Atomic layer deposition and biocompatibility of titanium nitride nano-coatings on cellulose fiber substrates. Biomed. Mater. 2009, 4, 025001. [Google Scholar] [CrossRef]
- Popescu, M.; Ungureanu, C.; Buse, E.; Nastase, F.; Tucureanu, V.; Suchea, M.P.; Draga, S. Antibacterial efficiency of cellulose-based fibers covered with ZnO and Al2O3 by atomic layer deposition. Appl. Surf. Sci. 2019, 481, 1287–1298. [Google Scholar] [CrossRef]
- Putkonen, M.; Sippola, P.; Svärd, L.; Sajavaara, T.; Vartiainen, J.; Buchanan, I.; Forsström, U.; Simell, P.; Tammelin, T. Low-temperature atomic layer deposition of SiO2/Al2O3 multilayer structures constructed on self-standing films of cellulose nanofibrils. Philos. Trans. R. Soc A Math. Phys. Eng. Sci. 2018, 376, 20170037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, N.D. (Ed.) Introduction to smart nanotextiles. In Smart Textiles: Wearable Nanotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 1–37. [Google Scholar]
- Pakdel, E.; Jian, F.; Sun, L.; Wang, X. Nanocoatings for smart textiles. In Smart Textiles: Wearable Nanotechnology; Yilmaz, N.D., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 247–300. [Google Scholar]
- Pulit-Prociak, J.; Chwastowski, J.; Kucharski, A.; Banach, M. Functionalization of textiles with silver and zinc oxide nanoparticles. Appl. Surf. Sci. 2016, 385, 543–553. [Google Scholar] [CrossRef]
- Riaz, S.; Ashraf, M.; Hussain, T.; Hussain, M.T.; Younus, A. Fabrication of Robust Multifaceted Textiles by Application of Functionalized TiO2 nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 581, 123799. [Google Scholar] [CrossRef]
- Mohamed, A.L.; El-Naggar, M.E.; Shaheen, T.I.; Hassabo, A.G. Laminating of chemically modified silan based nanosols for advanced functionalization of cotton textiles. Int. J. Biol. Macromol. 2017, 95, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Abidi, N.; Hequet, E.; Tarimala, S.; Dai, L.L. Cotton fabric surface modification for improved UV radiation protection using sol-gel process. J. Appl. Polym. Sci. 2007, 104, 111–117. [Google Scholar] [CrossRef]
- Daoud, W.A.; Xin, J.H. Low temperature sol-gel processed photocatalytic titania coating. J. Sol-Gel Sci. Technol. 2004, 29, 25–29. [Google Scholar] [CrossRef]
- Wang, P.; Dong, Y.; Li, B.; Li, Z.; Bian, L. A sustainable and cost effective surface functionalization of cotton fabric using TiO2 hydrosol produced in a pilot scale: Condition optimization, sunlight-driven photocatalytic activity and practical applications. Ind. Crop. Prod. 2018, 123, 197–207. [Google Scholar] [CrossRef]
- Abidi, N.; Kikens, P. Chemical functionalisation of cotton fabric to impart multifunctional properties. Tekstilec 2016, 59, 156–161. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Hequet, E. Functionalization of a cotton fabric surface with titania nanosols: Applications for self-cleaning and UV-protection properties. ACS Appl. Mater. Interfaces 2009, 1, 2141–2146. [Google Scholar] [CrossRef]
- Yuranova, T.; Mosteo, R.; Bandara, J.; Laub, D.; Kiwi, J. Self-cleaning cotton textiles surfaces modified by photoactive SiO2/TiO2 coating. J. Mol. Catal. A Chem. 2006, 244, 160–167. [Google Scholar] [CrossRef]
- Moiz, A.; Vijayan, A.; Padhye, R.; Wang, X. Chemical and water protective surface on cotton fabric by pad-knife-pad coating of WPU-PDMS-TMS. Cellulose 2016, 23, 3377–3388. [Google Scholar] [CrossRef]
- Abidi, N.; Aminayi, P.; Cabrales, L.; Fiber, E.H. Super-hydrophobic cotton fabric prepared using nanoparticles and molecular vapor deposition methods. In Functional Materials from Renewable Sources; Liebner, F., Rosenau, T., Eds.; American Chemical Society: Washington, DC, USA, 2012; Volume 1107, pp. 149–165. [Google Scholar]
- Aminayi, P.; Abidi, N. Imparting super hydro/oleophobic properties to cotton fabric by means of molecular and nanoparticles vapor deposition methods. Appl. Surf. Sci. 2013, 287, 223–231. [Google Scholar] [CrossRef]
