A Systematic Study of the Antioxidant Capacity of Humic Substances against Peroxyl Radicals: Relation to Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Humic Substances
2.2. Characterization of Humic Substances
2.3. Estimation of Oxygen Radical Absorbance Capacity (ORAC) of Humic Substances
2.4. Statistical Data Treatment
3. Results
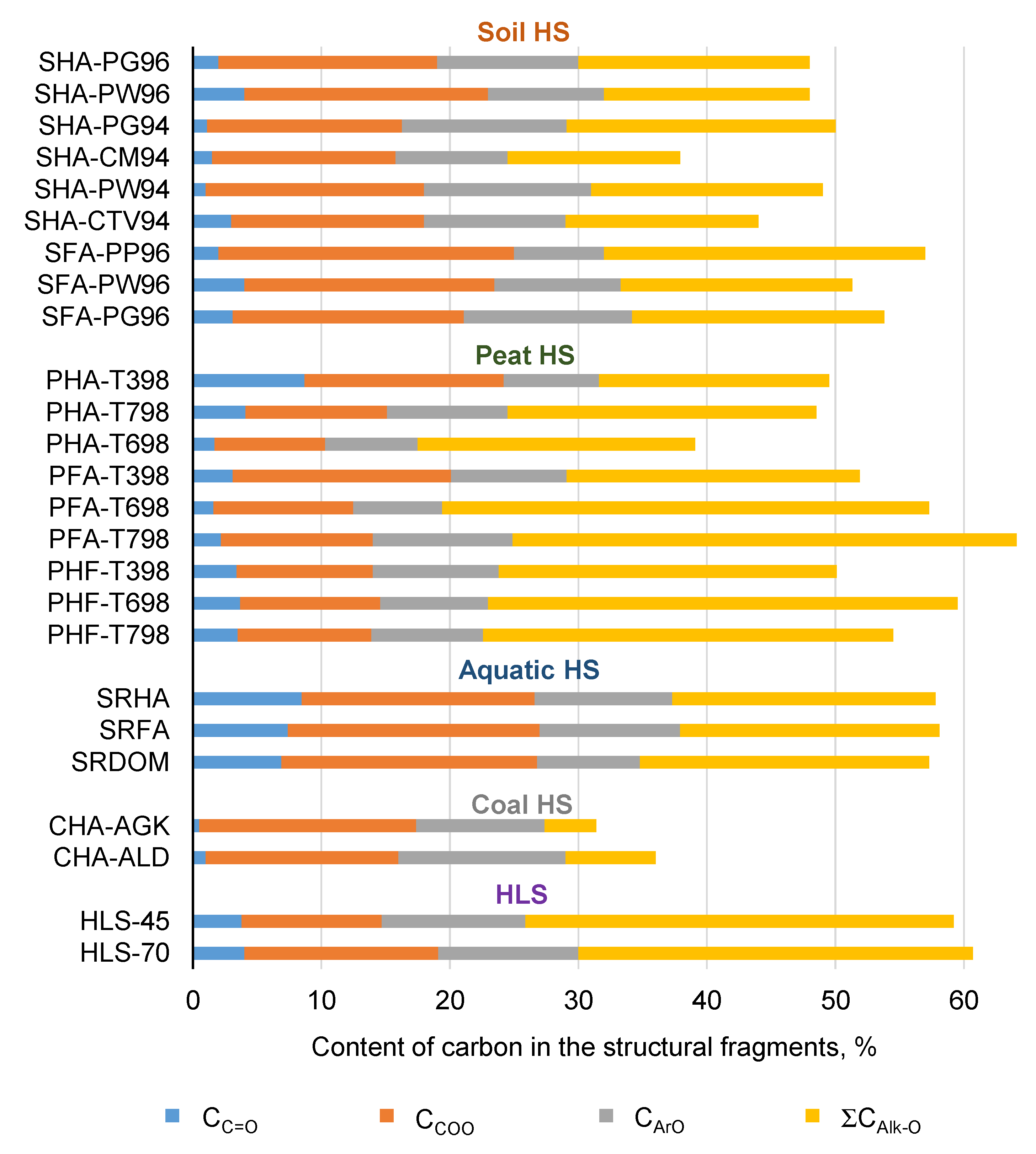
3.1. Structural Characteristics of HS
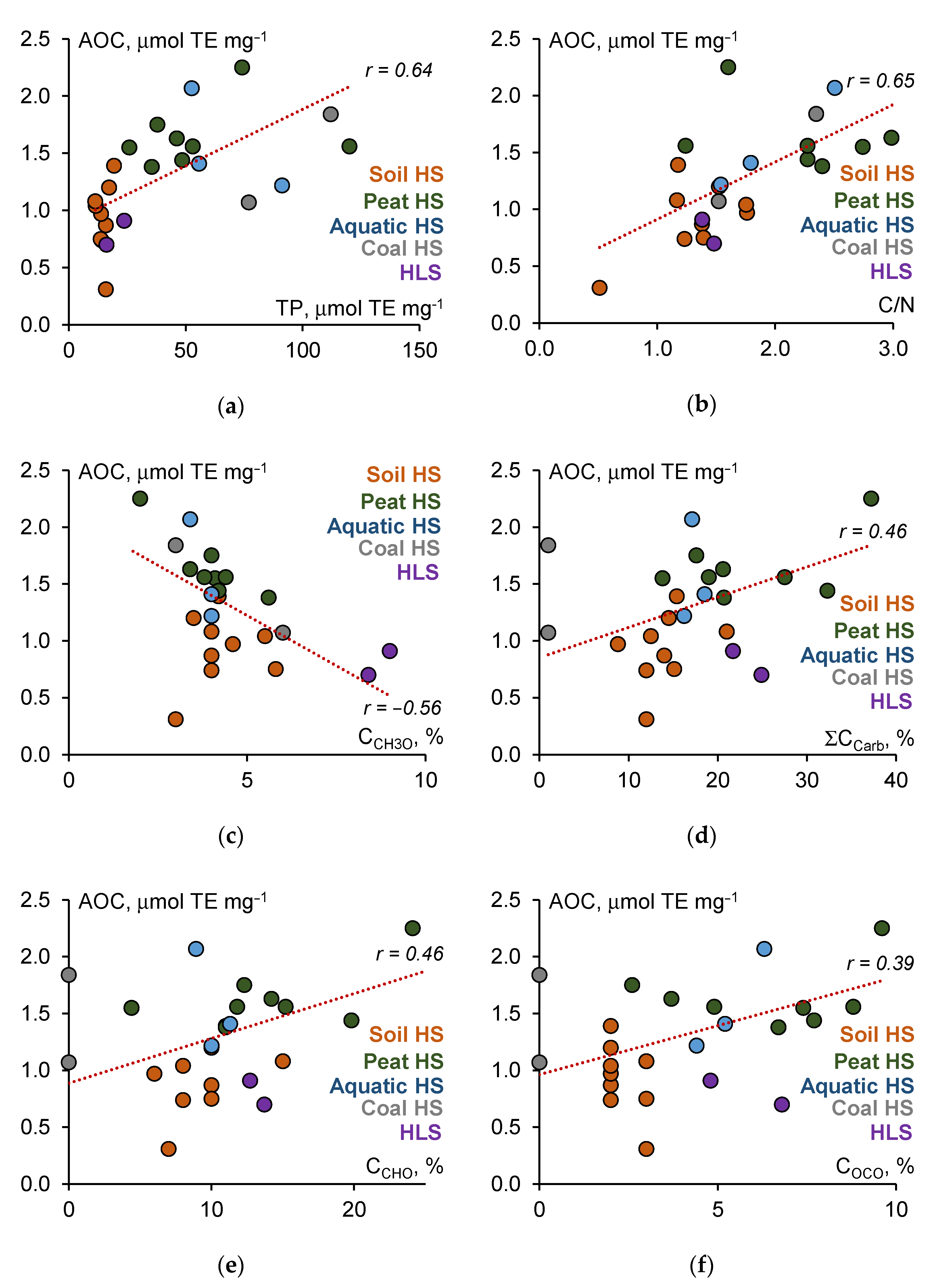
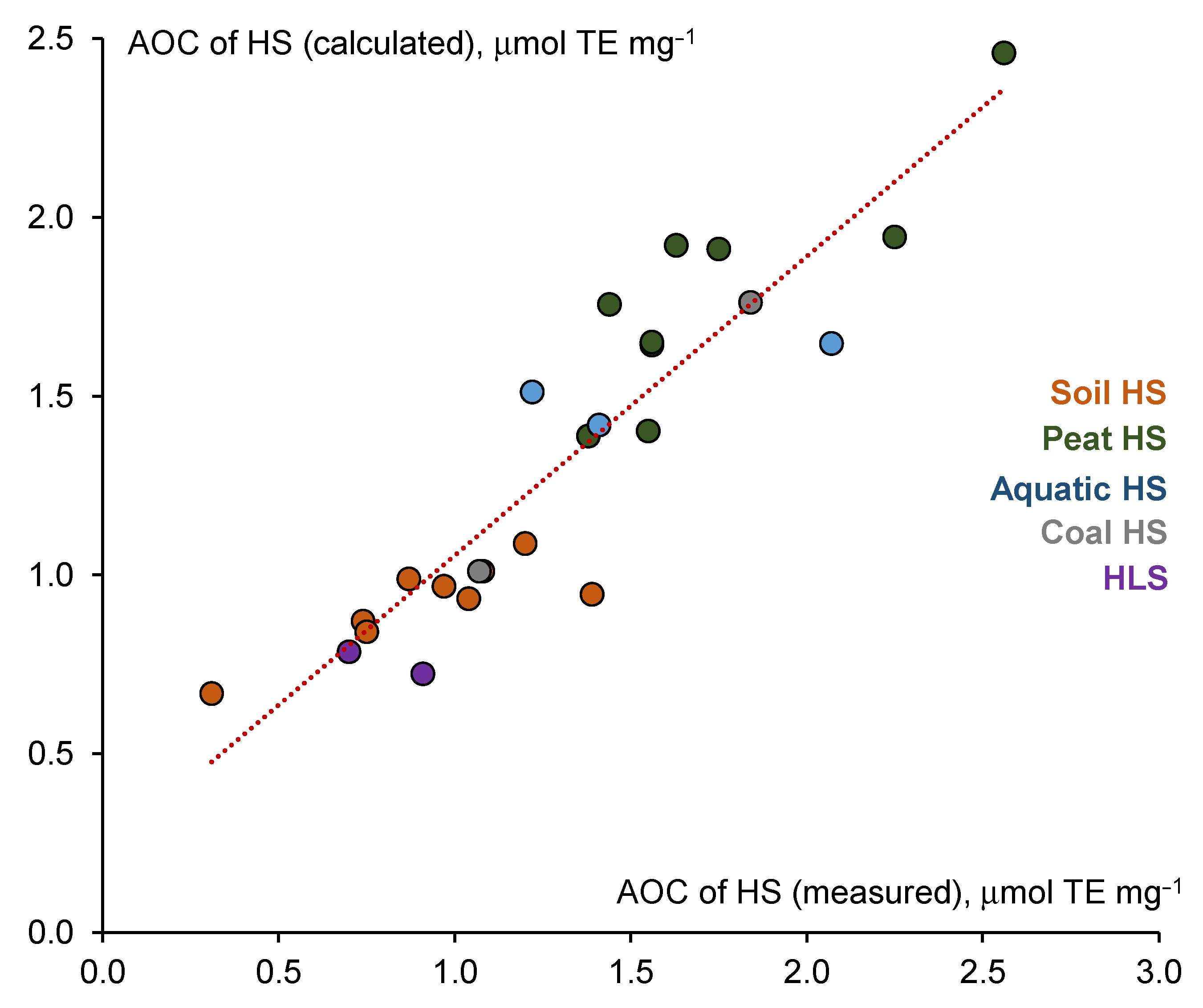
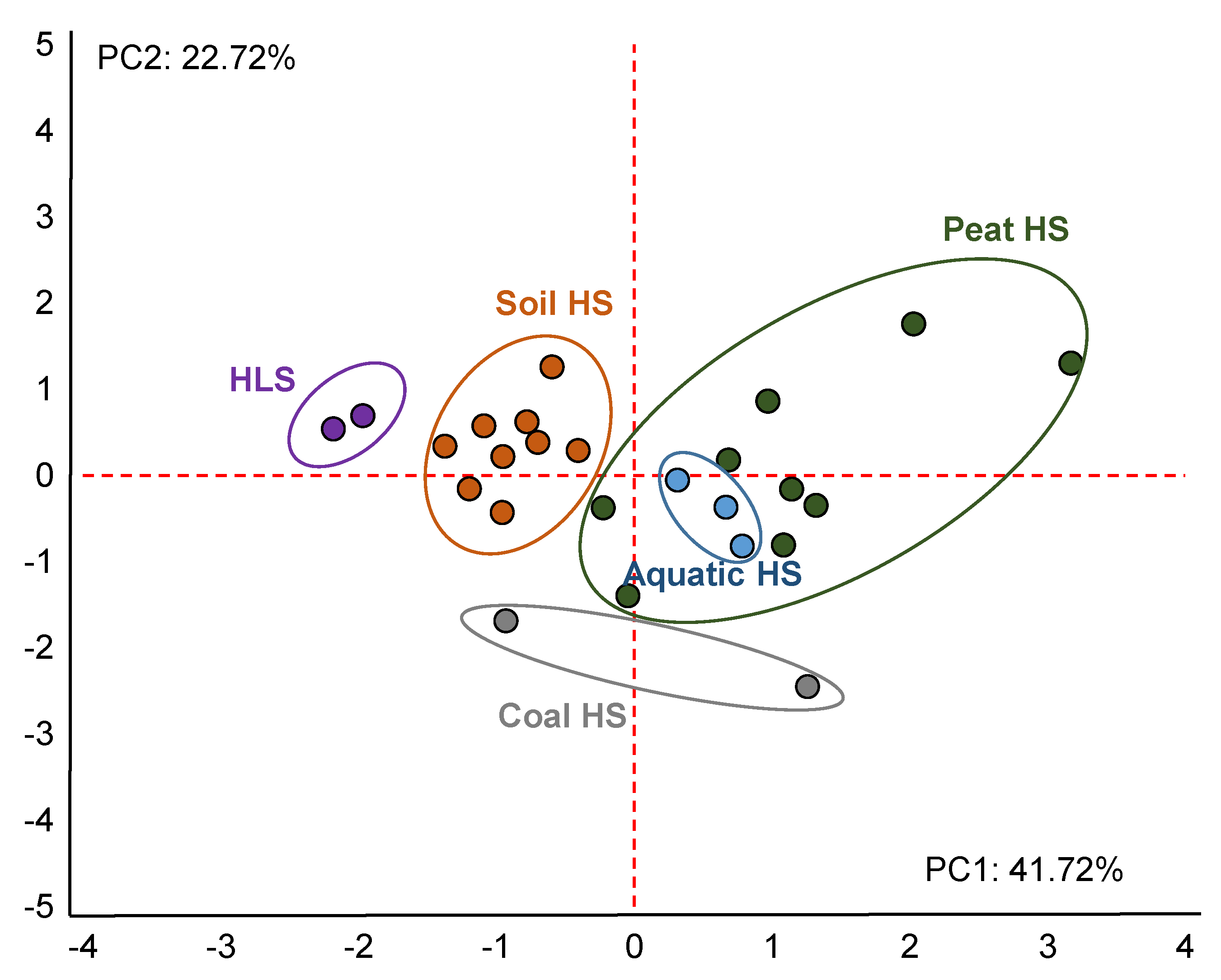
3.2. Antioxidant Capacity of HS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gaffney, J.S.; Marley, N.A.; Clarks, S.B. Humic and fulvic acids and organic colloidal materials in the environment. In Humic and Fulvic Acids: Isolation, Structure, and Environmental Role; Gaffney, J.S., Marley, N.A., Clarks, S.B., Eds.; American Chemical Society: Washington, DC, USA, 1996; pp. 2–16. [Google Scholar]
- Olk, D.C.; Bloom, P.R.; Perdue, E.M.; McKnight, D.M.; Chen, Y.; Farenhorst, A.; Senesi, N.; Chin, Y.-P.; Schmitt-Kopplin, P.; Hertkorn, N.; et al. Environmental and agricultural relevance of humic fractions extracted by alkali from soils and natural waters. J. Environ. Qual. 2019, 48, 217–232. [Google Scholar] [CrossRef]
- Quilty, J.R.; Cattle, S.R. Use and understanding of organic amendments in Australian agriculture: A review. Soil Res. 2011, 49, 1–26. [Google Scholar] [CrossRef]
- Field, J.A.; Cervantes, F.J.; van der Zee, F.P.; Lettinga, G. Role of quinones in the biodegradation of priority pollutants: A review. Water Sci. Technol. 2000, 42, 215–222. [Google Scholar] [CrossRef]
- Lehtonen, K.; Hanninen, K.; Ketola, M. Structurally bound lipids in peat humic acids. Org. Geochem. 2001, 32, 33–43. [Google Scholar] [CrossRef]
- van Trump, J.I.; Vega Fransheska, J.R.; Coates, J.D. Natural organic matter as global antennae for primary production. Astrobiology 2013, 3, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Orlov, A.A.; Zherebker, A.; Eletskaya, A.A.; Chernikov, V.S.; Kozlovskaya, L.I.; Zhernov, Y.V.; Kostyukevich, Y.; Palyulin, V.A.; Nikolaev, E.N.; Osolodkin, D.I.; et al. Examination of molecular space and feasible structures of bioactive components of humic substances by FTICR MS data mining in ChEMBL database. Sci. Rep. 2019, 9, 12066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley and Sons, Inc.: New York, NY, USA, 1994; 521p. [Google Scholar]
- De Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mat. Sci. Engineer. C 2016, 62, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Jooné, G.K.; van Rensburg, C.E. An in vitro investigation of the anti-inflammatory properties of potassium humate. Inflammation 2004, 28, 169–174. [Google Scholar] [CrossRef]
- van Rensburg, C.E. The anti-inflammatory properties of humic substances: A mini review. Phytother. Res. 2015, 29, 791–795. [Google Scholar] [CrossRef] [Green Version]
- Winkler, J.; Ghosh, S. Therapeutic potential of fulvic acid in chronic inflammatory diseases and diabetes. J. Diabetes Res. 2018, 2018, 5391014. [Google Scholar] [CrossRef] [Green Version]
- Schols, D.; Wutzler, P.; Klöcking, R.; Helbig, B.; De Clercq, E. Selective inhibitory action of polyhydroxycarboxylates derived from phenolic compounds against human immunodeficiency virus replication. J. Acquir. Immune Defic. Syndr. 1991, 7, 677–685. [Google Scholar]
