Peptide Conjugate on Multilayer Graphene Oxide Film for the Osteogenic Differentiation of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Designation of Peptide Sequences
2.2. Fabrication of m-GO and Peptide/m-GO Film
2.3. Characterization of m-GO and Peptide/m-GO Film
2.4. WJ-MSC Culture
2.5. Cell Viability Assay
2.6. Cell Morphology
2.7. Osteogenic Differentiation
2.8. Statistical Analysis
3. Results and Discussion
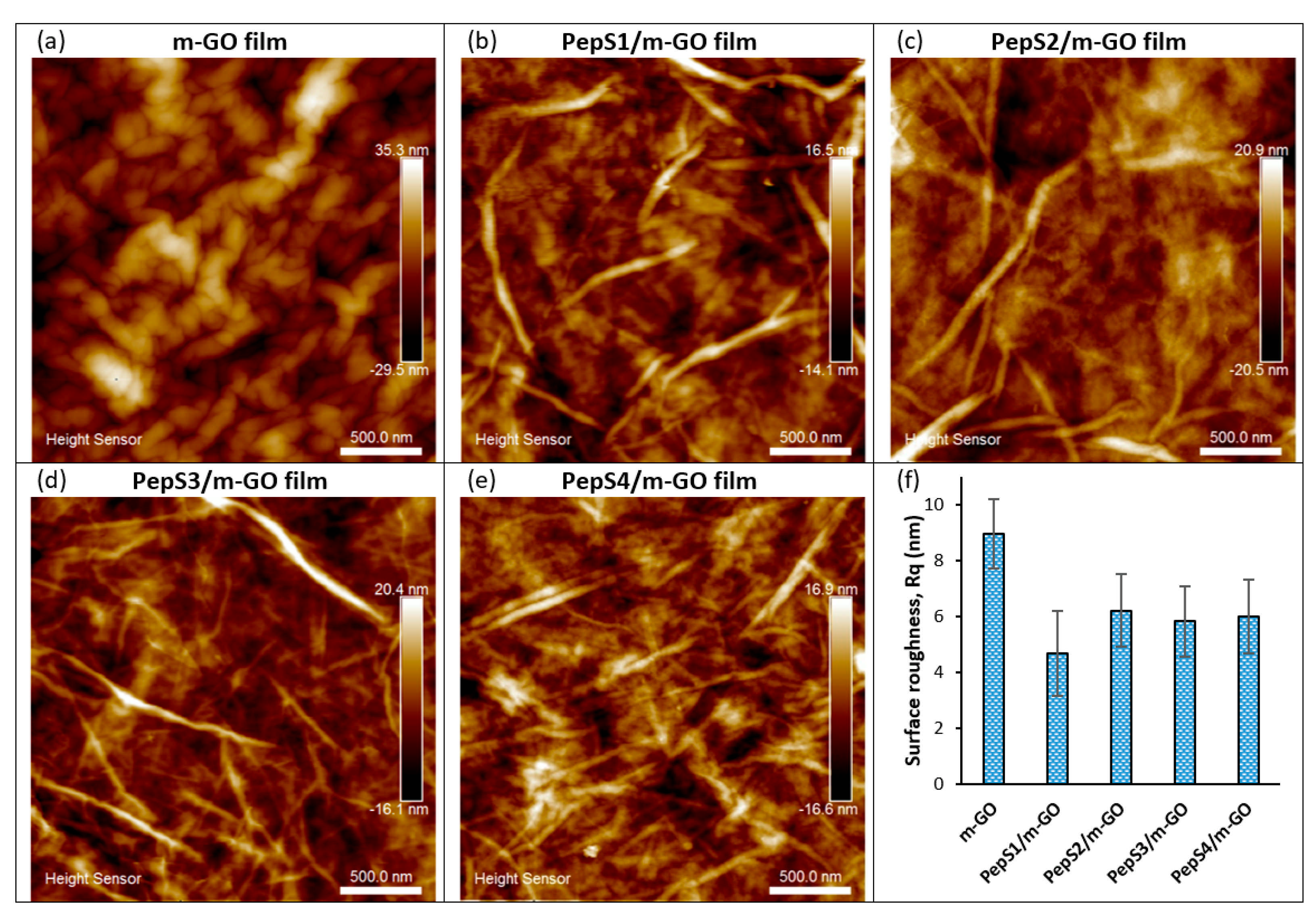
3.1. Characterization of Multilayer GO and Peptide/m-GO Films
3.2. WJ-MSC Morphology and Viability on the Multilayer GO and Peptide/m-GO Films
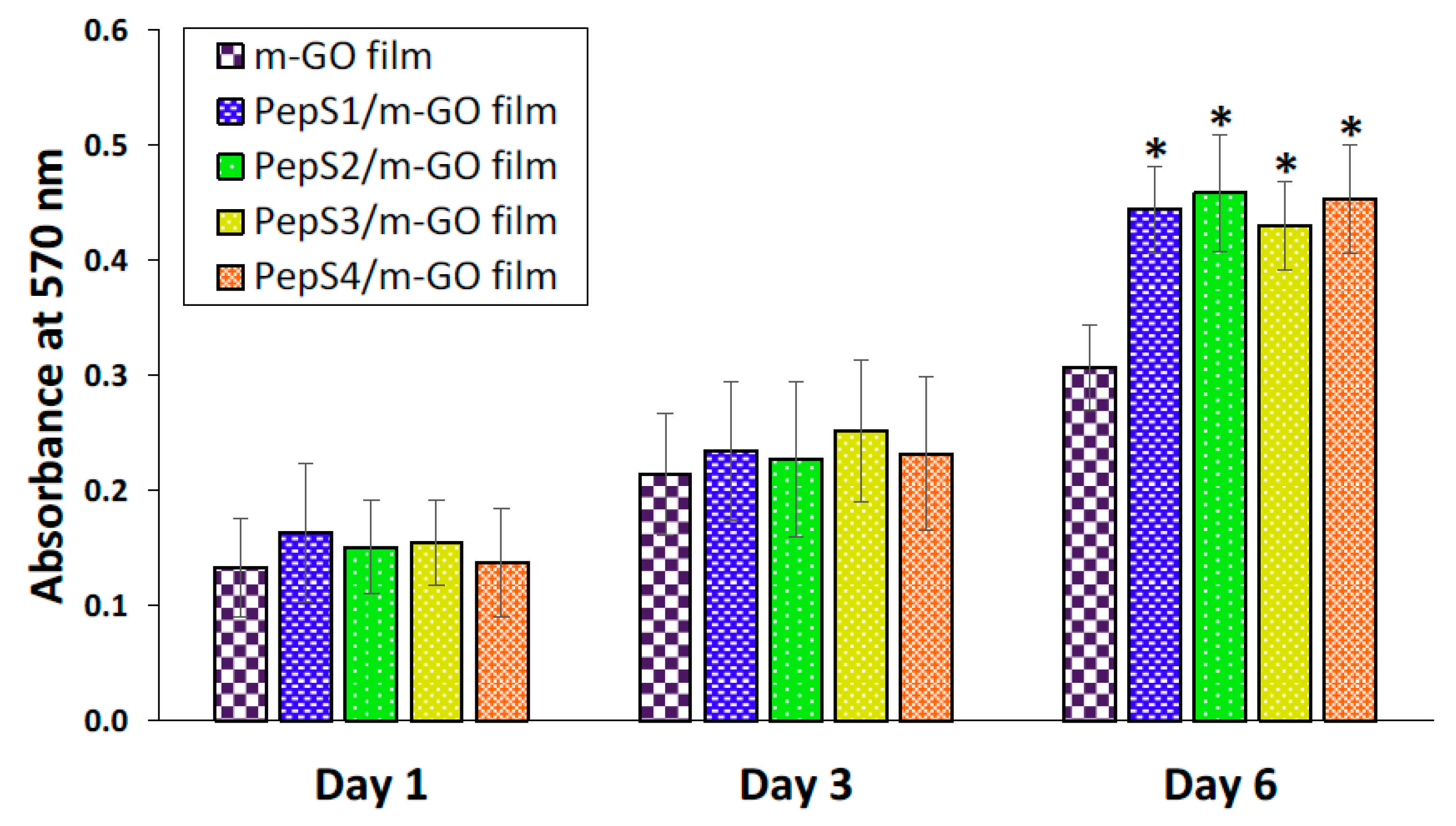
3.3. Osteogenic Differentiation of WJ-MSCs on the Multilayer GO and Peptide/m-GO Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suman, S.; Domingues, A.; Ratajczak, J.; Ratajczak, M.Z. Potential Clinical Applications of Stem Cells in Regenerative Medicine. In Stem Cells: Therapeutic Applications; Ratajczak, M.Z., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–22. [Google Scholar]
- Contaldo, M.; De Rosa, A.; Nucci, L.; Ballini, A.; Malacrinò, D.; La Noce, M.; Inchingolo, F.; Xhajanka, E.; Ferati, K.; Bexheti-Ferati, A.; et al. Titanium Functionalized with Polylysine Homopolymers: In Vitro Enhancement of Cells Growth. Materials 2021, 14, 3735. [Google Scholar] [CrossRef]
- Posa, F.; Colaianni, G.; Di Cosola, M.; Dicarlo, M.; Gaccione, F.; Colucci, S.; Grano, M.; Mori, G. The Myokine Irisin Promotes Osteogenic Differentiation of Dental Bud-Derived MSCs. Biology 2021, 10, 295. [Google Scholar] [CrossRef]
- Stefania, C.; Vito, C.; Antonio, B.; Antonio, E.U.; Michele, F.; Giuseppe, M.; Patrizio, B.; Chiara, D.; Francesca, F.; Andrea, B.; et al. Recent Advances in Endocrine, Metabolic and Immune Disorders: Mesenchymal Stem Cells (MSCs) and Engineered Scaffolds. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 466–469. [Google Scholar] [CrossRef]
- Charitos, I.A.; Ballini, A.; Cantore, S.; Boccellino, M.; Di Domenico, M.; Borsani, E.; Nocini, R.; Di Cosola, M.; Santacroce, L.; Bottalico, L. Stem Cells: A Historical Review about Biological, Religious, and Ethical Issues. Stem Cells Int. 2021, 2021, 9978837. [Google Scholar] [CrossRef]
- Kamal, M.M.; Kassem, D.H. Therapeutic Potential of Wharton’s Jelly Mesenchymal Stem Cells for Diabetes: Achievements and Challenges. Front. Cell Dev. Biol. 2020, 8, 16. [Google Scholar] [CrossRef]
- El Omar, R.; Beroud, J.; Stoltz, J.-F.; Menu, P.; Velot, E.; Decot, V. Umbilical Cord Mesenchymal Stem Cells: The New Gold Standard for Mesenchymal Stem Cell-Based Therapies? Tissue Eng. Part B Rev. 2014, 20, 523–544. [Google Scholar] [CrossRef]