- Cabrales, L.; Abidi, N. Microwave plasma induced grafting of oleic acid on cotton fabric surfaces. Appl. Surf. Sci. 2012, 258, 4636–4641. [Google Scholar] [CrossRef]
- Airoudj, A.; Gall, F.B.-L.; Roucoules, V. Textile with durable janus wetting properties produced by plasma polymerization. J. Phys. Chem. C 2016, 120, 29162–29172. [Google Scholar] [CrossRef]
- Qin, H.; Li, X.; Zhang, X.; Guo, Z. Preparation and performance testing of superhydrophobic flame retardant cotton fabric. N. J. Chem. 2019, 43, 5839–5848. [Google Scholar] [CrossRef]
- Nie, S.; Jin, D.; Yang, J.-N.; Dai, G.; Luo, Y. Fabrication of environmentally-benign flame retardant cotton fabrics with hydrophobicity by a facile chemical modification. Cellulose 2019, 26, 5147–5158. [Google Scholar] [CrossRef]
- Song, L.; Sun, L.; Zhao, J.; Wang, X.; Yin, J.; Luan, S.; Ming, W. Synergistic superhydrophobic and photodynamic cotton textiles with remarkable antibacterial activities. ACS Appl. Bio-Mater. 2019, 2, 2756–2765. [Google Scholar] [CrossRef]
- Montaser, A.; Rehan, M.; El-Senousy, W.; Zaghloul, S. Designing strategy for coating cotton gauze fabrics and its application in wound healing. Carbohydr. Polym. 2020, 244, 116479. [Google Scholar] [CrossRef]
- Kianfar, P.; Abate, M.; Trovato, V.; Rosace, G.; Ferri, A.; Bongiovanni, R.; Vitale, A. Surface functionalization of cotton fabrics by photo-grafting for pH sensing applications. Front. Mater. 2020, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Guido, E.; Colleoni, C.; De Clerck, K.; Plutino, M.R.; Rosace, G. Influence of catalyst in the synthesis of a cellulose-based sensor: Kinetic study of 3-glycidoxypropyltrimethoxysilane epoxy ring opening by Lewis acid. Sens. Actuators B Chem. 2014, 203, 213–222. [Google Scholar] [CrossRef]
- Rosace, G.; Guido, E.; Colleoni, C.; Brucale, M.; Piperopoulos, E.; Milone, C.; Plutino, M.R. Halochromic resorufin-GPTMS hybrid sol-gel: Chemical-physical properties and use as pH sensor fabric coating. Sens. Actuators B Chem. 2017, 241, 85–95. [Google Scholar] [CrossRef]
- Hoang-Van, C.; Zegaoui, O.; Pichat, P. Vanadia–titania aerogel deNOx catalysts. J. Non. Cryst. Solids 1998, 225, 157–162. [Google Scholar] [CrossRef]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose aerogels: Synthesis, applications, and prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef] [Green Version]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable cellulose-based hydrogels: Design and applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Matsumoto, Y.; Kuga, S. Cellulose gel and aerogel from LiCl/DMSO solution. Cellulose 2012, 19, 393–399. [Google Scholar] [CrossRef]
- de Moraes, J.O.; Scheibe, A.S.; Sereno, A.; Laurindo, J.B. Scale-up of the production of cassava starch based films using tape-casting. J. Food Eng. 2013, 119, 800–808. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L.; Zhou, J.; Zhang, L.; Kennedy, J.F. Structure and properties of hydrogels prepared from cellulose in NaOH/urea aqueous solutions. Carbohydr. Polym. 2010, 82, 122–127. [Google Scholar] [CrossRef]
- Kadokawa, J.-I.; Murakami, M.-A.; Kaneko, Y. A facile preparation of gel materials from a solution of cellulose in ionic liquid. Carbohydr. Res. 2008, 343, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Isobe, N.; Kim, U.-J.; Kimura, S.; Wada, M.; Kuga, S. Internal surface polarity of regenerated cellulose gel depends on the species used as coagulant. J. Colloid Interface Sci. 2011, 359, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chang, C.; Zhang, R.; Zhang, L. Hydrogels prepared from unsubstituted cellulose in NaOH/Urea aqueous solution. Macromol. Biosci. 2007, 7, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Ma, J.; Li, N. Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly(AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel. Carbohydr. Polym. 2011, 84, 76–82. [Google Scholar] [CrossRef]
