Layer-by-Layer Assembly of Polystyrene/Ag for a Highly Reproducible SERS Substrate and Its Use for the Detection of Food Contaminants
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Apparatus
2.3. Synthesis of Ag Colloid and PS Spheres
2.4. Fabrication of Colloidal PS/Ag Nanocomposite
2.5. Layer-by-Layer Assembly of Ag NPs and PS Spheres
3. Results and Discussion
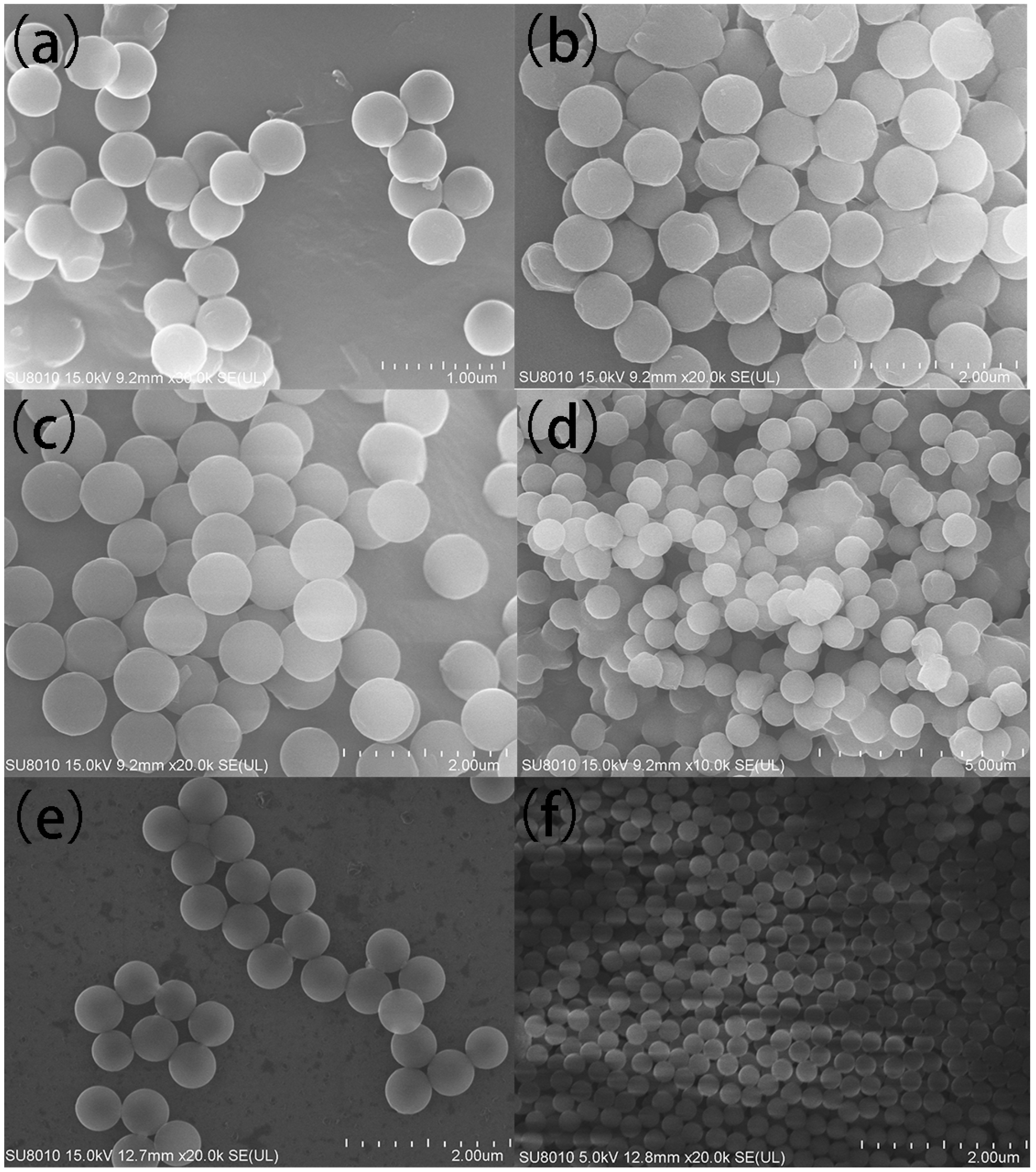
3.1. The Effect of Reaction Conditions on the Diameter of PS Spheres
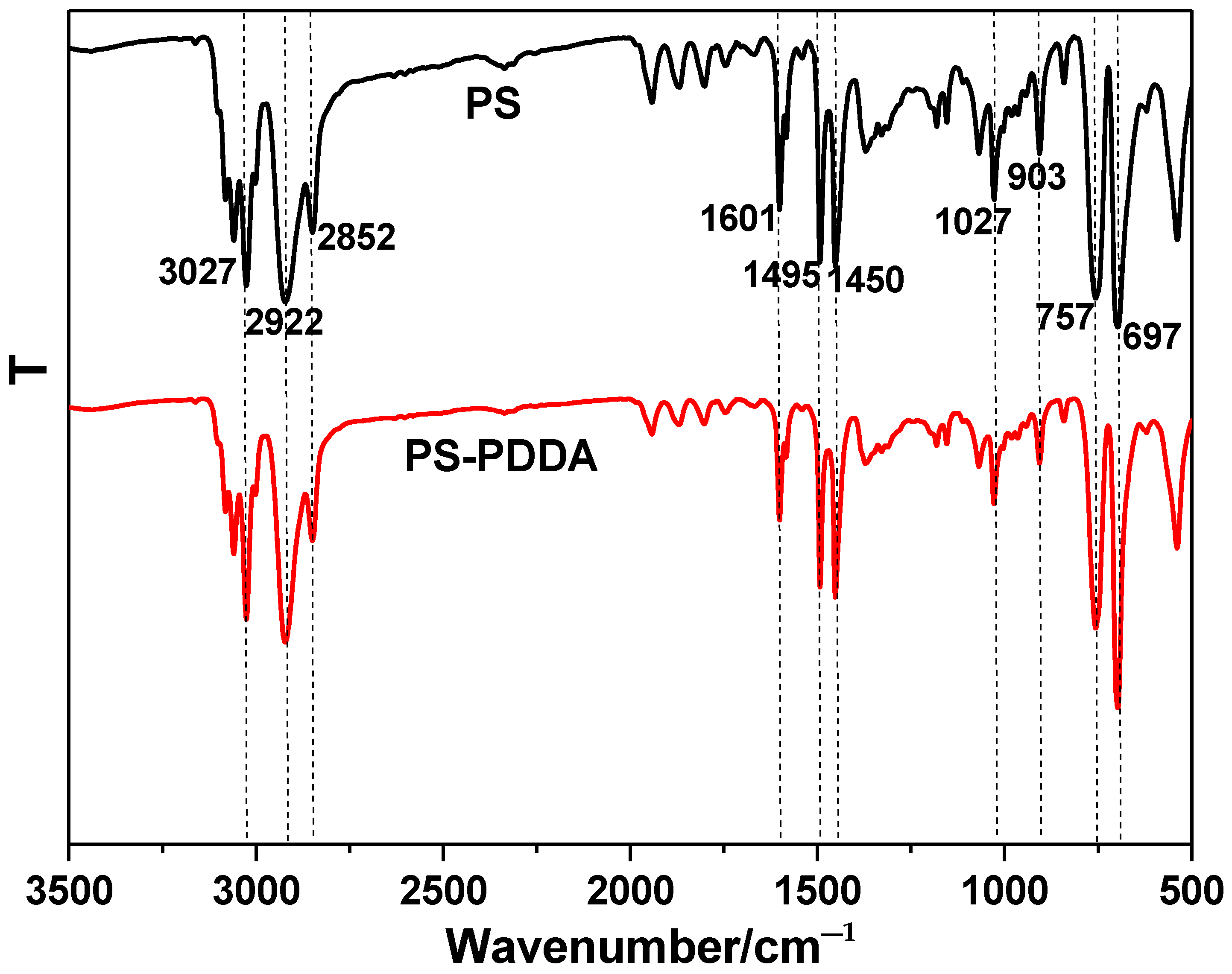
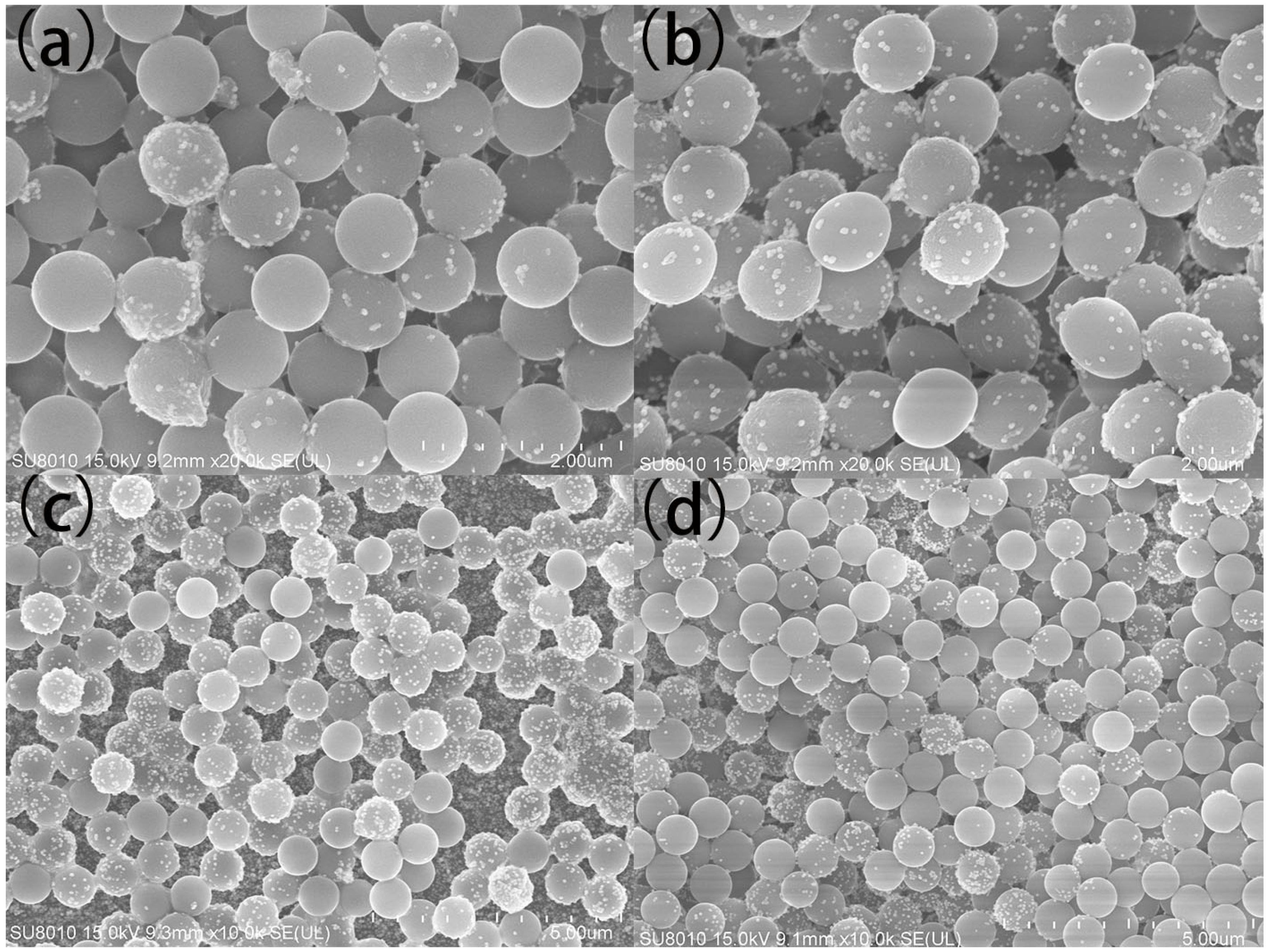
3.2. Characterization of PS/Ag Composite
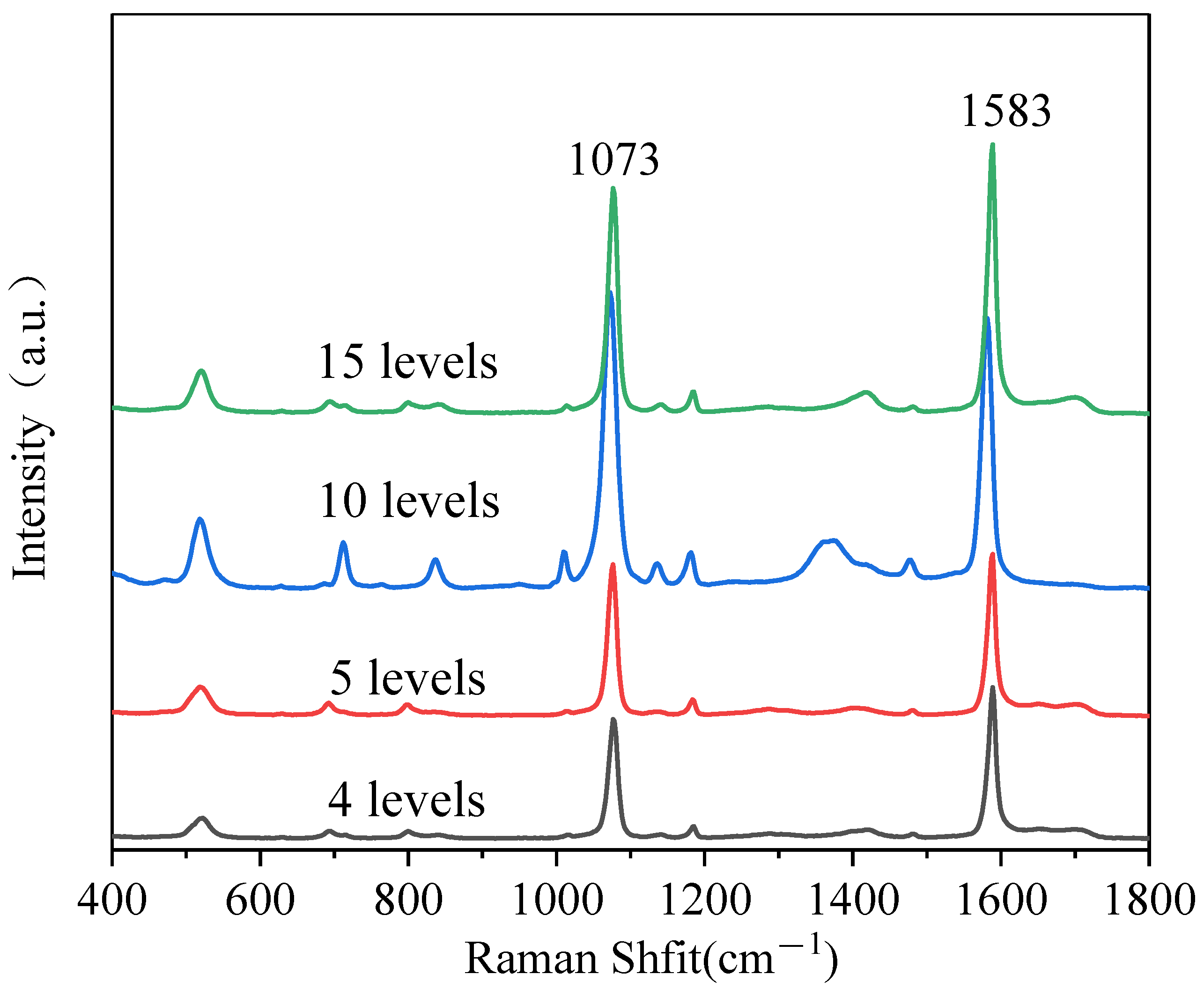
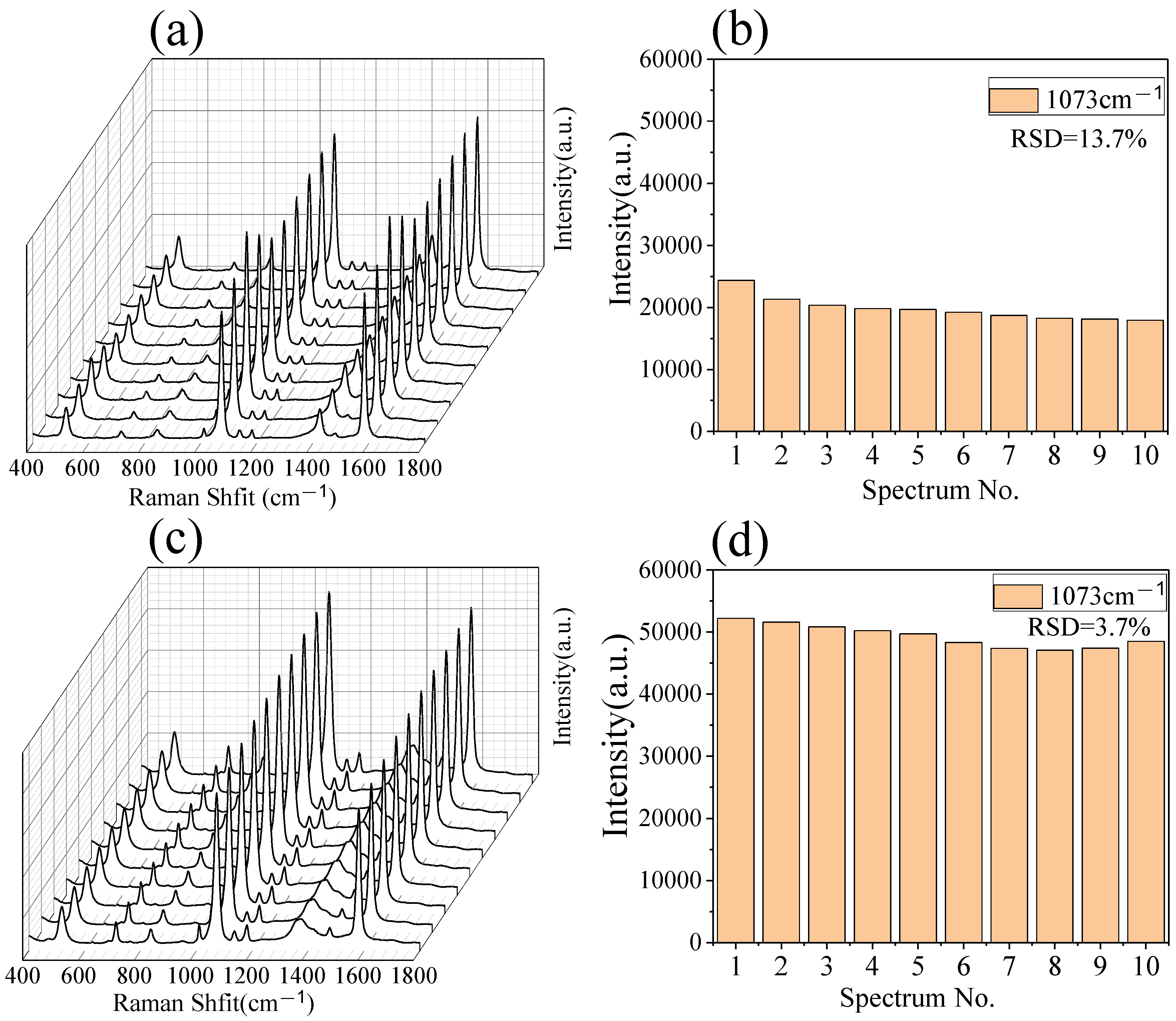
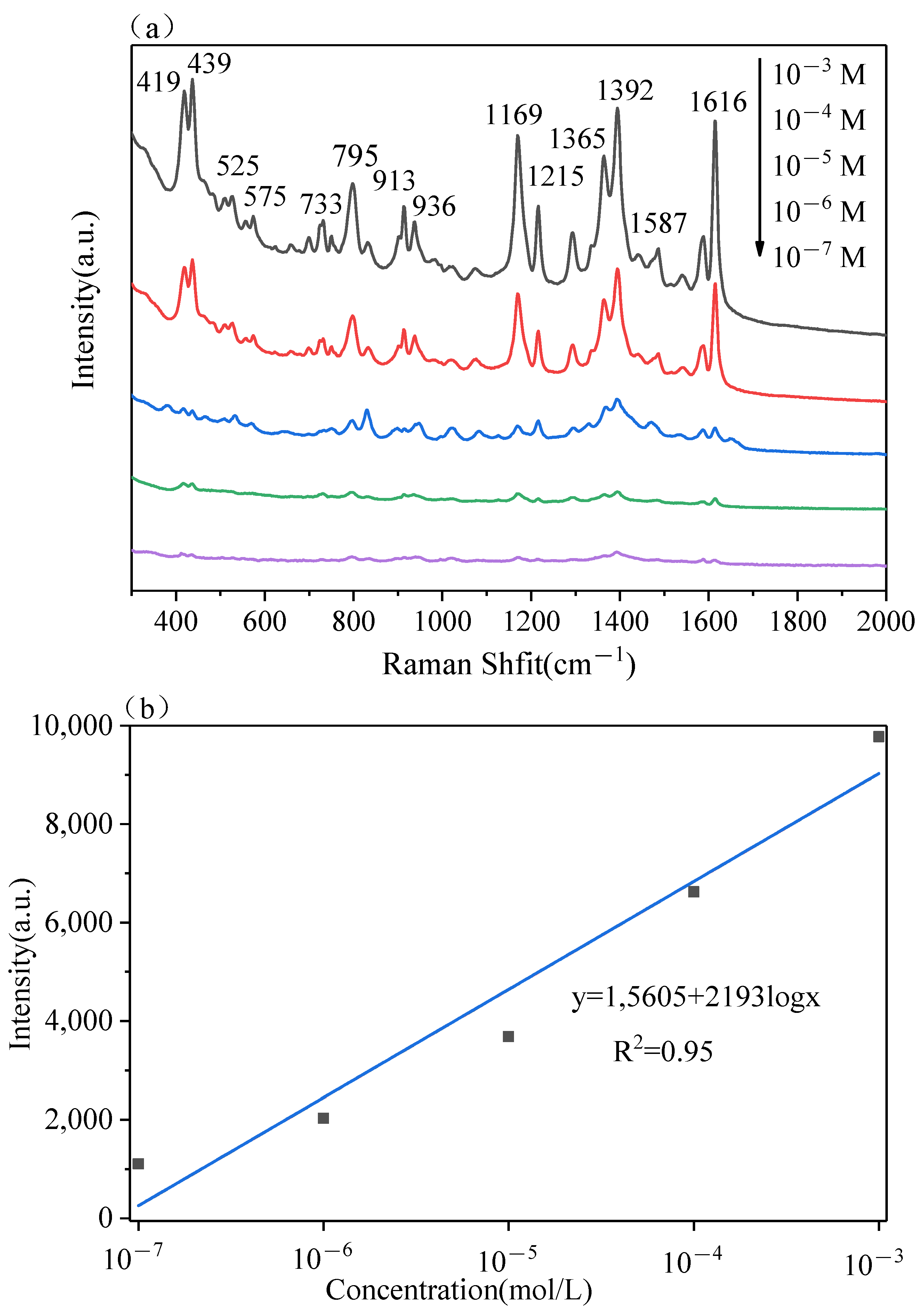
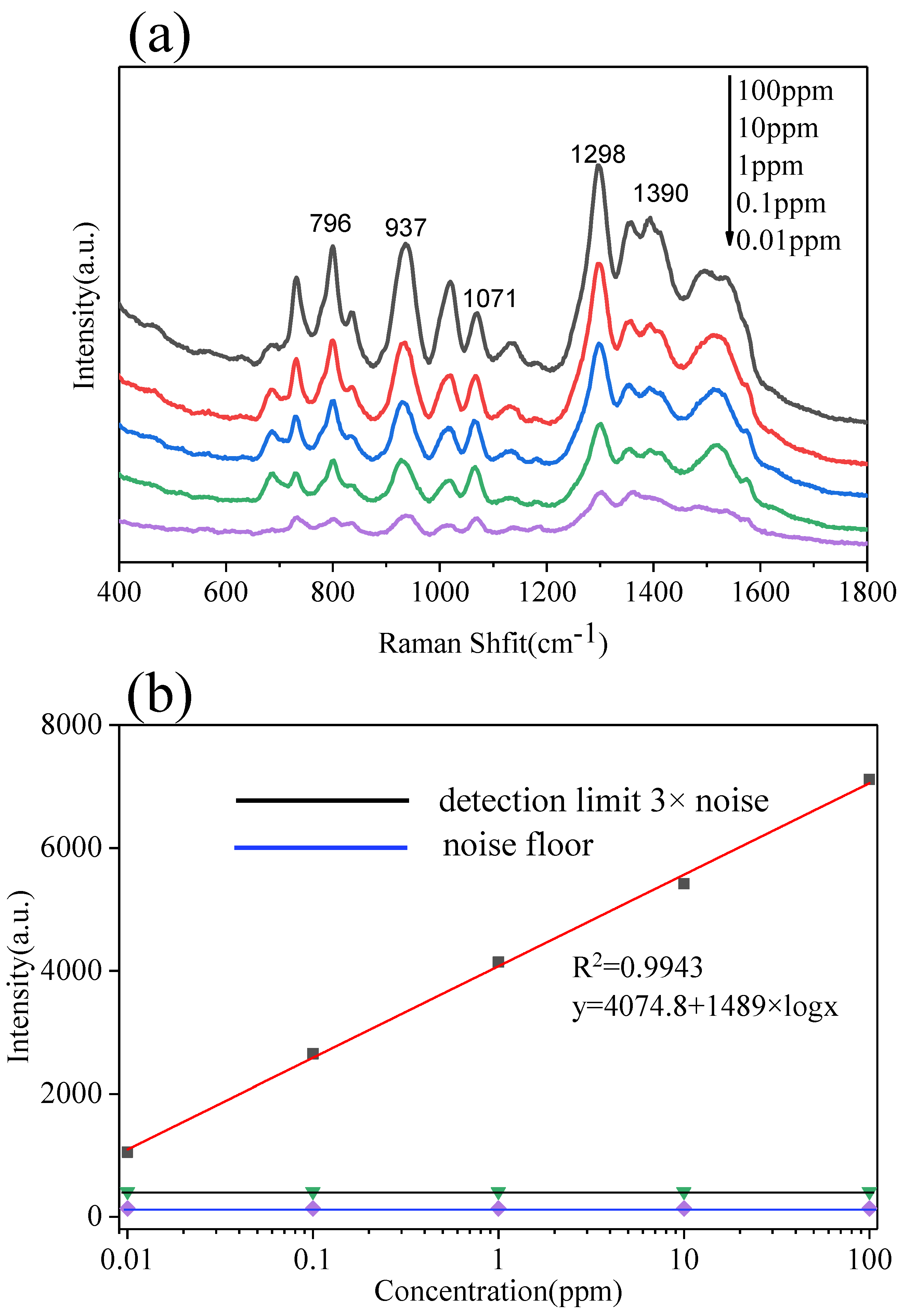
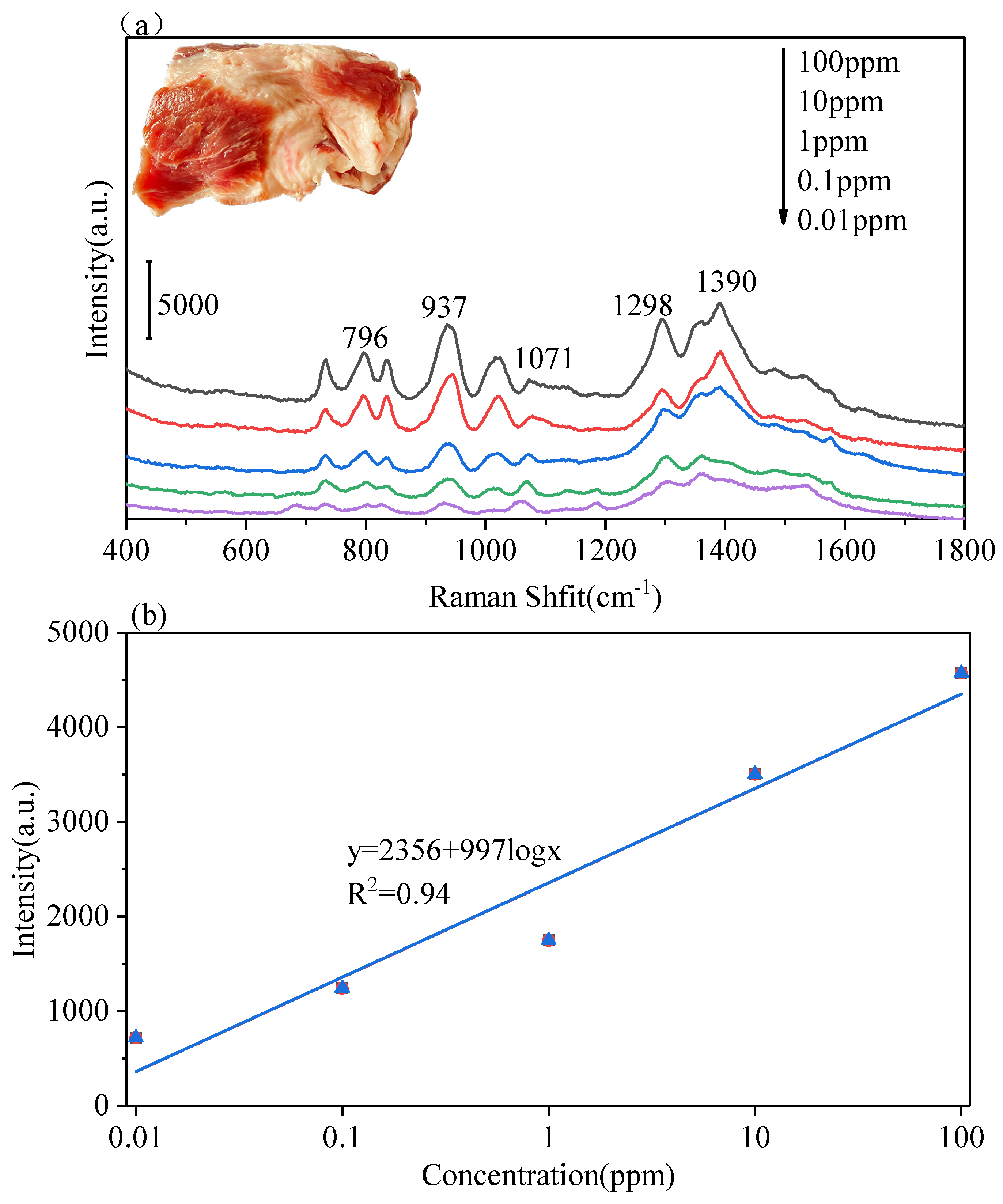
3.3. The Multilayer PS/Ag Substrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.; Guo, J.; Liang, Z.; Fan, D. Artificial micro/nanomachines for bioapplications: Biochemical delivery and diagnostic sensing. Adv. Funct. Mater. 2018, 28, 1705867. [Google Scholar] [CrossRef]
- Zhu, H.; Lin, X.; Su, Y.; Dong, H.; Wu, J. Screen-printed microfluidic dielectrophoresis chip for cell separation. Biosens. Bioelectron. 2015, 63, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jeong, J.H.; Woo, H.-C.; Kim, M.H. Synthesis of highly porous polymer microspheres with interconnected open pores for catalytic microreactors. Chem. Eng. J. 2021, 420, 127628. [Google Scholar] [CrossRef]