- Fong, C.-Y.; Chak, L.-L.; Biswas, A.; Tan, J.-H.; Gauthaman, K.; Chan, W.-K.; Bongso, A. Human Wharton’s Jelly Stem Cells Have Unique Transcriptome Profiles Compared to Human Embryonic Stem Cells and Other Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2011, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Aristea, K.B.; Maria-Christina, K.; Helen, A.P.; Charalampos, P. Mesenchymal Stem Cells Derived from Wharton’s Jelly of the Umbilical Cord: Biological Properties and Emerging Clinical Applications. Curr. Stem Cell Res. Ther. 2013, 8, 144–155. [Google Scholar] [CrossRef]
- Nagamura-Inoue, T.; He, H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J. Stem Cells 2014, 6, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.-Y.; Fu, Y.-S.; Chang, S.-J.; Tsuang, Y.-H.; Wang, H.-W. Functional Module Analysis Reveals Differential Osteogenic and Stemness Potentials in Human Mesenchymal Stem Cells from Bone Marrow and Wharton’s Jelly of Umbilical Cord. Stem Cells Dev. 2010, 19, 1895–1910. [Google Scholar] [CrossRef] [PubMed]
- Batsali, A.K.; Pontikoglou, C.; Koutroulakis, D.; Pavlaki, K.I.; Damianaki, A.; Mavroudi, I.; Alpantaki, K.; Kouvidi, E.; Kontakis, G.; Papadaki, H.A. Differential expression of cell cycle and WNT pathway-related genes accounts for differences in the growth and differentiation potential of Wharton’s jelly and bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Celiz, A.D.; Smith, J.G.W.; Langer, R.; Anderson, D.G.; Winkler, D.A.; Barrett, D.A.; Davies, M.C.; Young, L.E.; Denning, C.; Alexander, M.R. Materials for stem cell factories of the future. Nat. Mater. 2014, 13, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Ferroni, L.; Gardin, C.; Sbricoli, L.; Gobbato, L.; Ludovichetti, F.S.; Tocco, I.; Carraro, A.; Piattelli, A.; Zavan, B. Graphene based scaffolds effects on stem cells commitment. J. Transl. Med. 2014, 12, 296. [Google Scholar] [CrossRef]
- Liang, K.; Du, Y. Cell engineering techniques improve pharmacology of cellular therapeutics. Biomater. Biosyst. 2021, 2, 100016. [Google Scholar] [CrossRef]
- Jagiełło, J.; Sekuła-Stryjewska, M.; Noga, S.; Adamczyk, E.; Dźwigońska, M.; Kurcz, M.; Kurp, K.; Winkowska-Struzik, M.; Karnas, E.; Boruczkowski, D.; et al. Impact of Graphene-Based Surfaces on the Basic Biological Properties of Human Umbilical Cord Mesenchymal Stem Cells: Implications for Ex Vivo Cell Expansion Aimed at Tissue Repair. Int. J. Mol. Sci. 2019, 20, 4561. [Google Scholar] [CrossRef]
