Relaxation Phenomena in Chitosan-Au Nanoparticle Thin Films
Abstract
1. Introduction
2. Materials and Methods
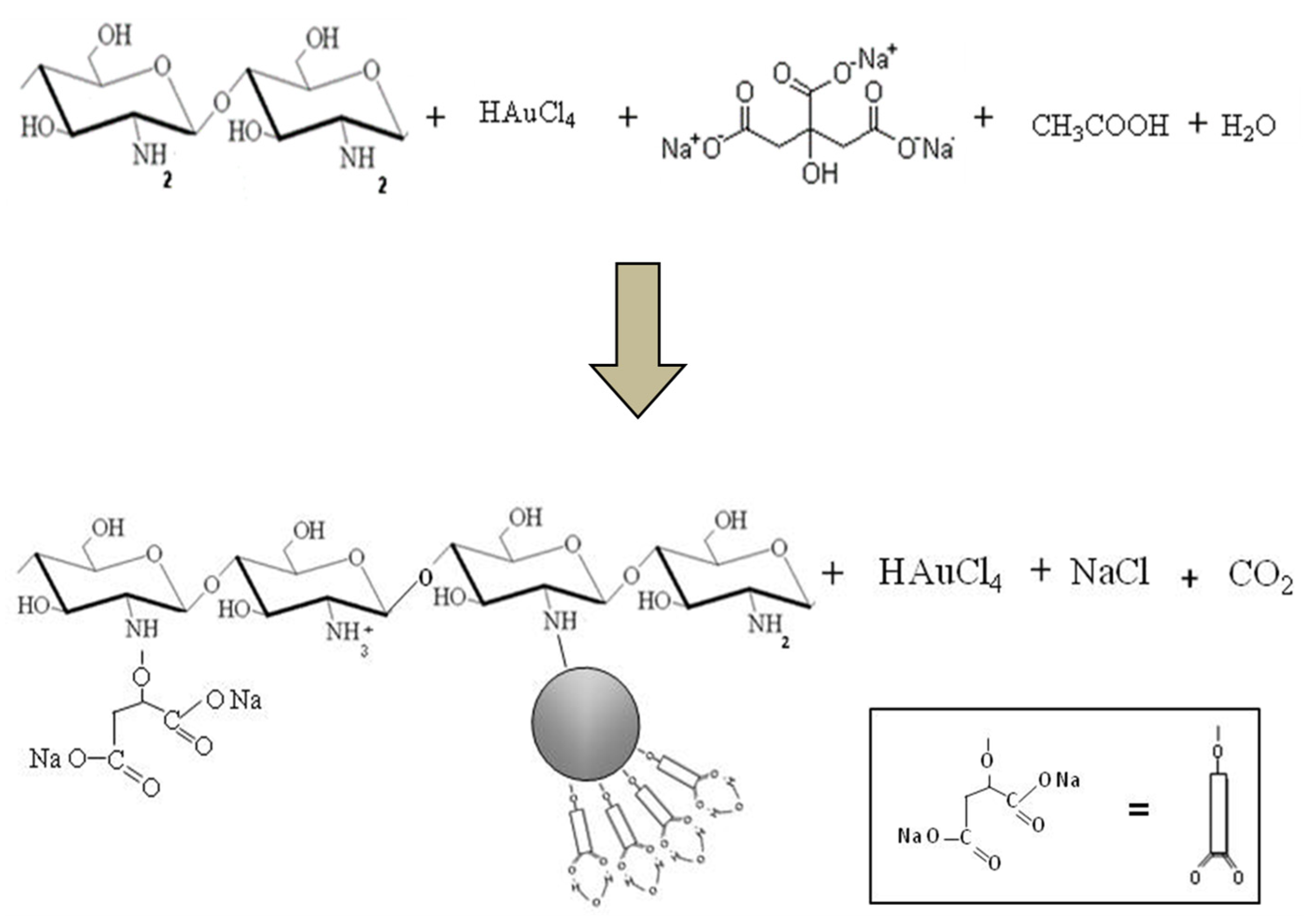
2.1. Synthesis of Nanocomposite
2.2. Characterization Studies
2.2.1. Infrared Measurements and Morphology Analysis
2.2.2. Thermal Measurements
2.2.3. Conductivity Measurements
- A non-linear behavior, well described by the Vogel–Fulcher–Tammann (VFT) relationship:where σ0 is the pre-exponential factor, D is a material constant, and T0 is the so-called Vogel temperature.
- An Arrhenius-type linear dependance:where is the activation energy.
