Influence of Newly Organosolv Lignin-Based Interface Modifier on Mechanical and Thermal Properties, and Enzymatic Degradation of Polylactic Acid/Chitosan Biocomposites
Abstract
:1. Introduction
2. Materials and Method
2.1. Raw Materials
2.2. Extraction of Organosolv Lignin (OSL) from Lignocellulosic Fiber
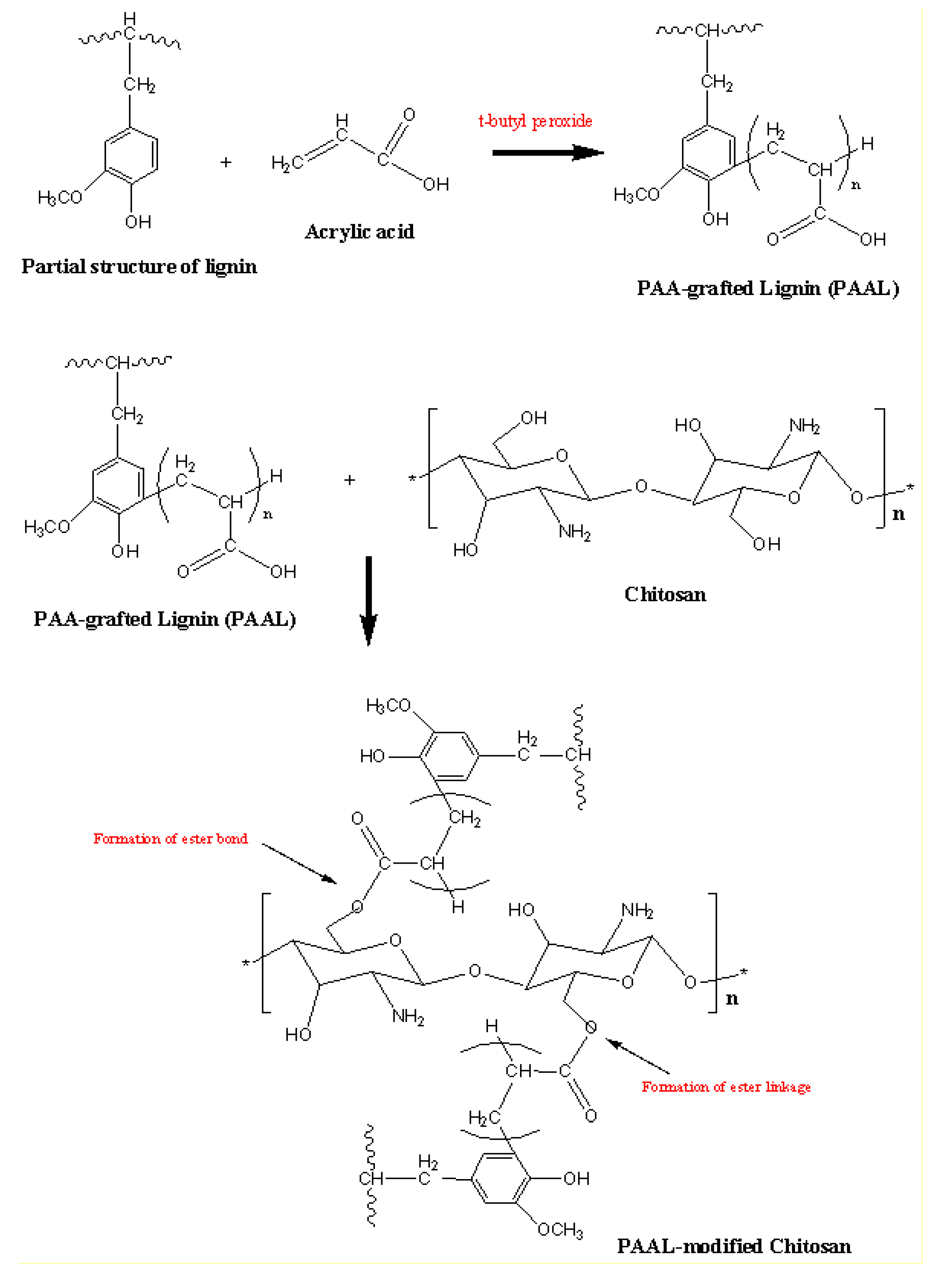
2.3. Preparation of PAA-grafted OSL
2.4. Chemical Modification of Chitosan
2.5. Preparation of PLA/Chitosan Biocomposites
2.6. Characterization
3. Results and Discussion
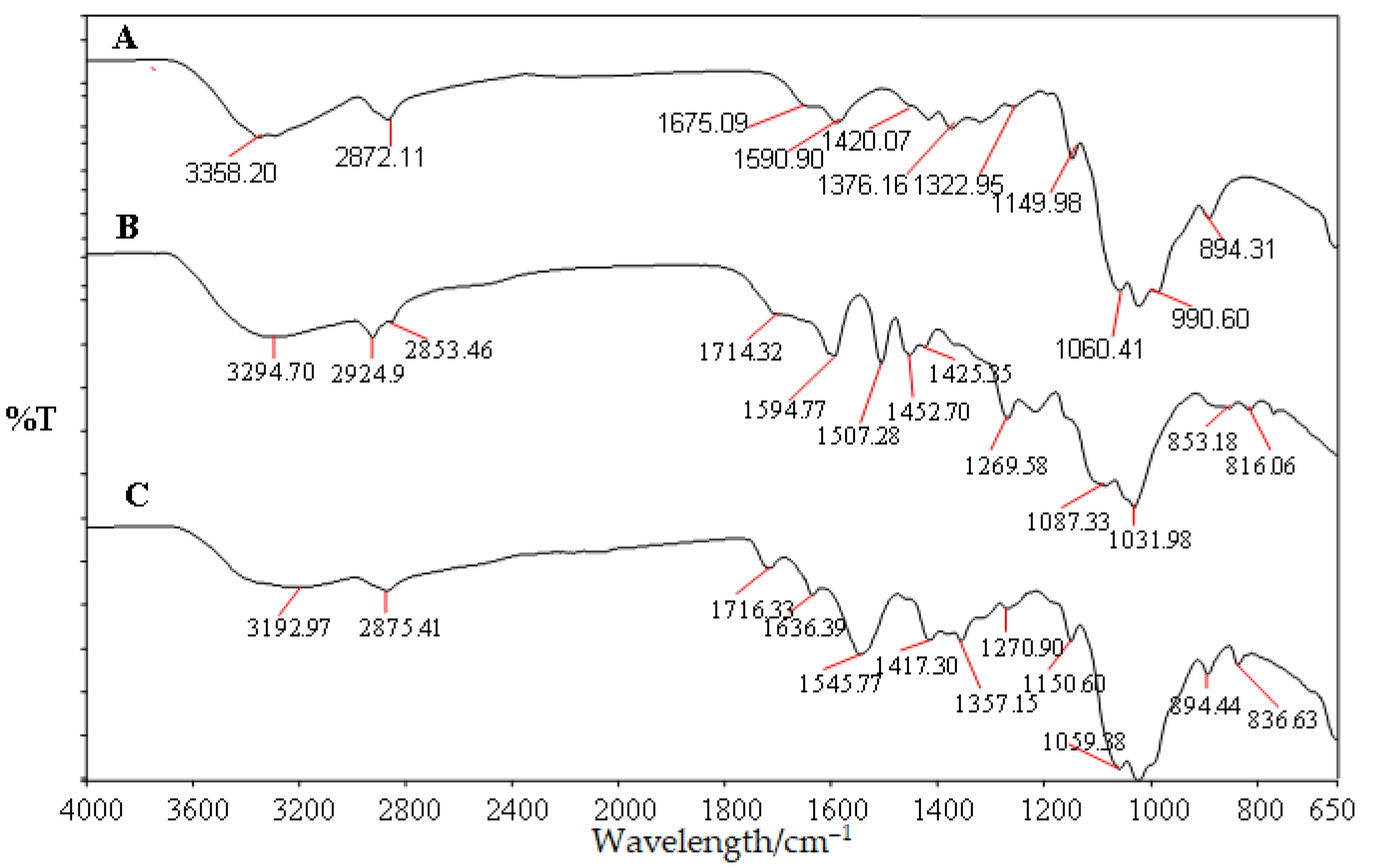
3.1. Structural Analysis of Modified Chitosan
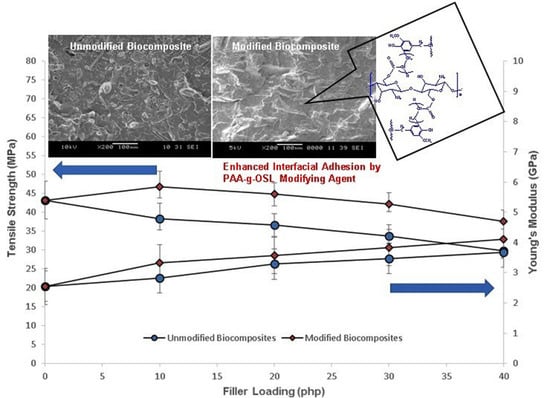
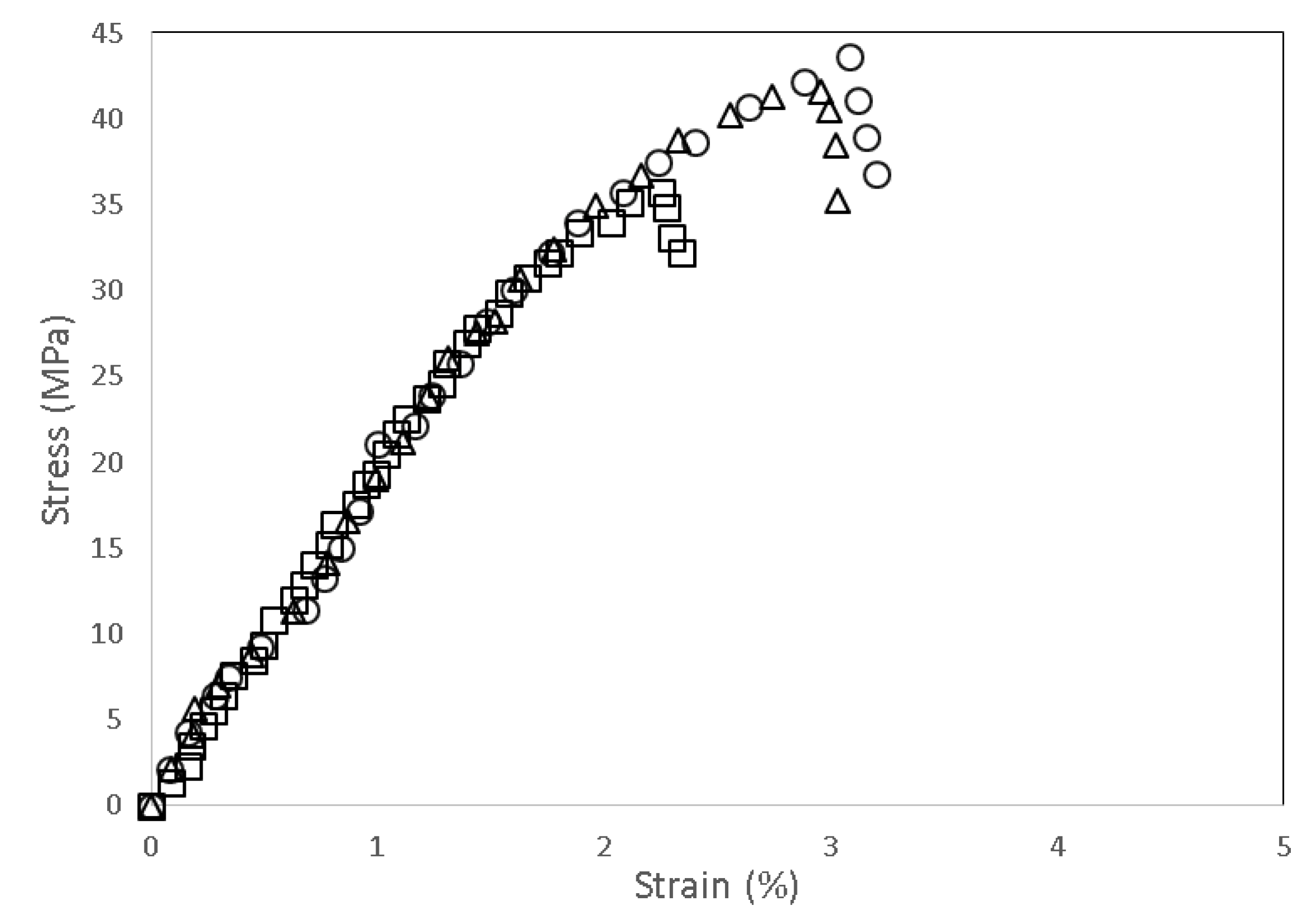
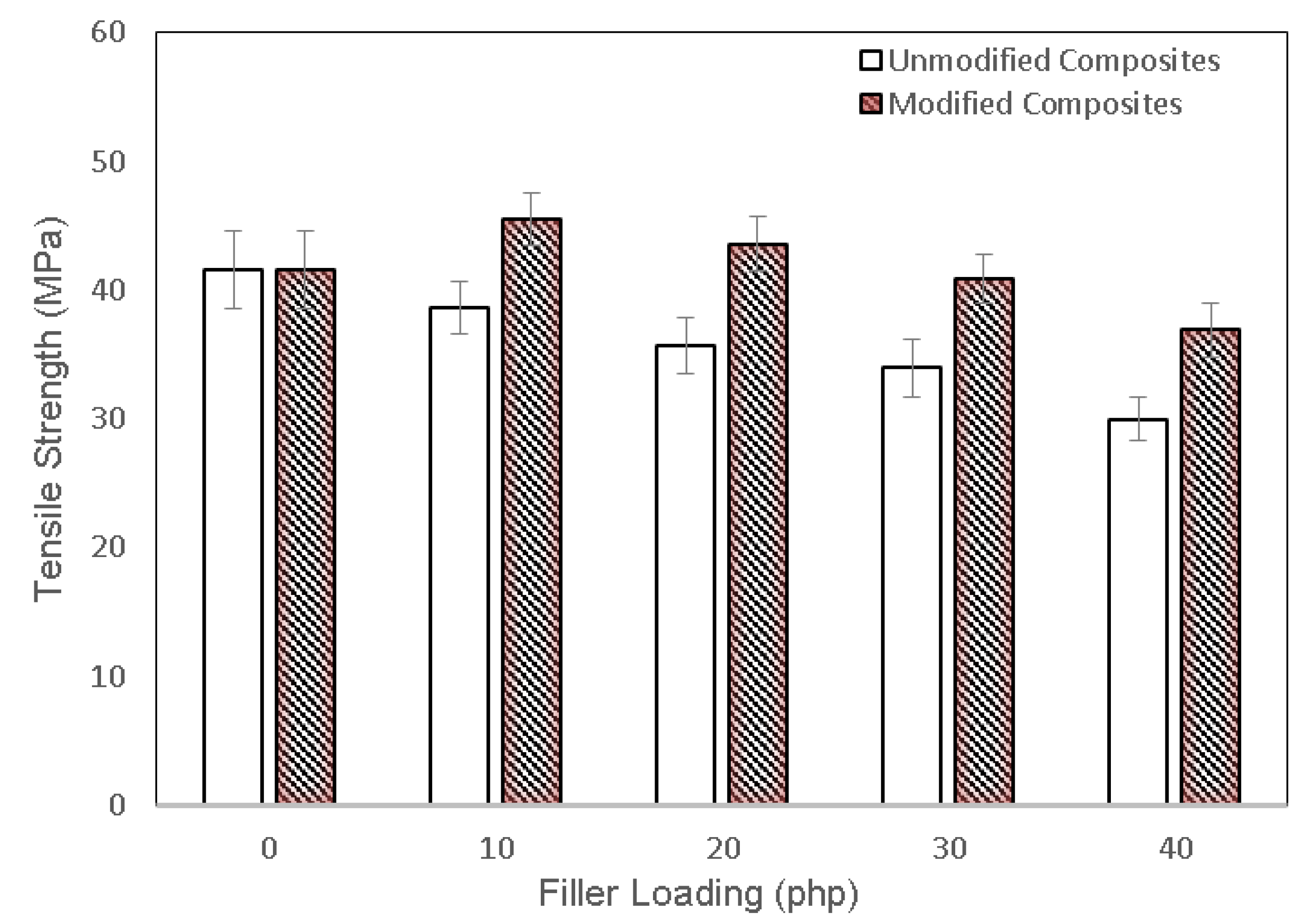
3.2. Mechanical Properties of PLA/Chitosan Biocomposites
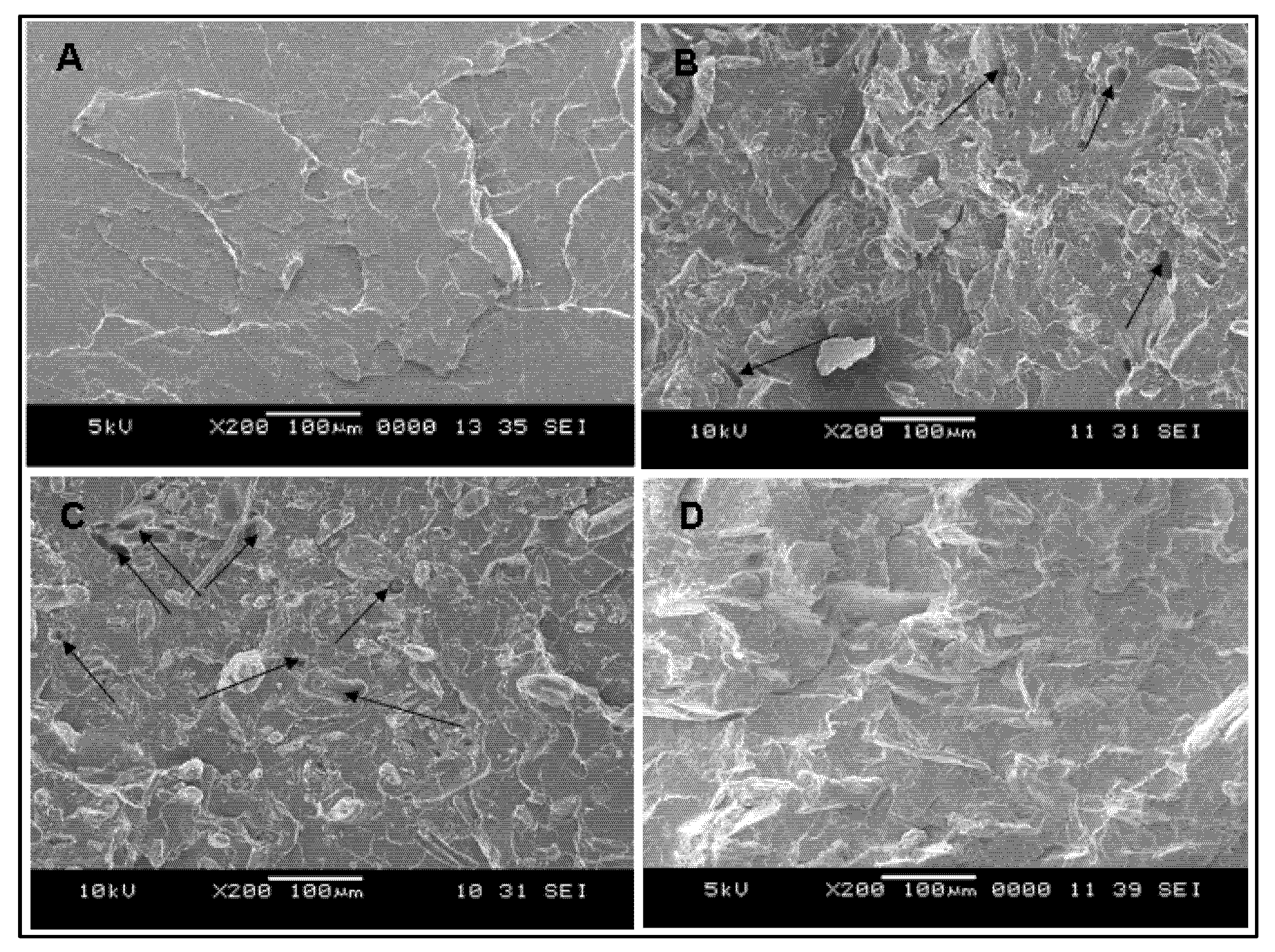
3.3. SEM Image Analysis
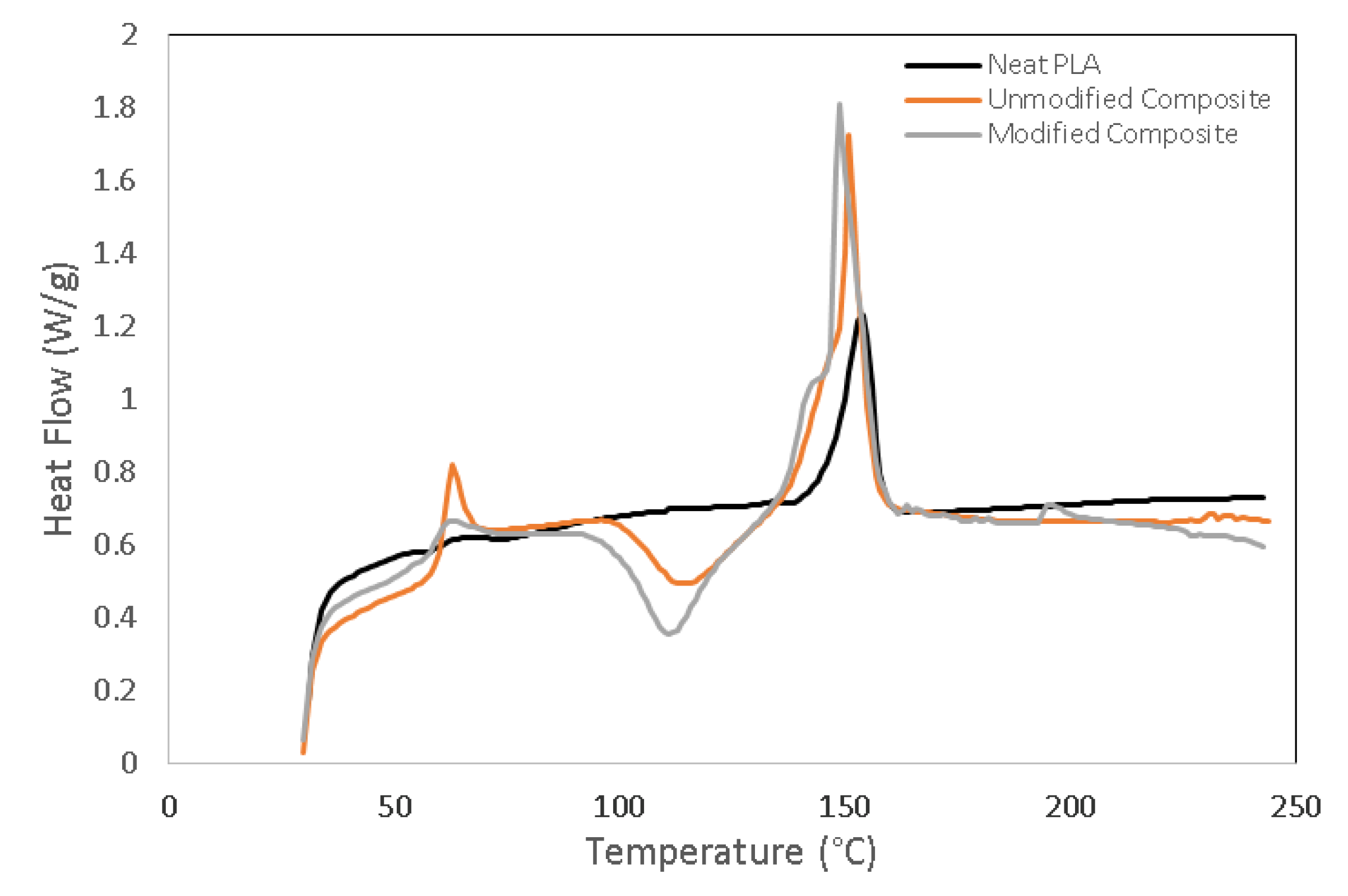
3.4. Thermal Properties of PLA/Chitosan Biocomposites
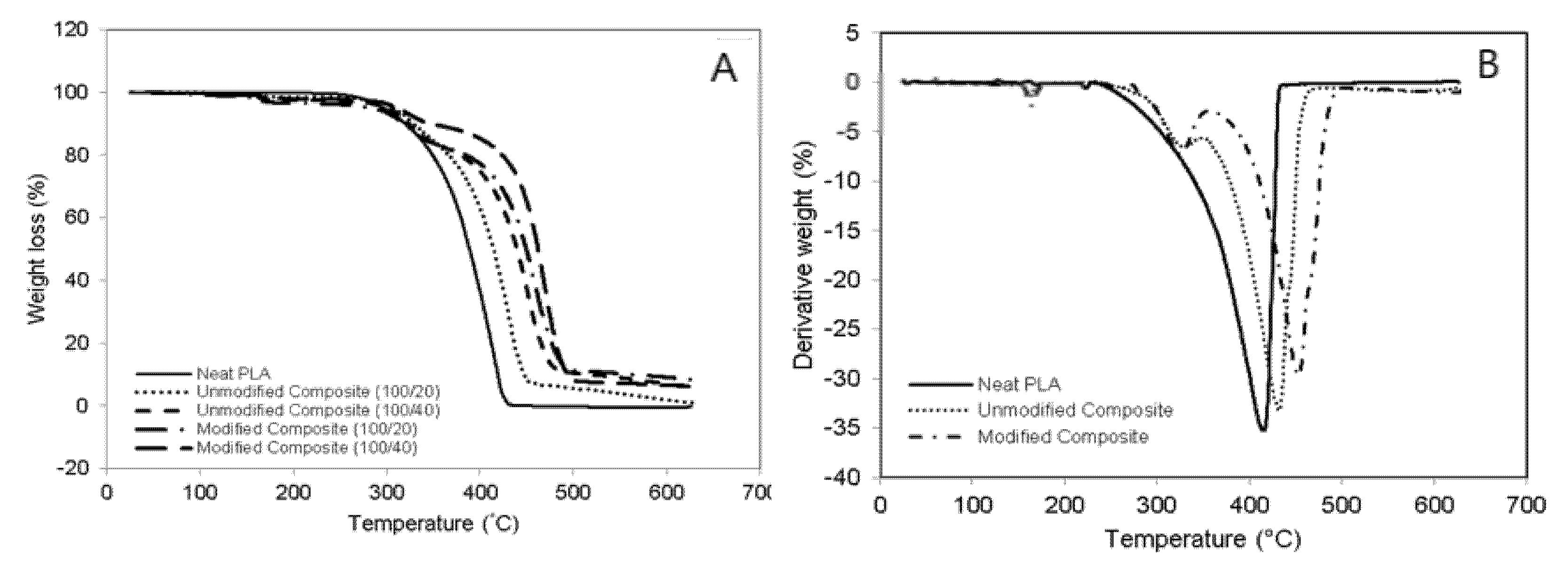
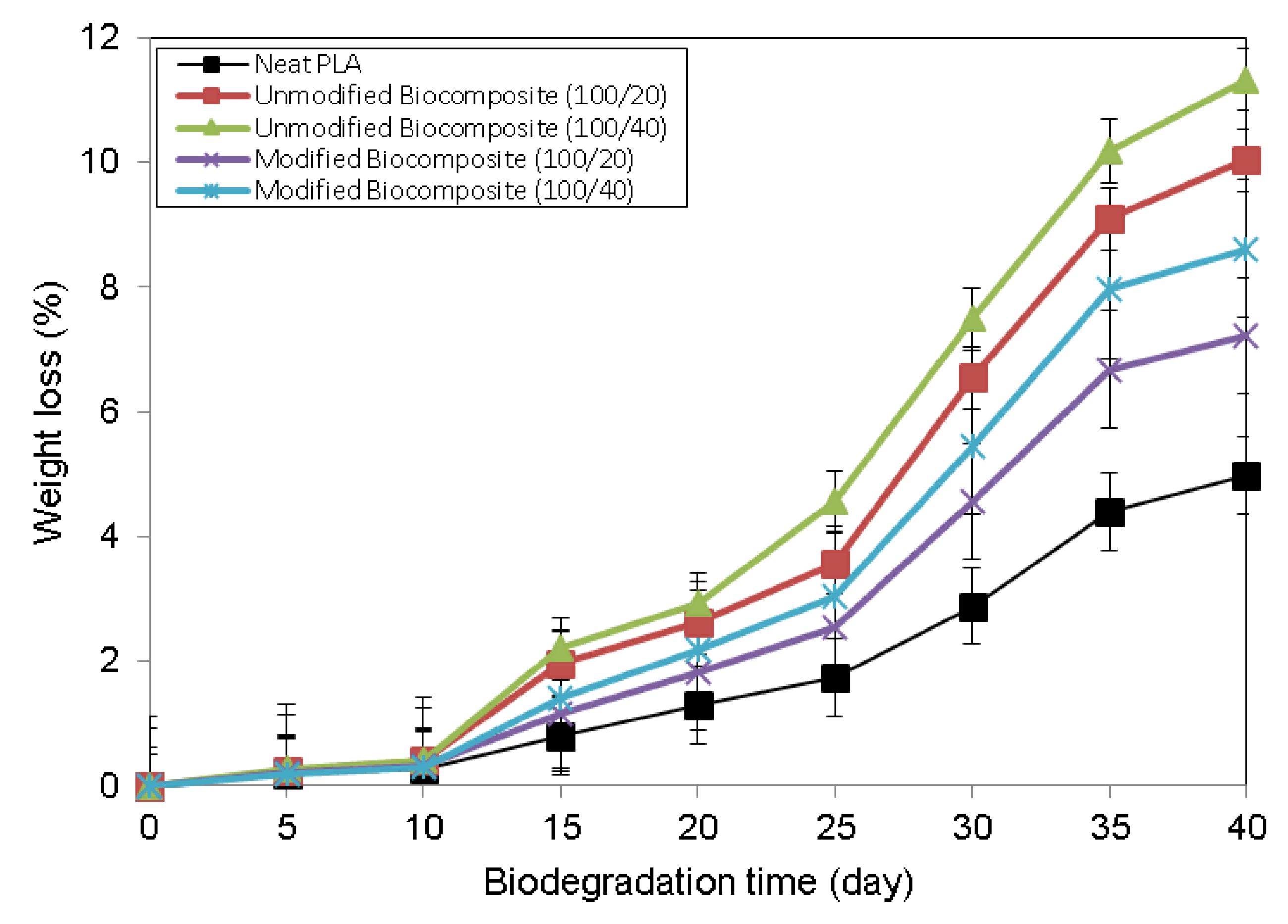
3.5. Weight Loss Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akonda, M.A.; Shah, D.U.; Gong, R.H. Natural fiber thermoplastic tapes to enhance reinforcing effects in composite structures. Compos. Part A 2020, 131, 105822. [Google Scholar] [CrossRef]
- Kun, D.; Kárpáti, Z.; Fekete, E.; Móczó, J. The role of interfacial adhesion in polymer composites engineered from lignocellulosic agricultural waste. Polymers 2021, 13, 3099. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Varadarajan, K.; Kumar, S. 3D-printed pol(ylactic acid) nanocomposite scaffolds for tissue engineering applications. Polym. Test. 2020, 81, 106203. [Google Scholar] [CrossRef]