- Lee, W.C.; Lim, C.H.Y.X.; Shi, H.; Tang, L.A.L.; Wang, Y.; Lim, C.T.; Loh, K.P. Origin of Enhanced Stem Cell Growth and Differentiation on Graphene and Graphene Oxide. ACS Nano 2011, 5, 7334–7341. [Google Scholar] [CrossRef]
- Garcia-Alegria, E.; Iliut, M.; Stefanska, M.; Silva, C.; Heeg, S.; Kimber, S.J.; Kouskoff, V.; Lacaud, G.; Vijayaraghavan, A.; Batta, K. Graphene Oxide promotes embryonic stem cell differentiation to haematopoietic lineage. Sci. Rep. 2016, 6, 25917. [Google Scholar] [CrossRef]
- Rosa, V.; Xie, H.; Dubey, N.; Madanagopal, T.T.; Rajan, S.S.; Morin, J.L.P.; Islam, I.; Neto, A.H.C. Graphene oxide-based substrate: Physical and surface characterization, cytocompatibility and differentiation potential of dental pulp stem cells. Dent. Mater. 2016, 32, 1019–1025. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene Oxide, Highly Reduced Graphene Oxide, and Graphene: Versatile Building Blocks for Carbon-Based Materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, W.; Yu, X.; Wang, Z.; Su, Z.; Wei, G. When biomolecules meet graphene: From molecular level interactions to material design and applications. Nanoscale 2016, 8, 19491–19509. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef]
- Plachá, D.; Jampilek, J. Graphenic Materials for Biomedical Applications. Nanomaterials 2019, 9, 1758. [Google Scholar] [CrossRef]
- Weaver, C.L.; LaRosa, J.M.; Luo, X.; Cui, X.T. Electrically Controlled Drug Delivery from Graphene Oxide Nanocomposite Films. ACS Nano 2014, 8, 1834–1843. [Google Scholar] [CrossRef]
- Kim, J.; Choi, K.S.; Kim, Y.; Lim, K.-T.; Seonwoo, H.; Park, Y.; Kim, D.-H.; Choung, P.-H.; Cho, C.-S.; Kim, S.Y.; et al. Bioactive effects of graphene oxide cell culture substratum on structure and function of human adipose-derived stem cells. J. Biomed. Mater. Res. Part A 2013, 101, 3520–3530. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, C.; Tang, L.; Wang, C.; Chen, Q.; Zheng, Q.; Yang, S.; Sheng, S.; Zan, X. Lysozyme (Lys), Tannic Acid (TA), and Graphene Oxide (GO) Thin Coating for Antibacterial and Enhanced Osteogenesis. ACS Appl. Bio Mater. 2020, 3, 673–684. [Google Scholar] [CrossRef]
- Xie, C.; Lu, X.; Han, L.; Xu, J.; Wang, Z.; Jiang, L.; Wang, K.; Zhang, H.; Ren, F.; Tang, Y. Biomimetic Mineralized Hierarchical Graphene Oxide/Chitosan Scaffolds with Adsorbability for Immobilization of Nanoparticles for Biomedical Applications. ACS Appl. Mater. Interfaces 2016, 8, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.; Kim, S.-H.; Kim, J.; Lee, J.-R.; Jeong, G.-J.; Yoon, J.-K.; Kang, S.; Bhang, S.H.; Yoon, H.H.; Lee, J.