- Kim, U.-J.; Kuga, S. Polyallylamine-grafted cellulose gel as high-capacity anion-exchanger. J. Chromatogr. A 2002, 946, 283–289. [Google Scholar] [CrossRef]
- RodrÍguez, R.; Alvarez-Lorenzo, C.; Concheiro, A. Cationic cellulose hydrogels: Kinetics of the cross-linking process and characterization as pH-/ion-sensitive drug delivery systems. J. Control. Release 2003, 86, 253–265. [Google Scholar] [CrossRef]
- Way, A.E.; Hsu, L.; Shanmuganathan, K.; Weder, C.; Rowan, S.J. pH-responsive cellulose nanocrystal gels and nanocomposites. ACS Macro Lett. 2012, 1, 1001–1006. [Google Scholar] [CrossRef]
- Ali, A.E.-H.; El-Rehim, H.A.A.; Kamal, H.; Hegazy, D.E.A. Synthesis of carboxymethyl cellulose based drug carrier hydrogel using ionizing radiation for possible use as site specific delivery system. J. Macromol. Sci. Part A 2008, 45, 628–634. [Google Scholar] [CrossRef]
- Aulin, C.; Netrval, J.; Wågberg, L.; Lindström, T. Aerogels from nanofibrillated cellulose with tunable oleophobicity. Soft Matter 2010, 6, 3298–3305. [Google Scholar] [CrossRef]
- Gustavsson, P.-E.; Larsson, P.-O. Monolithic Polysaccharide materials. In Monolithic Materials: Preparation, Properties and Applications; Svec, F., Tennikova, T.B., Deyl, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 67, pp. 121–141. [Google Scholar]
- Potter, O.G.; Hilder, E.F. Porous polymer monoliths for extraction: Diverse applications and platforms. J. Sep. Sci. 2008, 31, 1881–1906. [Google Scholar] [CrossRef]
- Schestakow, M.; Karadagli, I.; Ratke, L. Cellulose aerogels prepared from an aqueous zinc chloride salt hydrate melt. Carbohydr. Polym. 2016, 137, 642–649. [Google Scholar] [CrossRef]
- Innerlohinger, J.; Weber, H.K.; Kraft, G. Aerocellulose: Aerogels and aerogel-like materials made from cellulose. Macromol. Symp. 2006, 244, 126–135. [Google Scholar] [CrossRef]
- Strong, E.B.; Schultz, S.A.; Martinez, A.W.; Martinez, N.W. Fabrication of miniaturized paper-based microfluidic devices (MicroPADs). Sci. Rep. 2019, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Xin, Y.; Xiong, Q.; Bai, Q.; Miyamoto, M.; Li, C.; Shen, Y.; Uyama, H. A hierarchically porous cellulose monolith: A template-free fabricated, morphology-tunable, and easily functionalizable platform. Carbohydr. Polym. 2017, 157, 429–437. [Google Scholar] [CrossRef]
- Pan, X.; Jinka, S.; Singh, V.K.; Ramkumar, S.; Karim, M.N. Cellulose monolith with tunable functionality for immobilization of influenza virus. J. Bioeng. Biomed. Sci. 2018, 8, 1000246. [Google Scholar] [CrossRef]
- He, X.; Cheng, L.; Wang, Y.; Zhao, J.; Zhang, W.; Lu, C. Aerogels from quaternary ammonium-functionalized cellulose nanofibers for rapid removal of Cr(VI) from water. Carbohydr. Polym. 2014, 111, 683–687. [Google Scholar] [CrossRef]
- Gericke, M.; Trygg, J.; Fardim, P. Functional cellulose beads: Preparation, characterization, and applications. Chem. Rev. 2013, 113, 4812–4836. [Google Scholar] [CrossRef]
- Voon, L.K.; Pang, S.C.; Chin, S.F. Porous cellulose beads fabricated from regenerated cellulose as potential drug delivery carriers. J. Chem. 2017, 2017, 1943432. [Google Scholar] [CrossRef] [Green Version]
- Qi, H. Novel Functional Materials Based on Cellulose, 1st ed.; Springer Briefs in Applied Sciences and Technology; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 25–43. [Google Scholar]
- Trygg, J.; Fardim, P.; Gericke, M.; Mäkilä, E.; Salonen, J. Physicochemical design of the morphology and ultrastructure of cellulose beads. Carbohydr. Polym. 2013, 93, 291–299. [Google Scholar] [CrossRef]
- Karbstein, H.P.; Schubert, H. Developments in the continuous mechanical production of oil-in-water macro-emulsions. Chem. Eng. Process. Process. Intensif. 1995, 34, 205–211. [Google Scholar] [CrossRef]