- Liang, L.; Li, G.; Mei, Z.; Shi, J.; Mao, Y.; Pan, T.; Liao, C.; Zhang, J.; Tian, Y. Preparation and application of ratiometric polystyrene-based microspheres as oxygen sensors. Anal. Chim. Acta 2018, 1030, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.S.; Kaur, H. Au NPs@ polystyrene resin for mild and selective aerobic oxidation of 1,4 dioxane to 1,4 dioxan-2-ol. Catal. Commun. 2017, 90, 56–59. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Li, Y.; Chen, Y.; Tong, Z. Facile fabrication of nanocomposite microspheres with polymer cores and magnetic shells by Pickering suspension polymerization. React. Funct. Polym. 2009, 69, 750–754. [Google Scholar] [CrossRef]
- Du, X.; He, J. Facile size-controllable syntheses of highly monodisperse polystyrene nano-and microspheres by polyvinylpyrrolidone-mediated emulsifier-free emulsion polymerization. J. Appl. Polym. Sci. 2008, 108, 1755–1760. [Google Scholar] [CrossRef]
- Yang, W.; Ming, W.; Hu, J.; Lu, X.; Fu, S. Morphological investigations of crosslinked polystyrene microspheres by seeded polymerization. Colloid Polym. Sci. 1998, 276, 655–661. [Google Scholar] [CrossRef]
- Abdollahi, A.; Roghani-Mamaqani, H.; Salami-Kalajahi, M. Morphology evolution of functionalized styrene and methyl methacrylate copolymer latex nanoparticles by one-step emulsifier-free emulsion polymerization. Eur. Polym. J. 2020, 133, 109790. [Google Scholar] [CrossRef]