- Lu, F.J.; Tseng, S.N.; Li, M.L.; Shih, S.R. In vitro anti-influenza virus activity of synthetic humate analogues derived from protocatechuic acid. Arch. Virol. 2002, 147, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Zhernov, Y.V.; Konstantinov, A.I.; Zherebker, A.; Nikolaev, E.; Orlov, A.; Savinykh, M.I.; Kornilaeva, G.V.; Karamov, E.V.; Perminova, I.V. Antiviral activity of natural humic substances and shilajit materials against HIV-1: Relation to structure. Environ. Res. 2021, 193, 110312. [Google Scholar] [CrossRef]
- Jurcsik, I. Possibilities of applying humic acids in medicine (wound healing and cancer therapy). In Humic Substances in the Global Environment; Senesi, N., Milano, T.M., Eds.; Elsevier: Amsterdam, The Netherlands; London, UK; New York, NY, USA; Tokyo, Japan, 1994; pp. 1331–1336. [Google Scholar]
- Ji, Y.; Zhang, A.; Chen, X.; Che, X.; Zhou, K.; Wang, Z. Sodium humate accelerates cutaneous wound healing by activating TGF-β/Smads signaling pathway in rats. Acta Pharm. Sin. B 2016, 6, 132–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litvin, V.A.; Minaev, B.F. Spectroscopy study of silver nanoparticles fabrication using synthetic humic substances and their antimicrobial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 108, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Krempaská, K.; Vaško, L.; Vašková, J. Humic acids as therapeutic compounds in lead intoxication. Curr. Clin. Pharmacol. 2016, 11, 159–167. [Google Scholar] [CrossRef]
- Ozkan, A.; Sen, H.M.; Sehitoglu, I.; Alacam, H.; Guven, M.; Aras, A.B.; Akman, T.; Silan, C.; Cosar, M.; Karaman, H.I.O. Neuroprotective effect of humic acid on focal cerebral ischemia injury: An experimental study in rats. Inflammation 2015, 38, 32–39. [Google Scholar] [CrossRef]
- Zykova, M.V.; Schepetkin, I.A.; Belousov, M.V.; Krivoshchekov, S.V.; Logvinova, L.A.; Bratishko, K.A.; Yusubov, M.S.; Romanenko, S.V.; Quinn, M.T. Physicochemical characterization and antioxidant activity of humic acids isolated from peat of various origins. Molecules 2018, 23, 753. [Google Scholar] [CrossRef] [Green Version]
- Dell’Anno, M.; Hejna, M.; Sotira, S.; Caprarulo, V.; Reggi, S.; Pilu, R.; Miragoli, F.; Callegari, M.L.; Panseri, S.; Rossi, L. Evaluation of leonardite as a feed additive on lipid metabolism and growth of weaned piglets. Anim. Feed Sci. Technol. 2020, 266, 114519. [Google Scholar] [CrossRef]
- Piotrowska, D.; Dlugosz, A.; Witkiewicz, K.; Pak, J. The research on the antioxidant properties of Tolpa peat preparation and its fractions. Acta Pol. Pharm. 2000, 57, 127–129. [Google Scholar]
- Vetvicka, V.; Vashishta, A.; Fuentes, M.; Baigorri, R.; Jose, M.G.-M.; Yvin, J.-C. The relative abundance of oxygen alkyl-related groups in aliphatic domains is involved in the main pharmacological-pleiotropic effects of humic acids. J. Med. Food 2013, 16, 625–632. [Google Scholar] [CrossRef] [Green Version]
- Maslinski, C.; Fogel, W.A.; Andjewski, W. The influence of Tolpa peat preparation on rat liver regeneration. Acta Pol. Pharm. 1995, 50, 413–416. [Google Scholar]
- Belousov, M.V.; Akhmedzhanov, R.R.; Zykova, M.V.; Gur’ev, A.M.; Yusubov, M.S. Hepatoprotective properties of native humic acids isolated from lowland peat of Tomsk. Pharm. Chem. J. 2014, 48, 249–252. [Google Scholar] [CrossRef]
- Akbas, A.; Silan, C.; Gulpinar, M.T.; Sancak, E.B.; Ozkanli, S.S.; Cakir, D.U. Renoprotective effect of humic acid on renal ischemia-reperfusion injury: An experimental study in rats. Inflammation 2015, 38, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U. The response of Erythematous rosacea to ondasentron. Br. J. Dermatol. 1999, 140, 561–562. [Google Scholar] [CrossRef] [PubMed]
- Lasukova, T.V.; Zykova, M.V.; Belousov, M.V.; Logvinova, L.A.; Dygai, A.M. The role of NO synthase in the cardioprotective effect of substances of humic origin on the model of ischemia and reperfusion of isolated rat heart. Bull. Exp. Biol. Med. 2019, 166, 598–601. [Google Scholar] [CrossRef]
