Tuning the Activity of a Hybrid Polymer–Oxocluster Catalyst: A Composition—Selectivity Correlation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Zr4O2(OMc)12
2.2. Preparation of Hybrid Materials
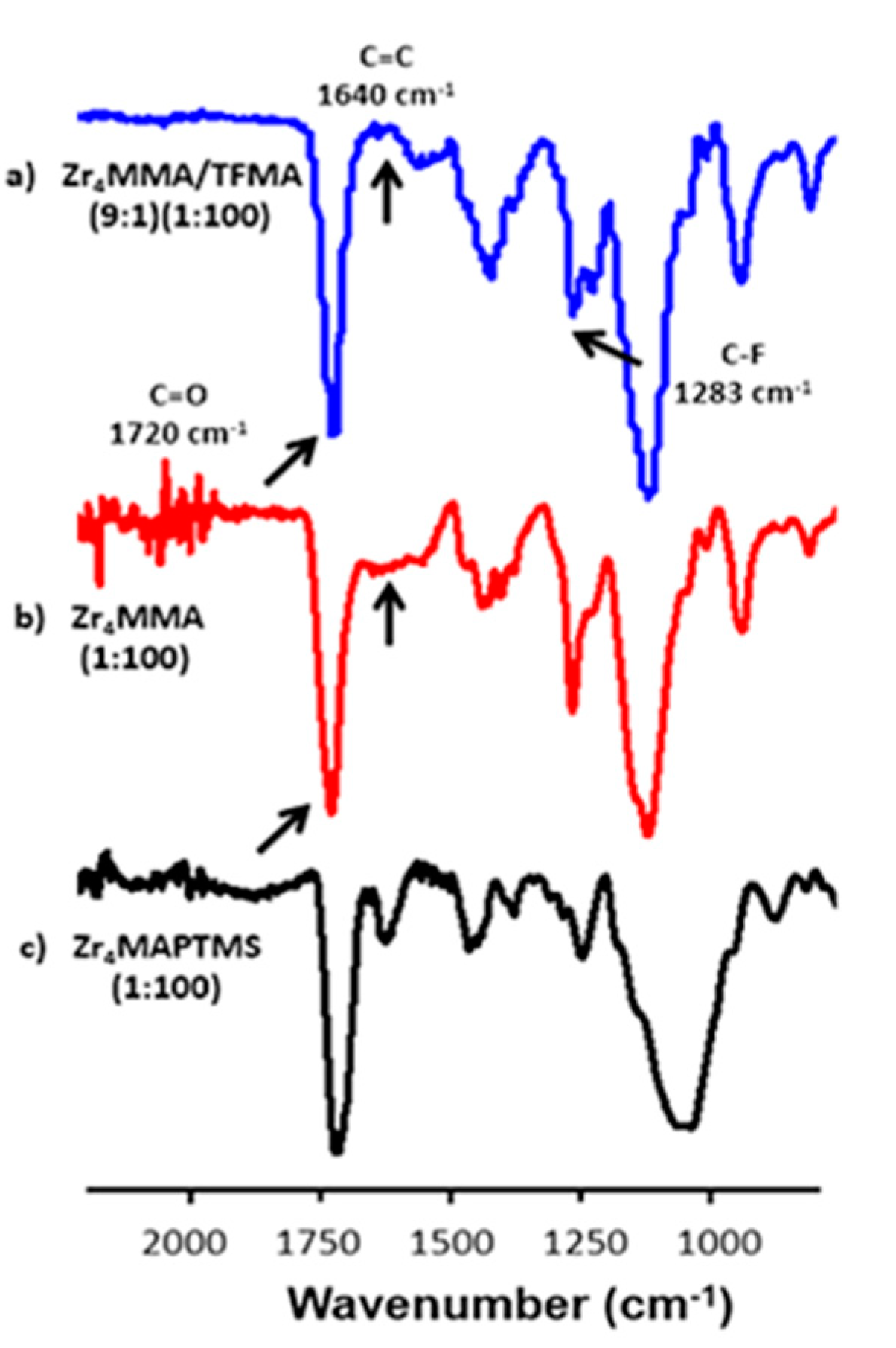
2.3. FT-IR Measurements
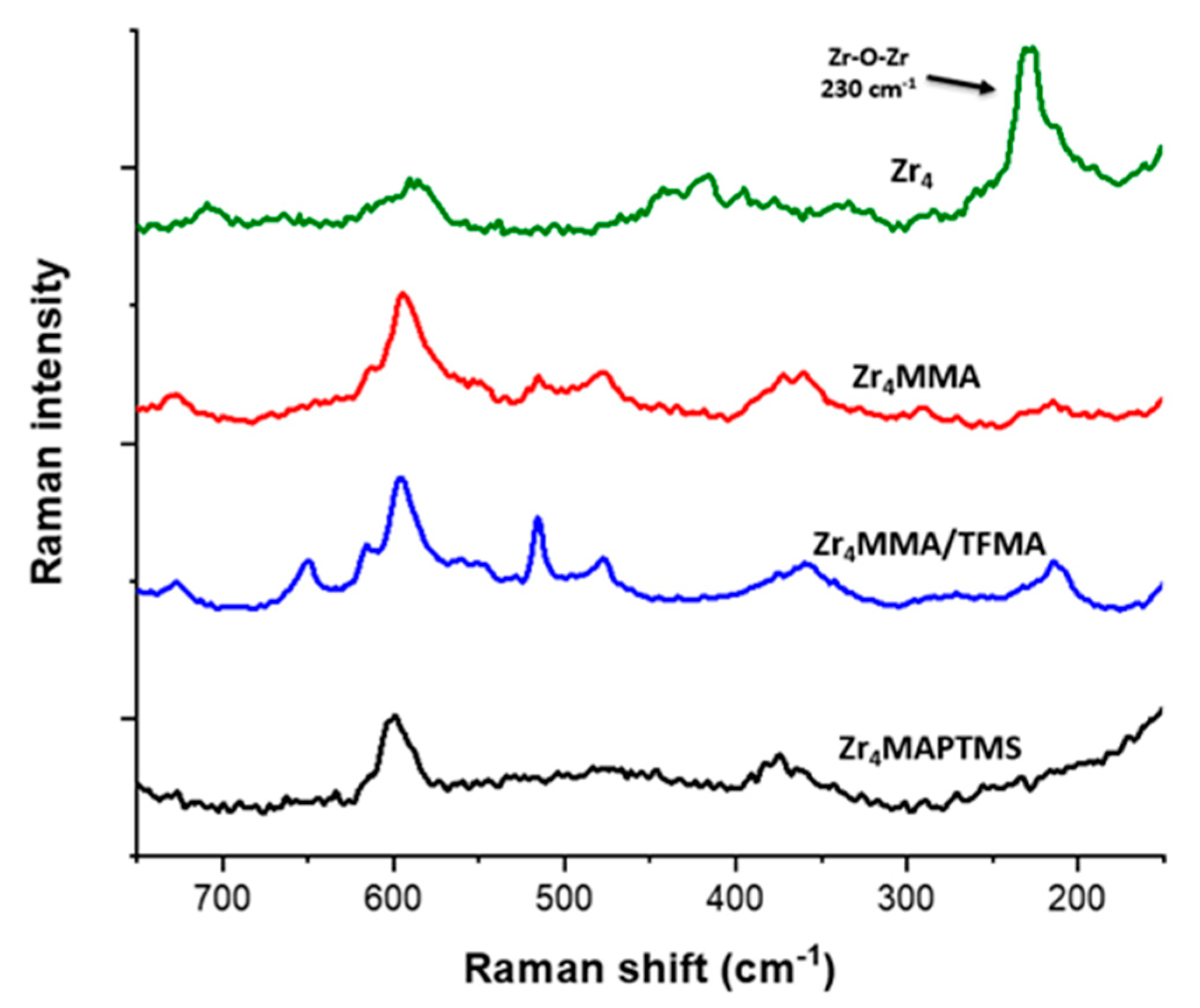
2.4. Raman Measurements
2.5. Thermogravimetric Analysis (TGA)
2.6. Swelling Measurements
2.7. Solid State NMR Spectroscopy
2.8. XAS Measurements
2.9. GC Analysis
2.10. Catalytic Tests and Catalytic Recycles
2.11. SEM-EDX Analysis
3. Results and Discussion
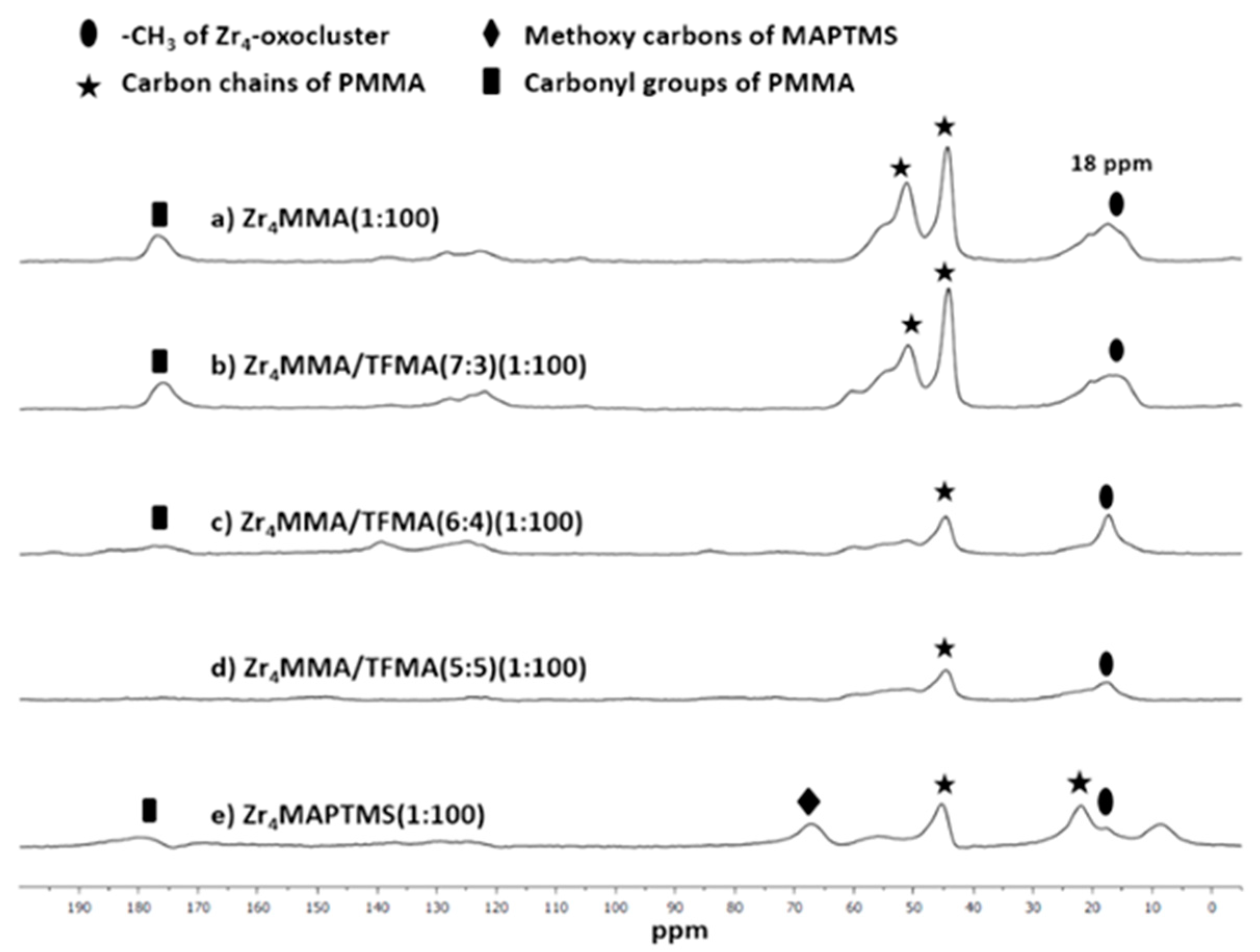
3.1. Synthesis and Characterization of the Hybrid Materials
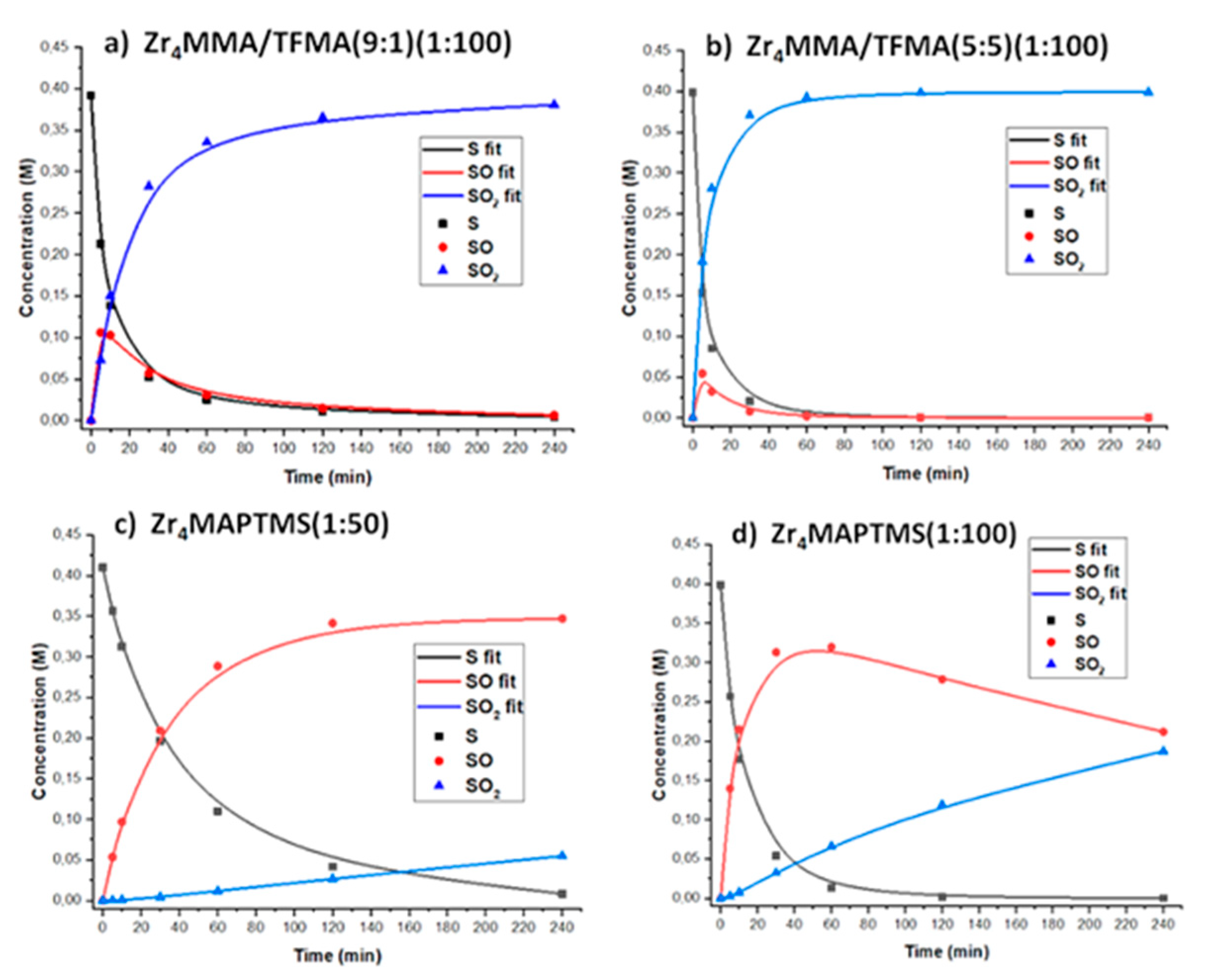
3.2. Catalytic Tests
3.3. Characterisation of Hybrid Materials after Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kickelbick, G. Hybrid Materials—Past, Present and Future. Hybrid Mater. 2014, 1, 39–51. [Google Scholar] [CrossRef]
- Gross, S. Oxocluster-reinforced organic–inorganic hybrid materials: Effect of transition metal oxoclusters on structural and functional properties. J. Mater. Chem. 2011, 21, 15853–15861. [Google Scholar] [CrossRef]
- Cordes, D.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef]
- Ayandele, E.; Sarkar, B.; Alexandridis, P. Polyhedral Oligomeric Silsesquioxane (POSS)-Containing Polymer Nanocomposites. Nanomaterials 2012, 2, 445–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, W.; Wu, L. Polyoxometalate/polymer hybrid materials: Fabrication and properties. Polym. Int. 2009, 58, 1217–1225. [Google Scholar] [CrossRef]
- Proust, A.; Matt, B.; Villanneau, R.; Guillemot, G.; Gouzerh, P.; Izzet, G. Functionalization and post-functionalization: A step towards polyoxometalate-based materials The Royal Society of Chemistry. Chem. Soc. Rev. 2012, 41, 7605–7622. [Google Scholar] [CrossRef] [PubMed]
- Carraro, M.; Sandei, L.; Sartorel, A.; Scorrano, G.; Bonchio, M. “Hybrid polyoxotungstates as second-generation POM-based catalysts for microwave-assisted H2O2 activation. Org. Lett. 2006. [Google Scholar] [CrossRef]
