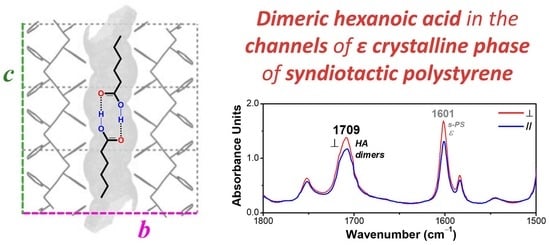
Monomeric and Dimeric Carboxylic Acid in Crystalline Cavities and Channels of Delta and Epsilon Forms of Syndiotactic Polystyrene
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Techniques and Methods
3. Results and Discussion
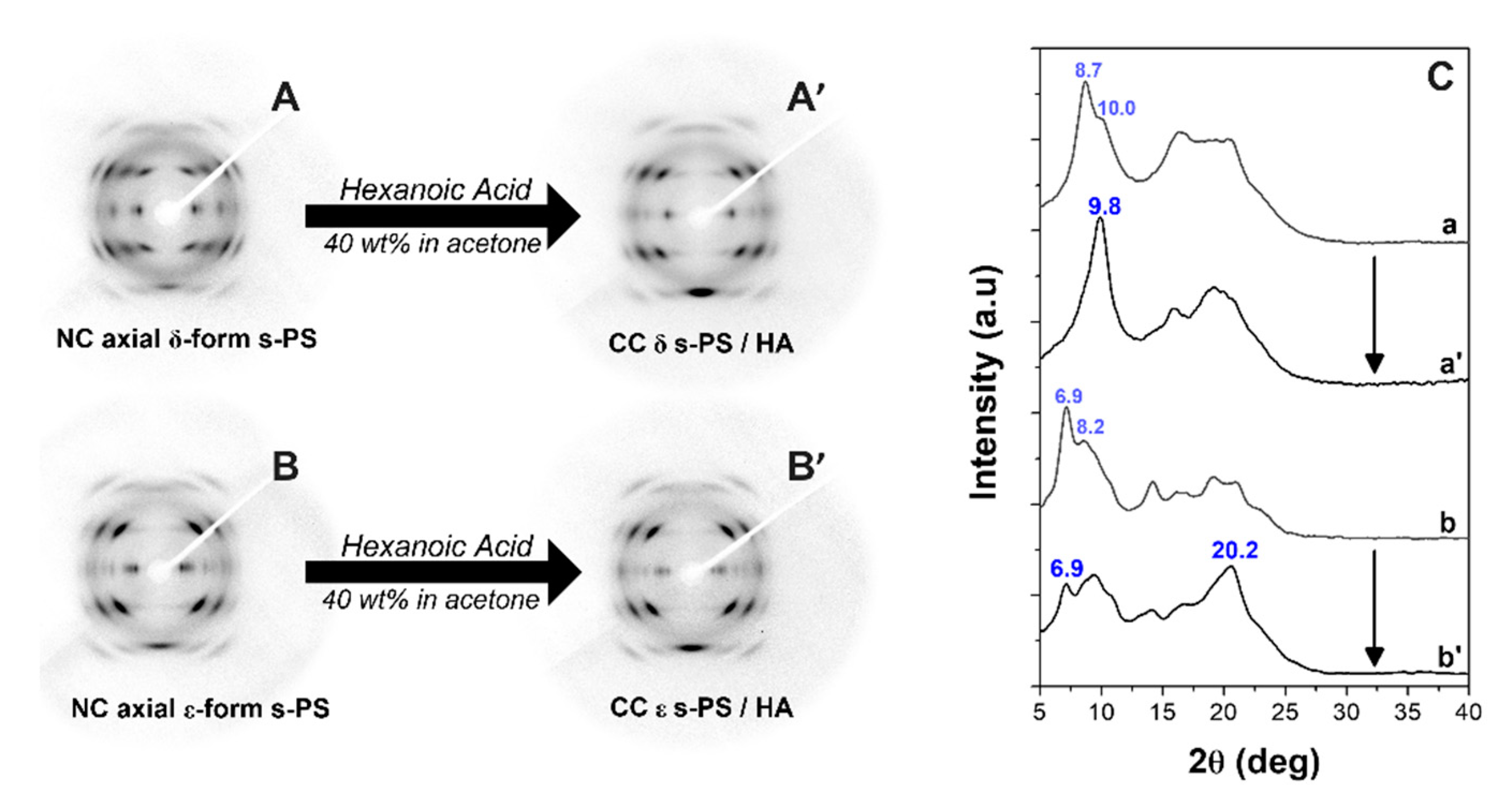
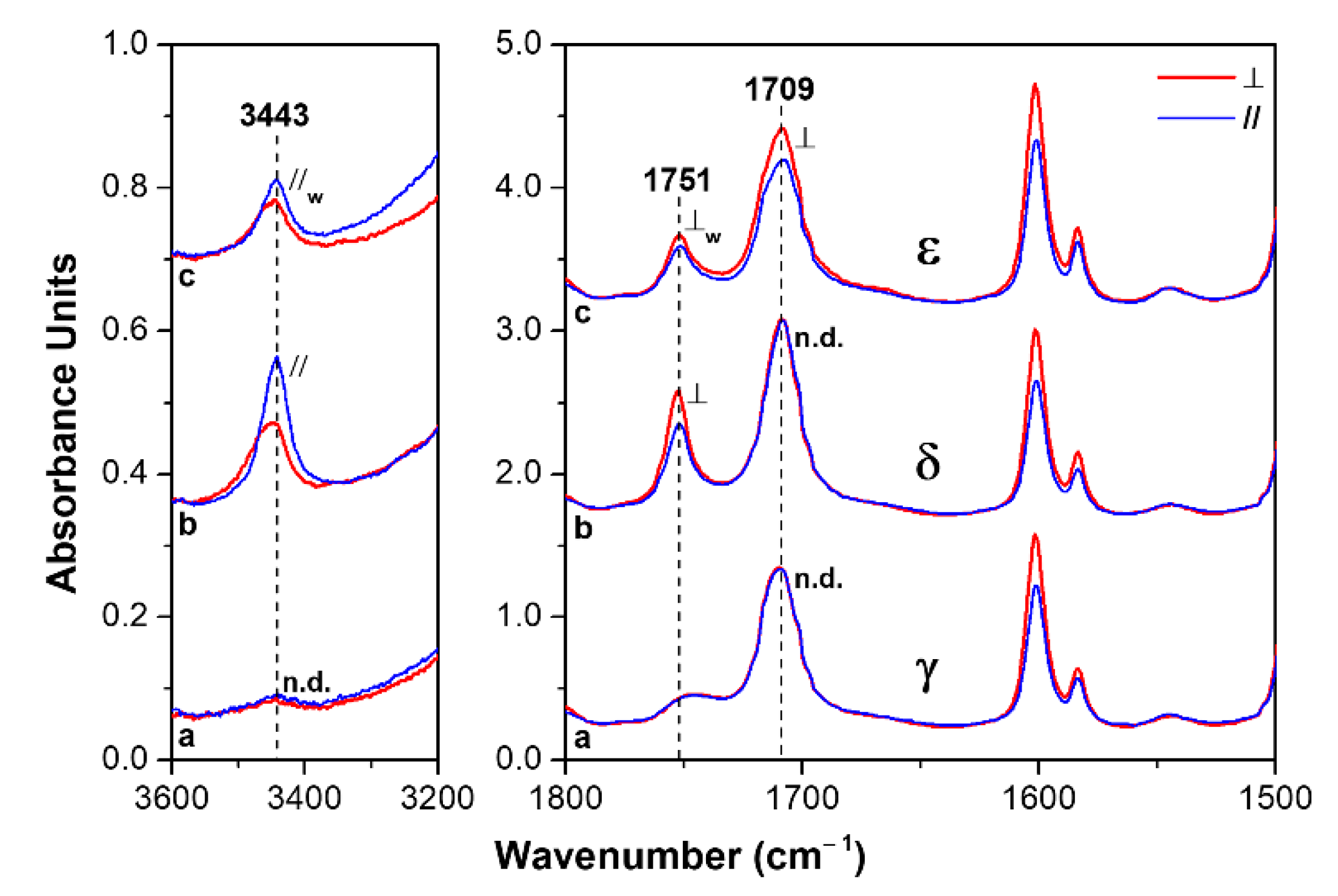
3.1. δ and ε Co-Crystalline Forms of s-PS with Hexanoic Acid
3.2. Dichroism of FTIR Peaks of HA in Axially Oriented s-PS Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guerra, G.; Daniel, C.; Rizzo, P.; Tarallo, O. Advanced materials based on polymer cocrystalline forms. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 305–322. [Google Scholar] [CrossRef]
- Atkins, E.D.T.; Isaac, D.H.; Keller, A.; Miyasaka, K. Analysis of anomalous X-ray diffraction effects of isotactic polystyrene gels and its implications for chain conformation and isomeric homogeneity. J. Polym. Sci. Polym. Phys. Ed. 1977, 15, 211–226. [Google Scholar] [CrossRef]
- El Hasri, S.; Ray, B.; Thierry, A.; Guenet, J.M. Solvent-Induced Organization of Amorphous Isotactic Polystyrene. Macromolecules 2004, 37, 4124–4129. [Google Scholar] [CrossRef]
- Iuliano, M.; Guerra, G.; Petraccone, V.; Corradini, P.; Pellecchia, C. Polymorphism of syndiotactic poly(p-methylstyrene) unoriented samples. New Polym. Mater. 1992, 3, 133–144. [Google Scholar]
- Tarallo, O.; Esposito, G.; Passarelli, U.; Petraccone, V. A Clathrate Form of Syndiotactic Poly(p-methylstyrene) Containing Two Different Types of Cavities. Macromolecules 2007, 40, 5471–5478. [Google Scholar] [CrossRef]