- Tumnantong, D.; Rempel, G.L.; Prasassarakich, P. Polyisoprene-Silica Nanoparticles Synthesized via RAFT Emulsifier-Free Emulsion Polymerization Using Water-Soluble Initiators. Polymers 2017, 9, 637. [Google Scholar] [CrossRef] [Green Version]
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Bjerneld, E.J.; Käll, M.; Börjesson, L. Spectroscopy of single hemoglobin molecules by surface enhanced Raman scattering. Phys. Rev. Lett. 1999, 83, 4357. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; You, T.; Yang, N.; Gao, Y.; Jiang, L.; Yin, P. Hydrophobic paper-based SERS platform for direct-droplet quantitative determination of melamine. Food Chem. 2019, 287, 363–368. [Google Scholar] [CrossRef]
- Lin, X.; Fang, G.; Liu, Y.; He, Y.; Wang, L.; Dong, B. Marangoni Effect-Driven Transfer and Compression at Three-Phase Interfaces for Highly Reproducible Nanoparticle Monolayers. J. Phys. Chem. Lett. 2020, 2020. 8, 2100412. [Google Scholar] [CrossRef]
- Kong, X.; Chong, X.; Squire, K.; Wang, A.X. Microfluidic diatomite analytical devices for illicit drug sensing with ppb-Level sensitivity. Sens. Actuators B Chem. 2018, 259, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, X.; Lin, S.; Zhou, S.; Fang, G.; Zhao, H.; Wang, L.; Cong, S. From Dilute to Multiple Layers: Bottom-Up Self-Assembly of Rough Gold Nanorods as SERS Platform for Quantitative Detection of Thiram in Soil. Adv. Mater. Interfaces 2021, 8, 2100412. [Google Scholar] [CrossRef]