- Carraro, M.; Gross, S. Hybrid Materials Based on the Embedding of Organically Modified Transition Metal Oxoclusters or Polyoxometalates into Polymers for Functional Applications: A Review. Materials 2014, 7, 3956–3989. [Google Scholar] [CrossRef] [Green Version]
- Schubert, U. Polymers Reinforced by Covalently Bonded Inorganic Clusters. Chem. Mater. 2001, 13, 3487–3494. [Google Scholar] [CrossRef]
- Schubert, U. Inorganic-Organic Hybrid Polymers Based on Surface-Modified Metal Oxide Clusters. Macromol. Symp. 2008, 267, 1–8. [Google Scholar] [CrossRef]
- Albinati, A.; Faccini, F.; Gross, S.; Kickelbick, G.; Rizzato, S.; Venzo, A. New Methacrylate-Functionalized Ba and Ba−Ti Oxoclusters as Potential Nanosized Building Blocks for Inorganic−Organic Hybrid Materials: Synthesis and Characterization. Inorg. Chem. 2007, 46, 3459–3466. [Google Scholar] [CrossRef]
- Schubert, U. Cluster-based inorganic–organic hybrid materials. Chem. Soc. Rev. 2011, 40, 575–582. [Google Scholar] [CrossRef]
- Carraro, M.; Nsouli, N.; Oelrich, H.; Sartorel, A.; Sorarù, A.; Mal, S.S.; Scorrano, G.; Walder, L.; Kortz, U.; Bonchio, M. Reactive ZrIV and HfIV Butterfly Peroxides on Polyoxometalate Surfaces: Bridging the Gap between Homogeneous and Heterogeneous Catalysis. Chem.-A Eur. J. 2011, 17, 8371–8378. [Google Scholar] [CrossRef]
- Vigolo, M.; Borsacchi, S.; Sorarù, A.; Geppi, M.; Smarsly, B.M.; Dolcet, P.; Rizzato, S.; Carraro, M.; Gross, S. Engineering of oxoclusters-reinforced polymeric materials with application as heterogeneous oxydesulfurization catalysts. Appl. Catal. B Environ. 2016, 182, 636–644. [Google Scholar] [CrossRef]
- Faccioli, F.; Bauer, M.; Pedron, D.; Sorarù, A.; Carraro, M.; Gross, S. Hydrolytic Stability and Hydrogen Peroxide Activation of Zirconium-Based Oxoclusters. Eur. J. Inorg. Chem. 2014, 2015, 210–225. [Google Scholar] [CrossRef]
- Legros, J.; Dehli, J.R.; Bolm, C. Applications of Catalytic Asymmetric Sulfide Oxidations to the Syntheses of Biologically Active Sulfoxides. Adv. Synth. Catal. 2005, 347, 19–31. [Google Scholar] [CrossRef]
- Martín, J.M.C.; Sánchez, M.D.C.C.; Presas, P.P.; Fierro, J.L.G. Oxidative processes of desulfurization of liquid fuels. J. Chem. Technol. Biotechnol. 2010, 85, 879–890. [Google Scholar] [CrossRef] [Green Version]