- Ruan, C.-Q.; Strømme, M.; Lindh, J. Preparation of porous 2,3-dialdehyde cellulose beads crosslinked with chitosan and their application in adsorption of Congo red dye. Carbohydr. Polym. 2018, 181, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Benhalima, T.; Ferfera-Harrar, H.; Lerari, D. Optimization of carboxymethyl cellulose hydrogels beads generated by an anionic surfactant micelle templating for cationic dye uptake: Swelling, sorption and reusability studies. Int. J. Biol. Macromol. 2017, 105, 1025–1042. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, L.; Wang, Y. Polymer fractionation using chromatographic column packed with novel regenerated cellulose beads modified with silane. J. Chromatogr. A 2005, 1063, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, D.; Morimoto, Y.; Saito, H. Influences of chemical modifications on the mechanical strength of cellulose beads. Cellulose 1998, 5, 135–151. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Zhang, N.; Song, P.; Hao, J.-Y.; Wan, Y.; Yao, X.-H.; Chen, T.; Li, L. Functionalized cellulose beads with three dimensional porous structure for rapid adsorption of active constituents from Pyrola incarnata. Carbohydr. Polym. 2018, 181, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Garnier, G.; Wright, J.; Godbout, L.; Yu, L. Wetting mechanism of alkyl ketene dimers on cellulose films. Colloids Surfaces A Physicochem. Eng. Asp. 1998, 145, 153–165. [Google Scholar] [CrossRef]
- Da Róz, A.; Leite, F.; Pereiro, L.; Nascente, P.; Zucolotto, V.; Oliveira, O.; Carvalho, A. Adsorption of chitosan on spin-coated cellulose films. Carbohydr. Polym. 2010, 80, 65–70. [Google Scholar] [CrossRef]
- Turner, M.B.; Spear, S.K.; Holbrey, J.; Rogers, R.D. Production of bioactive cellulose films reconstituted from ionic liquids. Biomacromolecules 2004, 5, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Khare, V.P.; Greenberg, A.R.; Kelley, S.S.; Pilath, H.; Roh, I.J.; Tyber, J. Synthesis and characterization of dense and porous cellulose films. J. Appl. Polym. Sci. 2007, 105, 1228–1236. [Google Scholar] [CrossRef]
- Cai, J.; Wang, L.; Zhang, L. Influence of coagulation temperature on pore size and properties of cellulose membranes prepared from NaOH–urea aqueous solution. Cellulose 2007, 14, 205–215. [Google Scholar] [CrossRef]
- Yang, Q.; Fukuzumi, H.; Saito, T.; Isogai, A.; Zhang, L. Transparent cellulose films with high gas barrier properties fabricated from aqueous alkali/urea solutions. Biomacromolecules 2011, 12, 2766–2771. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Mei, Y.; Cheng, L.; Zhou, J.; Zhang, L. Preparation of copper nanoparticles coated cellulose films with antibacterial properties through one-step reduction. ACS Appl. Mater. Interfaces 2012, 4, 2897–2902. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, L.; Xu, M. Novel regenerated cellulose films prepared by coagulating with water: Structure and properties. Carbohydr. Polym. 2012, 87, 95–100. [Google Scholar] [CrossRef]
- Bedane, A.H.; Eić, M.; Farmahini-Farahani, M.; Xiao, H. Water vapor transport properties of regenerated cellulose and nanofibrillated cellulose films. J. Membr. Sci. 2014, 493, 46–57. [Google Scholar] [CrossRef]
- Voicu, S.I.; Muhulet, A.; Antoniac, I.V.; Corobea, M.S. Cellulose derivatives based membranes for biomedical applications. Key Eng. Mater. 2015, 638, 27–30. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, Y.; Zhou, J.; Cai, J. Effects of coagulation conditions on the properties of regenerated cellulose films prepared in NaOH/urea aqueous solution. Ind. Eng. Chem. Res. 2005, 44, 522–529. [Google Scholar] [CrossRef]
- Rumi, S.S.; Liyanage, S.; Abidi, N. Conversion of low-quality cotton to bioplastics. Cellulose 2021, 28, 2021–2038. [Google Scholar] [CrossRef]