- Fang, Y.Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Kim, E.-A.; Lee, S.-H.; Ko, C.; Cha, S.-H.; Kang, M.-C.; Kang, S.-M.; Ko, S.-C.; Lee, W.-W.; Ko, J.-Y.; Lee, J.-H.; et al. Protective effect of fucoidan against AAPH-induced oxidative stress in zebrafish model. Carbohydr. Polym. 2014, 102, 185–191. [Google Scholar] [CrossRef]
- Whayne, F.T.; Saha, P.S.; Mukherjee, D. Antioxidants in the practice of medicine; what should the clinician know? Cardiovasc. Hematol. Disord. Drug Targets 2016, 16, 13–20. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T.; Biondi, D.M.; Amico, V. Antioxidant activity of extracts of the marine algal genus Cystoseira in a micellar model system. J. Appl. Phycol. 2001, 13, 403–407. [Google Scholar] [CrossRef]
- Foti, M.C.; Amorati, R. Non-phenolic radical-trapping antioxidants. J. Pharm. Pharmacol. 2009, 61, 1435–1448. [Google Scholar] [CrossRef]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef]
- Prior, R.L. Oxygen radical absorbance capacity (ORAC): New horizons in relating dietary antioxidants/bioactives and health benefits. J. Funct. Foods 2015, 18, 797–810. [Google Scholar] [CrossRef]
- Aeschbacher, M.; Graf, C.; Schwarzenbach, R.P.; Sander, M. Antioxidant properties of humic substances. Environ. Sci. Technol. 2012, 46, 4916–4925. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Yamada, K.; Maekawa, T.; Takeda, Y.; Yamaguchi, H. Antioxidant mechanism studies on ferulic acid: Identification of oxidative coupling products from methyl ferulate and linoleate. J. Agric. Food Chem. 2006, 54, 6069–6074. [Google Scholar] [CrossRef] [PubMed]
- Asikin, Y.; Takahashi, M.; Mishima, T.; Mizu, M.; Takara, K.; Wada, K. Antioxidant activity of sugarcane molasses against 2,2’-azobis(2-amidinopropane) dihydrochloride-induced peroxyl radicals. Food Chem. 2013, 141, 466–472. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid. Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Ding, F.; Xiao, L.; Shi, R.; Wang, H.; Han, W.; Huang, Z. Food-derived antioxidant polysaccharides and their pharmacological potential in neurodegenerative diseases. Nutrients 2017, 9, 778. [Google Scholar] [CrossRef] [Green Version]
- Kulikova, N.A.; Perminova, I.V. Interactions between humic substances and microorganisms and their implications for nature-like bioremediation technologies. Molecules 2021, 26, 2706. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Somogyi, A.; Rosta, K.; Pusztai, P.; Tulassay, Z.; Nagy, G. Antioxidant measurements. Physiol. Meas. 2007, 28, R41. [Google Scholar] [CrossRef]
- Frankel, E.N.; Finley, J.W. How to standardize the multiplicity of methods to evaluate natural antioxidants. J. Agric. Food Chem. 2008, 56, 4901–4908. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4620. [Google Scholar] [CrossRef] [PubMed]
- Dorta, E.; Fuentes-Lemus, E.; Aspée, A.; Atala, E.; Speisky, H.; Bridi, R.; Lissi, E.; López-Alarcón, C. The ORAC (oxygen radical absorbance capacity) index does not reflect the capacity of antioxidants to trap peroxyl radicals. RSC Adv. 2015, 5, 39899–39902. [Google Scholar] [CrossRef]
- Amić, A.; Lučić, B.; Stepanić, V.; Marković, Z.; Marković, S.; Dimitrić Marković, J.M.; Amić, D. Free radical scavenging potency of quercetin catecholic colonic metabolites: Thermodynamics of 2H+/2e− processes. Food Chem. 2017, 218, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Di Meo, F.; Lemaur, V.; Cornil, J.; Lazzaroni, R.; Duroux, J.-L.; Olivier, Y.; Trouillas, P. Free radical scavenging by natural polyphenols: Atom versus electron transfer. J. Phys. Chem. A 2013, 117, 2082–2092. [Google Scholar] [CrossRef]
