Poly-ε-Caprolactone/Fibrin-Alginate Scaffold: A New Pro-Angiogenic Composite Biomaterial for the Treatment of Bone Defects
Abstract
1. Introduction
2. Materials and Methods
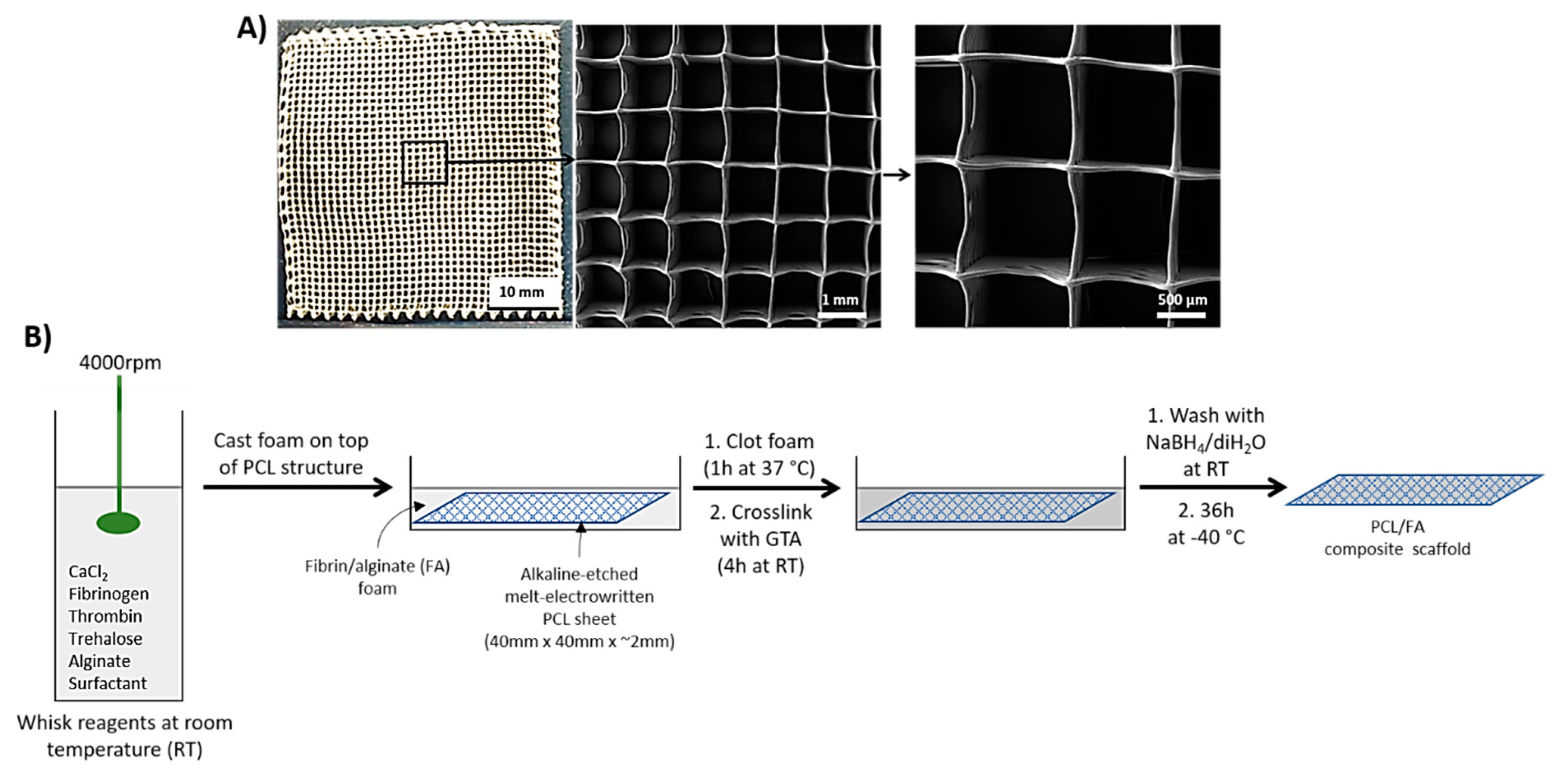
2.1. Melt-Electrowritten (MEW) PCL
2.2. PCL Surface Treatment
2.3. Casting of Fibrin/Alginate Matrix through the 3D PCL Structure
2.4. Scanning Electron Microscopy (SEM)
2.5. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.6. Differential Scanning Calorimetry (DSC)
2.7. Cell Seeding
2.8. Cell Viability by Live/Dead Assay
2.9. Cell Proliferation by Alamarblue™ Assay
2.10. Cell Infiltration through the Scaffolds
2.11. Angiogenesis by an Ex Ovo Chorioallantoic Membrane (CAM) Assay
2.12. Surgical Procedure of In Vivo Assessment of Scaffold in a Rat Cranial Defect Model
2.13. Microcomputed Tomography (µCT)
2.14. Histology: Paraffin Embedding and Sectioning
2.15. Haematoxylin and Eosin Stain (H&E)
2.16. Immunohistochemistry (IHC)
2.17. Histology: Resin Embedding and Sectioning
2.18. Von Kossa Stain
2.19. Goldner’s Trichrome Stain
2.20. Statistical Analysis
3. Results
3.1. PCL Surface Treatment
3.2. SEM
3.3. ATR-FTIR
3.4. DSC
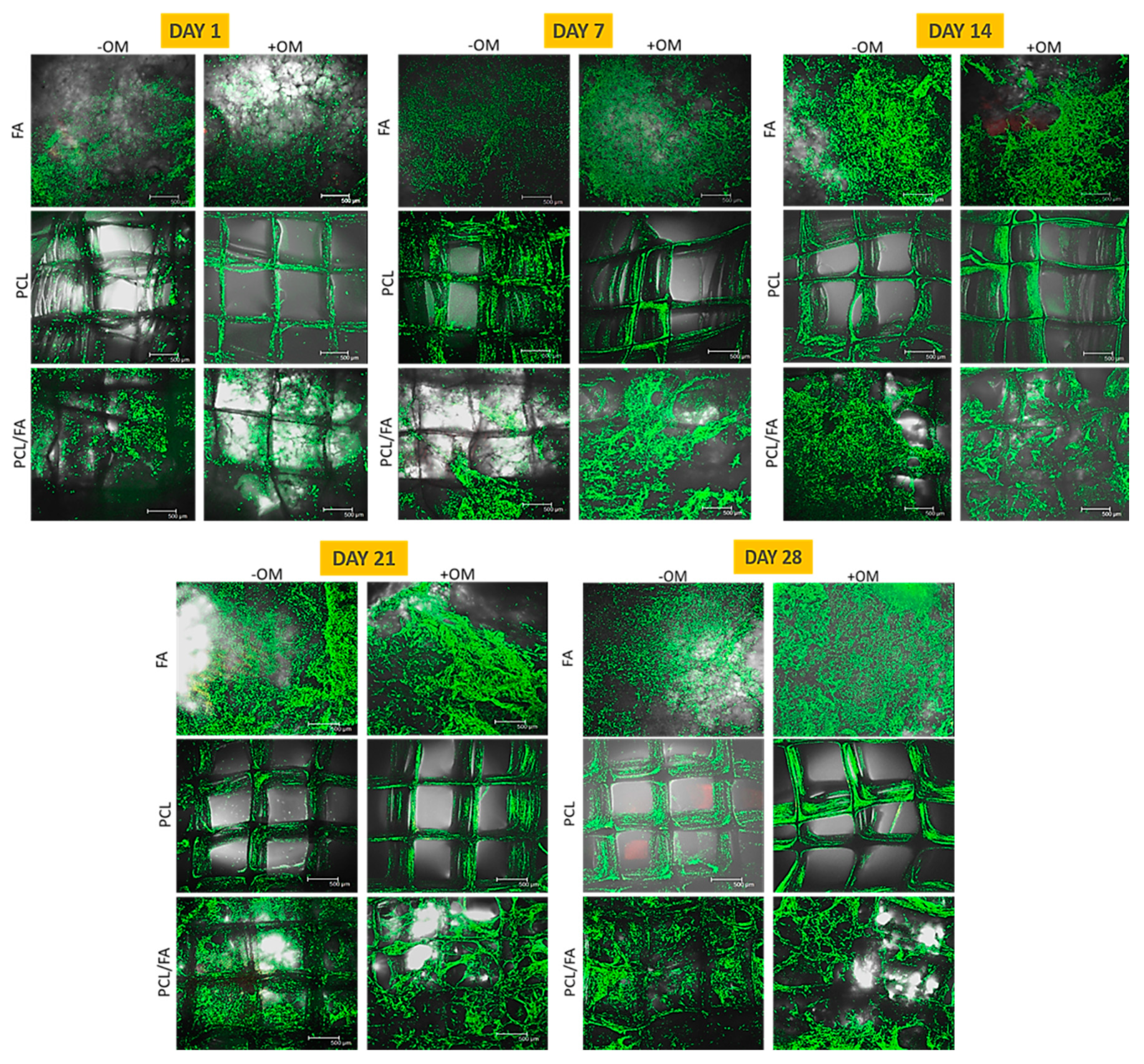
3.5. Cell Viability by Live/Dead Assay
3.6. Cell Proliferation by Alamarblue™ Assay
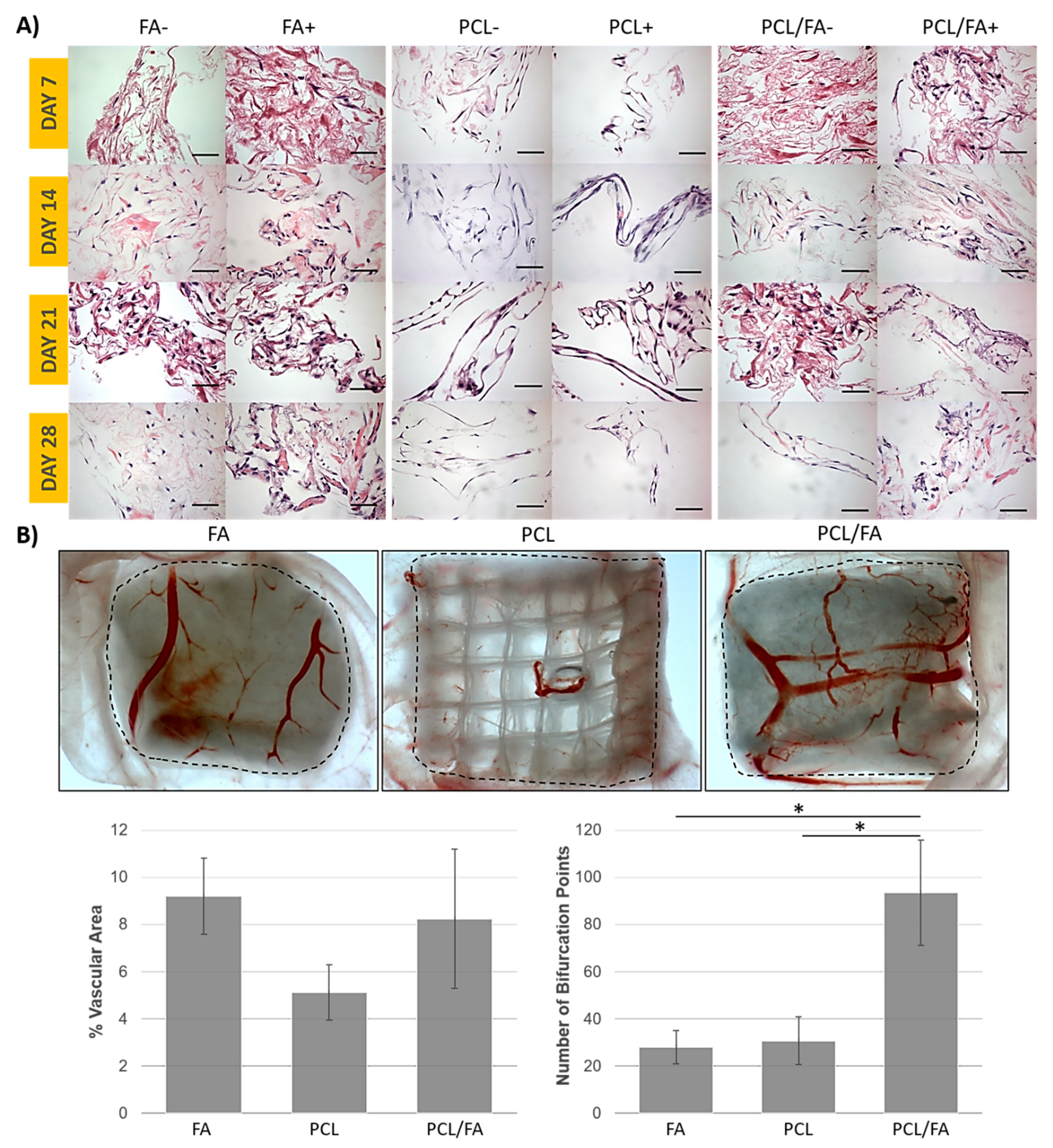
3.7. Angiogenesis by an Ex Ovo CAM Assay
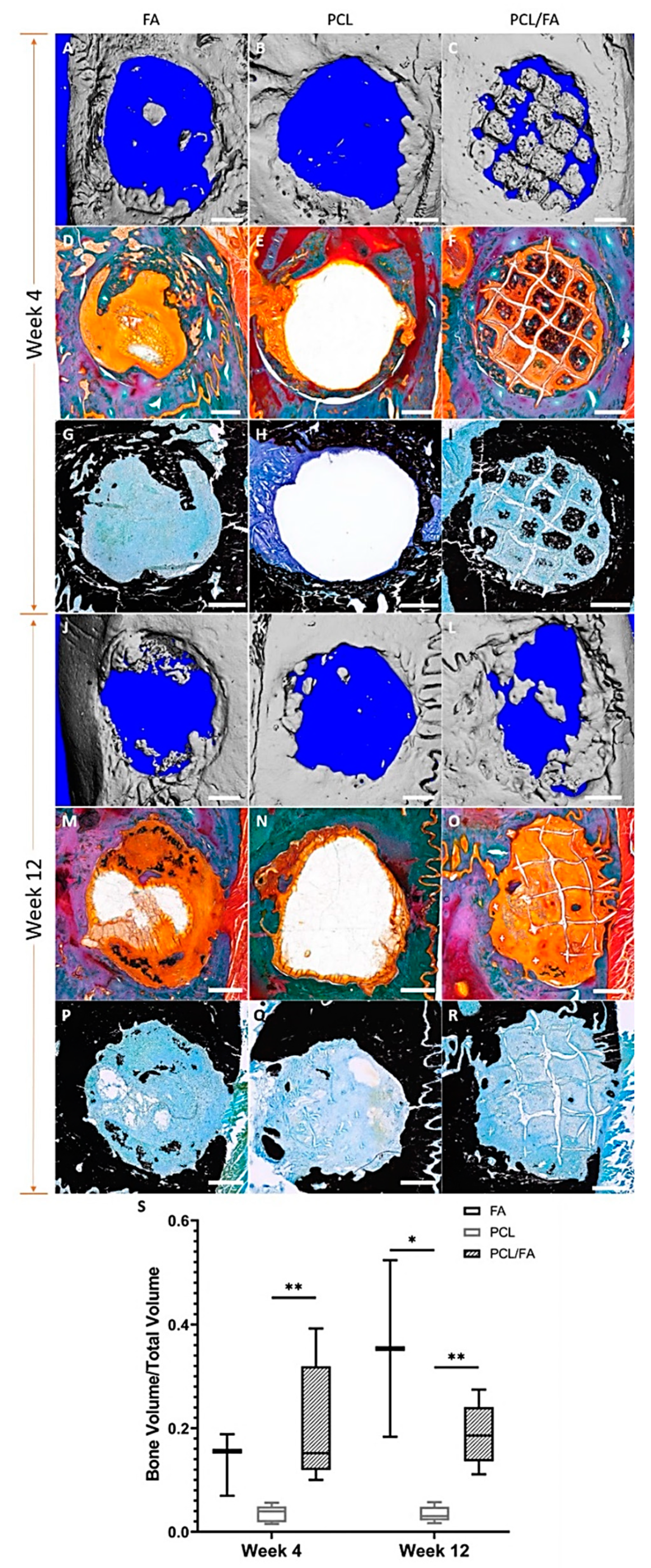
3.8. In Vivo Bone Regeneration by µCT
3.9. Goldner’s Trichrome and Von Kossa Stains
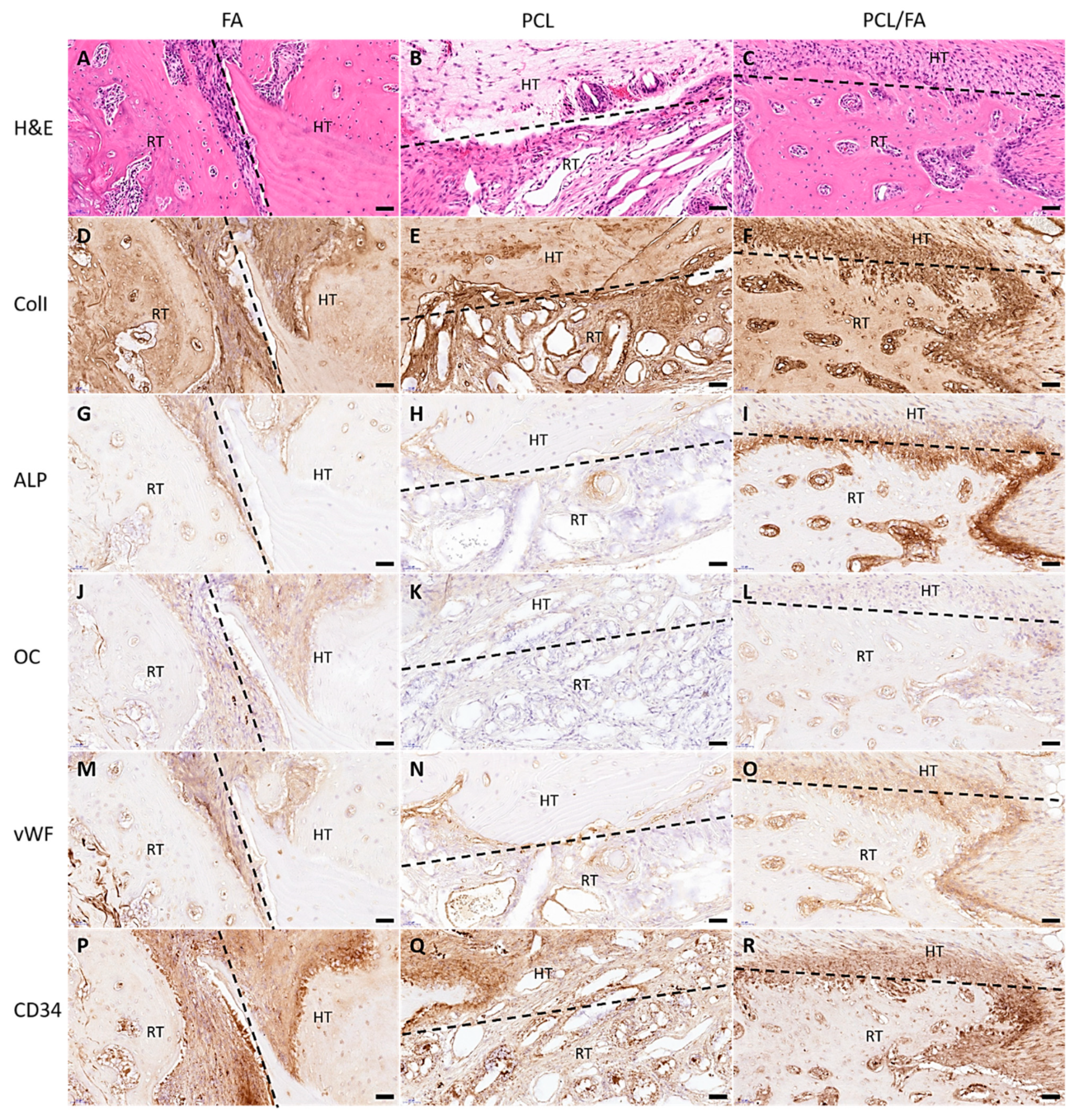
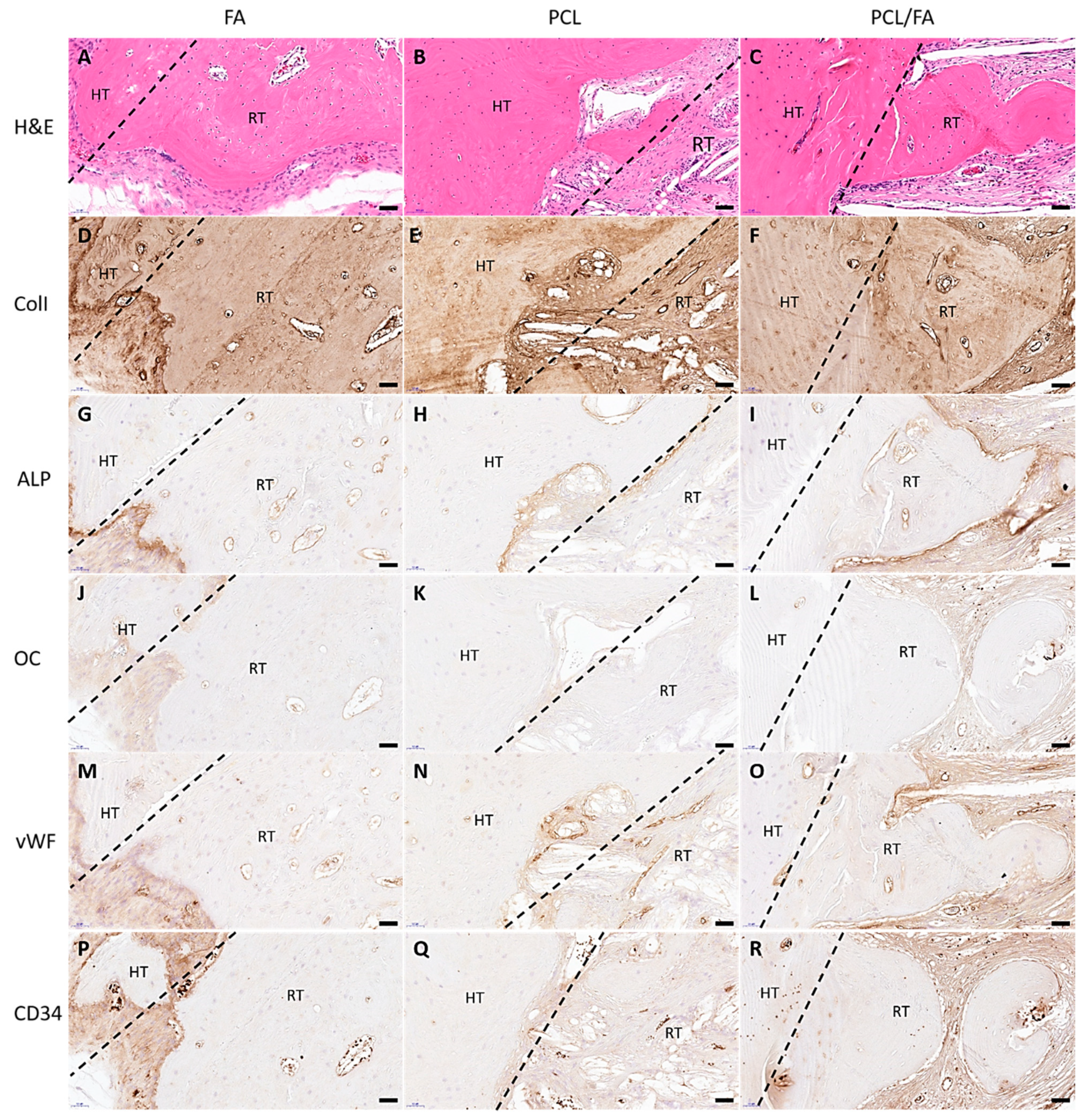
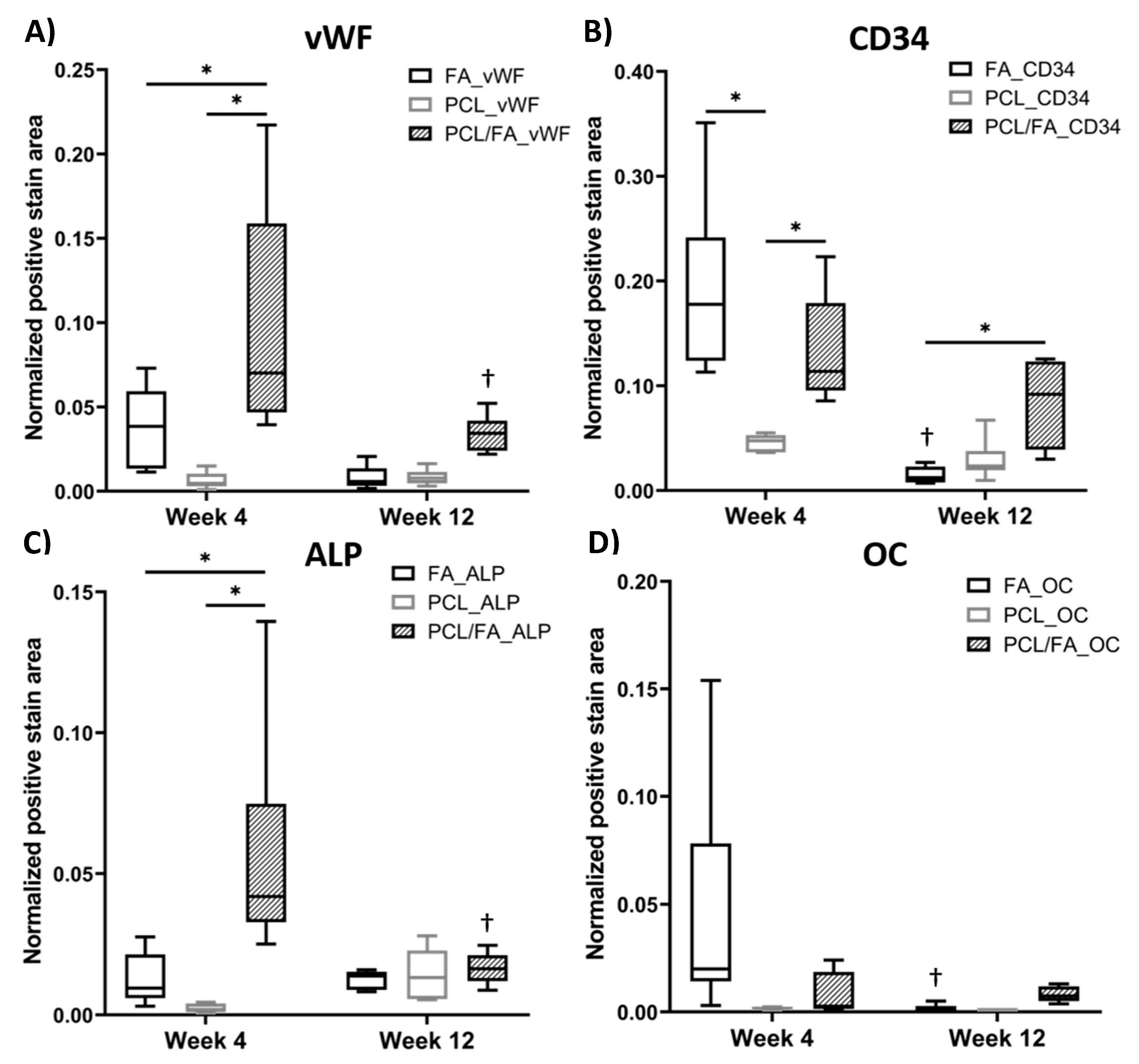
3.10. H&E Stain and Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef]
- Graziani, G.; Govoni, M.; Vivarelli, L.; Boi, M.; De Carolis, M.; Bianchi, M.; Sassoni, E.; Bignozzi, M.C.; Carnevale, G.; Marmi, F.; et al. A Comprehensive microstructural and compositional characterization of allogenic and xenogenic bone: Application to bone grafts and nanostructured biomimetic coatings. Coatings 2020, 10, 522. [Google Scholar] [CrossRef]