- Fernandes, S.C.; Sadocco, P.; Alonso-Varona, A.; Palomares, T.; Eceiza, A.; Silvestre, A.; Mondragon, I.; Freire, C. Bioinspired antimicrobial and biocompatible bacterial cellulose membranes obtained by surface functionalization with aminoalkyl groups. ACS Appl. Mater. Interfaces 2013, 5, 3290–3297. [Google Scholar] [CrossRef]
- Wei, Y.-T.; Zheng, Y.-M.; Chen, J.P. Functionalization of regenerated cellulose membrane via surface initiated atom transfer radical polymerization for boron removal from aqueous solution. Langmuir 2011, 27, 6018–6025. [Google Scholar] [CrossRef]
- Wittmar, A.S.M.; Ulbricht, M. Ionic liquid-based route for the preparation of catalytically active cellulose—TiO2 porous films and spheres. Ind. Eng. Chem. Res. 2017, 56, 2967–2975. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef] [Green Version]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Rahatekar, S.S.; Vignolini, S.; Windle, A.H. New horizons for cellulose nanotechnology. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170200. [Google Scholar] [CrossRef] [Green Version]
- Koshani, R.; Madadlou, A. A viewpoint on the gastrointestinal fate of cellulose nanocrystals. Trends Food Sci. Technol. 2018, 71, 268–273. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, A.; Ravishankar, K.; Dhamodharan, R. Preparation of nanofibrillated cellulose and nanocrystalline cellulose from surgical cotton and cellulose pulp in hot-glycerol medium. Cellulose 2019, 26, 3127–3141. [Google Scholar] [CrossRef]
- Kumar, V.; Elfving, A.; Koivula, H.M.; Bousfield, D.W.; Toivakka, M. Roll-to-roll processed cellulose nanofiber coatings. Ind. Eng. Chem. Res. 2016, 55, 3603–3613. [Google Scholar] [CrossRef]
- Li, J.; Cha, R.; Mou, K.; Zhao, X.; Long, K.; Luo, H.; Zhou, F.; Jiang, X. Nanocellulose-based antibacterial materials. Adv. Health Mater. 2018, 7, e1800334. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Thomas, V.; Vinod, V.; Abraham, R.; Abraham, S. Nanocellulose based functional materials for supercapacitor applications. J. Sci. Adv. Mater. Devices 2019, 4, 333–340. [Google Scholar] [CrossRef]
- Agate, S.; Joyce, M.; Lucia, L.; Pal, L. Cellulose and nanocellulose-based flexible-hybrid printed electronics and conductive composites—A review. Carbohydr. Polym. 2018, 198, 249–260. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Athira, B.K.; Rubiya, H.M.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Abidi, N. Distinct chiral nematic self-assembling behavior caused by different size-unified cellulose nanocrystals via a multistage separation. Langmuir 2016, 32, 9863–9872. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From fundamentals to advanced applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Ahmad, S.R. Polysaccharides and their derivatives for versatile tissue engineering application. Macromol. Biosci. 2013, 13, 395–421. [Google Scholar] [CrossRef] [PubMed]
- Eyley, S.; Thielemans, W. Surface modification of cellulose nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Missoum, K.; Belgacem, M.N.; Bras, J. Nanofibrillated cellulose surface modification: A review. Materials 2013, 6, 1745–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef]
- Gupta, P.; Singh, B.; Agrawal, A.; Maji, P.K. Low density and high strength nanofibrillated cellulose aerogel for thermal insulation application. Mater. Des. 2018, 158, 224–236. [Google Scholar] [CrossRef]
- Beyene, D.; Chae, M.; Dai, J.; Danumah, C.; Tosto, F.; DeMesa, A.G.; Bressler, D.C. Characterization of cellulase-treated fibers and resulting cellulose nanocrystals generated through acid hydrolysis. Materials 2018, 11, 1272. [Google Scholar] [CrossRef] [Green Version]
- Niamsap, T.; Lam, N.T.; Sukyai, P. Production of hydroxyapatite-bacterial nanocellulose scaffold with assist of cellulose nanocrystals. Carbohydr. Polym. 2019, 205, 159–166. [Google Scholar] [CrossRef]