- Houda, S.; Lancelot, C.; Blanchard, P.; Poinel, L.; Lamonier, C. Oxidative Desulfurization of Heavy Oils with High Sulfur Content: A Review. Catalysts 2018, 8, 344. [Google Scholar] [CrossRef] [Green Version]
- Kickelbick, G.; Puchberger, M.; Schubert, U.; Gross, S. Mono-, Di-, and Trimetallic Methacrylate-substituted Metal Oxide Clusters Derived from Hafnium Butoxide. Mon. Fur Chem. 2003, 134, 1053–1063. [Google Scholar] [CrossRef]
- Trimmel, G.; Gross, S.; Kickelbick, G.; Schubert, U. Swelling behavior and thermal stability of poly(methylmethacrylate) crosslinked by the oxozirconium cluster Zr4O2(methacrylate)12. Appl. Organomet. Chem. 2001, 15, 401–406. [Google Scholar] [CrossRef]
- Trimmel, G.; Fratzl, P.; Schubert, U. Cross-Linking of poly(methyl methacrylate) by the Methacrylate-Substituted Oxozirconium Cluster Zr6(OH)4O4(Methacrylate)12. Chem. Mater. 2000, 12, 602–604. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. Athena, Artemis, Hephaestus: Data analysis for X-ray absorption spectroscopy using Ifeffit. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [Green Version]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Final Report of the Cosmetic Ingredient Review Expert Panel Safety Assessment of Polymethyl Methacrylate (PMMA), Methyl Methacrylate Crosspolymer, and Methyl Methacrylate/Glycol Dimethacrylate Crosspolymer. Int. J. Toxicol. 2011, 30, 54S–65S. [Google Scholar] [CrossRef] [PubMed]
- Cracowski, J.-M.; Montembault, V.; Améduri, B. Free-radical copolymerization of 2,2,2-trifluoroethyl methacrylate and 2,2,2-trichloroethyl α-fluoroacrylate: Synthesis, kinetics of copolymerization, and characterization. J. Polym. Sci. Part. A Polym. Chem. 2010, 48, 2154–2161. [Google Scholar] [CrossRef]
- Ramos-Fernández, J.M.; Beleña, I.; Romero-Sánchez, M.D.; Fuensanta, M.; Guillem, C.; López-Buendía, M. Study of the film formation and mechanical properties of the latexes obtained by miniemulsion co-polymerization of butyl acrylate, methyl acrylate and 3-methacryloxypropyltrimethoxysilane. Prog. Org. Coat. 2012, 75, 86–91. [Google Scholar] [CrossRef]