- Jiashen, L.; Yi, L.; Lin, L.; Frank, K.F.T.; Ling, Q. Fabrication and degradation of poly(l-lactic acid) scaffolds with wool keratin. Compos. Part B Eng. 2009, 13, 664–667. [Google Scholar]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef]
- Sanchez-Olivares, G.; Sanchez-Solis, A.; Calderas, F.; Alongi, J. Keratin fibres derived from tannery industry wastes for flame retarded PLA composites. Polym. Degrad. Stab. 2017, 140, 42–54. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Spiridon, I.; Paduraru, O.M.; Zaltariov, M.F.; Darie, R.N. Influence of keratin on polylactic acid/chitosan composite properties. behavior upon accelerated weathering. Ind. Eng. Chem. Res. 2013, 52, 9822–9833. [Google Scholar] [CrossRef]
- Suh, H.; Hwang, Y.S.; Lee, J.E.; Han, C.D.; Park, J.C. Behavior of osteoblasts on a type I atelocollagen grafted ozone oxidized poly-L-lactic acid membrane. Biomaterials 2001, 22, 219–230. [Google Scholar] [CrossRef]
- Tanase, C.E.; Spiridon, I. PLA/chitosan/keratin composites for biomedical applications. Mater. Sci. Eng. C 2014, 40, 242–247. [Google Scholar] [CrossRef]
- Bonilla, J.; Fortunati, E.; Vargas, M.; Chiralt, A.; Kenny, J.M. Effects of chitosan on the physicochemical and antimicrobial properties of PLA films. J. Food Eng. 2013, 119, 236–243. [Google Scholar] [CrossRef]
- Quirk, R.A.; Chan, W.C.; Davies, M.C.; Tendler, S.J.B.; Shakesheff, K.M. Poly(L-lysine)-GRGDS as a biomimetic surface modifier for poly(lactic acid). Biomaterials 2001, 22, 865–872. [Google Scholar] [CrossRef]