-C.; et al. Graphene oxide reinforced hydrogels for osteogenic differentiation of human adipose-derived stem cells. RSC Adv. 2017, 7, 20779–20788. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, H.; Fang, Y.; Cao, Y.; Huang, J.; Zhang, M.; Dai, J.; Shi, X.; Zhang, Z. Enhanced Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells on Graphene Oxide-Incorporated Electrospun Poly(lactic-co-glycolic acid) Nanofibrous Mats. ACS Appl. Mater. Interfaces 2015, 7, 6331–6339. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, J.; Xu, Z.; Zou, Q.; Yang, L.; Ma, M.; Shu, L.; He, Z.; Ye, C. Osteoblastic differentiation of stem cells induced by graphene oxide-hydroxyapatite-alginate hydrogel composites and construction of tissue-engineered bone. J. Mater. Sci. Mater. Med. 2020, 31, 125. [Google Scholar] [CrossRef]
- Shuai, Y.; Mao, C.; Yang, M. Protein Nanofibril Assemblies Templated by Graphene Oxide Nanosheets Accelerate Early Cell Adhesion and Induce Osteogenic Differentiation of Human Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 31988–31997. [Google Scholar] [CrossRef]
- Qi, W.; Yuan, W.; Yan, J.; Wang, H. Growth and accelerated differentiation of mesenchymal stem cells on graphene oxide/poly-l-lysine composite films. J. Mater. Chem. B 2014, 2, 5461–5467. [Google Scholar] [CrossRef]
- Ahmed, M.; ffrench-Constant, C. Extracellular Matrix Regulation of Stem Cell Behavior. Curr. Stem Cell Rep. 2016, 2, 197–206. [Google Scholar] [CrossRef]
- Ma, P.X. Biomimetic Materials for Tissue Engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef]
- Yeo, I.-S.; Min, S.-K.; Ki Kang, H.; Kwon, T.-K.; Jung, S.; Min, B.-M. Identification of a bioactive core sequence from human laminin and its applicability to tissue engineering. Biomaterials 2015, 73, 96–109. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Josey, B.; Chou, C.J.; Tan, Y.; Zhang, N.; Wen, X. Short Laminin Peptide for Improved Neural Stem Cell Growth. Stem Cells Transl. Med. 2014, 3, 662–670. [Google Scholar] [CrossRef]
- Tatman, P.D.; Muhonen, E.G.; Wickers, S.T.; Gee, A.O.; Kim, E.-S.; Kim, D.-H. Self-Assembling Peptides for Stem Cell and Tissue Engineering. Biomater. Sci. 2016, 4, 543–554. [Google Scholar] [CrossRef]
- Ma, R.; Ren, Z.; Li, B.; Siu, S.W.I.; Chen, G.; Kwok, H.F. Novel venom-based peptides (P13 and its derivative—M6) to maintain self-renewal of human embryonic stem cells by activating FGF and TGFβ signaling pathways. Stem Cell Res. Ther. 2020, 11, 243. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Shi, L.; Pan, S.; He, L.; Niu, Y.; Wang, X. A Customized Self-Assembling Peptide Hydrogel-Wrapped Stem Cell Factor Targeting Pulp Regeneration Rich in Vascular-Like Structures. ACS Omega 2020, 5, 16568–16574. [Google Scholar] [CrossRef] [PubMed]