- Yokoyama, M.; Ishihara, H.; Iwamoto, R.; Tadokoro, H. Structure of poly (ethylene oxide) complexes. III. Poly (ethylene oxide)-mercuric chloride complex. Type II. Macromolecules 1969, 2, 184–192. [Google Scholar] [CrossRef]
- Paternostre, L.; Damman, P.; Dosière, M. Polymorphism and Crystal Morphology of Poly(ethylene oxide)-2-Methyl Resorcinol Supramolecular Complexes. Macromolecules 1999, 32, 153–161. [Google Scholar] [CrossRef]
- Dikshit, A.K.; Nandi, A.K. Thermoreversible gelation of poly (vinylidene fluoride) in diesters: Influence of intermittent length on morphology and thermodynamics of gelation. Macromolecules 2000, 33, 2616–2625. [Google Scholar] [CrossRef]
- Dasgupta, D.; Malik, S.; Thierry, A.; Guenet, J.M.; Nandi, A.K. Thermodynamics, Morphology, and Structure of the Poly(vinylidene fluoride)−Ethyl Acetoacetate System. Macromolecules 2006, 39, 6110–6114. [Google Scholar] [CrossRef]
- Daniel, C.; Vitillo, J.G.; Fasano, G.; Guerra, G. Aerogels and Polymorphism of Isotactic Poly(4-methyl-pentene-1). ACS Appl. Mater. Interfaces 2011, 3, 969–977. [Google Scholar] [CrossRef]
- Marubayashi, H.; Asai, S.; Sumita, M. Complex Crystal Formation of Poly(l-lactide) with Solvent Molecules. Macromolecules 2012, 45, 1384–1397. [Google Scholar] [CrossRef]
- Marubayashi, H.; Asai, S.; Sumita, M. Guest-Induced Crystal-to-Crystal Transitions of Poly(l-lactide) Complexes. J. Phys. Chem. B 2013, 117, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, P.; Ianniello, G.; Venditto, V.; Tarallo, O.; Guerra, G. Poly(L-lactic acid): Uniplanar Orientation in Cocrystalline Films and Structure of the Cocrystalline Form with Cyclopentanone. Macromolecules 2015, 48, 7513–7520. [Google Scholar] [CrossRef]