- Carraro, M.; Gardan, M.; Sartorel, A.; Maccato, C.; Bonchio, M. Hydrogen peroxide activation by fluorophilic polyoxotungstates for fast and selective oxygen transfer catalysis. Dalton Trans. 2016, 45, 14544–14548. [Google Scholar] [CrossRef] [PubMed]
- Graziola, F.; Girardi, F.; di Maggio, R.; Callone, E.; Miorin, E.; Negri, M.; Müller, K.; Gross, S. Three-components organic–inorganic hybrid materials as protective coatings for wood: Optimisation, synthesis, and characterisation. Prog. Org. Coat. 2012, 74, 479–490. [Google Scholar] [CrossRef]
- Raihane, M.; Ameduri, B. Radical copolymerization of 2,2,2-trifluoroethyl methacrylate with cyano compounds for dielectric materials: Synthesis and characterization. J. Fluor. Chem. 2006, 127, 391–399. [Google Scholar] [CrossRef]
- Wardhani, G.A.P.K.; Nurlela, N.; Azizah, M. Silica Content and Structure from Corncob Ash with Various Acid Treatment (HCl, HBr, and Citric Acid). Molekul 2017, 12, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Bautista, Y.; Gozalbo, A.; Mestre, S.; Sanz, V. Improvement in Char Strength with an Open Cage Silsesquioxane Flame Retardant. Materials 2017, 10, 567. [Google Scholar] [CrossRef] [Green Version]
- Armelao, L.; Bertagnolli, H.; Gross, S.; Krishnan, V.; Lavrencic-Stangar, U.; Müller, K.; Orel, B.; Srinivasan, G.; Tondello, E.; Zattin, A. Zr and Hf oxoclusters as building blocks for the preparation of nanostructured hybrid materials and binary oxides MO2–SiO2 (M = Hf, Zr). J. Mater. Chem. 2005, 15, 1954–1965. [Google Scholar] [CrossRef] [Green Version]
- Pantoja, M.; Velasco, F.; Broekema, D.; Abenojar, J.; del Real, J.C. The Influence of pH on the Hydrolysis Process of γ-Methacryloxypropyltrimethoxysilane, Analyzed by FT-IR, and the Silanization of Electrogalvanized Steel. J. Adhes. Sci. Technol. 2010, 24, 1131–1143. [Google Scholar] [CrossRef]
- Walther, P.; Puchberger, M.; Kogler, F.R.; Schwarz, K.; Schubert, U. Ligand dynamics on the surface of zirconium oxo clusters. Phys. Chem. Chem. Phys. 2009, 11, 3640–3647. [Google Scholar] [CrossRef] [PubMed]
- Kickelbick, G.; Feth, M.P.; Bertagnolli, H.; Puchberger, M.; Holzinger, D.; Gross, S. Formation of organically surface-modified metal oxo clusters from carboxylic acids and metal alkoxides: A mechanistic study. J. Chem. Soc. Dalton Trans. 2002, 3892–3898. [Google Scholar] [CrossRef]