- Marchini, A.; Favoino, C.; Gelain, F. Multi-Functionalized Self-Assembling Peptides as Reproducible 3D Cell Culture Systems Enabling Differentiation and Survival of Various Human Neural Stem Cell Lines. Front. Neurosci. 2020, 14, 413. [Google Scholar] [CrossRef]
- Hellmund, K.S.; Koksch, B. Self-Assembling Peptides as Extracellular Matrix Mimics to Influence Stem Cell’s Fate. Front. Chem. 2019, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Tsou, Y.-H.; Khoneisser, J.; Huang, P.-C.; Xu, X. Hydrogel as a bioactive material to regulate stem cell fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Tung, V.C.; Allen, M.J.; Yang, Y.; Kaner, R.B. High-throughput solution processing of large-scale graphene. Nat. Nanotechnol. 2008, 4, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Madl, C.M.; Lee, S.; Duda, G.N.; Mooney, D.J. The collagen I mimetic peptide DGEA enhances an osteogenic phenotype in mesenchymal stem cells when presented from cell-encapsulating hydrogels. J. Biomed. Mater. Res. Part A 2015, 103, 3516–3525. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.E.; Mills, R.J.; Hudson, J.E.; Cooper-White, J.J. Tailored Integrin–Extracellular Matrix Interactions to Direct Human Mesenchymal Stem Cell Differentiation. Stem Cells Dev. 2012, 21, 2442–2456. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Jekarl, D.W.; Kim, M.; Oh, E.-J.; Kim, Y.; Park, I.Y.; Shin, J.C. Effects of ECM Protein Mimetics on Adhesion and Proliferation of Chorion Derived Mesenchymal Stem Cells. Int. J. Med Sci. 2014, 11, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Castro, N.J.; Zhu, W.; Cui, H.; Aliabouzar, M.; Sarkar, K.; Zhang, L.G. Improved Human Bone Marrow Mesenchymal Stem Cell Osteogenesis in 3D Bioprinted Tissue Scaffolds with Low Intensity Pulsed Ultrasound Stimulation. Sci. Rep. 2016, 6, 32876. [Google Scholar] [CrossRef]
- Chollet, C.; Bareille, R.; Rémy, M.; Guignandon, A.; Bordenave, L.; Laroche, G.; Durrieu, M.-C. Impact of Peptide Micropatterning on Endothelial Cell Actin Remodeling for Cell Alignment under Shear Stress. Macromol. Biosci. 2012, 12, 1648–1659. [Google Scholar] [CrossRef]
- Katayama, N.; Kato, H.; Taguchi, Y.; Tanaka, A.; Umeda, M. The Effects of Synthetic Oligopeptide Derived from Enamel Matrix Derivative on Cell Proliferation and Osteoblastic Differentiation of Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2014, 15, 14026–14043. [Google Scholar] [CrossRef] [PubMed]