2.2.4. Dielectric Measurements
2.2.5. X-ray Diffraction
3. Results
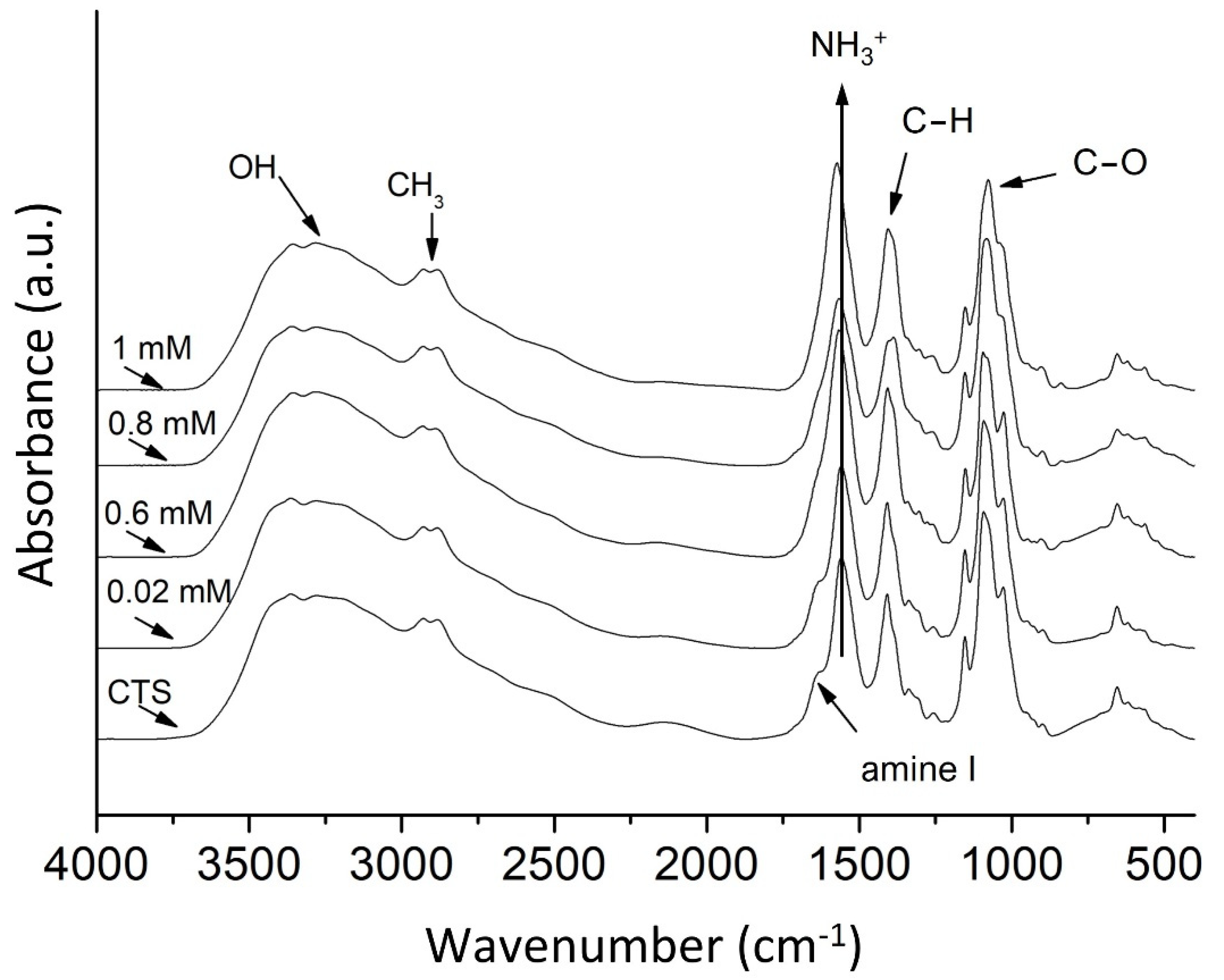
3.1. Infrared Spectroscopy
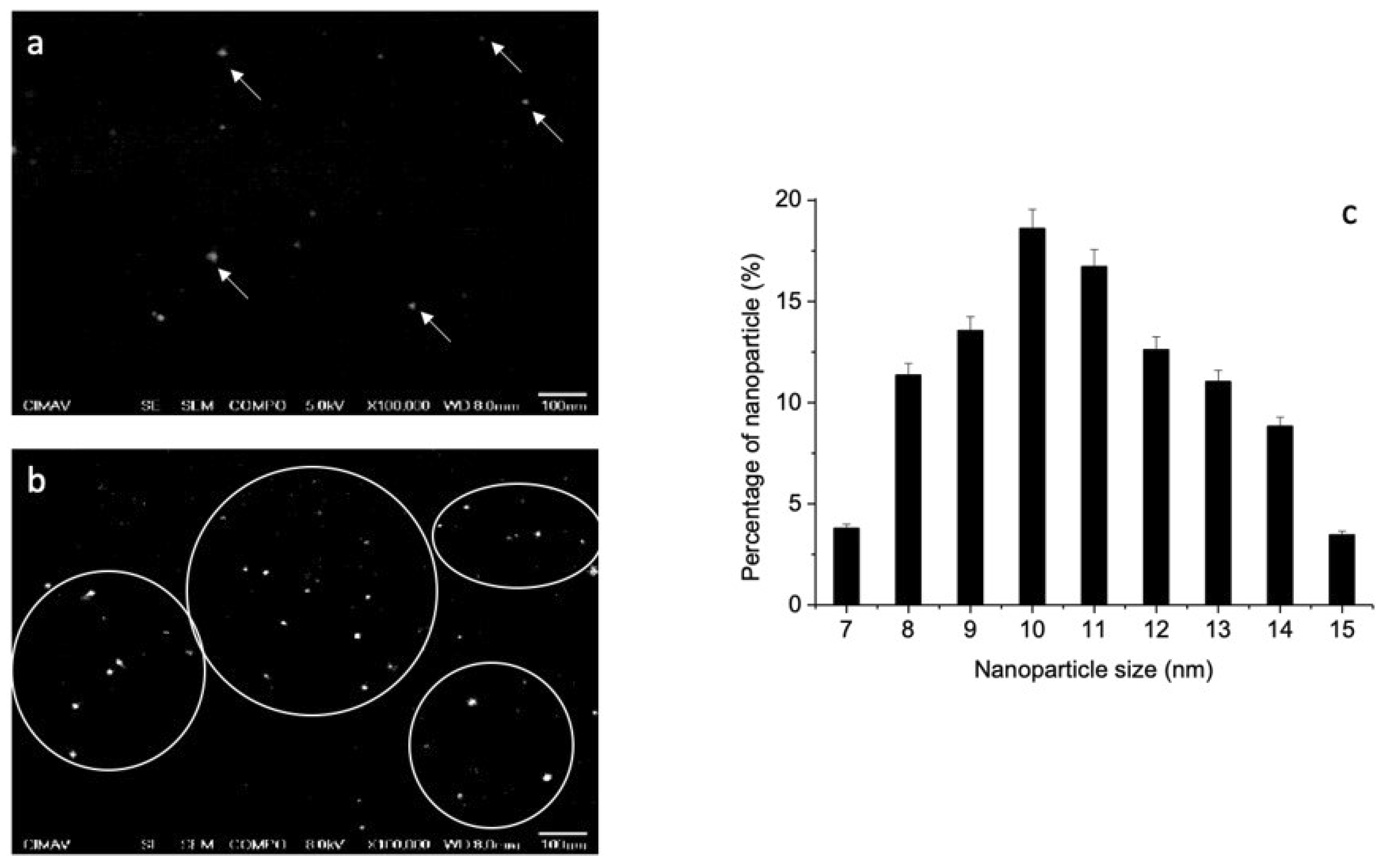