- Hu, S.; Yin, J.; Nie, S.; Wang, J.; Phillips, G.O.; Xie, M.; Cui, S.W. In vitro evaluation of the antioxidant activities of carbohydrates. Bioact. Carbohydr. Diet. Fibre 2016, 7, 19–27. [Google Scholar] [CrossRef]
- Klein, O.I.; Kulikova, N.A.; Filimonov, I.S.; Koroleva, O.V.; Konstantinov, A.I. Long-term kinetics study and quantitative characterization of the antioxidant capacities of humic and humic-like substances. J. Soils Sediments 2016, 18, 1355–1364. [Google Scholar] [CrossRef]
- Vašková, J.; Veliká, B.; Pilátová, M.; Kron, I.; Vaško, L. Effects of humic acids in vitro. In Vitro Cell. Dev. Biol. Anim. 2011, 47, 376–382. [Google Scholar] [CrossRef]
- Tarasova, A.S.; Stom, D.I.; Kudryasheva, N.S. Antioxidant activity of humic substances via bioluminescent monitoring in vitro. Environ. Monit. Assess. 2015, 187, 89. [Google Scholar] [CrossRef]
- Karadirek, Ş.; Kanmaz, N.; Balta, Z.; Demirçivi, P.; Üzer, A.; Hızal, J.; Apak, R. Determination of total antioxidant capacity of humic acids using CUPRAC, Folin–Ciocalteu, noble metal nanoparticle- and solid–liquid extraction-based methods. Talanta 2016, 153, 120–129. [Google Scholar] [CrossRef]
- Avvakumova, N.P.; Gerchikov, A.Y.; Khairullina, V.R.; Zhdanova, A.V. Antioxidant properties of humic substances isolated from peloids. Pharm. Chem. J. 2011, 45, 192. [Google Scholar] [CrossRef]
- Khil’ko, S.L.; Efimova, I.V.; Smirnova, O.V. Antioxidant properties of humic acids from brown coal. Solid Fuel Chem. 2011, 45, 367–371. [Google Scholar] [CrossRef]
- Kholodov, V.A.; Konstantinov, A.I.; Kudryavtsev, A.V.; Perminova, I.V. Structure of humic acids in zonal soils from 13C NMR data. Euras. Soil Sci. 2011, 44, 976–983. [Google Scholar] [CrossRef]
- Lowe, L.E. Studies on the nature of sulfur in peat humic acids from Frazer River delta, British Columbia. Sci. Total Environ. 1992, 113, 133–145. [Google Scholar] [CrossRef]
- Koroleva, O.V.; Kulikova, N.A.; Alekseeva, T.N.; Stepanova, E.V.; Davidchik, V.N.; Belyaeva, E.Y.; Tsvetkova, E.A. A comparative characterization of fungal melanin and the humin-like substances synthesized by Cerrena maxima 0275. Appl. Biochem. Microbiol. 2007, 43, 61–67. [Google Scholar] [CrossRef]
- Hertkorn, N.; Claus, H.; Schmitt-Kopplin, P.; Perdue, E.M.; Filip, Z. Utilization and transformation of aquatic humic substances by autochthonous microorganisms. Environ. Sci. Technol. 2002, 36, 4334–4345. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J. Colorimetry of total phenolics with phospohmolibdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Rice, J.A.; MacCarthy, P. Statistical evaluation of the elemental composition of humic substances. Org. Geochem. 1991, 17, 635–648. [Google Scholar] [CrossRef]
- Barahona, T.; Chandía, N.P.; Encinas, M.V.; Matsuhiro, B.; Zúñiga, E.A. Antioxidant capacity of sulfated polysaccharides from seaweeds. A kinetic approach. Food Hydrocoll. 2011, 25, 529–535. [Google Scholar] [CrossRef]
- Petera, B.; Delattre, C.; Pierre, G.; Wadouachi, A.; Elboutachfaiti, R.; Engel, E.; Poughon, L.; Michaud, P.; Fenoradosoa, T.A. Characterization of arabinogalactan-rich mucilage from Cereus triangularis cladodes. Carbohydr. Polym. 2015, 127, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.F.R.; Heleno, S.A.; Reis, F.S.; Stojkovic, D.; Queiroz, M.J.R.P.; Vasconcelos, M.H.; Sokovic, M. Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochemistry 2015, 114, 38–55. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Xue, X.; Zhang, Z. Structural, physicochemical, antioxidant and antitumor property of an acidic polysaccharide from Polygonum multiflorum. Int. J. Biol. Macromol. 2017, 96, 494–500. [Google Scholar] [CrossRef]