- Mayer, C.R.; Thouvenot, R.; Lalot, T. New Hybrid Covalent Networks Based on Polyoxometalates: Part 1. Hybrid Networks Based on poly(ethyl methacrylate) Chains Covalently Cross-linked by Heteropolyanions: Synthesis and Swelling Properties. Chem. Mater. 2000, 12, 257–260. [Google Scholar] [CrossRef]
- Sperling, L. Introduction to Physical Polymer Science; Wiley: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- Cerón-Carrasco, J.P.; Jacquemin, D.; Laurence, C.; Planchat, A.; Reichardt, C.; Sraïdi, K. Solvent polarity scales: Determination of newET(30) values for 84 organic solvents. J. Phys. Org. Chem. 2014, 27, 512–518. [Google Scholar] [CrossRef]
- Reichardt, C. Pyridinium-N-phenolate betaine dyes as empirical indicators of solvent polarity: Some new findings. Pure Appl. Chem. 2008, 80, 1415–1432. [Google Scholar] [CrossRef]
- Elhrari, W.; Assumption, H.; Mallon, P.E. Correlation between positron annihilation lifetime parameters and T1ρ relaxation times determined from solid state NMR at the compositional phase segregation point of graft copolymers. Polymer 2015, 77, 95–101. [Google Scholar] [CrossRef]
- Gandhi, S.; Melian, C.; Demco, D.E.; Brar, A.S.; Blümich, B.; Bluemich, B. Morphology and Motional Heterogeneity in PS/PMMA Diblock Copolymers Studied by 1 H and 13 C Solid-State NMR Spectroscopy. Macromol. Chem. Phys. 2008, 209, 1576–1585. [Google Scholar] [CrossRef]
- Armelao, L.; Gross, S.; Müller, K.; Pace, G.; Tondello, E.; Tsetsgee, O.; Zattin, A. Structural Evolution upon Thermal Heating of Nanostructured Inorganic−Organic Hybrid Materials to Binary Oxides MO2−SiO2(M = Hf, Zr) as Evaluated by Solid-State NMR and FTIR Spectroscopy. Chem. Mater. 2006, 18, 6019–6030. [Google Scholar] [CrossRef]
- Gross, S.; Bauer, M. EXAFS as Powerful Analytical Tool for the Investigation of Organic-Inorganic Hybrid Materials. Adv. Funct. Mater. 2010, 20, 4026–4047. [Google Scholar] [CrossRef]