- Shimao, M. Biodegradation of plastics. Curr. Opin. Biotechnol. 2001, 12, 242–247. [Google Scholar] [CrossRef]
- Olaiya, N.G.; Surya, I.; Oke, P.K.; Rizal, S.; Sadiku, E.R.; Ray, S.S.; Farayibi, P.K.; Hossain, M.S.; Khalil, H.P.S.A. Properties and characterization of a PLA-chitin-starch biodegradable polymer composite. Polymers 2019, 11, 1656. [Google Scholar] [CrossRef] [Green Version]
- Rigotti, D.; Checchetto, R.; Tarter, S.; Caretti, D.; Rizzuto, M.; Fambri, L.; Pegoretti, A. Polylactic acid-lauryl functionalized nanocellulose nanocomposites: Microstructural, thermo-mechanical and gas transport properties. Express Polym. Lett. 2019, 13, 858–876. [Google Scholar] [CrossRef]
- Mania, S.; Partyka, K.; Pilch, J.; Augustin, E.; Cieślik, M.; Ryl, J.; Jinn, J.-R.; Wang, Y.-J.; Michałowska, A.; Tylingo, R. Obtaining and characterization of the PLA/chitosan foams with antimicrobial properties achieved by the emulsification combined with the dissolution of chitosan by CO2 saturation. Molecules 2019, 24, 4532. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, H.; Bhowmik, N. Structure and stability analysis of biocompatible hydroxyapatite reinforced chitosan nanocomposite. Polym. Compos. 2018, 39, E573–E583. [Google Scholar] [CrossRef]
- Tanjung, F.A.; Husseinsyah, S.; Hussin, K.; Hassan, A. Mechanical and thermal properties of organosolv lignin/sodium dodecyl sulphate binary agent-treated polypropylene/chitosan composites. Polym. Bull. 2016, 73, 1427–1445. [Google Scholar] [CrossRef]