- Mooradian, A.D.; Haas, M.J.; Onstead-Haas, L.; Tani, Y.; Iida, T.; Tokuda, M. Naturally occurring rare sugars are free radical scavengers and can ameliorate endoplasmic reticulum stress. Int. J. Vitam. Nutr. Res. 2020, 90, 210–220. [Google Scholar] [CrossRef] [PubMed]
- García, A.C.; de Souza, L.G.A.; Pereira, M.G.; Castro, R.N.; García-Mina, J.M.; Zonta, E.; Lisboa, F.G.J.; Berbara, R.L.L. Structure-property-function relationship in humic substances to explain the biological activity in plants. Sci. Rep. 2016, 6, 20798. [Google Scholar] [CrossRef] [Green Version]
- Ussiri, D.A.; Johnson, C.E. Characterization of organic matter in a northern hardwood forest soil by 13C NMR spectroscopy and chemical methods. Geoderma 2003, 111, 123–149. [Google Scholar] [CrossRef]
- Fong, S.S.; Mohamed, M. Chemical characterization of humic substances occurring in the peats of Sarawak, Malaysia. Org. Geochem. 2007, 38, 967–976. [Google Scholar] [CrossRef]
- Wilson, M.A.; Barron, P.F.; Gillam, A.H. The structure of freshwater humic substances as revealed by 13C-NMR spectroscopy. Geochim. Cosmochim. Acta 1981, 45, 1743–1750. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, N.; Li, J.; Wang, Y.; Liu, Y.; Cao, M.; Yan, Q. Characterization of humic acids from original coal and its oxidization production. Sci. Rep. 2021, 11, 15381. [Google Scholar] [CrossRef]
- Kurková, M.; Klika, Z.; Kliková, C.; Havel, J. Humic acids from oxidized coals. Chemosphere 2004, 54, 1237–1245. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Gijsman, P. Polymer Stabilization. In Handbook of Environmental Degradation of Materials, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherland, 2012; pp. 673–714. [Google Scholar] [CrossRef]
- Rimmer, D.L.; Smith, A.M. Antioxidants in soil organic matter and in associated plant materials. Eur. J. Soil Sci. 2009, 60, 170–175. [Google Scholar] [CrossRef]
- Rimmer, D.L.; Abbott, G.D. Phenolic compounds in NaOH extracts of UK soils and their contribution to antioxidant capacity. Eur. J. Soil Sci. 2011, 62, 285–294. [Google Scholar] [CrossRef]
- Rodríguez-Bonilla, P.; Gandía-Herrero, F.; Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. Comparative study of the antioxidant capacity of four stilbenes using ORAC, ABTS+, and FRAP techniques. Food Anal. Methods 2017, 10, 2994–3000. [Google Scholar] [CrossRef]
- Korotkova, E.I.; Karbainov, Y.A.; Avramchik, O.A. Investigation of antioxidant and catalytic properties of some biologically active substances by voltammetry. Anal. Bioanal. Chem. 2003, 375, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Hegab, R.H.; Fawy, H.A.; Habib, A.A. Evaluates effect of amino acids, humic acid and antioxidants as foliar application on the biochemical content and productivity of wheat under North Sinai soils conditions. Am. J. Agric. For. 2020, 8, 167–174. [Google Scholar] [CrossRef]
- Bratishko, K.A.; Zykova, M.V.; Ivanov, V.V.; Perminova, I.V.; Belousov, M.V. Peat humic acids-prospective biologically active substances with antioxidant activity for the development of protective agents. Khimiya Rastitel’nogo Syr’ya 2021, 1, 287–298. [Google Scholar] [CrossRef]
- Sakr, M.T.; Sarkassy, N.M.; Fuller, M.P. Exogenously applied antioxidants and biostimulants counteract the adverse effect of biotic stress in wheat plant. Agric. Res. Technol. Open Access J. 2017, 12, 555853. [Google Scholar] [CrossRef]