- Nyberg, E.; Rindone, A.; Dorafshar, A.; Grayson, W.L. Comparison of 3D-Printed Poly-ɛ-Caprolactone Scaffolds Functionalized with Tricalcium Phosphate, Hydroxyapatite, Bio-Oss, or Decellularized Bone Matrix. Tissue Eng. Part. A 2017, 23, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Govoni, M.; Vivarelli, L.; Mazzotta, A.; Stagni, C.; Maso, A.; Dallari, D. Commercial Bone Grafts Claimed as an Alternative to Autografts: Current Trends for Clinical Applications in Orthopaedics. Mater 2021, 14, 3290. [Google Scholar] [CrossRef] [PubMed]
- Munir, A.; Døskeland, A.; Avery, S.J.; Fuoco, T.; Mohamed-Ahmed, S.; Lygre, H.; Finne-Wistrand, A.; Sloan, A.J.; Waddington, R.J.; Mustafa, K.; et al. Efficacy of copolymer scaffolds delivering human demineralised dentine matrix for bone regeneration. J. Tissue Eng. 2019, 10, 2041731419852703. [Google Scholar] [CrossRef] [PubMed]
- Kohli, N.; Sharma, V.; Orera, A.; Sawadkar, P.; Owji, N.; Frost, O.G.; Bailey, R.J.; Snow, M.; Knowles, J.C.; Blunn, G.W.; et al. Pro-angiogenic and osteogenic composite scaffolds of fibrin, alginate and calcium phosphate for bone tissue engineering. J. Tissue Eng. 2021, 12, 20417314211005610. [Google Scholar] [CrossRef]
- Chou, J.; Hao, J.; Kuroda, S.; Ben-Nissan, B.; Milthopre, B.; Otsuka, M. Bone regeneration of calvarial defect using marine calcareous-derived beta-tricalcium phosphate macrospheres. J. Tissue Eng. 2014, 5, 2041731414523441. [Google Scholar] [CrossRef]
- García-Gareta, E.; Hua, J.; Orera, A.; Kohli, N.; Knowles, J.C.; Blunn, G.W. Biomimetic surface functionalization of clinically relevant metals used as orthopaedic and dental implants. Biomed. Mater. 2018, 13, 015008. [Google Scholar] [CrossRef]
- Dubey, N.; Bentini, R.; Islam, I.; Cao, T.; Castro Neto, A.H.; Rosa, V. Graphene: A Versatile Carbon-Based Material for Bone Tissue Engineering. Stem Cells Int. 2015, 2015, 804213. [Google Scholar] [CrossRef]
- Jang, Y.S.; Moon, S.H.; Nguyen, T.D.T.; Lee, M.H.; Oh, T.J.; Han, A.L.; Bae, T.S. In vivo bone regeneration by differently designed titanium membrane with or without surface treatment: A study in rat calvarial defects. J. Tissue Eng. 2019, 10, 2041731419831466. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Hassanajili, S.; Karami-Pour, A.; Oryan, A.; Talaei-Khozani, T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mater. Sci. Eng. C 2019, 104, 109960. [Google Scholar] [CrossRef] [PubMed]