- De Rosa, C.; Guerra, G.; Petraccone, V.; Pirozzi, B. Crystal structure of the emptied clathrate form (δe form) of syndiotactic polystyrene. Macromolecules 1997, 30, 4147–4152. [Google Scholar] [CrossRef]
- Gowd, E.B.; Tashiro, K.; Ramesh, C. Structural phase transitions of syndiotactic polystyrene. Prog. Polym. Sci. 2009, 34, 280–315. [Google Scholar] [CrossRef]
- Tarallo, O.; Petraccone, V.; Albunia, A.R.; Daniel, C.; Guerra, G. Monoclinic and Triclinic δ-Clathrates of Syndiotactic Polystyrene. Macromolecules 2010, 43, 8549–8558. [Google Scholar] [CrossRef]
- Acocella, M.R.; Rizzo, P.; Daniel, C.; Tarallo, O.; Guerra, G. Nanoporous triclinic δ modification of syndiotactic polystyrene. Polymer 2015, 63, 230–236. [Google Scholar] [CrossRef]
- Joseph, A.M.; Nagendra, B.; Shaiju, P.; Surendran, K.P.; Gowd, E.B. Aerogels of hierarchically porous syndiotacttic polystyrene with a dielectric constant near to air. J. Mater. Chem. C 2018, 6, 360–368. [Google Scholar] [CrossRef]
- Petraccone, V.; Ruiz de Ballesteros, O.; Tarallo, O.; Rizzo, P.; Guerra, G. Nanoporous Polymer Crystals with Cavities and Channels. Chem. Mater. 2008, 20, 3663–3668. [Google Scholar] [CrossRef]
- Daniel, C.; Longo, S.; Fasano, G.; Vitillo, J.G.; Guerra, G. Nanoporous Crystalline Phases of Poly(2,6-Dimethyl-1,4-phenylene)oxide. Chem. Mater. 2011, 23, 3195–3200. [Google Scholar] [CrossRef]
- Nagendra, B.; Cozzolino, A.; Daniel, C.; Rizzo, P.; Guerra, G.; Auriemma, F.; De Rosa, C.; D’Alterio, M.C.; Tarallo, O.; Nuzzo, A. Two nanoporous-crystalline forms of PPO and related co-crystalline forms. Macromolecules 2019, 52, 9646–9656. [Google Scholar] [CrossRef]
- Daniel, C.; Galdi, N.; Montefusco, T.; Guerra, G. Syndiotactic Polystyrene Clathrates with Polar Guest Molecules. Chem. Mater. 2007, 19, 3302–3308. [Google Scholar] [CrossRef]