- Khalil, H.A.; Davoudpour, Y.; Islam, N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Mahrouz, M.; Vignon, M.R. Microfibrillated cellulose from the peel of prickly pear fruits. Food Chem. 2009, 115, 423–429. [Google Scholar] [CrossRef]
- Zimmermann, T.; Bordeanu, N.; Strub, E. Properties of nanofibrillated cellulose from different raw materials and its reinforcement potential. Carbohydr. Polym. 2010, 79, 1086–1093. [Google Scholar] [CrossRef]
- Wang, Q.; Du, H.; Zhang, F.; Zhang, Y.; Wu, M.; Yu, G.; Liu, C.; Li, B.; Peng, H. Flexible cellulose nanopaper with high wet tensile strength, high toughness and tunable ultraviolet blocking ability fabricated from tobacco stalk via a sustainable method. J. Mater. Chem. A 2018, 6, 13021–13030. [Google Scholar] [CrossRef]
- Mou, K.; Li, J.; Wang, Y.; Cha, R.; Jiang, X. 2,3-Dialdehyde nanofibrillated cellulose as a potential material for the treatment of MRSA infection. J. Mater. Chem. B 2017, 5, 7876–7884. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, R.; Heuberger, L.; Siqueira, G.; Gutt, B.; Zimmermann, T.; Maniura-Weber, K.; Salentinig, S.; Faccio, G. Enhanced antimicrobial activity and structural transitions of a nanofibrillated cellulose–nisin biocomposite suspension. ACS Appl. Mater. Interfaces 2018, 10, 20170–20181. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, A.; Asghari, S.; Lakouraj, M.M.; Mohseni, M. Nanofibrillated cellulose surface modification: A review. Int. J. Biol. Macromol. 2018, 115, 528–539. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Osorio, D.A.; Cranston, E.D. Optimization of cellulose nanocrystal length and surface charge density through phosphoric acid hydrolysis. Philos. Trans. R. Soc. A 2018, 376, 20170041. [Google Scholar] [CrossRef] [Green Version]
- Jordan, J.H.; Easson, M.W.; Dien, B.; Thompson, S.; Condon, B.D. Extraction and characterization of nanocellulose crystals from cotton gin motes and cotton gin waste. Cellulose 2019, 26, 5959–5979. [Google Scholar] [CrossRef]
- Pirich, C.L.; Picheth, G.F.; Machado, J.P.E.; Sakakibara, C.N.; Martin, A.A.; de Freitas, R.A.; Sierakowski, M.R. Influence of mechanical pretreatment to isolate cellulose nanocrystals by sulfuric acid hydrolysis. Int. J. Biol. Macromol. 2019, 130, 622–626. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, J.Y.; Reiner, R.S.; Verrill, S.P.; Baxa, U.; McNeil, S.E. Approaching zero cellulose loss in cellulose nanocrystal (CNC) production: Recovery and characterization of cellulosic solid residues (CSR) and CNC. Cellulose 2012, 19, 2033–2047. [Google Scholar] [CrossRef]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef]
- Acharya, S.; Rumi, S.S.; Parajuli, P.; Abidi, N. Cellulose nanocrystals-sources, preparation, and applications: Research Advances. In Cellulose Nanocrystals: Advances in Research and Applications; Croteau, O., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2019; pp. 1–54. [Google Scholar]
- Peyre, J.; Pääkkönen, T.; Reza, M.; Kontturi, E. Simultaneous preparation of cellulose nanocrystals and micron-sized porous colloidal particles of cellulose by TEMPO-mediated oxidation. Green Chem. 2015, 17, 808–811. [Google Scholar] [CrossRef]
- Chen, D.; van de Ven, T.G.M. Morphological changes of sterically stabilized nanocrystalline cellulose after periodate oxidation. Cellulose 2016, 23, 1051–1059. [Google Scholar] [CrossRef]
- Bashar, M.M.; Zhu, H.; Yamamoto, S.; Mitsuishi, M. Highly carboxylated and crystalline cellulose nanocrystals from jute fiber by facile ammonium persulfate oxidation. Cellulose 2019, 26, 3671–3684. [Google Scholar] [CrossRef]
- Zhou, L.; Li, N.; Shu, J.; Liu, Y.; Wang, K.; Cui, X.; Yuan, Y.; Ding, B.; Geng, Y.; Wang, Z.; et al. One-pot preparation of carboxylated cellulose nanocrystals and their liquid crystalline behaviors. ACS Sustain. Chem. Eng. 2018, 6, 12403–12410. [Google Scholar] [CrossRef]