- Drobnik, J.; Stebel, A. Central European ethnomedical and officinal uses of peat, with special emphasis on the Tołpa peat preparation (TPP): An historical review. J. Ethnopharmac. 2019, 112248. [Google Scholar] [CrossRef] [PubMed]
- Jouraiphy, A.; Amir, S.; Winterton, P.; El Gharous, M.; Revel, J.-C.; Hafidi, M. Structural study of the fulvic fraction during composting of activated sludge-plant matter: Elemental analysis, FTIR and 13C NMR. Bioresour. Technol. 2008, 99, 1066–1072. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y. Protection of wheat bran feruloyl oligosaccharides against free radical-induced oxidative damage in normal human erythrocytes. Food Chem. Toxicol. 2009, 47, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Cordova-Gomez, M.; Galano, A.; Alvarez-Idaboy, J.R. Piceatannol, a better peroxyl radical scavenger than resveratrol. RSC Adv. 2013, 3, 20209. [Google Scholar] [CrossRef]
- Efimova, I.V.; Khil’ko, S.L.; Smirnova, O.V. Antioxidant activity of humic acids in radical-chain oxidation processes. Russ. J. Appl. Chem. 2020, 85, 1351–1354. [Google Scholar] [CrossRef]
- Catalá, T.S.; Rossel, P.E.; Álvarez-Gómez, F.; Tebben, J.; Figueroa, F.L.; Dittmar, T. Antioxidant activity and phenolic content of marine dissolved organic matter and their relation to molecular composition. Front. Mar. Sci. 2020, 7, 603447. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J. Agric. Food Chem. 2010, 58, 6706–6714. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, B.-T.; Xu, X.-R.; Qin, X.-S.; Gan, R.-Y.; Li, H.-B. Antioxidant capacities and total phenolic contents of 56 wild fruits from South China. Molecules 2010, 15, 8602–8617. [Google Scholar] [CrossRef] [Green Version]
- Aspée, A.; Aliaga, C.; Maretti, L.; Zúñiga-Núñez, D.; Godoy, J.; Pino, E.; Cárdenas-Jirón, G.; Lopez-Alarcon, C.; Scaiano, J.C.; Alarcon, E.I. Reaction kinetics of phenolic antioxidants toward photoinduced pyranine free radicals in biological models. J. Phys. Chem. B 2017, 121, 6331–6340. [Google Scholar] [CrossRef]
- Jha, N.S.; Mishra, S.; Jha, S.K.; Surolia, A. Antioxidant activity and electrochemical elucidation of the enigmatic redox behavior of curcumin and its structurally modified analogues. Electrochim. Acta 2015, 151, 574–583. [Google Scholar] [CrossRef]
- Laguerre, M.; Lecomte, J.; Villeneuvene, P. The physico-chemical basis of phenolic antioxidant activity. Lipid Technol. 2014, 26, 59–62. [Google Scholar] [CrossRef]
- Acid Strength and pKa. Available online: https://chem.libretexts.org/@go/page/225794 (accessed on 19 August 2021).
- Mayans, B.; Pérez-Esteban, J.; Escolástico, C.; Eymar, E.; Masaguer, A. Evaluation of commercial humic substances and other organic amendments for the immobilization of copper through 13C CPMAS NMR, FT-IR, and DSC analyses. Agronomy 2019, 9, 762. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 5666. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Marin, E.; Martínez, A. Carbohydrates and their free radical scavenging capability: A theoretical study. J. Phys. Chem. B 2012, 116, 9668–9675. [Google Scholar] [CrossRef] [PubMed]
- Tsiapali, E.; Whaley, S.; Kalbfleisch, J.; Ensley, H.E.; Browder, I.W.; Williams, D.L. Glucans exhibit weak antioxidant activity, but stimulate macrophage free radical activity. Free Radic. Biol. Med. 2001, 30, 393–402. [Google Scholar] [CrossRef]
- Shyama Prasad Rao, R.; Muralikrishna, G. Water soluble feruloyl arabinoxylans from rice and ragi: Changes upon malting and their consequence on antioxidant activity. Phytochemistry 2006, 67, 91–99. [Google Scholar] [CrossRef]
- Source Materials for IHSS Samples. Available online: https://humic-substances.org/source-materials-for-ihss-samples/ (accessed on 19 August 2021).