- Tarallo, O.; Schiavone, M.M.; Petraccone, V.; Daniel, C.; Rizzo, P.; Guerra, G. Channel Clathrate of Syndiotactic Polystyrene with p-nitroaniline. Macromolecules 2010, 43, 1455–1466. [Google Scholar] [CrossRef]
- Uda, Y.; Kaneko, F.; Tanigaki, N.; Kawaguchi, T. The First Example of a Polymer-Crystal–Organic-Dye Composite Material: The Clathrate Phase of Syndiotactic Polystyrene with Azulene. Adv. Mater. 2005, 17, 1846–1850. [Google Scholar] [CrossRef]
- De Girolamo Del Mauro, A.; Carotenuto, M.; Venditto, V.; Petraccone, V.; Scoponi, M.; Guerra, G. Fluorescence of Syndiotactic Polystyrene / Trimethylbenzene Clathrate and Intercalate Co-Crystals. Chem. Mater. 2007, 19, 6041–6046. [Google Scholar] [CrossRef]
- Albunia, A.R.; Rizzo, P.; Coppola, M.; De Pascale, M.; Guerra, G. Azobenzene Isomerization in Polymer Co-crystalline Phases. Polymer 2012, 53, 2727–2735. [Google Scholar] [CrossRef]
- Albunia, A.R.; D’Aniello, C.; Guerra, G.; Gatteschi, D.; Mannini, M.; Sorace, L. Ordering Magnetic Molecules within Nanoporous Crystalline Polymers. Chem. Mater. 2009, 21, 4750–4752. [Google Scholar] [CrossRef]
- Daniel, C.; Rufolo, C.; Bobba, F.; Scarfato, A.; Cucolo, A.M.; Guerra, G. Ferroelectric co-crystalline polymers. J. Mater. Chem. 2011, 21, 19074–19079. [Google Scholar] [CrossRef]
- Albunia, A.R.; Rizzo, P.; Ianniello, G.; Rufolo, C.; Guerra, G. Syndiotactic polystyrene films with a cocrystalline phase including carvacrol guest molecules. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 657–665. [Google Scholar] [CrossRef]
- Albunia, A.R.; Guerra, G. Spectroscopic Investigation of Guest-Guest Interactions in the Nanoporous-Crystalline δ and ε Forms of Syndiotactic Polystyrene. J. Phys. Chem. C 2014, 118, 11774–11783. [Google Scholar] [CrossRef]