- Felice, B.; Sánchez, M.A.; Socci, M.C.; Sappia, L.D.; Gómez, M.I.; Cruz, M.K.; Felice, C.J.; Martí, M.; Pividori, M.I.; Simonelli, G.; et al. Controlled degradability of PCL-ZnO nanofibrous scaffolds for bone tissue engineering and their antibacterial activity. Mater. Sci. Eng. C 2018, 93, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Ristovski, N.; Bock, N.; Liao, S.; Powell, S.K.; Ren, J.; Kirby, G.T.; Blackwood, K.A.; Woodruff, M.A. Improved fabrication of melt electrospun tissue engineering scaffolds using direct writing and advanced electric field control. Biointerphases 2015, 10, 011006. [Google Scholar] [CrossRef] [PubMed]
- Röder, A.; García-Gareta, E.; Theodoropoulos, C.; Ristovski, N.; Blackwood, K.; Woodruff, M. An Assessment of Cell Culture Plate Surface Chemistry for in Vitro Studies of Tissue Engineering Scaffolds. J. Funct. Biomater. 2015, 6, 1054–1063. [Google Scholar] [CrossRef]
- Paxton, N.C.; Ren, J.; Ainsworth, M.J.; Solanki, A.K.; Jones, J.R.; Allenby, M.C.; Stevens, M.M.; Woodruff, M.A. Rheological Characterization of Biomaterials Directs Additive Manufacturing of Strontium-Substituted Bioactive Glass/Polycaprolactone Microfibers. Macromol. Rapid Commun. 2019, 40, 1900019. [Google Scholar] [CrossRef]
- Recek, N.; Resnik, M.; Motaln, H.; Lah-Turnšek, T.; Augustine, R.; Kalarikkal, N.; Thomas, S.; Mozetič, M. Cell Adhesion on Polycaprolactone Modified by Plasma Treatment. Int. J. Polym. Sci. 2016, 2016, 7354396. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, J.M.; Lee, S.J.; Lee, S.G.; Jeong, J.K.; Kim, S.E.; Lee, S.C. Surface Hydrolysis Of Fibrous Poly(ε-caprolactone) scaffolds for enhanced osteoblast adhesion and proliferation. Macromol. Res. 2007, 15, 424–429. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lim, H.; Ahn, J.W.; Jang, D.; Lee, S.H.; Park, K.; Kim, S.E. Design of a 3D BMP-2-Delivering Tannylated PCL Scaffold and Its Anti-Oxidant, Anti-Inflammatory, and Osteogenic Effects In Vitro. Int. J. Mol. Sci. 2018, 19, 3602. [Google Scholar] [CrossRef]
- Ferracini, R.; Bistolfi, A.; Garibaldi, R.; Furfaro, V.; Battista, A.; Perale, G. Composite Xenohybrid Bovine Bone-Derived Scaffold as Bone Substitute for the Treatment of Tibial Plateau Fractures. Appl. Sci. 2019, 9, 2675. [Google Scholar] [CrossRef]