- Ji, W.; Li, L.; Song, W.; Wang, X.; Zhao, B.; Ozaki, Y. Enhanced Raman scattering by ZnO superstructures: Synergistic effect of charge transfer and Mie resonances. Angew. Chem. Int. Ed. 2019, 58, 14452–14456. [Google Scholar] [CrossRef]
- Abdulazeez, I.; Popoola, S.A.; Saleh, T.A.; Al-Saadi, A.A. Spectroscopic, DFT and trace detection study of procaine using surface-enhanced Raman scattering technique. Chem. Phys. Lett. 2019, 730, 617–622. [Google Scholar] [CrossRef]
- Onawole, A.T.; Popoola, S.A.; Saleh, T.A.; Al-Saadi, A.A. Silver-loaded graphene as an effective SERS substrate for clotrimazole detection: DFT and spectroscopic studies. Spectrochim Acta Part. A Mol. Biomol. Spectrosc. 2018, 201, 354–361. [Google Scholar] [CrossRef]
- Kahraman, M.; Sur, İ.; Çulha, M. Label-Free Detection of Proteins from Self-Assembled Protein-Silver Nanoparticle Structures using Surface-Enhanced Raman Scattering. Analytical Chemistry 2010, 82, 7596–7602. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, W.; Kanda, P.; Cheng, H.; Chen, Y.; Wang, S.; Huan, S. Silver deposited polystyrene (PS) microspheres for surface-enhanced Raman spectroscopic-encoding and rapid label-free detection of melamine in milk powder. Talanta 2013, 113, 7–13. [Google Scholar] [CrossRef]
- Li, P.; Dong, R.; Wu, Y.; Liu, H.; Kong, L.; Yang, L. Polystyrene/Ag nanoparticles as dynamic surface-enhanced Raman spectroscopy substrates for sensitive detection of organophosphorus pesticides. Talanta 2014, 127, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, M.