- Dallabrida, S.M.; Ismail, N.; Oberle, J.R.; Himes, B.E.; Rupnick, M.A. Angiopoietin-1 Promotes Cardiac and Skeletal Myocyte Survival Through Integrins. Circ. Res. 2005, 96, e8–e24. [Google Scholar] [CrossRef]
- Hasenbein, M.E.; Andersen, T.T.; Bizios, R. Micropatterned surfaces modified with select peptides promote exclusive interactions with osteoblasts. Biomaterials 2002, 23, 3937–3942. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, B.-C.; Wang, X.-F.; Ye, B.-C. Interaction of peptides with graphene oxide and its application for real-time monitoring of protease activity. Chem. Commun. 2011, 47, 2399–2401. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zhang, Y.; Feng, T.; Shi, W.; Li, X.; Ma, H. A graphene oxide-peptide fluorescence sensor tailor-made for simple and sensitive detection of matrix metalloproteinase 2. Chem. Commun. 2011, 47, 10680–10682. [Google Scholar] [CrossRef]
- Puah, P.Y.; Yusoff, U.H.; Lee, P.C.; Moh, P.Y.; How, S.E. Surface characterization, biocompatibility and osteogenic differentiation of drop-casted multilayer graphene oxide film towards human wharton’s jelly derived mesenchymal stem cells. Mater. Technol. 2020, 35, 238–247. [Google Scholar] [CrossRef]
- Puah, P.Y.; Moh, P.Y.; Lee, P.C.; How, S.E. Spin-coated graphene oxide as a biomaterial for Wharton’s Jelly derived mesenchymal stem cell growth: A preliminary study. Mater. Technol. 2018, 33, 835–843. [Google Scholar] [CrossRef]
- Duffy, C.R.E.; Zhang, R.; How, S.-E.; Lilienkampf, A.; Tourniaire, G.; Hu, W.; West, C.C.; de Sousa, P.; Bradley, M. A high-throughput polymer microarray approach for identifying defined substrates for mesenchymal stem cells. Biomater. Sci. 2014, 2, 1683–1692. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, P.; Bhirde, A.; Jin, A.; Ma, Y.; Niu, G.; Neamati, N.; Chen, X. A nanoscale graphene oxide–peptide biosensor for real-time specific biomarker detection on the cell surface. Chem. Commun. 2012, 48, 9768–9770. [Google Scholar] [CrossRef] [PubMed]
- Puah, P.Y. ID2015 Preparation of graphene oxide/oligopeptides composite for promoting mesenchymal stem cell proliferation. Biomed. Res. Ther. 2017, 4, 48. [Google Scholar] [CrossRef][Green Version]
- Kim, F.; Cote, L.J.; Huang, J. Graphene Oxide: Surface Activity and Two-Dimensional Assembly. Adv. Mater. 2010, 22, 1954–1958. [Google Scholar] [CrossRef]