3.2. Scanning Electron Microscope Measurements
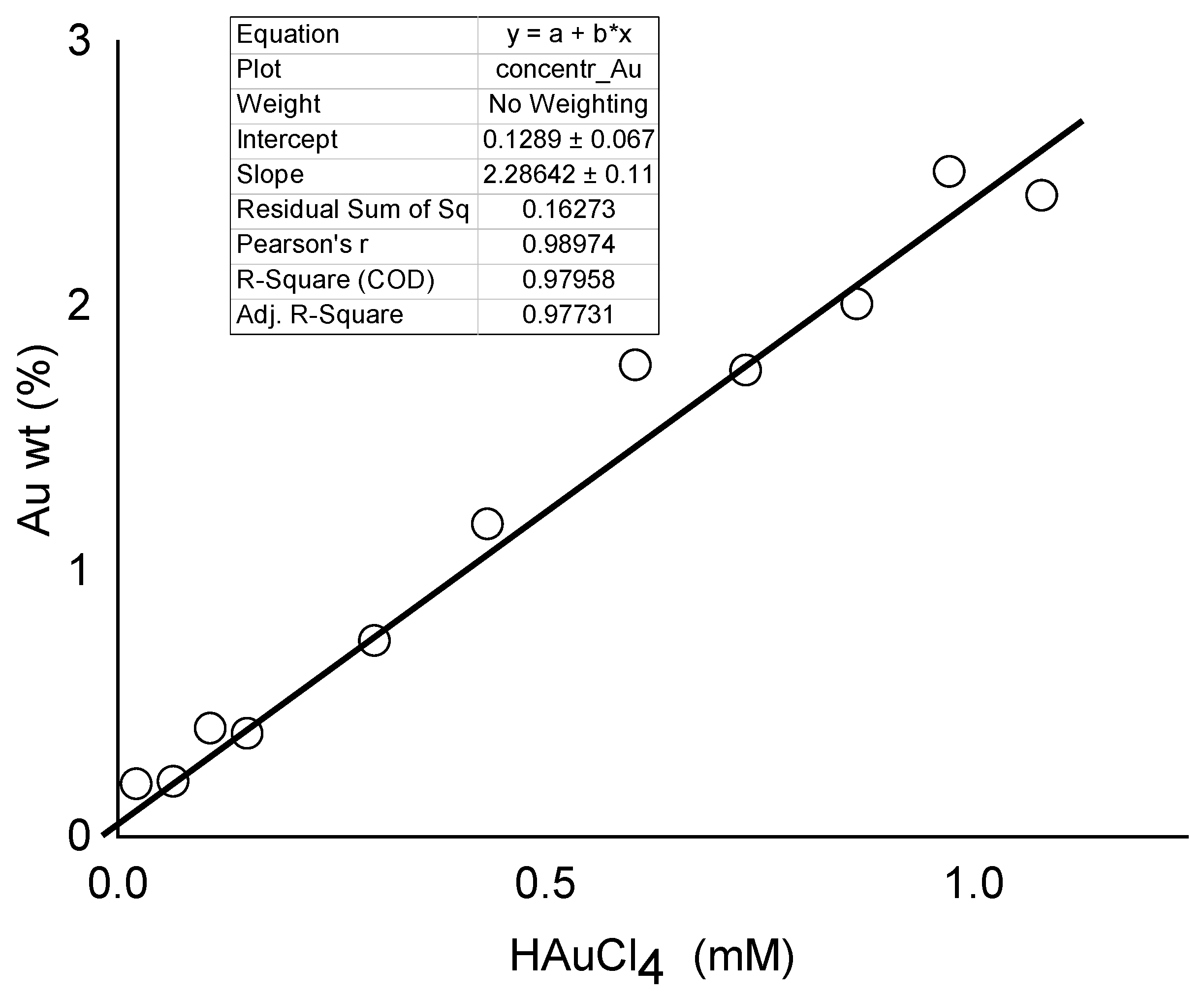
3.3. Thermogravimetric Analysis Measurements
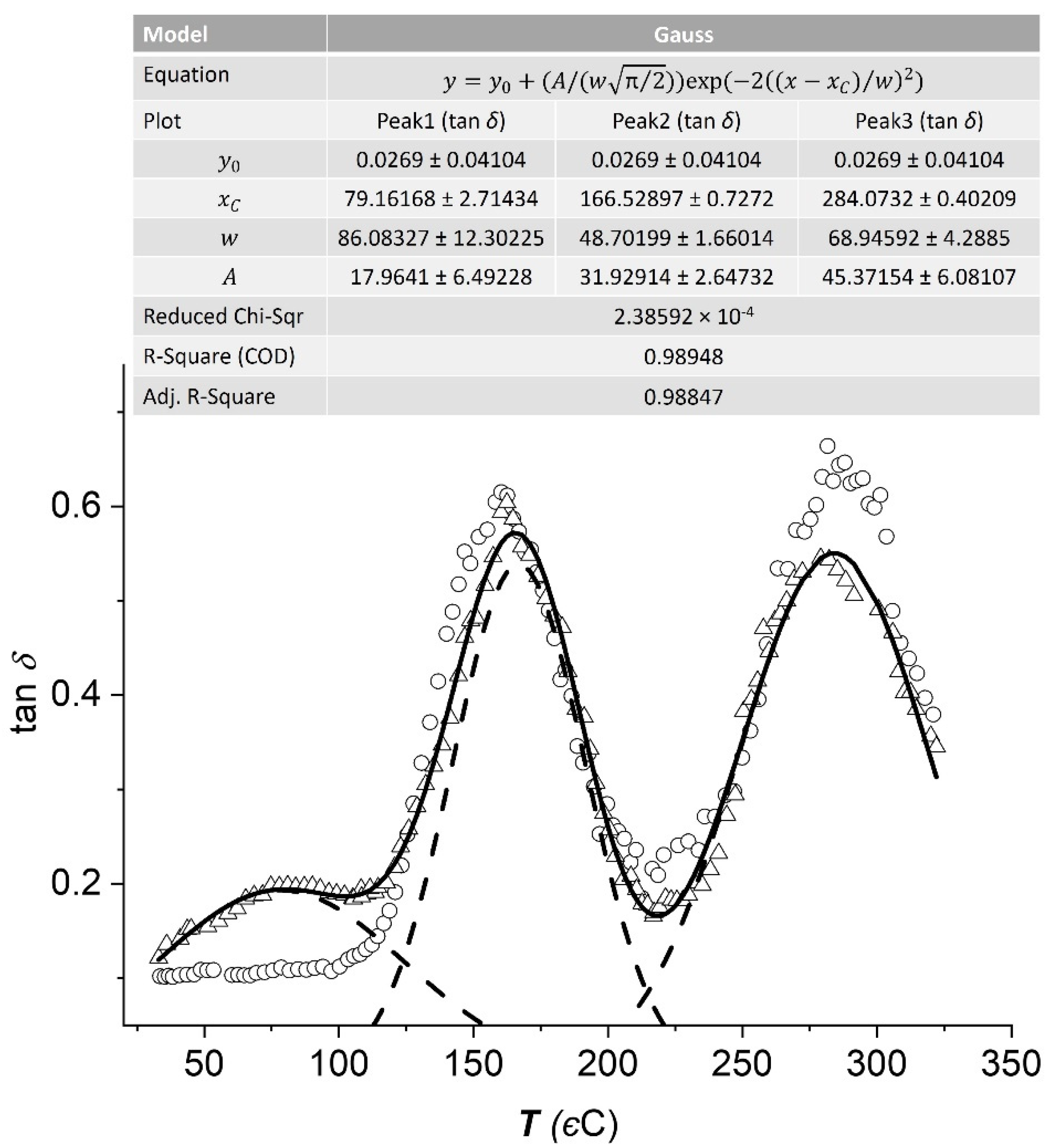
