Characterization of Collagen/Beta Glucan Hydrogels Crosslinked with Tannic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collagen Isolation
2.2. Hydrogel Preparation
2.3. Infrared Spectroscopy
2.4. Mechanical Properties
2.5. Release of Tannic Acid from Hydrogels
2.6. Antioxidant Activity
2.7. The Effects of the Materials on Bacterial Cellular Processes
2.7.1. The Influence of the Materials on Dehydrogenase Activity
2.7.2. The Influence of Materials on ATP Level
2.8. Effects of the Material Extracts on HaCaT Cultures
2.9. Statistical Analysis
3. Results
3.1. Infrared Spectroscopy
3.2. Mechanical Properties
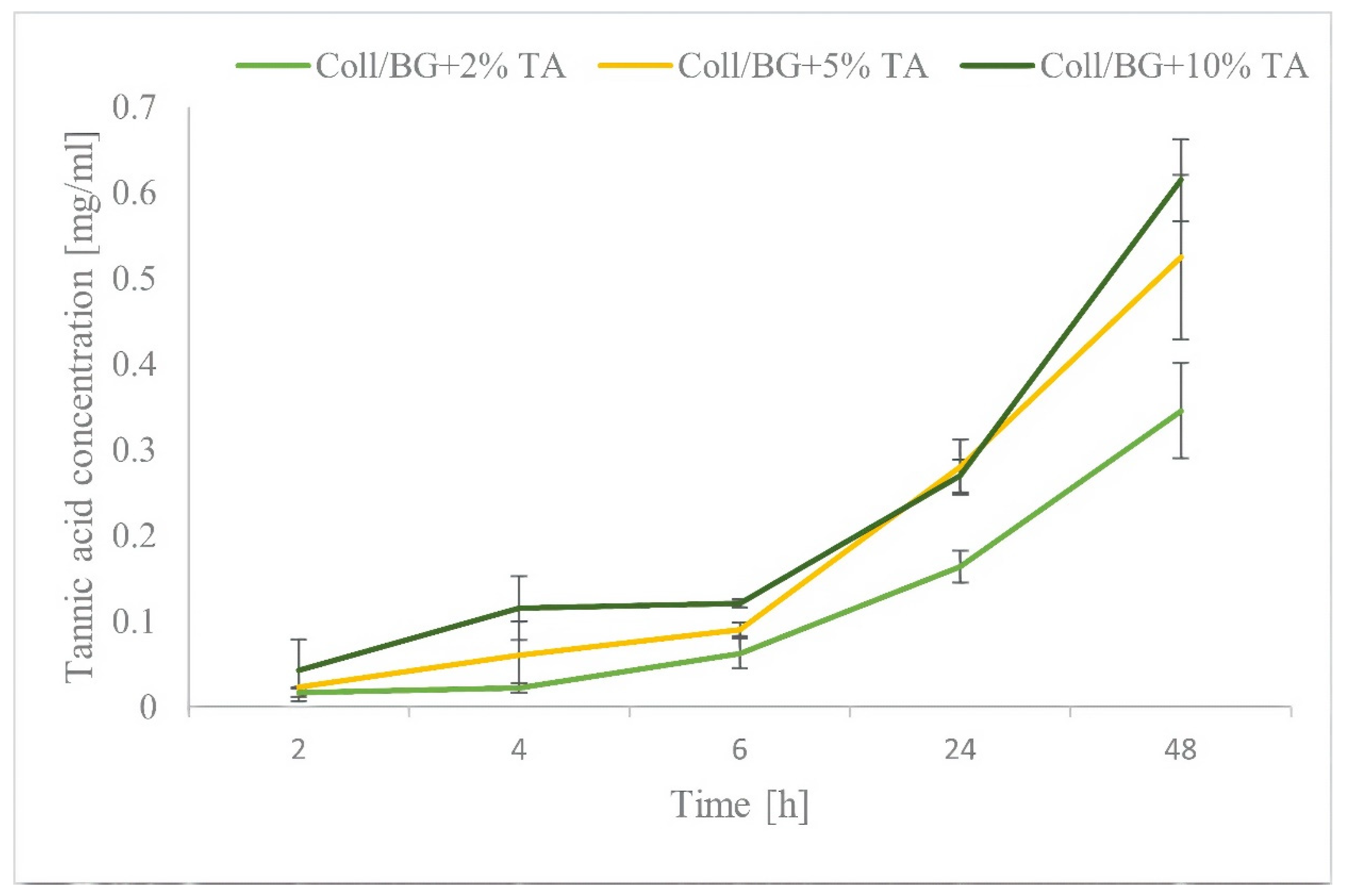
3.3. Tannic Acid Release
3.4. Antioxidant Activity
3.5. The Influence of the Materials on the Dehydrogenase Activity of Bacteria Cells
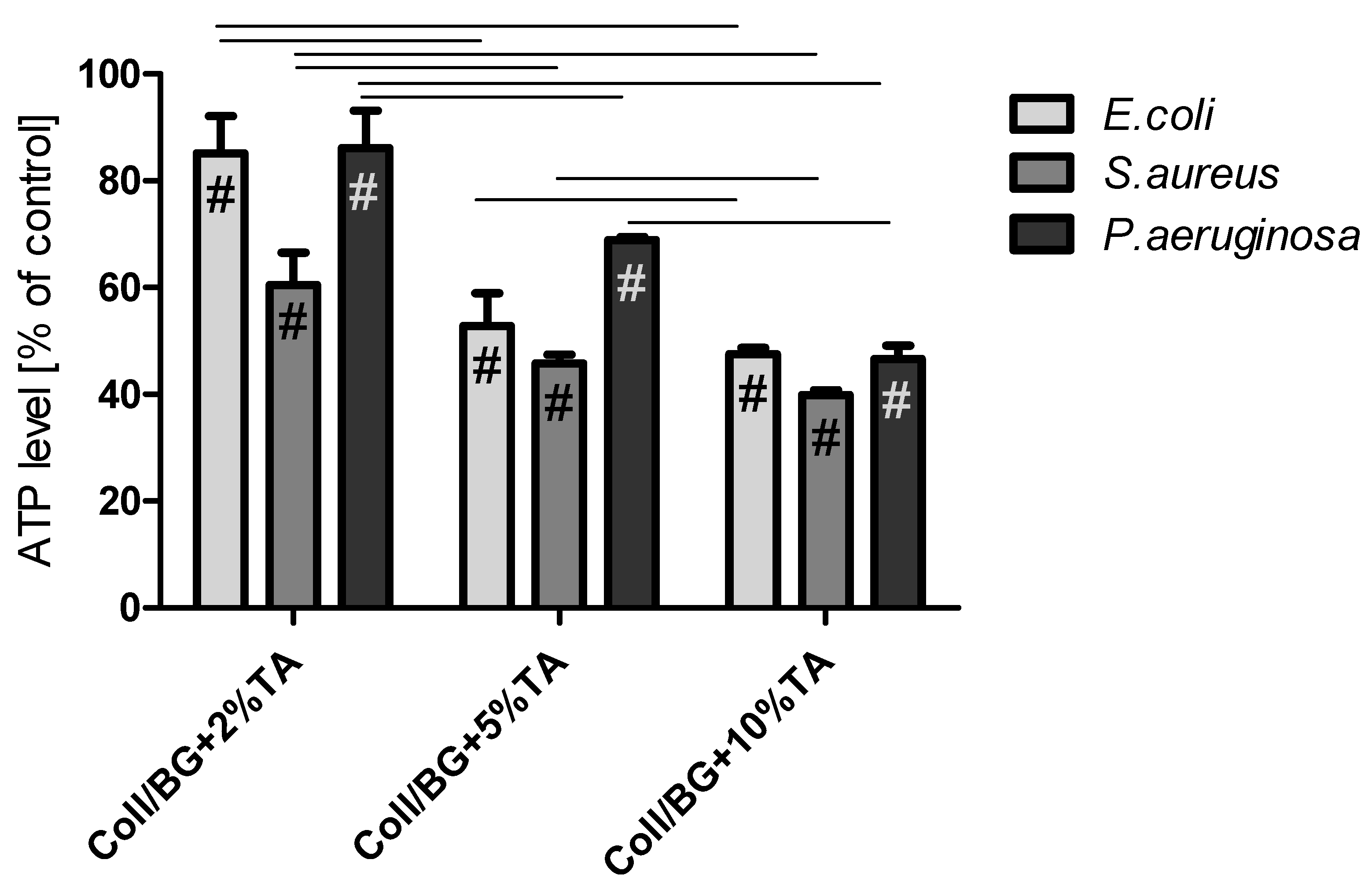
3.6. The Influence of the Materials on ATP Level in Selected Bacteria Strains
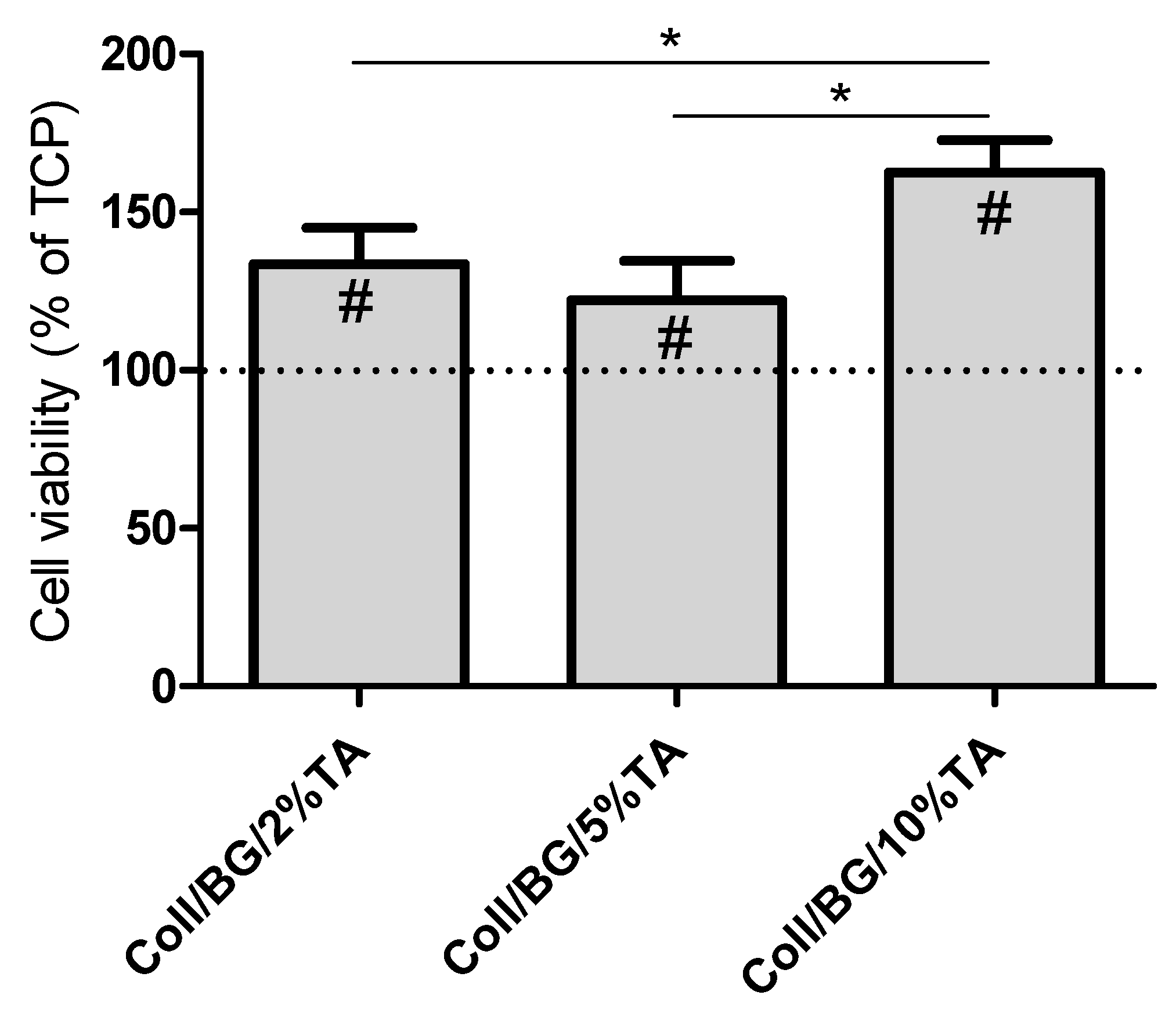
3.7. Biocompatibility Studies in HaCaT Cultures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhargava Reddy, M.S.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative of Natural and Synthetic Biopolymer Composite Scaffold. Polymer 2021, 13, 1105. [Google Scholar] [CrossRef]
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2010, 339, 247–257. [Google Scholar] [CrossRef]
- Richard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [Green Version]
- Biswal, T. Biopolymers for tissue engineering applications: A review. Mater. Today Proc. 2021, 41, 397–402. [Google Scholar] [CrossRef]
- Mudgil, D. The Interaction between Insoluble and Soluble Fiber, Dietary Fiber for the Prevention of Cardivascular Didease; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 35–59. [Google Scholar]
- Kaur, R.; Sharma, M.; Ji, D.; Xu, E. Structural Features, Modification, and Functionalities of Beta-Glucan. Fibers 2020, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. Structure-functional activity relationship of β-glucans from the perspective of immunomodulation: A mini-review. Front. Immunol. 2020, 11, 658. [Google Scholar] [CrossRef] [Green Version]