- Poh, P.S.P.; Hutmacher, D.W.; Stevens, M.M.; Woodruff, M.A. Fabrication and in vitro characterization of bioactive glass composite scaffolds for bone regeneration. Biofabrication 2013, 5, 045005. [Google Scholar] [CrossRef]
- Ren, J.; Blackwood, K.A.; Doustgani, A.; Poh, P.P.; Steck, R.; Stevens, M.M.; Woodruff, M.A. Melt-electrospun polycaprolactone strontium-substituted bioactive glass scaffolds for bone regeneration. J. Biomed. Mater. Res. Part. A 2014, 102, 3140–3153. [Google Scholar] [CrossRef]
- Lee, J.H.; Parthiban, P.; Jin, G.Z.; Knowles, J.C.; Kim, H.W. Materials roles for promoting angiogenesis in tissue regeneration. Prog. Mater. Sci. 2021, 117, 100732. [Google Scholar] [CrossRef]
- Ahmed, T.A.E.; Dare, E.V.; Hincke, M. Fibrin: A Versatile Scaffold for Tissue Engineering Applications. Tissue Eng. Part. B Rev. 2008, 14, 110306231744007. [Google Scholar] [CrossRef]
- Mishra, R.; Roux, B.M.; Posukonis, M.; Bodamer, E.; Brey, E.M.; Fisher, J.P.; Dean, D. Effect of prevascularization on in vivo vascularization of poly(propylene fumarate)/fibrin scaffolds. Biomaterials 2016, 77, 255–266. [Google Scholar] [CrossRef]
- Ignjatovic, N.; Ajdukovic, Z.; Uskokovic, D. New biocomposite [biphasic calcium phosphate/poly-DL-lactide-co-glycolide/biostimulative agent] filler for reconstruction of bone tissue changed by osteoporosis. J. Mater. Sci. Mater. Med. 2005, 16, 621–626. [Google Scholar] [CrossRef]
- Schagemann, J.C.; Chung, H.W.; Mrosek, E.H.; Stone, J.J.; Fitzsimmons, J.S.; O’Driscoll, S.W.; Reinholz, G.G. Poly-ϵ-caprolactone/gel hybrid scaffolds for cartilage tissue engineering. J. Biomed. Mater. Res. Part. A 2010, 93A, 454–463. [Google Scholar] [CrossRef]
- Panadero, J.A.; Vikingsson, L.; Gomez Ribelles, J.L.; Sencadas, V.; Lanceros-Mendez, S. Fatigue prediction in fibrin poly-ε-caprolactone macroporous scaffolds. J. Mech. Behav. Biomed. Mater. 2013, 28, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Van Lieshout, M.; Peters, G.; Rutten, M.; Baaijens, F. A Knitted, Fibrin-Covered Polycaprolactone Scaffold for Tissue Engineering of the Aortic Valve. Tissue Eng. 2006, 12, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, K.; Storck, K.; Muhr, C.; Mayer, H.; Regn, S.; Staudenmaier, R.; Wiese, H.; Maier, G.; Bauer-Kreisel, P.; Blunk, T. Development of volume-stable adipose tissue constructs using polycaprolactone-based polyurethane scaffolds and fibrin hydrogels. J. Tissue Eng. Regen. Med. 2016, 10, E409–E418. [Google Scholar] [CrossRef]