- Panariello, L.; Coltelli, M.B.; Buchignani, M.; Lazzeri, A. Chitosan and nano-structured chitin for biobased anti-microbial treatments onto cellulose based materials. Eur. Polym. J. 2019, 113, 328–339. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Sionkowska, A.; Walczak, M.; Sionkowska, M.M. Preparation and characterization of collagen/chitosan composites with silver nanoparticles. Polym. Compos. 2020, 41, 951–957. [Google Scholar] [CrossRef]
- Mir, S.; Yasin, T.; Halley, P.J.; Siddiq, H.M.; Nicholson, T. Thermal, rheological, mechanical and morphological behavior of HDPE/chitosan blend. Carbohydr. Polym. 2011, 83, 414–421. [Google Scholar] [CrossRef]
- Taufik, M.J.; Mansor, M.R.; Mustafa, Z. Characterization of wood plastic composite manufactured from kenaf fiber reinforced recycled-unused plastic blend. Compos. Struct. 2018, 189, 510–515. [Google Scholar] [CrossRef]
- Tanjung, F.A.; Husseinsyah, S.; Hussin, K. Chitosan filled-polypropylene composites: The effect of filler loading and organosolv lignin on mechanical, morphological and thermal properties. Fiber Polym. 2014, 15, 800–808. [Google Scholar] [CrossRef]
- Mishra, S.B.; Mishra, A.K.; Kaushik, N.K.; Khan, M.A. Study of performance properties of lignin-based polyblends with polyvinyl chloride. J. Mater. Process. Technol. 2007, 183, 273–276. [Google Scholar] [CrossRef]
- Shikinaka, K.; Nakamura, M.; Otsuka, Y. Strong UV absorption by nanoparticulated lignin in polymer films with reinforcement of mechanical properties. Polymer 2020, 190, 122254. [Google Scholar] [CrossRef]