- Tan, X.Y.; Hamid, S.B.A.; Lai, C.W. Preparation of high crystallinity cellulose nanocrystals (CNCs) by ionic liquid solvolysis. Biomass.-Bioenergy 2015, 81, 584–591. [Google Scholar] [CrossRef]
- Abushammala, H.; Krossing, I.; Laborie, M.-P. Ionic liquid-mediated technology to produce cellulose nanocrystals directly from wood. Carbohydr. Polym. 2015, 134, 609–616. [Google Scholar] [CrossRef]
- Filson, P.B.; Dawson-Andoh, B.E.; Schwegler-Berry, D. Enzymatic-mediated production of cellulose nanocrystals from recycled pulp. Green Chem. 2009, 11, 1808–1814. [Google Scholar] [CrossRef]
- Juárez-Luna, G.N.; Favela-Torres, E.; Quevedo, I.; Batina, N. Enzymatically assisted isolation of high-quality cellulose nanoparticles from water hyacinth stems. Carbohydr. Polym. 2019, 220, 110–117. [Google Scholar] [CrossRef]
- Sacui, I.A.; Nieuwendaal, R.; Burnett, D.J.; Stranick, S.J.; Jorfi, M.; Weder, C.; Foster, J.; Olsson, R.T.; Gilman, J.W. Comparison of the properties of cellulose nanocrystals and cellulose nanofibrils isolated from bacteria, tunicate, and wood processed using acid, enzymatic, mechanical, and oxidative methods. ACS Appl. Mater. Interfaces 2014, 6, 6127–6138. [Google Scholar] [CrossRef] [PubMed]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Hussin, M.H.; Haafiz, M.K.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef] [Green Version]
- Kaboorani, A.; Riedl, B.; Blanchet, P.; Fellin, M.; Hosseinaei, O.; Wang, S. Nanocrystalline cellulose (NCC): A renewable nano-material for polyvinyl acetate (PVA) adhesive. Eur. Polym. J. 2012, 48, 1829–1837. [Google Scholar] [CrossRef]
- Kaboorani, A.; Riedl, B. Surface modification of cellulose nanocrystals (CNC) by a cationic surfactant. Ind. Crop. Prod. 2015, 65, 45–55. [Google Scholar] [CrossRef]
- Pinheiro, I.; Ferreira, F.; Souza, D.D.H.S.; Gouveia, R.; Lona, L.; Morales, A.R.; Mei, L.H.I. Mechanical, rheological and degradation properties of PBAT nanocomposites reinforced by functionalized cellulose nanocrystals. Eur. Polym. J. 2017, 97, 356–365. [Google Scholar] [CrossRef]
- Ogunsona, E.O.; Panchal, P.; Mekonnen, T.H. Surface grafting of acrylonitrile butadiene rubber onto cellulose nanocrystals for nanocomposite applications. Compos. Sci. Technol. 2019, 184, 107884. [Google Scholar] [CrossRef]
- Grishkewich, N.; Mohammed, N.; Tang, J.; Tam, M.K. Recent advances in the application of cellulose nanocrystals. Curr. Opin. Colloid Interface Sci. 2017, 29, 32–45. [Google Scholar] [CrossRef]
- Abol-Fotouh, D.; Hassan, M.A.; Shokry, H.; Roig, A.; Azab, M.S.; Kashyout, A.E.-H.B. Bacterial nanocellulose from agro-industrial wastes: Low-cost and enhanced production by Komagataeibacter saccharivorans MD1. Sci. Rep. 2020, 10, 879. [Google Scholar] [CrossRef] [Green Version]
- Saichana, N.; Matsushita, K.; Adachi, O.; Frébort, I.; Frebortova, J. Acetic acid bacteria: A group of bacteria with versatile biotechnological applications. Biotechnol. Adv. 2015, 33, 1260–1271. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial cellulose as a raw material for food and food packaging applications. Front. Sustain. Food Syst. 2019, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Zhang, J.; Xiong, G.; Wan, Y. Evolution of morphology of bacterial cellulose scaffolds during early culture. Carbohydr. Polym. 2014, 111, 722–728. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Siddaramaiah, J. High performance edible nanocomposite films containing bacterial cellulose nanocrystals. Carbohydr. Polym. 2012, 87, 2031–2037. [Google Scholar] [CrossRef]
- Rovera, C.; Ghaani, M.; Santo, N.; Trabattoni, S.; Olsson, R.T.; Romano, D.; Farris, S. Enzymatic hydrolysis in the green production of bacterial cellulose nanocrystals. ACS Sustain. Chem. Eng. 2018, 6, 7725–7734. [Google Scholar] [CrossRef]
- Gatenholm, P.; Klemm, D. Bacterial nanocellulose as a renewable material for biomedical applications. MRS Bull. 2010, 35, 208–213. [Google Scholar] [CrossRef]