- Perez, R.A.; Kim, M.; Kim, T.-H.; Kim, J.-H.; Lee, J.H.; Park, J.-H.; Knowles, J.C.; Kim, H.-W. Utilizing Core–Shell Fibrous Collagen-Alginate Hydrogel Cell Delivery System for Bone Tissue Engineering. Tissue Eng. Part. A 2014, 20, 103–114. [Google Scholar] [CrossRef]
- Sharma, V.; Patel, N.; Kohli, N.; Ravindran, N.; Hook, L.; Mason, C.; García-Gareta, E. Viscoelastic, physical, and bio-degradable properties of dermal scaffolds and related cell behaviour. Biomed. Mater. 2016, 11, 055001. [Google Scholar] [CrossRef]
- García-Gareta, E.; Ravindran, N.; Sharma, V.; Samizadeh, S.; Dye, J.F. A Novel Multiparameter In Vitro Model of Three-Dimensional Cell Ingress Into Scaffolds for Dermal Reconstruction to Predict In Vivo Outcome. BioRes. Open Access. 2013, 2, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Kohli, N.; Moulding, D.; Afolabi, H.; Hook, L.; Mason, C.; García-Gareta, E. Design of a Novel Two-Component Hybrid Dermal Scaffold for the Treatment of Pressure Sores. Macromol. Biosci. 2017, 17, 1700185. [Google Scholar] [CrossRef]
- Levin, A.; Sharma, V.; Hook, L.; García-Gareta, E. The importance of factorial design in tissue engineering and biomaterials science: Optimisation of cell seeding efficiency on dermal scaffolds as a case study. J. Tissue Eng. 2018, 9, 204173141878169. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Blackwood, K.A.; Haddow, D.; Hook, L.; Mason, C.; Dye, J.F.; García-Gareta, E. Method for estimating protein binding capacity of polymeric systems. Biochim. Open 2015, 1, 40–50. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pitt, C.G.; Chasalow, F.I.; Hibionada, Y.M.; Klimas, D.M.; Schindler, A. Aliphatic Polyesters. I. The Degradation of Poly (e-caprolactone) In Vivo. J. Appl. Polym. Sci. 1981, 26, 3779–3787. [Google Scholar] [CrossRef]
- Kohli, N.; Sawadkar, P.; Ho, S.; Sharma, V.; Snow, M.; Powell, S.; Woodruff, M.A.; Hook, L.; García-Gareta, E. Pre-screening the intrinsic angiogenic capacity of biomaterials in an optimised ex ovo chorioallantoic membrane model. J. Tissue Eng. 2020, 11, 2041731420901621. [Google Scholar] [CrossRef] [PubMed]