- Rizzo, P.; Cozzolino, A.; Guerra., G. Chemical Stabilization of Molecules by Inclusion as Guests of Nanoporous-Crystalline Polymer Phases. Macromolecules 2019, 52, 2255–2264. [Google Scholar] [CrossRef]
- Golla, M.; Nagendra, B.; Daniel, C.; Rizzo, P.; Guerra, G. Isolated and Aggregated Carvacrol Guest Molecules in Co-crystalline Poly(2,6-dimethyl-1,4-phenylene)oxide Films. Polymer J. 2021, 53, 1093–1100. [Google Scholar]
- Cozzolino, A.; Daniel, C. Active yarns and textiles for the stabilization and controlled release of active compounds. WO 2020026164A1, 6 February 2020. [Google Scholar]
- Sato, S.; Kawaguchi, T.; Kaneko, F. ATR FTIR Spectroscopic Study on Complexation of Sydiotactic Polystyrene with n-Alkyl Carboxylic Acids. Macromol. Symp. 2016, 369, 114–118. [Google Scholar] [CrossRef]
- Hismiogullari, S.E.; Hismiogullari, A.A.; Sahin, F.; Oner, E.T.; Yenice, S.M.; Karasartova, D. Investigation of antibacterial and cytotoxic effects of organic acids including ascorbic acid, lactic acid and acetic acids on mammalian cells. J. Anim. Vet. Adv. 2008, 7, 681–684. [Google Scholar]
- Leyva, M.O.; Vicedo, B.; Finiti, I.; Flors, V.; Del Amo, G.; Real, M.D.; García-Agustín, P.; González-Bosch, C. Preventive and post-infection control of Botrytis cinerea in tomato plants by hexanoic acid. Plant Pathol. 2008, 57, 1038–1046. [Google Scholar] [CrossRef]
- Creager, S.E.; Steiger, C.M. Conformational Rigidity in a Self-Assembled Monolayer of 4-Mercaptobenzoic Acid on Gold. Langmuir 1995, 11, 1852–1854. [Google Scholar] [CrossRef]