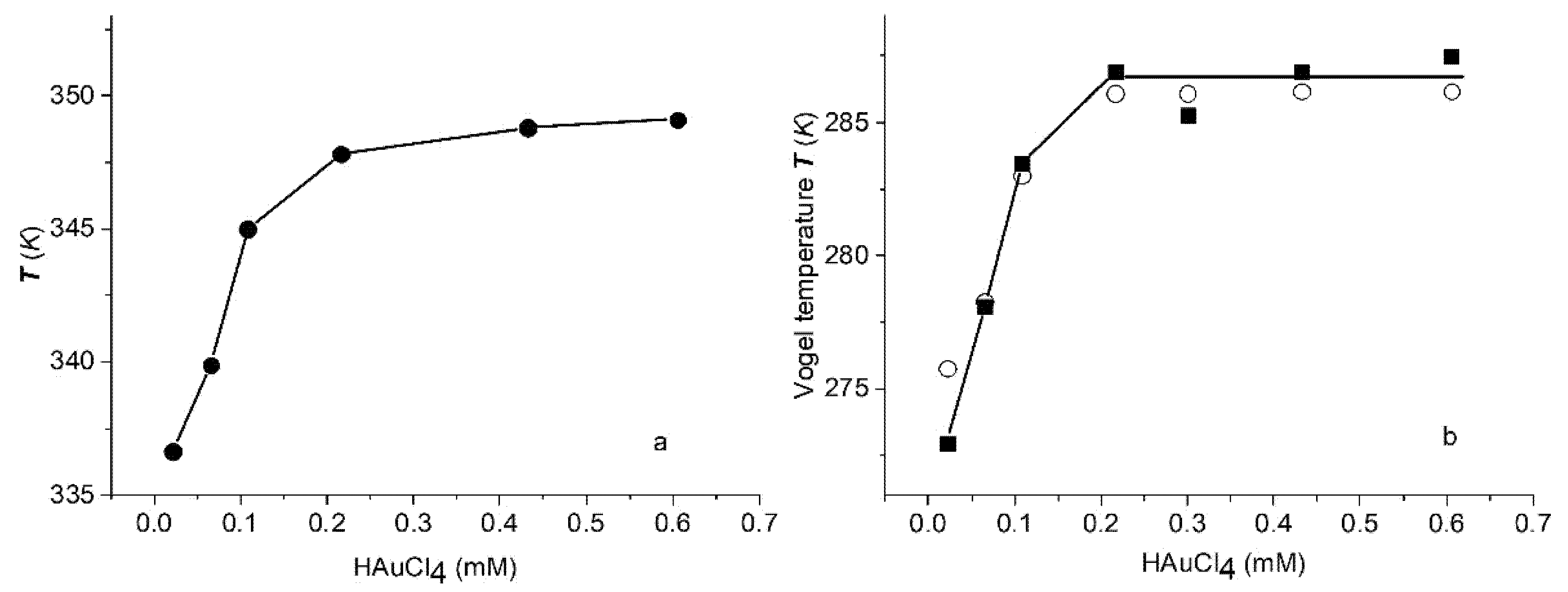
3.4. Dynamic Mechanical Analysis
3.5. X-ray Diffraction
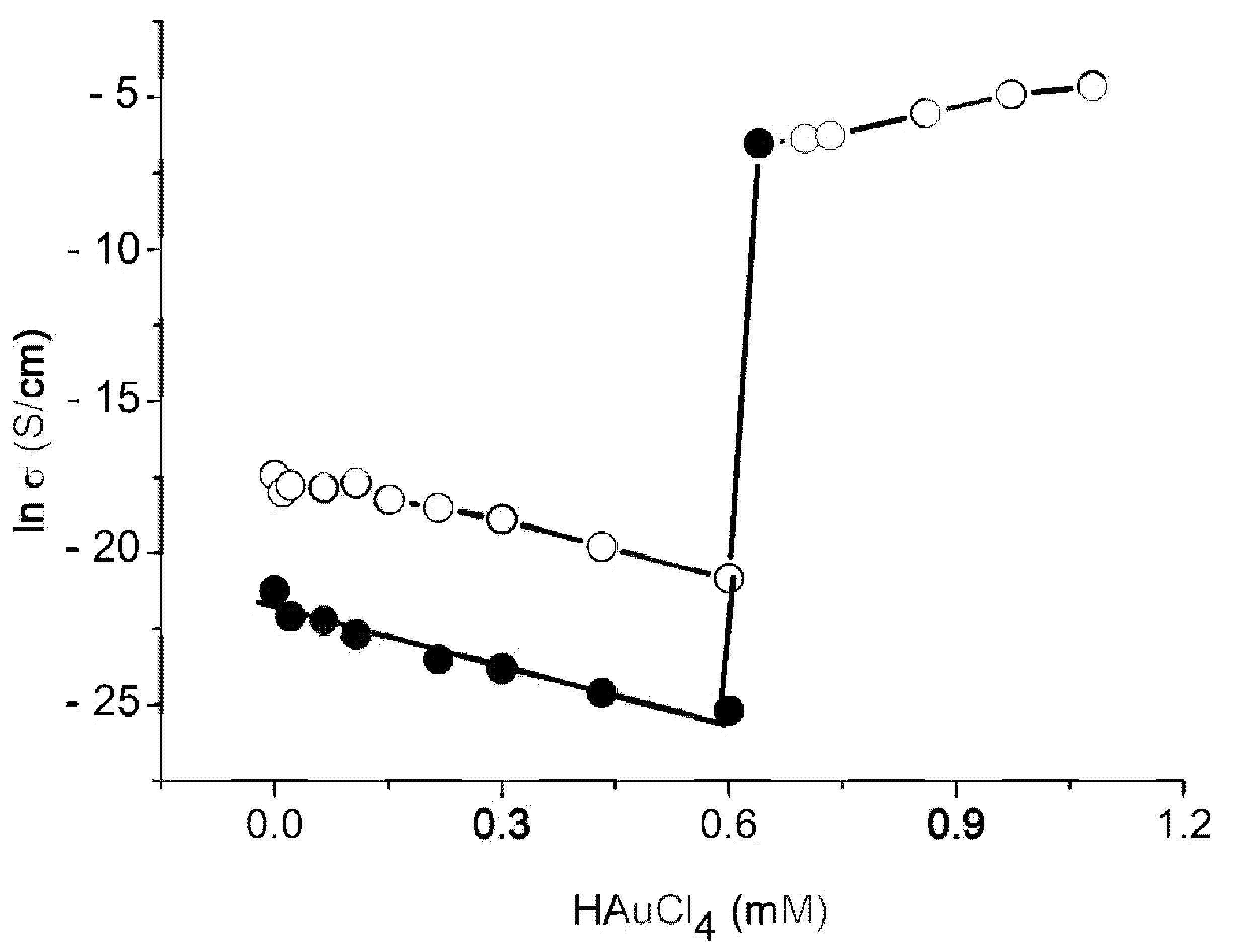
3.6. Conductivity Measurements
- A nonlinear behavior in the as-prepared film with water content between 8.5% and 10.4% in the temperature range of 25–70 °C. The nonlinear VFT behavior observed in the temperature conductivity measurements is a well-known feature of the α-relaxation process related to glass transition (see Equation (2)).