- Singhsa, P.; Narain, R.; Manuspiya, H. Bacterial cellulose nanocrystals (BCNC) preparation and characterization from three bacterial cellulose sources and development of functionalized BCNCs as nucleic acid delivery systems. ACS Appl. Nano Mater. 2017, 1, 209–221. [Google Scholar] [CrossRef]
- Badshah, M.; Ullah, H.; Khan, A.R.; Khan, S.; Park, J.K.; Khan, T. Surface modification and evaluation of bacterial cellulose for drug delivery. Int. J. Biol. Macromol. 2018, 113, 526–533. [Google Scholar] [CrossRef]
- Gao, M.; Li, J.; Bao, Z.; Hu, M.; Nian, R.; Feng, D.; An, D.; Li, X.; Xian, M.; Zhang, H. A natural in situ fabrication method of functional bacterial cellulose using a microorganism. Nat. Commun. 2019, 10, 437. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Zhang, M.; Shen, J.; He, Z.; Fatehi, P.; Ni, Y. Applications of cellulose-based materials in sustained drug delivery systems. Curr. Med. Chem. 2019, 26, 2485–2501. [Google Scholar] [CrossRef]
- Vatankhah, E.; Prabhakaran, M.P.; Jin, G.; Mobarakeh, L.G.; Ramakrishna, S. Development of nanofibrous cellulose acetate/gelatin skin substitutes for variety wound treatment applications. J. Biomater. Appl. 2014, 28, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, Y.; Wen, X.; Lin, Q.; Chen, X.; Wu, Z. Silver nanoparticle/bacterial cellulose gel membranes for antibacterial wound dressing: Investigation in vitro and in vivo. Biomed. Mater. 2014, 9, 035005. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, Y.; Song, W.; Luan, J.; Wen, X.; Wu, Z.; Chen, X.; Wang, Q.; Guo, S. In situ synthesis of silver-nanoparticles/bacterial cellulose composites for slow-released antimicrobial wound dressing. Carbohydr. Polym. 2014, 102, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Rostami, J.; Gordeyeva, K.; Benselfelt, T.; Lahchaichi, E.; Hall, S.A.; Riazanova, A.V.; Larsson, P.A.; Ciftci, G.C.; Wågberg, L. Hierarchical build-up of bio-based nanofibrous materials with tunable metal–organic framework biofunctionality. Mater. Today 2021. [Google Scholar] [CrossRef]
- Rendon, J.G.T.; Femmer, T.; De Laporte, L.; Tigges, T.; Rahimi, K.; Gremse, F.; Zafarnia, S.; Lederle, W.; Ifuku, S.; Wessling, M.; et al. Bioactive gyroid scaffolds formed by sacrificial templating of nanocellulose and nanochitin hydrogels as instructive platforms for biomimetic tissue engineering. Adv. Mater. 2015, 27, 2989–2995. [Google Scholar] [CrossRef]
- Wickström, S.A.; Niessen, C.M. Cell adhesion and mechanics as drivers of tissue organization and differentiation: Local cues for large scale organization. Curr. Opin. Cell Biol. 2018, 54, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-Y.; Pei, Y.; Xie, M.; Jin, Z.-H.; Xiao, Y.-S.; Wang, Y.; Zhang, L.-N.; Li, Y.; Huang, W.-H. An artificial blood vessel implanted three-dimensional microsystem for modeling transvascular migration of tumor cells. Lab Chip 2015, 15, 1178–1187. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liyanage, S.; Acharya, S.; Parajuli, P.; Shamshina, J.L.; Abidi, N. Production and Surface Modification of Cellulose Bioproducts. Polymers 2021, 13, 3433. https://doi.org/10.3390/polym13193433
Liyanage S, Acharya S, Parajuli P, Shamshina JL, Abidi N. Production and Surface Modification of Cellulose Bioproducts. Polymers. 2021; 13(19):3433. https://doi.org/10.3390/polym13193433
Chicago/Turabian StyleLiyanage, Sumedha, Sanjit Acharya, Prakash Parajuli, Julia L. Shamshina, and Noureddine Abidi. 2021. "Production and Surface Modification of Cellulose Bioproducts" Polymers 13, no. 19: 3433. https://doi.org/10.3390/polym13193433
APA StyleLiyanage, S., Acharya, S., Parajuli, P., Shamshina, J. L., & Abidi, N. (2021). Production and Surface Modification of Cellulose Bioproducts. Polymers, 13(19), 3433. https://doi.org/10.3390/polym13193433