- Halupka, M.; Sander, W. A simple method for the matrix isolation of monomeric and dimeric carboxylic acids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1998, 54, 495–500. [Google Scholar] [CrossRef]
- Yamamoto, M.; Iwai, Y.; Nakajima, T.; Arai, Y. Fourier Transform Infrared Study on Hydrogen Bonding Species of Carboxylic Acids in Supercritical Carbon Dioxide with Ethanol. J. Phys. Chem. A 1999, 103, 3525–3529. [Google Scholar] [CrossRef]
- Eliason, T.L.; Havey, D.K.; Vaida, V. Gas phase infrared spectroscopic observation of the organic acid dimers (CH3(CH2)6COOH)2, (CH3(CH2)7COOH)2, and (CH3(CH2)8COOH)2. Chem. Phys. Lett. 2005, 402, 239–244. [Google Scholar] [CrossRef]
- Macoas, E.M.S.; Myllyperkio, P.; Kunttu, H.; Pettersson, M. Vibrational Relaxation of Matrix-Isolated Carboxylic Acid Dimers and Monomers. J. Phys. Chem. A 2009, 113, 7227–7234. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.A.E.; Suhm, M.A. Vibrational exciton coupling in homo and hetero dimers of carboxylic acids studied by linear infrared and Raman jet spectroscopy. J. Chem. Phys. 2018, 149, 104307. [Google Scholar] [CrossRef]
- Musto, P.; Mensitieri, G.; Cotugno, S.; Guerra, G.; Venditto, V. Probing by Time-Resolved FTIR Spectroscopy Mass Transport, Molecular Interactions, and Conformational Ordering in the System Chloroform-Syndiotactic Polystyrene. Macromolecules 2002, 35, 2296–2304. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Austin, A.; Petersson, G.A.; Frisch, M.J.; Dobek, F.J.; Scalmani, G.; Throssell, K. A Density Functional with Spherical Atom Dispersion Terms. J. Chem. Theory Comput. 2012, 8, 4989–5007. [Google Scholar] [CrossRef] [PubMed]