- Krueger, M.; Berg, S.; Stone, D.A.; Strelcov, E.; Dikin, D.A.; Kim, J.; Cote, L.J.; Huang, J.; Kolmakov, A. Drop-Casted Self-Assembling Graphene Oxide Membranes for Scanning Electron Microscopy on Wet and Dense Gaseous Samples. ACS Nano 2011, 5, 10047–10054. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Montazeri, A.; Rafii-Tabar, H. Molecular dynamics study of the interfacial mechanical properties of the graphene–collagen biological nanocomposite. Comput. Mater. Sci. 2013, 69, 29–39. [Google Scholar] [CrossRef]
- Kumar, S.; Parekh, S.H. Linking graphene-based material physicochemical properties with molecular adsorption, structure and cell fate. Commun. Chem. 2020, 3, 8. [Google Scholar] [CrossRef]
- Eckhart, K.E.; Schmidt, S.J.; Starvaggi, F.A.; Wolf, M.E.; Vickery, W.M.; Sydlik, S.A. Peptide- and Protein-Graphene Oxide Conjugate Materials for Controlling Mesenchymal Stem Cell Fate. Regen. Eng. Transl. Med. 2020, 1–25. [Google Scholar] [CrossRef]
- Maity, S.; Zanuy, D.; Razvag, Y.; Das, P.; Alemán, C.; Reches, M. Elucidating the mechanism of interaction between peptides and inorganic surfaces. Phys. Chem. Chem. Phys. 2015, 17, 15305–15315. [Google Scholar] [CrossRef]
- Saiani, A.; Mohammed, A.; Frielinghaus, H.; Collins, R.; Hodson, N.; Kielty, C.M.; Sherratt, M.J.; Miller, A.F. Self-assembly and gelation properties of α-helix versus β-sheet forming peptides. Soft Matter 2009, 5, 193–202. [Google Scholar] [CrossRef]
- Wychowaniec, J.K.; Iliut, M.; Zhou, M.; Moffat, J.; Elsawy, M.A.; Pinheiro, W.A.; Hoyland, J.A.; Miller, A.F.; Vijayaraghavan, A.; Saiani, A. Designing Peptide/Graphene Hybrid Hydrogels through Fine-Tuning of Molecular Interactions. Biomacromolecules 2018, 19, 2731–2741. [Google Scholar] [CrossRef]
- Chen, C.-M.; Huang, J.-Q.; Zhang, Q.; Gong, W.-Z.; Yang, Q.-H.; Wang, M.-Z.; Yang, Y.-G. Annealing a graphene oxide film to produce a free standing high conductive graphene film. Carbon 2012, 50, 659–667. [Google Scholar] [CrossRef]
- Chen, C.; Yang, Q.-H.; Yang, Y.; Lv, W.; Wen, Y.; Hou, P.-X.; Wang, M.; Cheng, H.-M. Self-Assembled Free-Standing Graphite Oxide Membrane. Adv. Mater. 2009, 21, 3007–3011. [Google Scholar] [CrossRef]
- Eckhart, K.E.; Holt, B.D.; Laurencin, M.G.; Sydlik, S.A. Covalent conjugation of bioactive peptides to graphene oxide for biomedical applications. Biomater. Sci. 2019, 7, 3876–3885. [Google Scholar] [CrossRef]
- Joshi, S.; Siddiqui, R.; Sharma, P.; Kumar, R.; Verma, G.; Saini, A. Green synthesis of peptide functionalized reduced graphene oxide (rGO) nano bioconjugate with enhanced antibacterial activity. Sci. Rep. 2020, 10, 9441. [Google Scholar] [CrossRef]