- Seo, G.; Hyun, C.; Choi, S.; Mee Kim, Y.; Cho, M. The wound healing effect of four types of beta-glucan. Appl. Biol. Chem. 2019, 62, 1. [Google Scholar] [CrossRef] [Green Version]
- Cao, N.; Fu, Y.; He, Y. Mechanical properties of gelatin films cross-linked, respectively, by ferulic acid and tannin acid. Food Hydrocoll. 2007, 21, 575–584. [Google Scholar] [CrossRef]
- Picchio, M.L.; Linck, Y.G.; Monti, G.A.; Gugliotta, L.M.; Minari, R.J.; Alvarez Igarzabal, C.I. Casein films crosslinked by tannic acid for food packaging applications. Food Hydrocoll. 2018, 84, 424–434. [Google Scholar] [CrossRef]
- Rivero, S.; García, M.A.; Pinotti, A. Crosslinking capacity of tannic acid in plasticized chitosan films. Carbohydr. Polym. 2010, 82, 270–276. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [Green Version]
- Pizzi, A. Tannins: Prospectives and Actual Industrial Applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef] [Green Version]
- Michalska-Sionkowska, M.; Warżyńska, O.; Kaczmarek-Szczepańska, B.; Łukowicz, K.; Osyczka, A.M.; Walczak, M. Preparation and Characterization of Fish Skin Collagen Material Modified with B-Glucan as Potential Wound Dressing. Materials 2021, 14, 1322. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Miłek, O.; Michalska-Sionkowska, M.; Zasada, L.; Twardowska, M.; Warżyńska, O.; Kleszczyński, K.; Osyczka, A.M. Novel Eco-Friendly Tannic Acid-Enriched Hydrogels Preparation and Characterization for Biomedical Application. Materials 2020, 13, 4572. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, J.; Stachowiak, N.; Prus, W. Stability studies of collagen-based microspheres with Calendula officinalis flower extract. Polym. Degrad. Stab. 2019, 163, 214–219. [Google Scholar] [CrossRef]