- Immirzi, A.; De Candia, F.; Iannelli, P.; Zambelli, A.; Vittoria, V. Solvent-induced polymorphism in syndiotactic polystyrene. Makromol. Chem. Rapid Commun. 1988, 9, 761–764. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; Wiley: Hoboken, NJ, USA, 2004; p. 18. [Google Scholar]
- Itagaki, H.; Sano, T.; Okabe, T.; Sano, S.; Ebihara, H.; Tomono, F.; Dohra, H. Polymerization of Aniline in Tubular Cavities of the Crystalline Phase of syndiotactic polystyrene: Proposal of a Preparation Method of Sophisticated Polymer Composites. ACS Macro Lett. 2017, 6, 1099–1103. [Google Scholar] [CrossRef]
- Sano, T.; Ebihara, H.; Sano, S.; Okabe, T.; Itagaki, H. The ways of connecting crystalline phases having tubular cavities like stringing beads: New conductive polymer composites prepared by the polymerization of aniline in highly oriented ε crystalline phase of syndiotactic polystyrene. Eur. Polym. J. 2020, 138, 109975. [Google Scholar] [CrossRef]
- Banerjee, P.; Bhattacharya, I.; Chakraborty, T. Matrix Isolation Infrared Spectroscopy of an O–H···π Hydrogen-Bonded Complex between Formic Acid and Benzene. J. Phys. Chem. A 2016, 120, 3731–3739. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, A.; Rizzo, P.; Gallo, C.; Bianchi, R.; Daniel, C.; Guerra, G. Axially oriented guest induced crystallization in syndiotactic polystyrene unstretched fibers. Polymer 2021, 228, 123908. [Google Scholar] [CrossRef]
- Albunia, A.R.; Di Masi, S.; Rizzo, P.; Milano, G.; Musto, P.; Guerra, G. Chlorinated Guest Orientation and Mobility in Clathrate Structures Formed with Syndiotactic Polystyrene. Macromolecules 2003, 36, 8695–8703. [Google Scholar] [CrossRef]
- Golla, M.; Nagendra, B.; Fierro, F.; Rizzo, P.; Daniel, C.; Guerra, G. Axially Oriented Nanoporous Crystalline Phases of Poly(2,6-dimethyl-1,4-phenylene)oxide. ACS Appl. Polym. Mater. 2020, 2, 3518–3524. [Google Scholar] [CrossRef]