- Graziola, F.; Girardi, F.; Bauer, M.; Di Maggio, R.; Rovezzi, M.; Bertagnolli, H.; Sada, C.; Rossetto, G.; Gross, S. UV-photopolymerisation of poly(methyl methacrylate)-based inorganic–organic hybrid coatings and bulk samples reinforced with methacrylate-modified zirconium oxocluster. Polymer 2008, 49, 4332–4343. [Google Scholar] [CrossRef]
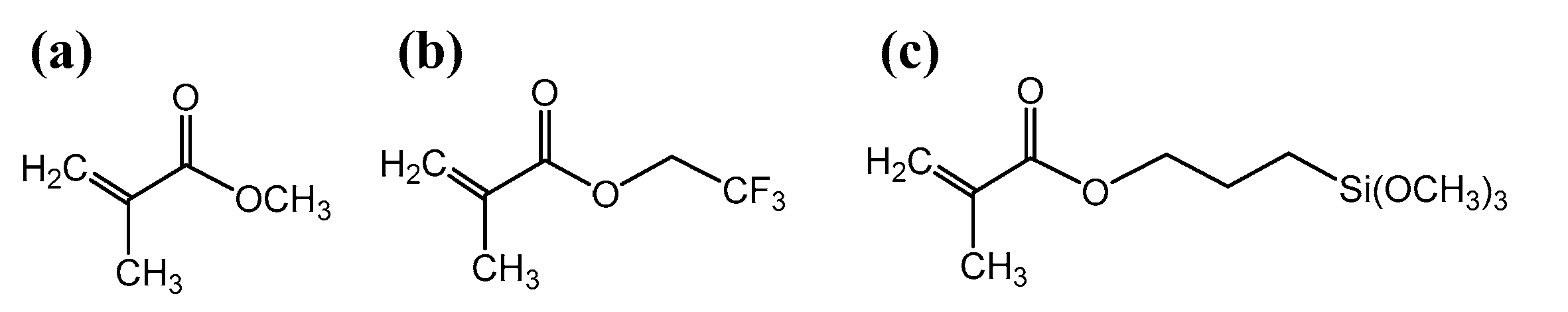

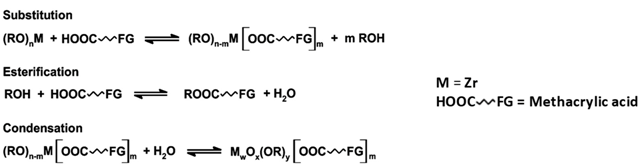
| Sample | Monomer (M1) | Co-Monomer (M2) | M1:M2 Molar Ratio | Oxocluster: (M1 + M2) Molar Ratio |
|---|---|---|---|---|
| Zr4MAPTMS (1:50) | MAPTMS | - | - | 1:50 |
| Zr4MMA (1:50) | MMA | - | ||
| Zr4MMA/TFMA (9:1) (1:50) | MMA | TFMA | 9:1 | |
| Zr4MMA/TFMA (8:2) (1:50) | 8:2 | |||
| Zr4MMA/TFMA (7:3) (1:50) | 7:3 | |||
| Zr4MMA/TFMA (6:4) (1:50) | 6:4 | |||
| Zr4MMA/TFMA (5:5) (1:50) | 5:5 | |||
| Zr4MAPTMS (1:100) | MAPTMS | - | - | 1:100 |
| Zr4MMA (1:100) | MMA | - | ||
| Zr4MMA/TFMA (9:1) (1:100) | MMA | TFMA | 9:1 | |
| Zr4MMA/TFMA (8:2) (1:100) | 8:2 | |||
| Zr4MMA/TFMA (7:3) (1:100) | 7:3 | |||
| Zr4MMA/TFMA (6:4) (1:100) | 6:4 | |||
| Zr4MMA/TFMA (5:5) (1:100) | 5:5 |
| Hybrid | Experimental Residue (% wt.) | Theoretical Residue (% wt.) |
|---|---|---|
| Zr4MMA (1:100) | 3.2 | 4.3 |
| Zr4MMA/TFMA (6:4) (1:100) | 8.0 | 7.4 |
| Zr4MAPTMS (1:100) | 38.5 a | 35.3 a |
| Samples | Isw EtOAc ET(30) = 38 [37] | Isw ACN ET(30) = 46 [37] | Isw EtOH ET(30) = 52 [37] | Isw H2O ET(30) = 63 [37] |
|---|---|---|---|---|
| Zr4MAPTMS (1:50) | soluble | Soluble | 40 | 25 |
| Zr4MMA (1:50) | 72 | 33 | 29 | 2 |
| Zr4MMA/TFMA (9:1) (1:50) | 120 | 62 | 29 | 7 |
| Zr4MMA/TFMA (8:2) (1:50) | 119 | 50 | 24 | 3 |
| Zr4MMA/TFMA (7:3) (1:50) | 130 | 54 | 30 | 4 |
| Zr4MMA/TFMA (6:4) (1:50) | 174 | 61 | 29 | 2 |
| Zr4MMA/TFMA (5:5) (1:50) | 140 | 63 | 22 | 18 |
| Zr4MAPTMS (1:100) | soluble | soluble | 29 | 21 |
| Zr4MMA (1:100) | 188 | 84 | 14 | 0 |
| Zr4MMA/TFMA (9:1) (1:100) | 324 | 116 | 16 | 0 |
| Zr4MMA/TFMA (8:2) (1:100) | 406 | 109 | 13 | 0 |
| Zr4MMA/TFMA (7:3) (1:100) | 250 | 151 | 85 | 2 |
| Zr4MMA/TFMA (6:4) (1:100) | 353 | 162 | 50 | 0 |
| Zr4MMA/TFMA (5:5) (1:100) | 300 | 102 | 17 | 7 |
| PMMA | soluble | soluble | 24 | 6 |
| PMMA/TFMA (6:4) | soluble | soluble | 3 | 1 |
| MAPTMS | soluble | soluble | soluble | soluble |