-Y.; Wang, K.; Sun, R.; Zhao, S.; Zhang, Z.; He, Y.-P.; Yu, F. Geometry transformation of ionic surfactants and adsorption behavior on water/n-decane-interface: Calculation by molecular dynamics simulation and DFT study. RSC Advances 2021, 11, 28286–28294. [Google Scholar] [CrossRef]
- Su, T.; Hong, K.H.; Zhang, W.; Li, F.; Li, Q.; Yu, F.; Luo, G.; Gao, H.; He, Y.-P. Scaleable two-component gelator from phthalic acid derivatives and primary alkyl amines: Acid–base interaction in the cooperative assembly. Soft Matter 2017, 13, 4066–4073. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yan, Z.; Bai, L. Layer-by-layer assembly of polyelectrolyte and gold nanoparticle for highly reproducible and stable SERS substrate. Appl. Surf. Sci. 2016, 360, 437–441. [Google Scholar] [CrossRef]
- Hua, F.; Cui, T.; Lvov, Y. Lithographic Approach to Pattern Self-Assembled Nanoparticle Multilayers. Langmuir 2002, 18, 6712–6715. [Google Scholar] [CrossRef]
- Kahraman, M.; Zamaleeva, A.I.; Fakhrullin, R.F.; Culha, M. Layer-by-layer coating of bacteria with noble metal nanoparticles for surface-enhanced Raman scattering. Anal. Bioanal. Chem. 2009, 395, 2559. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Deng, J.; Zhao, Y.; Xia, L.; Huang, K.; Ju, S.; Xiao, N. A novel core–shell magnetic nano-sorbent with surface molecularly imprinted polymer coating for the selective solid phase extraction of dimetridazole. Food Chem. 2014, 158, 366–373. [Google Scholar] [CrossRef]