- Kanters, J.A.; Kroon, J.; Peerdeman, A.F.; Schoone, J.C. Conformation of some carboxylic acids and their derivatives. Tetrahedron 1967, 23, 4027–4033. [Google Scholar] [CrossRef][Green Version]

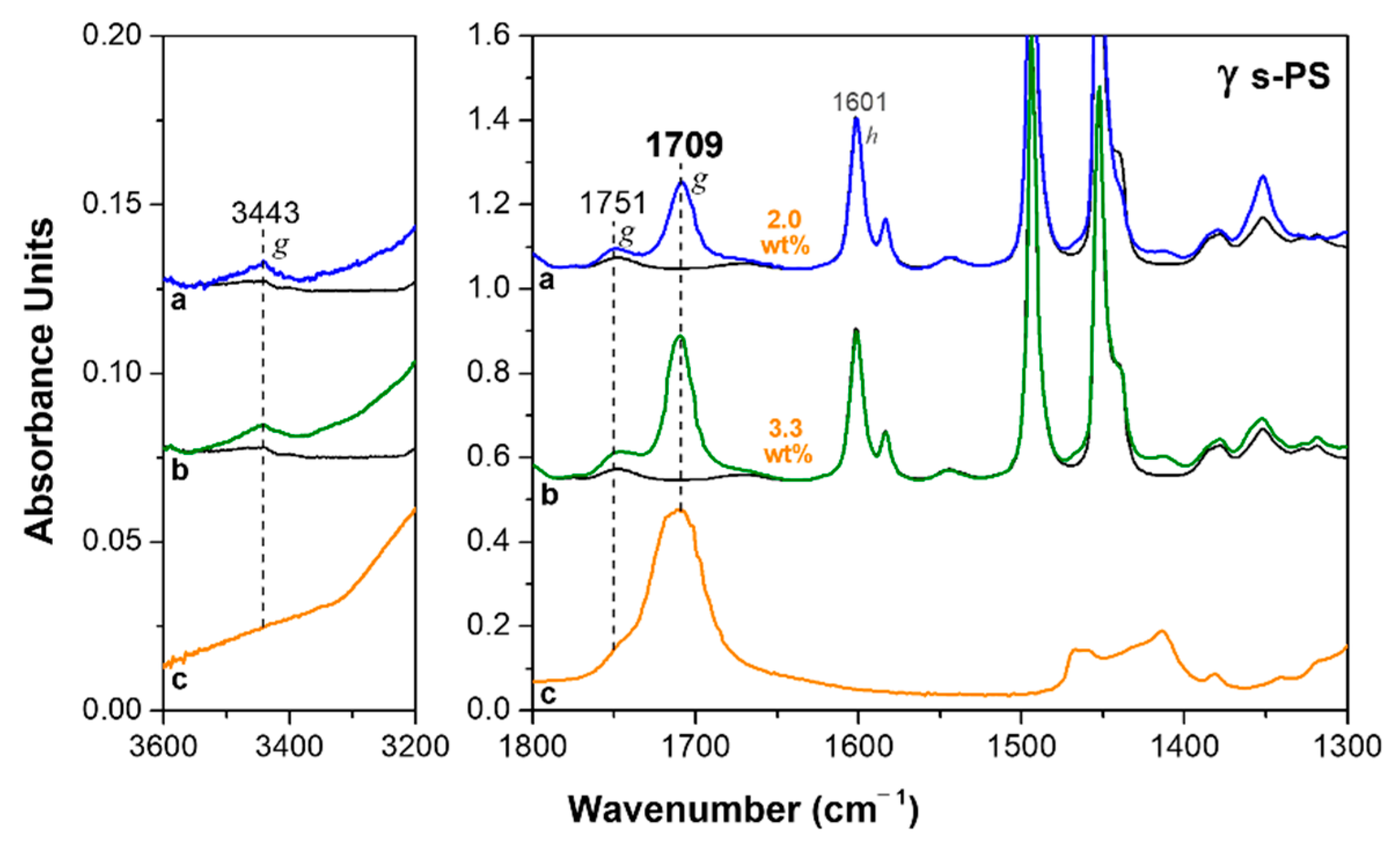

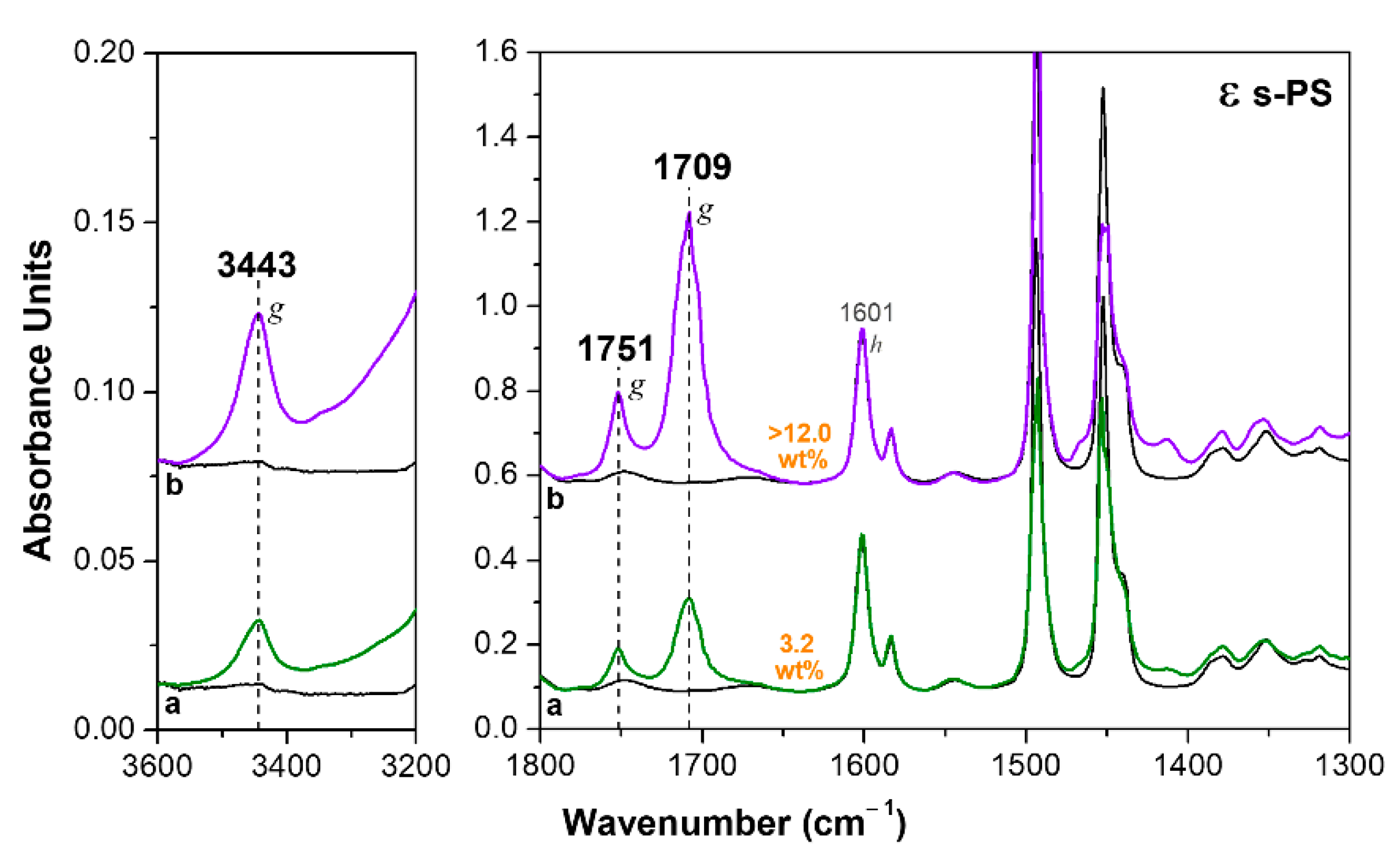


| Form | I1601 | I1709 | I1751 | %mon |
|---|---|---|---|---|
| δ | 17.8 | - | 2.0 | |
| δ + HA | 15.2 | 30.7 | 12.7 | 43 |
| ε | 29.2 | - | 6.3 | |
| ε + HA | 33.2 | 35.6 | 11.2 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozzolino, A.; Monaco, G.; Daniel, C.; Rizzo, P.; Guerra, G. Monomeric and Dimeric Carboxylic Acid in Crystalline Cavities and Channels of Delta and Epsilon Forms of Syndiotactic Polystyrene. Polymers 2021, 13, 3330. https://doi.org/10.3390/polym13193330
Cozzolino A, Monaco G, Daniel C, Rizzo P, Guerra G. Monomeric and Dimeric Carboxylic Acid in Crystalline Cavities and Channels of Delta and Epsilon Forms of Syndiotactic Polystyrene. Polymers. 2021; 13(19):3330. https://doi.org/10.3390/polym13193330
Chicago/Turabian StyleCozzolino, Antonietta, Guglielmo Monaco, Christophe Daniel, Paola Rizzo, and Gaetano Guerra. 2021. "Monomeric and Dimeric Carboxylic Acid in Crystalline Cavities and Channels of Delta and Epsilon Forms of Syndiotactic Polystyrene" Polymers 13, no. 19: 3330. https://doi.org/10.3390/polym13193330
APA StyleCozzolino, A., Monaco, G., Daniel, C., Rizzo, P., & Guerra, G. (2021). Monomeric and Dimeric Carboxylic Acid in Crystalline Cavities and Channels of Delta and Epsilon Forms of Syndiotactic Polystyrene. Polymers, 13(19), 3330. https://doi.org/10.3390/polym13193330