| Entry | ACN Volume (mL) | Catalyst | Yield (%) (1 h) | Yield (%) (4 h) | SO:SO2 (1 h) | SO:SO2 (4 h) | R0 (Ms−1) |
|---|---|---|---|---|---|---|---|
| 1 | 1.2 | Zr4 | 49 | 69 | 69:31 | 50:50 | 4.3 × 10−5 |
| 2 | 1.2 | Zr4MMA (1:50) | 86 | 95 | 19:81 | 7:93 | 1.1 × 10−4 |
| 3 | 1.2 | Zr4MMA/TFMA (9:1) (1:50) | 74 | 90 | 32:68 | 15:85 | 8.1 × 10−5 |
| 4 | 1.2 | Zr4MMA/TFMA (8:2) (1:50) | 88 | 98 | 19:81 | 3:97 | 9.1 × 10−5 |
| 5 | 1.2 | Zr4MMA (1:100) | 84 | 95 | 21:79 | 9:91 | 1.1 × 10−4 |
| 6 | 1.2 | Zr4MMA/TFMA (9:1) (1:100) | 93 | 97 | 11:89 | 5:95 | 1.4 × 10−4 |
| 7 | 1.2 | Zr4MMA/TFMA (8:2) (1:100) | 95 | 99 | 8:92 | 2:98 | 1.6 × 10−4 |
| 8 | 2.2 | Zr4MMA (1:100) | 84 | 95 | 21:79 | 9:91 | 1.1 × 10−4 |
| 9 | 2.2 | Zr4MMA/TFMA (9:1) (1:100) | 93 | 97 | 11:89 | 5:95 | 1.4 × 10−4 |
| 10 | 2.2 | Zr4MMA/TFMA (8:2) (1:100) | 95 | 99 | 8:92 | 2:98 | 1.6 × 10−4 |
| 11 | 2.2 | Zr4MMA/TFMA (7:3) (1:100) | 91 | 92 | 7:93 | 8:92 | 4.1 × 10−4 |
| 12 | 2.2 | Zr4MMA/TFMA (6:4) (1:100) | 97 | 98 | 1:99 | 1:99 | 4.8 × 10−4 |
| 13 | 2.2 | Zr4MMA/TFMA (5:5) (1:100) | 99 | >99 | 1:99 | 0:100 | 5.0 × 10−4 |
| 14 | 2.2 | Zr4MAPTMS (1:50) | 39 | 55 | 96:4 | 87:13 | 2.0 × 10−4 |
| 15 | 2.2 | Zr4MAPTMS (1:100) | 57 | 76 | 85:15 | 47:53 | 2.8 × 10−4 |
| Samples | k1 (M−1s−1) | k2 (M−1s−1) | S (k1/k2) |
|---|---|---|---|
| Zr4MMA (1:100) | 0.0055 | 0.0099 | 0.6 |
| Zr4MMA/TFMA (9:1) (1:100) | 0.003 | 0.0051 | 0.6 |
| Zr4MMA/TFMA (5:5) (1:100) | 0.0045 | 0.0162 | 0.3 |
| Zr4MAPTMS (1:50) | 0.0006 | 0.0003 | 2.0 |
| Zr4MAPTMS (1:100) | 0.002 | 0.0015 | 1.3 |
| Sample | Scatterer | N | R (Å) | σ (10−3Å) | Eo (eV) | R Factor |
|---|---|---|---|---|---|---|
| Zr4 | O1 | 3.2 ±1.3 | 2.13 ±0.01 | 3.9 ±1.0 | 2.28 ± 1.00 | 12.2 |
| O2 | 4.1 ±0.4 | 2.27 ±0.01 | 4.1 ± 1.0 | |||
| Zr | 3.0 ±0.8 | 3.53 ±0.01 | 4.7 ± 0.5 | |||
| Zr4MMA (1:100) | O1 | 3.7 ±1.3 | 2.12 ±0.02 | 4.9 ± 1.4 | 2.92 ± 1.42 | 23.3 |
| O2 | 4.1 ±1.1 | 2.25 ±0.02 | 5.1 ±1.6 | |||
| Zr | 1.4 ±1.0 | 3.47 ±0.03 | 14.3 ± 4.3 | |||
| Zr4MMA (1:100) after catalysis | O1 | 2.8 ±1.0 | 2.12 ±0.02 | 3.0 ± 0.6 | 3.84 ± 1.77 | 20.4 |
| O2 | 4.1 ±1.2 | 2.26 ±0.02 | 7.0 ± 0.9 | |||
| Zr4MMA/TFMA (5:5) (1:100) | O1 | 3.3 ±1.6 | 2.14 ±0.01 | 3.0 ± 0.6 | 3.45 ± 1.27 | 19.4 |