- Kong, X.; Yu, Q.; Zhang, X.; Du, X.; Gong, H.; Jiang, H. Synthesis and application of surface enhanced Raman scattering (SERS) tags of Ag@ SiO 2 core/shell nanoparticles in protein detection. J. Mater. Chem. 2012, 22, 7767–7774. [Google Scholar] [CrossRef]
- Fang, J.; Xuan, Y.; Li, Q. Preparation of polystyrene spheres in different particle sizes and assembly of the PS colloidal crystals. Sci. China Technol. Sci. 2010, 53, 3088–3093. [Google Scholar] [CrossRef]
- Yang, B.; Xu, L.; Liu, Y.; Liu, B.; Zhang, M. Preparation of monodisperse polystyrene microspheres with different functional groups using soap-free emulsion polymerization. Colloid Polym. Sci. 2021, 299, 1095–1102. [Google Scholar] [CrossRef]
- Arunbabu, D.; Sanga, Z.; Seenimeera, K.M.; Jana, T. Emulsion copolymerization of styrene and sodium styrene sulfonate: Kinetics, monomer reactivity ratios and copolymer properties. Polym. Int. 2009, 58, 88–96. [Google Scholar] [CrossRef]
- Gulmine, J.V.; Janissek, P.R.; Heise, H.M.; Akcelrud, L. Polyethylene characterization by FTIR. Polym. Test. 2002, 21, 557–563. [Google Scholar] [CrossRef]
- Yang, B.; Agrios, A.G. Attachment of Pt nanoparticles to a metal oxide surface using a thiol–carboxyl bifunctional molecule. J. Colloid Interface Sci. 2018, 513, 464–469. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, X.; Li, P.; Han, R.; Liu, Y.; Han, X.; Zhao, B. Surface-enhanced Raman scattering (SERS) as a probe for detection of charge-transfer between TiO2 and CdS nanoparticles. New J. Chem. 2019, 43, 230–237. [Google Scholar] [CrossRef]