- Ali, B.M.; Boothapandi, M.; Nasar, A.S.S. Nitric oxide, DPPH and hydrogen peroxide radical scavenging activity of TEMPO terminated polyurethane dendrimers: Data supporting antioxidant activity of radical dendrimers. Data Brief 2020, 28, 104972. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 421–422. [Google Scholar] [CrossRef] [Green Version]
- Walczak, M.; Michalska-Sionkowska, M.; Kaczmarek, B.; Sionkowska, A. Surface and antibacterial properties of thin films based on collagen and thymol. Mater. Today Commun. 2020, 22, 100949. [Google Scholar] [CrossRef]
- Phale, P.S.; Sharma, A.; Gautam, K. Microbial degradation of xenobiotics like aromatic pollutants from the terrestrial environments. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Prasad, M.N.V., Vithanage, M., Kapley, A., Eds.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 259–278. [Google Scholar]
- Wang, X.; Cao, W.; Xiang, Q.; Jin, F.; Peng, X.; Li, Q.; Jiang, M.; Hu, B.; Xing, X. Silver nanoparticle and lysozyme/tannic acid layer-by-layer assembly antimicrobial multilayer on magnetic nanoparticle by an eco-friendly route. Mater. Sci. Eng. C 2017, 76, 886–896. [Google Scholar] [CrossRef]
- Doll, K.; Jongsthaphongpun, K.L.; Stumpp, N.S.; Winkel, A.; Stiesch, M. Quantifying implant-associated biofilms: Comparison of microscopic, microbiologic and biochemical methods. J. Microbiol. Methods 2016, 130, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Kaczmarek, B.; Gnatowska, M.; Kowalonek, J. The influence of UV-irradiation on chitosan modified by the tannic acid addition. J. Photochem. Photobiol. B 2015, 148, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Muhoza, B.; Xia, S.; Zhang, X. Gelatin and high methyl pectin coacervates crosslinked with tannic acid: The characterization, rheological properties, and application for peppermint oil microencapsulation. Food Hydrocoll. 2019, 97, 105174. [Google Scholar] [CrossRef]