| HS Index | HA Type | Source |
|---|---|---|
| Soil | ||
| SHA-PG96 | HA | Albic Retisol |
| SHA-PW96 | HA | Albic Retisol |
| SHA-PG94 | HA | Albic Retisol |
| SHA-CM94 | HA | Gleyic Chernozem |
| SHA-PW94 | HA | Albic Retisol |
| SHA-CTV94 | HA | Endocalcic Chernozem |
| SFA-PP96 | FA | Albic Retisol |
| SFA-PW96 | FA | Albic Retisol |
| SFA-PG96 | FA | Albic Retisol |
| Peat | ||
| PHA-T398 | HA | Lowland peat, sedge type |
| PHA-T798 | HA | Lowland peat, woody type |
| PHA-T698 | HA | Highland peat, sedge type |
| PFA-T398 | FA | Lowland peat, sedge type |
| PFA-T698 | FA | Highland peat, sedge type |
| PFA-T798 | FA | Lowland peat, woody type |
| PHF-T398 | HA+FA | Lowland peat, sedge type |
| PHF-T698 | HA+FA | Highland peat, sedge type |
| PHF-T798 | HA+FA | Lowland peat, woody type |
| Natural water | ||
| SRHA | HA | Suwannee River (IHSS standard) |
| SRFA | FA | Suwannee River (IHSS standard) |
| SRDOM | DOM | Suwannee River (IHSS standard) |
| Coal | ||
| СHA-AGK | HA | Biotechnology Ltd. (RF) |
| CHA-ALD | HA | Aldrich (Germany) |
| Humic-like substances | ||
| HLS-45 | HA | Oat straw solubilized by Trametes maxima 0275 |
| HLS-70 | HA | Oat straw solubilized by Trametes maxima 0275 |
| HS Index | Atomic Ratio 1 | Content of Carbon in Structural Fragments Determined by 13CNMR Spectroscopy as Integral Intensity (%) 2 | TP 3, μmol TE mg−1 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O/C | H/C | C/N | CC=O | CCOO | СArO | СAr | COCO | CCHO | CCH2O | CCH3O | CCHn | ∑СAr | ∑CСarb | ∑CAlk | ∑CAlk-O | ∑CAr/∑CAlk | ||
| Soil | ||||||||||||||||||
| SHA-PG96 | 0.48 | 0.93 | 15.9 | 2.0 | 17.0 | 11.0 | 31.0 | 2.0 | 10.0 | 2.0 | 4.0 | 22.0 | 42.0 | 14.0 | 40.0 | 18.0 | 1.05 | 1.378 ± 0.001 |
| SHA-PW96 | 0.46 | 1.10 | 14.0 | 4.0 | 19.0 | 9.0 | 25.0 | 2.0 | 8.0 | 2.0 | 4.0 | 26.0 | 34.0 | 12.0 | 42.0 | 16.0 | 0.81 | 1.230 ± 0.005 |
| SHA-PG94 | 0.45 | 1.00 | 13.6 | 1.1 | 15.2 | 12.8 | 32.8 | 3.0 | 10.0 | 2.1 | 5.8 | 17.5 | 45.6 | 15.1 | 38.4 | 20.9 | 1.19 | 1.392 ± 0.001 |
| SHA-CM94 | 0.34 | 0.66 | 13.8 | 1.5 | 14.3 | 8.7 | 48.1 | 2.0 | 6.0 | 0.8 | 4.6 | 14.2 | 56.8 | 8.8 | 27.6 | 13.4 | 2.06 | 1.760 ± 0.001 |
| SHA-PW94 | 0.46 | 0.92 | 11.5 | 1.0 | 17.0 | 13.0 | 33.0 | 2.0 | 8.0 | 2.5 | 5.5 | 17.0 | 46.0 | 12.5 | 35.0 | 18.0 | 1.31 | 1.753 ± 0.001 |
| SHA-CTV94 | 0.42 | 0.62 | 15.9 | 3.0 | 15.0 | 11.0 | 43.0 | 3.0 | 7.0 | 2.0 | 3.0 | 13.0 | 54.0 | 12.0 | 28.0 | 15.0 | 1.93 | 0.510 ± 0.001 |
| SFA-PP96 | 0.54 | 0.91 | 11.3 | 2.0 | 23.0 | 7.0 | 19.0 | 3.0 | 15.0 | 3.0 | 4.0 | 22.0 | 26.0 | 21.0 | 47.0 | 25.0 | 0.55 | 1.167 ± 0.001 |
| SFA-PW96 | 0.58 | 0.94 | 17.2 | 4.0 | 19.5 | 9.8 | 23.5 | 2.0 | 10.0 | 2.5 | 3.5 | 25.1 | 33.3 | 14.5 | 43.1 | 18.0 | 0.77 | 1.523 ± 0.001 |
| SFA-PG96 | 0.61 | 0.88 | 19.4 | 3.1 | 18.0 | 13.1 | 28.2 | 2.0 | 11.0 | 2.4 | 4.2 | 17.6 | 41.3 | 15.4 | 37.2 | 19.6 | 1.11 | 1.176 ± 0.001 |
| Peat | ||||||||||||||||||
| PHA-T398 | 0.44 | 0.87 | 25.9 | 8.7 | 15.5 | 7.4 | 32.4 | 7.4 | 4.4 | 2.0 | 4.1 | 18.2 | 39.7 | 13.8 | 36.1 | 17.9 | 1.10 | 2.743 ± 0.001 |
| PHA-T798 | 0.49 | 0.87 | 46.2 | 4.1 | 11.0 | 9.4 | 31.7 | 3.7 | 14.2 | 2.7 | 3.4 | 19.8 | 41.1 | 20.6 | 43.8 | 24.0 | 0.94 | 2.986 ± 0.001 |
| PHA-T698 | 0.55 | 0.91 | 37.9 | 1.7 | 8.6 | 7.2 | 35.4 | 2.6 | 12.3 | 2.7 | 4.0 | 25.7 | 42.5 | 17.6 | 47.3 | 21.6 | 0.90 | 3.353 ± 0.001 |
| PFA-T398 | 0.66 | 0.76 | 120 | 3.1 | 17.0 | 9.0 | 28.1 | 4.9 | 11.8 | 2.3 | 3.8 | 20.0 | 37.1 | 19.0 | 42.8 | 22.8 | 0.87 | 1.239 ± 0.001 |
| PFA-T698 | 0.51 | 1.03 | 101 | 1.6 | 10.9 | 6.9 | 24.4 | 6.9 | 26.1 | 3.1 | 1.8 | 18.3 | 31.3 | 36.1 | 56.2 | 37.9 | 0.56 | 2.368 ± 0.001 |
| PFA-T798 | 0.60 | 1.00 | 74.1 | 2.2 | 11.8 | 10.9 | 24.0 | 9.6 | 24.1 | 3.5 | 2 | 11.9 | 34.9 | 37.2 | 51.1 | 39.2 | 0.68 | 1.602 ± 0.001 |
| PHF-T398 | 0.49 | 1.10 | 35.5 | 3.4 | 10.6 | 9.8 | 32.9 | 6.7 | 11.0 | 3.0 | 5.6 | 16.9 | 42.7 | 20.7 | 43.2 | 26.3 | 0.99 | 2.398 ± 0.001 |
| PHF-T698 | 0.54 | 0.91 | 48.5 | 3.7 | 10.9 | 8.4 | 24.4 | 7.7 | 19.8 | 4.8 | 4.2 | 16.2 | 32.8 | 32.3 | 52.7 | 36.5 | 0.62 | 2.275 ± 0.001 |