- Wang, H.-S.; Hung, S.-C.; Peng, S.-T.; Huang, C.-C.; Wei, H.-M.; Guo, Y.-J.; Fu, Y.-S.; Lai, M.-C.; Chen, C.-C. Mesenchymal Stem Cells in the Wharton’s Jelly of the Human Umbilical Cord. Stem Cells 2004, 22, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.K.; Whitehead, J. Materials-Directed Differentiation of Mesenchymal Stem Cells for Tissue Engineering and Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, U.H.; Puah, P.Y.; Lee, P.C.; Teoh, P.L.; How, S.E. A Mini Review: Interaction of Graphene Oxide with Wharton’s Jelly Derived Mesenchymal Stem Cells. Trans. Sci. Technol. 2019, 6, 357–365. [Google Scholar]
- Brafman, D.A. Constructing stem cell microenvironments using bioengineering approaches. Physiol. Genom. 2013, 45, 1123–1135. [Google Scholar] [CrossRef] [PubMed]



| Code | Short Bioactive Peptide Sequence | Number of Positively Charged Amino Acids | Number of Aromatic Rings Amino Acids | Reference |
|---|---|---|---|---|
| PepS1 | YIGSRWYQNMIRIKVAV | 3 (Arg, Arg, Lys) | 3 (Trp, Tyr, Tyr) | [45,49] |
| PepS2 | QHREDGSYIGSRIKVAV | 4 (His, Arg, Arg, Lys) | 2 (His, Tyr) | [45,50] |
| PepS3 | WQPPRARIYIGSRIKVAV | 4 (Arg, Arg, Lys, Arg) | 2 (Trp, Tyr) | [45,48] |
| PepS4 | DGEARGDSPKRSR | 4 (Lys, Arg, Arg, Arg) | 0 | [45,46,47,51] |
| Element | Weight (%) | ||||
|---|---|---|---|---|---|
| m-GO Film | PepS1/m-GO Film | PepS2/m-GO Film | PepS3/m-GO Film | PepS4/m-GO Film | |
| C | 34.48 | 42.96 | 45.64 | 21.00 | 29.59 |
| O | 65.52 | 55.04 | 52.23 | 74.70 | 66.80 |
| N | 0.00 | 2.00 | 2.13 | 4.30 | 3.61 |
| Total | 100 | 100 | 100 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puah, P.Y.; Moh, P.Y.; Sipaut, C.S.; Lee, P.C.; How, S.E. Peptide Conjugate on Multilayer Graphene Oxide Film for the Osteogenic Differentiation of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells. Polymers 2021, 13, 3290. https://doi.org/10.3390/polym13193290
Puah PY, Moh PY, Sipaut CS, Lee PC, How SE. Peptide Conjugate on Multilayer Graphene Oxide Film for the Osteogenic Differentiation of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells. Polymers. 2021; 13(19):3290. https://doi.org/10.3390/polym13193290
Chicago/Turabian StylePuah, Perng Yang, Pak Yan Moh, Coswald Stephen Sipaut, Ping Chin Lee, and Siew Eng How. 2021. "Peptide Conjugate on Multilayer Graphene Oxide Film for the Osteogenic Differentiation of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells" Polymers 13, no. 19: 3290. https://doi.org/10.3390/polym13193290
APA StylePuah, P. Y., Moh, P. Y., Sipaut, C. S., Lee, P. C., & How, S. E. (2021). Peptide Conjugate on Multilayer Graphene Oxide Film for the Osteogenic Differentiation of Human Wharton’s Jelly-Derived Mesenchymal Stem Cells. Polymers, 13(19), 3290. https://doi.org/10.3390/polym13193290