- An Arrhenius-type linear dependence (see Equation (3)) in the temperature range of 70–150 °C for the as-prepared film and in the temperature range 33–150 °C for the annealed film, with the same activation energy of 103.2 kJ/mol.
- Nonlinear dependence for the annealed film in the temperature range 0–33 °C.
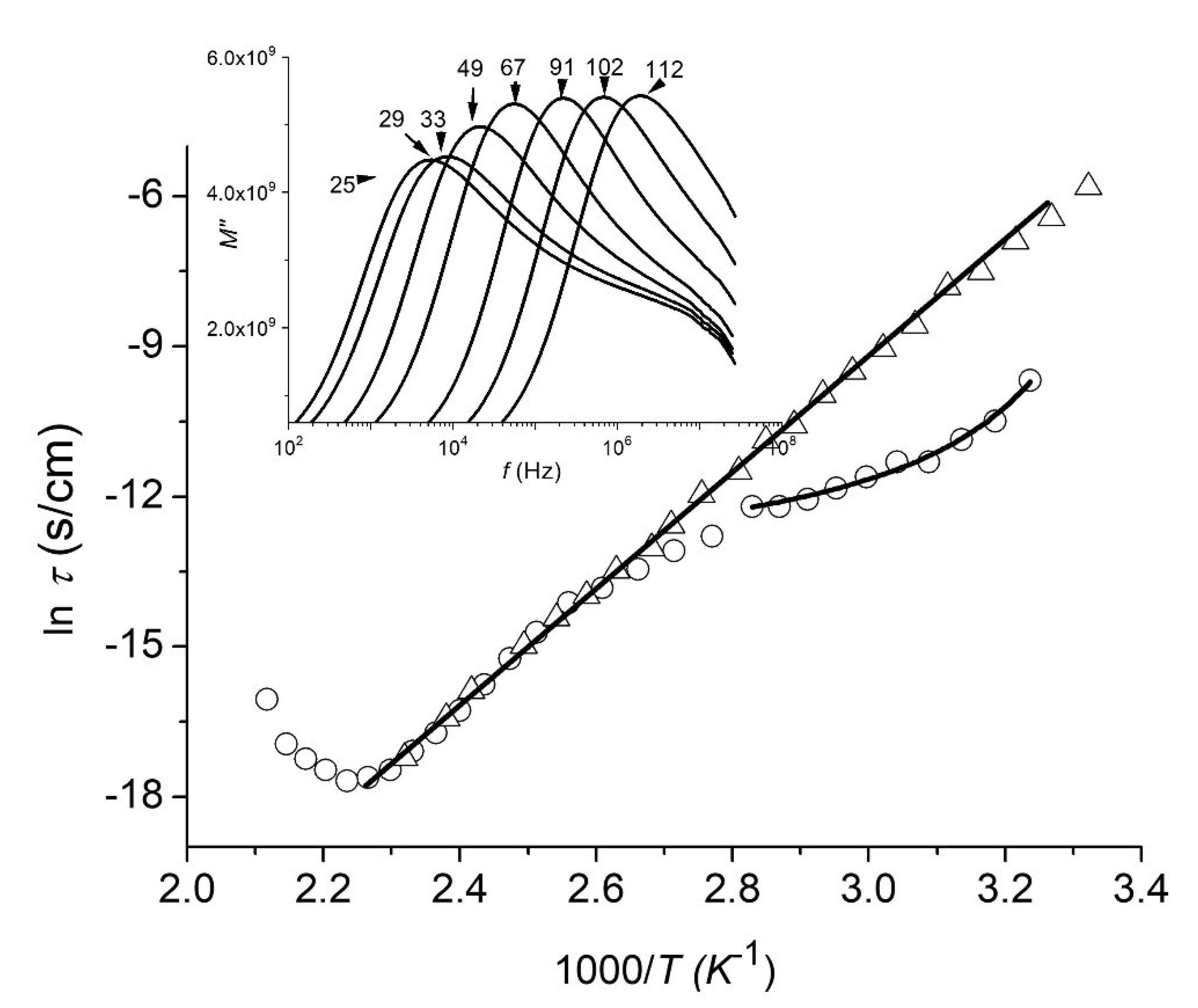
3.7. Dielectric Measurements
- (1)
- Nonlinear behaviors in the temperature range of 22–70 °C (for peaks with lower amplitude), which is well described by the VFT relationship (see Equation (2) with , instead of , respectively).
- (2)
- Arrhenius-type linear dependencies in the temperature range of 70–150 °C for the as-prepared film and in the temperature range of 22–150 °C for the annealed film, with an activation energy of 102.4 kJ/mol (see Equation (3) with , instead of , respectively).