- Shokoohmand, A.; Ren, J.; Baldwin, J.; Atack, A.; Shafiee, A.; Theodoropoulos, C.; Wille, M.L.; Tran, P.A.; Bray, L.J.; Smith, D.; et al. Microenvironment engineering of osteoblastic bone metastases reveals osteomimicry of patient-derived prostate cancer xenografts. Biomaterials 2019, 220, 119402. [Google Scholar] [CrossRef]
- Brierly, G.I.; Ren, J.; Baldwin, J.; Saifzadeh, S.; Theodoropoulos, C.; Tsurkan, M.V.; Lynham, A.; Hsu, E.; Nikolarakos, D.; Werner, C.; et al. Investigation of Sustained BMP Delivery in the Prevention of Medication-Related Osteonecrosis of the Jaw (MRONJ) in a Rat Model. Macromol. Biosci. 2019, 19, 1900226. [Google Scholar] [CrossRef]
- Law, A.M.K.; Yin, J.X.M.; Castillo, L.; Young, A.I.J.; Piggin, C.; Rogers, S.; Caldon, C.E.; Burgess, A.; Millar, E.K.A.; O’Toole, S.A.; et al. Andy’s Algorithms: New automated digital image analysis pipelines for FIJI. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Sparks, D.S.; Saifzadeh, S.; Savi, F.M.; Dlaska, C.E.; Berner, A.; Henkel, J.; Reichert, J.C.; Wullschleger, M.; Ren, J.; Cipitria, A.; et al. A preclinical large-animal model for the assessment of critical-size load-bearing bone defect reconstruction. Nat. Protoc. 2020, 15, 877–924. [Google Scholar] [CrossRef]
- Kim, Y.B.; Kim, G.H. PCL/alginate composite scaffolds for hard tissue engineering: Fabrication, characterization, and cellular activities. ACS Comb. Sci. 2015, 17, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Liu, J.; Li, X.; Liang, Q.; Xu, X. Relationship between the Young’s Modulus and the Crystallinity of Cross-Linked Poly(ε-caprolactone) as an Immobilization Membrane for Cancer Radiotherapy. Glob. Chall. 2020, 4, 2000008. [Google Scholar] [CrossRef]
- Manuscript, A. NIH Public Access Angiogenesis in Bone Regeneration. Injury 2012, 42, 556. [Google Scholar] [CrossRef]
- Filipowska, J.; Tomaszewski, K.A.; Niedźwiedzki, Ł.; Walocha, J.A.; Niedźwiedzki, T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017, 20, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef]