- Cao, X.; Huang, J.; He, Y.; Hu, C.; Zhang, Q.; Yin, X.; Wu, W.; Li, R.K.Y. Biodegradable and renewable UV-shielding polylactide composites containing hierarchical structured POSS functionalized lignin. Int. J. Biol. Macromol. 2021, 188, 323–332. [Google Scholar] [CrossRef]
- Wu, W.; Liu, T.; Deng, X.; Sun, Q.; Cao, X.; Feng, Y.; Wang, B.; Roy, V.A.L.; Li, R.K.Y. Ecofriendly UV-protective films based on poly(propylene carbonate) biocomposites filled with TiO2 decorated lignin. Int. J. Biol. Macromol. 2019, 126, 1030–1036. [Google Scholar] [CrossRef]
- Toledano, A.; Garcia, A.; Mondragon, I.; Labidi, J. Lignin separation and fractionation by ultrafiltration. Sep. Purif. Technol. 2010, 71, 38–43. [Google Scholar] [CrossRef]
- Kabir, A.S.; Yuan, Z.S.; Kuboki, T.; Xu, C.B. Depolymerization of industrial lignins to improve the thermo-oxidative stability of polyolefins. Ind. Crop. Prod. 2018, 120, 238–249. [Google Scholar] [CrossRef]
- Hage, R.E.; Brosse, N.; Chrusciel, L.; Sanchez, L.; Sannigrahi, P.; Ragauskas, A. Characterization of milled wood lignin and ethanol organosolv lignin from miscanthus. Polym. Degrad. Stab. 2009, 94, 1632–1638. [Google Scholar] [CrossRef]
- Carrosco, F.; Pages, P.; Gamez, P.J.; Satana, O.O.; Maspoch, M.L. Processing of poly(lactic acid): Characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Huda, M.; Drzal, L.T.; Mohanty, A.K. Effect of fiber surface-treatments on the properties of laminated biocomposites from poly(lactic acid) (PLA) and kenaf fibers. Compos. Sci. Technol. 2008, 68, 424–432. [Google Scholar] [CrossRef]