| O2 | 4.1 ±0.4 | 2.28 ±0.01 | 5.3 ± 0.5 | |||
| Zr | 3.0 ±0.9 | 3.53 ±0.01 | 4.7 ± 0.5 | |||
| Zr4MMA/TFMA (5:5) (1:100) after catalysis | O1 | 3.5 ±1.3 | 2.14 ±0.01 | 3.2 ± 1.1 | 3.45 ± 1.27 | 21.3 |
| O2 | 4.0 ±1.3 | 2.27 ±0.01 | 5.1 ± 2.4 | |||
| Zr | 2.8 ±1.4 | 3.53 ±0.01 | 7.6 ± 3.4 | |||
| Zr4MAPTMS (1:50) | O1 | 3.5 ±1.2 | 2.13 ±0.01 | 4.7 ± 0.7 | 3.70 ± 0.78 | 9.6 |
| O2 | 4.1 ±1.1 | 2.28 ±0.03 | 5.3 ± 0.7 | |||
| Zr | 1.5 ±0.4 | 3.56 ±0.01 | 4.3 ± 1.4 | |||
| Zr4MAPTMS (1:50) after catalysis | O1 | 3.5 ±2.0 | 2.12 ±0.02 | 2.9 ± 0.7 | 2.11 ± 1.58 | 14.9 |
| O2 | 4.1 ±0.3 | 2.25 ±0.02 | 6.8 ± 1.7 | |||
| Zr4MAPTMS (1:100) | O1 | 1.5 ±1.2 | 2.13 ±0.02 | 2.3 ± 1.6 | 5.62 ± 1.13 | 15.7 |
| O2 | 4.0 ±1.3 | 2.27 ±0.01 | 6.1 ± 1.4 | |||
| Zr | 2.0 ±1.1 | 3.60 ±0.02 | 8.7 ± 4.1 | |||
| Zr4MAPTMS (1:100) after catalysis | O1 | 2.3 ±1.7 | 2.12 ±0.03 | 2.6 ± 0.9 | 2.33 ± 2.13 | 26.6 |
| O2 | 4.0 ±0.8 | 2.25 ±0.02 | 4.2 ± 0.8 | |||
| Zr | 0.3 ±1.2 | 3.58 ±0.02 | 8.7 ± 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bragaggia, G.; Beghetto, A.; Bassato, F.; Reichenbächer, R.; Dolcet, P.; Carraro, M.; Gross, S. Tuning the Activity of a Hybrid Polymer–Oxocluster Catalyst: A Composition—Selectivity Correlation. Polymers 2021, 13, 3268. https://doi.org/10.3390/polym13193268
Bragaggia G, Beghetto A, Bassato F, Reichenbächer R, Dolcet P, Carraro M, Gross S. Tuning the Activity of a Hybrid Polymer–Oxocluster Catalyst: A Composition—Selectivity Correlation. Polymers. 2021; 13(19):3268. https://doi.org/10.3390/polym13193268
Chicago/Turabian StyleBragaggia, Giulia, Anna Beghetto, Ferdinando Bassato, Rudi Reichenbächer, Paolo Dolcet, Mauro Carraro, and Silvia Gross. 2021. "Tuning the Activity of a Hybrid Polymer–Oxocluster Catalyst: A Composition—Selectivity Correlation" Polymers 13, no. 19: 3268. https://doi.org/10.3390/polym13193268
APA StyleBragaggia, G., Beghetto, A., Bassato, F., Reichenbächer, R., Dolcet, P., Carraro, M., & Gross, S. (2021). Tuning the Activity of a Hybrid Polymer–Oxocluster Catalyst: A Composition—Selectivity Correlation. Polymers, 13(19), 3268. https://doi.org/10.3390/polym13193268