- Swetha, M.; Sahithi, K.; Moorthi, A.; Srinivasan, N.; Ramasamy, K.; Selvamurugan, N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2010, 47, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Nansu, W.; Ross, S.; Ross, G.; Mahasaranon, S. Effect of crosslinking agent on the physical and mechanical properties of a composite foam based on cassava starch and coconut residue fiber. Mater. Today Proc. 2019, 17, 2010–2019. [Google Scholar] [CrossRef]
- Weiss, A.V.; Fischer, T.; Iturri, J.; Benitez, R.; Toca-Herrera, J.L.; Schneider, M. Mechanical properties of gelatin nanoparticles in dependency of crosslinking time and storage. Colloids Surf. B 2019, 175, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Weng, R.; Wang, W.; Wei, X.; Li, J.; Chen, X.; Liu, Y.; Lu, F.; Li, Y. Tunable physical and mechanical properties of gelatin hydrogel after transglutaminase crosslinking on two gelatin types. Int. J. Biol. Macromol. 2020, 162, 405–413. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepańska, B.; Mazur, O.; Michalska-Sionkowska, M.; Łukowicz, K.; Osyczka, A.M. The Preparation and Characterization of Chitosan-Based Hydrogels Cross-Linked by Glyoxal. Materials 2021, 14, 2449. [Google Scholar] [CrossRef]
- Ahammed, S.; Liu, F.; Wu, J.; Khin, M.N.; Yokoyama, W.H.; Zhong, F. Effect of transglutaminase crosslinking on solubility property and mechanical strength of gelatin-zein composite films. Food Hydrocoll. 2021, 116, 106649. [Google Scholar] [CrossRef]
- Gámez-Herrera, E.; García-Salinas, S.; Salido, S.; Sancho-Albero, M.; Andreu, V.; Pérez, M.; Luján, L.; Irusta, S.; Arruebo, M.; Mendoza, G. Drug-eluting wound dressings having sustained release of antimicrobial compounds. Eur. J. Pharm. Biopharm. 2020, 152, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Kuai, L.; Liu, F.; Ma, Y.; Goff, H.D.; Zhong, F. Regulation of nano-encapsulated tea polyphenol release from gelatin films with different loom values. Food Hydrocoll. 2020, 108, 106045. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, Z.; Liu, Y.; Wang, J.; Li, Z.; Wang, M. Shape memory polyacrylamide/gelatin hydrogel with controllable mechanical and drug release properties potential for wound dressing application. Polymer 2021, 226, 123786. [Google Scholar] [CrossRef]