- Leluk, K.; Frąckowiak, S.; Ludwiczak, J.; Rydzkowski, T.; Thakur, V.K. The impact of filler geometry on polylactic acid-based sustainable polymer composites. Molecules 2021, 26, 149. [Google Scholar] [CrossRef]
- Jang, W.; Shin, B.Y.; Lee, T.J. Thermal properties and morphology of biodegradable PLA/starch compatibilized blends. J. Ind. Eng. Chem. 2007, 13, 457–464. [Google Scholar]
- Ibrahim, M.N.M.; Haras, M.R.A.; Sipaut, C.S.; Aboul-Enein, H.Y.; Mohamed, A.A. Preparation and characterization of a newly water-soluble lignin graft copolymer from oil palm lignocellulosic waste. Carbohydr. Polym. 2010, 80, 1102–1110. [Google Scholar] [CrossRef]
- Perinovic, S.; Andricic, B.; Erceg, M. Thermal properties of poly(L-lactic)/olive stone flour composites. Acta 2010, 510, 97–102. [Google Scholar]
- Gregorova, A.; Hrabalova, M.; Wimmer, R.; Saake, B.; Altaner, C. Poly(lactide acid) composites reinforced with fibers obtained from different tissue type of Picea sitchensis. J. Appl. Polym. Sci. 2009, 114, 2616–2623. [Google Scholar] [CrossRef]
- Kijchavengkul, T.; Rafael, A.; Maria, R. Biodegradation and hydrolysis rate of aliphatic aromatic polyester. Polym. Degrad. Stab. 2010, 95, 2641–2647. [Google Scholar] [CrossRef]

| Item | Specification | Test Method |
|---|---|---|
| Appearance | Off-white powder | Visual |
| Particle size | 80 µm | Malvern Particle Size Analyzer |
| Solubility of 1% chitosan in 1% acetic acid | >99.0% | Dissolution & Filtration |
| Viscosity | 150–200 m Pa·s | Ubbelohde Viscometer |
| Moisture content | <10.0% | Infra-red drying |
| Ash content | <1.0% | Incineration |