- Berner, A.; Woodruff, M.A.; Lam, C.X.F.; Arafat, M.T.; Saifzadeh, S.; Steck, R.; Ren, J.; Nerlich, M.; Ekaputra, A.K.; Gibson, I.; et al. Effects of scaffold architecture on cranial bone healing. Int. J. Oral Maxillofac. Surg. 2014, 43, 506–513. [Google Scholar] [CrossRef]
- Kohli, N.; Ho, S.; Brown, S.J.; Sawadkar, P.; Sharma, V.; Snow, M.; García-Gareta, E. Bone remodelling in vitro: Where are we headed?: -A review on the current understanding of physiological bone remodelling and inflammation and the strategies for testing biomaterials in vitro. Bone 2018, 110, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Honma, T.; Itagaki, T.; Nakamura, M.; Kamakura, S.; Takahashi, I.; Echigo, S.; Sasano, Y. Bone formation in rat calvaria ceases within a limited period regardless of completion of defect repair. Oral Dis. 2008, 14, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Detsch, R.; Boccaccini, A.R. The role of osteoclasts in bone tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1133–1149. [Google Scholar] [CrossRef] [PubMed]
- Bensaïd, W.; Triffitt, J.T.; Blanchat, C.; Oudina, K.; Sedel, L.; Petite, H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials 2003, 24, 2497–2502. [Google Scholar] [CrossRef]
- Fickert, S.; Schroter-Bobsin, U.; Groß, A.F.; Hempel, U.; Wojciechowski, C.; Rentsch, C.; Corbeil, D.; Gunther, K.P. Human mesenchymal stem cell proliferation and osteogenic differentiation during long-term ex vivo cultivation is not age dependent. J. Bone Miner. Metab. 2011, 29, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Perka, C.; Schultz, O.; Spitzer, R.S.; Lindenhayn, K.; Burmester, G.R.; Sittinger, M. Segmental bone repair by tissue-engineered periosteal cell transplants with bioresorbable fleece and fibrin scaffolds in rabbits. Biomaterials 2000, 21, 1145–1153. [Google Scholar] [CrossRef]
- Hong, S.J.; Kim, C.S.; Han, D.K.; Cho, I.H.; Jung, U.W.; Choi, S.H.; Kim, C.K.; Cho, K.S. The effect of a fibrin-fibronectin/β-tricalcium phosphate/recombinant human bone morphogenetic protein-2 system on bone formation in rat calvarial defects. Biomaterials 2006, 27, 3810–3816. [Google Scholar] [CrossRef]