- Séon-Lutz, M.; Couffin, A.C.; Vignoud, S.; Schlatter, G.; Hébraud, A. Electrospinning in water and in situ crosslinking of hyaluronic acid/cyclodextrin nanofibers: Towards wound dressing with controlled drug release. Carbohydr. Polym. 2019, 207, 276–287. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Han, Y.; Guo, B. Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J. Colloid Interface Sci. 2019, 556, 514–528. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Kavoosi, G.; Dadfar, S.M.M.; Purfard, A.M. Mechanical, Physical, Antioxidant, and Antimicrobial Properties of Gelatin Films Incorporated with Thymol for Potential Use as Nano Wound Dressing. J. Food Sci. 2013, 78, E244–E250. [Google Scholar] [CrossRef]
- Fan, H.; Wang, L.; Feng, X.; Bu, Y.; Wu, D.; Jin, Z. Supramolecular Hydrogel Formation Based on Tannic Acid. Macromolecules 2017, 50, 666–676. [Google Scholar] [CrossRef]
- Ge, W.; Cao, S.; Shen, F.; Wang, Y.; Ren, J.; Wang, X. Rapid self-healing, stretchable, moldable, antioxidant and antibacterial tannic acid-cellulose nanofibril composite hydrogels. Carbohydr. Polym. 2019, 224, 115147. [Google Scholar] [CrossRef]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef]
- Shah, S.R.; Tatara, A.M.; D’Souza, R.N.; Mikos, A.G.; Kasper, F.K. Evolving strategies for preventing biofilm on implantable materials. Mater. Today 2013, 16, 177–182. [Google Scholar] [CrossRef]
- Tavoukjian, V. Faecal microbiota transplantation for the decolonization of antibiotic-resistant bacteria in the gut: A systematic review and meta-analysis. J. Hosp. Infect. 2019, 102, 174–188. [Google Scholar] [CrossRef]