| PHF-T798 | 048 | 0.87 | 53.0 | 3.5 | 10.4 | 8.7 | 28.1 | 8.8 | 15.2 | 3.5 | 4.4 | 17.4 | 36.8 | 27.5 | 49.3 | 31.9 | 0.75 | 2.275 ± 0.001 |
| Natural water | ||||||||||||||||||
| SRHA | 0.60 | 0.97 | 52.5 | 8.5 | 18.1 | 10.7 | 26.6 | 6.3 | 8.9 | 1.9 | 3.4 | 15.6 | 37.3 | 17.1 | 36.1 | 20.5 | 1.03 | 2.504 ± 0.001 |
| SRFA | 0.62 | 0.99 | 91.1 | 7.4 | 19.6 | 10.9 | 22.3 | 4.4 | 10.0 | 1.8 | 4.0 | 19.6 | 33.2 | 16.2 | 39.8 | 20.2 | 0.83 | 1.537 ± 0.001 |
| SRDOM | 0.61 | 0.95 | 55.6 | 6.9 | 19.9 | 8.0 | 23.7 | 5.2 | 11.3 | 2.0 | 4.0 | 19.0 | 31.8 | 18.5 | 41.5 | 22.5 | 0.76 | 1.791 ± 0.001 |
| Coal | ||||||||||||||||||
| СHA-AGK | 0.32 | 0.79 | 112 | 0.5 | 16.9 | 10.0 | 47.8 | 0 | 0 | 1.0 | 3.0 | 20.8 | 57.8 | 1.0 | 24.8 | 4.0 | 2.33 | 2.348 ± 0.001 |
| CHA-ALD | 0.31 | 0.81 | 77.0 | 1.0 | 15.0 | 13.0 | 43.0 | 0 | 0 | 1.0 | 6.0 | 21.0 | 56.0 | 1.0 | 28.0 | 7.0 | 2.00 | 1.521 ± 0.001 |
| Humic-like substances | ||||||||||||||||||
| HLS-45 | 0.37 | 1.22 | 16.1 | 3.8 | 10.9 | 11.2 | 25.6 | 6.8 | 13.7 | 4.4 | 8.4 | 15.1 | 36.8 | 24.9 | 48.4 | 33.3 | 0.76 | 1.481 ± 0.003 |
| HLS-70 | 0.55 | 1.03 | 23.7 | 4.0 | 15.1 | 10.9 | 26.8 | 4.8 | 12.7 | 4.2 | 9.0 | 12.5 | 37.6 | 21.7 | 43.2 | 30.7 | 0.87 | 1.381 ± 0.001 |
| HS Index | AOC, μmol TE mg−1 |
|---|---|
| Soil | |
| SHA-PG96 | 0.87 ± 0.08 bc |
| SHA-PW96 | 0.74 ± 0.04 bc |
| SHA-PG94 | 0.75 ± 0.03 bc |
| SHA-CM94 | 0.97 ± 0.05 d |
| SHA-PW94 | 1.04 ± 0.05 de |
| SHA-CTV94 | 0.31 ± 0.02 a |
| SFA-PP96 | 1.08 ± 0.06 def |
| SFA-PW96 | 1.20 ± 0.06 efg |
| SFA-PG96 | 1.39 ± 0.06 hi |
| Peat | |
| PHA-T398 | 1.55 ± 0.03 hij |
| PHA-T798 | 1.63 ± 0.05 jk |
| PHA-T698 | 1.75 ± 0.03 kl |
| PFA-T398 | 1.56 ± 0.08 hij |
| PFA-T698 | 2.56 ± 0.06 m |
| PFA-T798 | 2.25 ± 0.13 m |
| PHF-T398 | 1.38 ± 0.03 gh |
| PHF-T698 | 1.44 ± 0.05 hi |
| PHF-T798 | 1.56 ± 0.08 ij |
| Natural water | |
| SRHA | 2.07 ± 0.09 m |
| SRFA | 1.22 ± 0.04 fg |
| SRDOM | 1.41 ± 0.05 hi |
| Coal | |
| СHA-AGK | 1.84 ± 0.07 l |
| CHA-ALD | 1.07 ± 0.06 def |
| Humic-like substances | |
| HLS-45 | 0.70 ± 0.05 b |
| HLS-70 | 0.91 ± 0.08 cd |
| Compound | AOC, μmol TE mg−1 |
|---|---|
| Ascorbic acid | 2.32 ± 0.21 |
| Vitamin E | 2.95 ± 0.16 |
| Gallic acid | 6.35 ± 0.25 |
| Syringic acid | 7.67 ± 0.39 |
| Hydroxybenzoic acid | 13.11 ± 0.63 |
| Sinapic acid | 15.71 ± 1.01 |
| Vanillic acid | 20.46 ± 1.14 |
| Ferulic acid | 23.64 ± 1.18 |
| Coumaric acid | 30.58 ± 1.32 |
| Parameter | Value | −95% | +95% |
|---|---|---|---|
| Intercept | 0.432 | −0.087 | 0.952 |
| C/N | 0.006 | 0.003 | 0.009 |
| CCHO, % | 0.026 | 0.009 | 0.042 |
| CCH3O, % | −0.077 | −0.143 | −0.010 |
| TP, μmol mg−1 | 0.369 | 0.216 | 0.522 |
| Multiple determination coefficient | 0.91 | ||
| Adjusted multiple determination coefficient | 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, O.I.; Kulikova, N.A.; Konstantinov, A.I.; Zykova, M.V.; Perminova, I.V. A Systematic Study of the Antioxidant Capacity of Humic Substances against Peroxyl Radicals: Relation to Structure. Polymers 2021, 13, 3262. https://doi.org/10.3390/polym13193262
Klein OI, Kulikova NA, Konstantinov AI, Zykova MV, Perminova IV. A Systematic Study of the Antioxidant Capacity of Humic Substances against Peroxyl Radicals: Relation to Structure. Polymers. 2021; 13(19):3262. https://doi.org/10.3390/polym13193262
Chicago/Turabian StyleKlein, Olga I., Natalia A. Kulikova, Andrey I. Konstantinov, Maria V. Zykova, and Irina V. Perminova. 2021. "A Systematic Study of the Antioxidant Capacity of Humic Substances against Peroxyl Radicals: Relation to Structure" Polymers 13, no. 19: 3262. https://doi.org/10.3390/polym13193262
APA StyleKlein, O. I., Kulikova, N. A., Konstantinov, A. I., Zykova, M. V., & Perminova, I. V. (2021). A Systematic Study of the Antioxidant Capacity of Humic Substances against Peroxyl Radicals: Relation to Structure. Polymers, 13(19), 3262. https://doi.org/10.3390/polym13193262