| Materials | Unmodified Biocomposites | Modified Biocomposites with PAAL |
|---|---|---|
| Polylactic acid (PLA) (php) | 100 | 100 |
| Chitosan (php) | 0, 10, 20, 30, 40 | 10, 20, 30, 40 |
| PAA-g-OSL (PAAL) (wt%) | - | 3 |
| Temperature (°C) | Neat PLA | Unmodified Biocomposites | Modified Biocomposites | ||
|---|---|---|---|---|---|
| 20 php | 40 php | 20 php | 40 php | ||
| 100–150 | 0.002 | 0.039 | 0.277 | 0.342 | 0.600 |
| 150–200 | 0.016 | 0.271 | 0.555 | 0.464 | 0.773 |
| 200–250 | 0.150 | 1.017 | 1.894 | 0.943 | 1.561 |
| 250–300 | 0.476 | 0.157 | 0.212 | 0.428 | 0.673 |
| 300–350 | 4.993 | 0.495 | 1.262 | 2.102 | 3.419 |
| 350–400 | 14.813 | 4.540 | 5.080 | 3.988 | 2.005 |
| 400–450 | 43.150 | 13.539 | 13.105 | 10.197 | 9.663 |
| 450–500 | 36.400 | 40.499 | 36.512 | 38.738 | 31.997 |
| 500–550 | 0 | 33.153 | 32.855 | 32.834 | 29.461 |
| 550–600 | 0 | 1.494 | 1.428 | 1.359 | 9.302 |
| 600–630 | 0 | 1.886 | 1.692 | 0.348 | 0.674 |
| Total | 100 | 97.090 | 94.872 | 91.743 | 90.128 |
| Biocomposites | Tg (°C) | Tc(°C) | Tm (°C) | Xc (%) |
|---|---|---|---|---|
| Neat PLA | 57.6 | - | 152.0(0.2) | 32.74 |
| PLA/chitosan: 100/20 php (unmodified) | 63.4 | 117.7 | 151.3(0.3) | 27.41 |
| PLA/chitosan: 100/40 php (unmodified) | 62.5 | 117.2 | 151.1(0.1) | 24.25 |
| PLA/chitosan: 100/20 php (modified) | 61.3 | 115.3 | 150.4(0.5) | 39.42 |
| PLA/chitosan: 100/40 php (modified) | 61.2 | 114.5 | 150.2(0.3) | 33.80 |
| Biocomposites | 0 Day | 30 Day | ||
|---|---|---|---|---|
| Tensile Strength (MPa) | Young’s Modulus (MPa) | Tensile Strength (MPa) | Young’s Modulus (MPa) | |