- Pol, I.E.; Krommer, J.; Smid, E.J. Bioenergetic consequences of nisin combined with carvacrol towards Bacillus cereus. Innov. Food Sci. Emerg. Technol. 2002, 3, 55–61. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Silan, C.; Aktas, N.; Turk, M. Biocompatible and biodegradable poly(Tannic Acid) hydrogel with antimicrobial and antioxidant properties. Int. J. Biol. Macromol. 2016, 82, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Sun, Y.; Zheng, Y.D.; He, W.; Yang, Y.Y.; Xie, Y.J.; Feng, Z.X.; Qiao, K. A biocompatible bacterial cellulose/tannic acid composite with antibacterial and anti-biofilm activities for biomedical applications. Mater. Sci. Eng. C 2020, 106, 110249. [Google Scholar] [CrossRef] [PubMed]



| Specimen | After 1.5 h of Contact [%] | After 18 h of Contact [%] | After 24 h of Contact [%] |
|---|---|---|---|
| Coll/BG + 2%TA | 0.23 ± 0.01 | 0.84 ± 0.04 | 20.16 ± 0.08 |
| Coll/BG + 5%TA | 0.91 ± 0.03 | 62.08 ± 0.12 | 58.30 ± 0.17 |
| Coll/BG + 10%TA | 7.91 ± 0.02 | 74.83 ± 0.24 | 81.06 ± 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalska-Sionkowska, M.; Warżyńska, O.; Kaczmarek-Szczepańska, B.; Łukowicz, K.; Osyczka, A.M.; Walczak, M. Characterization of Collagen/Beta Glucan Hydrogels Crosslinked with Tannic Acid. Polymers 2021, 13, 3412. https://doi.org/10.3390/polym13193412
Michalska-Sionkowska M, Warżyńska O, Kaczmarek-Szczepańska B, Łukowicz K, Osyczka AM, Walczak M. Characterization of Collagen/Beta Glucan Hydrogels Crosslinked with Tannic Acid. Polymers. 2021; 13(19):3412. https://doi.org/10.3390/polym13193412
Chicago/Turabian StyleMichalska-Sionkowska, Marta, Oliwia Warżyńska, Beata Kaczmarek-Szczepańska, Krzysztof Łukowicz, Anna Maria Osyczka, and Maciej Walczak. 2021. "Characterization of Collagen/Beta Glucan Hydrogels Crosslinked with Tannic Acid" Polymers 13, no. 19: 3412. https://doi.org/10.3390/polym13193412
APA StyleMichalska-Sionkowska, M., Warżyńska, O., Kaczmarek-Szczepańska, B., Łukowicz, K., Osyczka, A. M., & Walczak, M. (2021). Characterization of Collagen/Beta Glucan Hydrogels Crosslinked with Tannic Acid. Polymers, 13(19), 3412. https://doi.org/10.3390/polym13193412