- Schützenberger, S.; Schultz, A.; Hausner, T.; Hopf, R.; Zanoni, G.; Morton, T.; Kropik, K.; Van Griensven, M.; Redl, H. The optimal carrier for BMP-2: A comparison of collagen versus fibrin matrix. Arch. Orthop. Trauma Surg. 2012, 132, 1363–1370. [Google Scholar] [CrossRef]
- Zhu, S.J.; Choi, B.H.; Huh, J.Y.; Jung, J.H.; Kim, B.Y.; Lee, S.H. A comparative qualitative histological analysis of tissue-engineered bone using bone marrow mesenchymal stem cells, alveolar bone cells, and periosteal cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, 164–169. [Google Scholar] [CrossRef]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef]


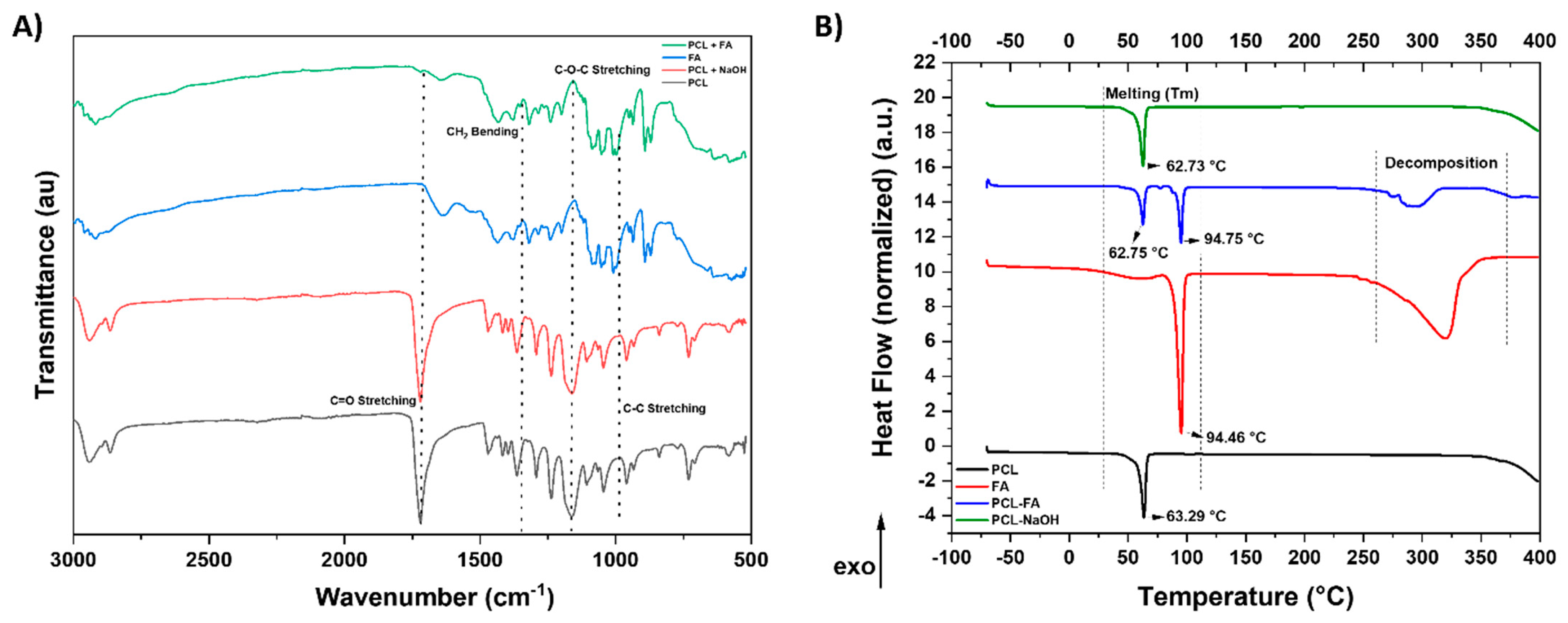

| Material | Onset °C | Tm Peak °C | Enthalpy (ΔH) J/g | Crystallinity (Xc) % |
|---|---|---|---|---|
| PCL | 56.44 | 63.29 | 127.64 | 91.47 |
| PCL–NaOH | 58.07 | 62.73 | 120.25 | 86.18 |
| PCL/FA | 59.22 | 62.75 | 67.49 | 48.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Kohli, N.; Sharma, V.; Shakouri, T.; Keskin-Erdogan, Z.; Saifzadeh, S.; Brierly, G.I.; Knowles, J.C.; Woodruff, M.A.; García-Gareta, E. Poly-ε-Caprolactone/Fibrin-Alginate Scaffold: A New Pro-Angiogenic Composite Biomaterial for the Treatment of Bone Defects. Polymers 2021, 13, 3399. https://doi.org/10.3390/polym13193399
Ren J, Kohli N, Sharma V, Shakouri T, Keskin-Erdogan Z, Saifzadeh S, Brierly GI, Knowles JC, Woodruff MA, García-Gareta E. Poly-ε-Caprolactone/Fibrin-Alginate Scaffold: A New Pro-Angiogenic Composite Biomaterial for the Treatment of Bone Defects. Polymers. 2021; 13(19):3399. https://doi.org/10.3390/polym13193399
Chicago/Turabian StyleRen, Jiongyu, Nupur Kohli, Vaibhav Sharma, Taleen Shakouri, Zalike Keskin-Erdogan, Siamak Saifzadeh, Gary I. Brierly, Jonathan C. Knowles, Maria A. Woodruff, and Elena García-Gareta. 2021. "Poly-ε-Caprolactone/Fibrin-Alginate Scaffold: A New Pro-Angiogenic Composite Biomaterial for the Treatment of Bone Defects" Polymers 13, no. 19: 3399. https://doi.org/10.3390/polym13193399
APA StyleRen, J., Kohli, N., Sharma, V., Shakouri, T., Keskin-Erdogan, Z., Saifzadeh, S., Brierly, G. I., Knowles, J. C., Woodruff, M. A., & García-Gareta, E. (2021). Poly-ε-Caprolactone/Fibrin-Alginate Scaffold: A New Pro-Angiogenic Composite Biomaterial for the Treatment of Bone Defects. Polymers, 13(19), 3399. https://doi.org/10.3390/polym13193399