- Wang, S.; Guo, J.; Ma, Y.; Wang, A.X.; Kong, X.; Yu, Q. Fabrication and Application of SERS-Active Cellulose Fibers Regenerated from Waste Resource. Polymers 2021, 13, 2142. [Google Scholar] [CrossRef]
- Tian, X.; Zhai, P.; Guo, J.; Yu, Q.; Xu, L.; Yu, X.; Wang, R.; Kong, X. Fabrication of plasmonic cotton gauze-Ag composite as versatile SERS substrate for detection of pesticides residue. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2021, 257, 119766. [Google Scholar] [CrossRef]
- Tian, X.; Fan, Q.; Guo, J.; Yu, Q.; Xu, L.; Kong, X. Surface-enhanced Raman scattering of flexible cotton fiber-Ag for rapid adsorption and detection of malachite green in fish. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2021, 263, 120174. [Google Scholar] [CrossRef]
- Yu, Q.; Kong, X.; Ma, Y.; Wang, R.; Liu, Q.; Hinestroza, J.P.; Wang, A.X.; Vuorinen, T. Multi-functional regenerated cellulose fibers decorated with plasmonic Au nanoparticles for colorimetry and SERS assays. Cellulose 2018, 25, 6041–6053. [Google Scholar] [CrossRef]
- Ravi, P. Experimental study and Ab-initio calculations on the molecular structure, infrared and Raman spectral properties of dinitroimidazoles. Chem. Data Collect. 2017, 9–10, 11–23. [Google Scholar] [CrossRef]
- Ho, C.; Sin, D.W.M.; Wong, K.M.; Tang, H.P.O. Determination of dimetridazole and metronidazole in poultry and porcine tissues by gas chromatography–electron capture negative ionization mass spectrometry. Anal. Chim. Acta 2005, 530, 23–31. [Google Scholar] [CrossRef]