| Neat PLA | 42.82 | 2674.68 | 35.67 | 2476.43 |
| PLA/chitosan: 100/20 (unmodified) | 37.74 | 3158.97 | 28.84 | 2847.91 |
| PLA/chitosan: 100/40 (unmodified) | 31.81 | 4125.86 | 25.65 | 3789.36 |
| PLA/chitosan: 100/20 (modified) | 45.64 | 3567.45 | 40.27 | 3254.29 |
| PLA/chitosan: 100/40 (modified) | 38.72 | 4180.87 | 32.85 | 3810.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanjung, F.A.; Arifin, Y.; Kuswardani, R.A. Influence of Newly Organosolv Lignin-Based Interface Modifier on Mechanical and Thermal Properties, and Enzymatic Degradation of Polylactic Acid/Chitosan Biocomposites. Polymers 2021, 13, 3355. https://doi.org/10.3390/polym13193355
Tanjung FA, Arifin Y, Kuswardani RA. Influence of Newly Organosolv Lignin-Based Interface Modifier on Mechanical and Thermal Properties, and Enzymatic Degradation of Polylactic Acid/Chitosan Biocomposites. Polymers. 2021; 13(19):3355. https://doi.org/10.3390/polym13193355
Chicago/Turabian StyleTanjung, Faisal Amri, Yalun Arifin, and Retna Astuti Kuswardani. 2021. "Influence of Newly Organosolv Lignin-Based Interface Modifier on Mechanical and Thermal Properties, and Enzymatic Degradation of Polylactic Acid/Chitosan Biocomposites" Polymers 13, no. 19: 3355. https://doi.org/10.3390/polym13193355
APA StyleTanjung, F. A., Arifin, Y., & Kuswardani, R. A. (2021). Influence of Newly Organosolv Lignin-Based Interface Modifier on Mechanical and Thermal Properties, and Enzymatic Degradation of Polylactic Acid/Chitosan Biocomposites. Polymers, 13(19), 3355. https://doi.org/10.3390/polym13193355