- Elzatahry, A.A.; Eldin, M.S.M. Preparation and characterization of metronidazole-loaded chitosan nanoparticles for drug delivery application. Polym. Adv. Technol. 2008, 19, 1787–1791. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Xu, Z.; Guo, J.; Wang, H.; Ma, Y.; Kong, X.; Fan, H.; Yu, Q. Layer-by-Layer Assembly of Polystyrene/Ag for a Highly Reproducible SERS Substrate and Its Use for the Detection of Food Contaminants. Polymers 2021, 13, 3270. https://doi.org/10.3390/polym13193270
Zhang S, Xu Z, Guo J, Wang H, Ma Y, Kong X, Fan H, Yu Q. Layer-by-Layer Assembly of Polystyrene/Ag for a Highly Reproducible SERS Substrate and Its Use for the Detection of Food Contaminants. Polymers. 2021; 13(19):3270. https://doi.org/10.3390/polym13193270
Chicago/Turabian StyleZhang, Sihan, Zhihua Xu, Jiaqi Guo, Haiying Wang, Yibo Ma, Xianming Kong, Hongtao Fan, and Qian Yu. 2021. "Layer-by-Layer Assembly of Polystyrene/Ag for a Highly Reproducible SERS Substrate and Its Use for the Detection of Food Contaminants" Polymers 13, no. 19: 3270. https://doi.org/10.3390/polym13193270
APA StyleZhang, S., Xu, Z., Guo, J., Wang, H., Ma, Y., Kong, X., Fan, H., & Yu, Q. (2021). Layer-by-Layer Assembly of Polystyrene/Ag for a Highly Reproducible SERS Substrate and Its Use for the Detection of Food Contaminants. Polymers, 13(19), 3270. https://doi.org/10.3390/polym13193270