- (3)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef]
- Sun, I.-C.; Na, J.H.; Jeong, S.Y.; Kim, D.-E.; Kwon, I.C.; Choi, K.; Ahn, C.-H.; Kim, K. Biocompatible Glycol Chitosan-Coated Gold Nanoparticles for Tumor-Targeting CT Imaging. Pharm. Res. 2013, 31, 1418–1425. [Google Scholar] [CrossRef]
- Collado-González, M.; Espín, V.F.; Montalbán, M.G.; Pamies, R.; Cifre, J.G.H.; Baños, F.G.D.; Víllora, G.; de la Torre, J.G. Aggregation behaviour of gold nanoparticles in presence of chitosan. J. Nanoparticle Res. 2015, 17, 268. [Google Scholar] [CrossRef]
- Katas, H.; Lim, C.S.; Azlan, A.Y.H.N.; Buang, F.; Busra, M.F.M. Antibacterial activity of biosynthesized gold nanoparticles using biomolecules from Lignosus rhinocerotis and chitosan. Saudi Pharm. J. 2019, 27, 283–292. [Google Scholar] [CrossRef]
- Virgili, A.H.; Laranja, D.C.; Malheiros, P.S.; Pereira, M.B.; Costa, T.M.H.; de Menezes, E.W. Nanocomposite film with antimicrobial activity based on gold nanoparticles, chitosan and aminopropylsilane. Surf. Coat. Technol. 2021, 415, 127086. [Google Scholar] [CrossRef]
- Regiel-Futyra, A.; Kus-Liśkiewicz, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Stochel, G.; Kyzioł, A. Development of Noncytotoxic Chitosan–Gold Nanocomposites as Efficient Antibacterial Materials. ACS Appl. Mater. Interfaces 2015, 7, 1087–1099. [Google Scholar] [CrossRef]
- Bhumkar, D.R.; Joshi, H.M.; Sastry, M.; Pokharkar, V.B. Chitosan Reduced Gold Nanoparticles as Novel Carriers for Transmucosal Delivery of Insulin. Pharm. Res. 2007, 24, 1415–1426. [Google Scholar] [CrossRef]
- Esther, J.; Sridevi, V. Synthesis and characterization of chitosan-stabilized gold nanoparticles through a facile and green approach. Gold Bull. 2017, 50, 1–5. [Google Scholar] [CrossRef]
- Jeyarani, S.; Vinita, N.M.; Puja, P.; Senthamilselvi, S.; Devan, U.; Velangani, A.J.; Biruntha, M.; Pugazhendhi, A.; Kumar, P. Biomimetic gold nanoparticles for its cytotoxicity and biocompatibility evidenced by fluorescence-based assays in cancer (MDA-MB-231) and non-cancerous (HEK-293) cells. J. Photochem. Photobiol. B Biol. 2020, 202, 111715. [Google Scholar] [CrossRef]
- da Silva, A.B.; Rufato, K.B.; de Oliveira, A.C.; Souza, P.R.; da Silva, E.P.; Muniz, E.C.; Vilsinski, B.H.; Martins, A.F. Composite materials based on chitosan/gold nanoparticles: From synthesis to biomedical applications. Int. J. Biol. Macromol. 2020, 161, 977–998. [Google Scholar] [CrossRef]
- Hu, L.; Fu, X.; Kong, G.; Yin, Y.; Meng, H.-M.; Ke, G.; Zhang, X.-B. DNAzyme–gold nanoparticle-based probes for biosensing and bioimaging. J. Mater. Chem. B 2020, 8, 9449–9465. [Google Scholar] [CrossRef]
- Falahati, M.; Attar, F.; Sharifi, M.; Saboury, A.A.; Salihi, A.; Aziz, F.M.; Kostova, I.; Burda, C.; Priecel, P.; Lopez-Sanchez, J.A.; et al. Gold nanomaterials as key suppliers in biological and chemical sensing, catalysis, and medicine. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129435. [Google Scholar] [CrossRef]
- Xu, Q.; Mao, C.; Liu, N.N.; Zhu, J.J.; Sheng, J. Direct electrochemistry of horseradish peroxidase based on biocompatible carboxymethyl chitosan–gold nanoparticle nanocomposite. Biosens. Bioelectron. 2006, 22, 768–773. [Google Scholar] [CrossRef]
- Zeng, J.; Gong, M.; Wang, D.; Li, M.; Xu, W.; Li, Z.; Li, S.; Zhang, D.; Yan, Z.; Yin, Y. Direct Synthesis of Water-Dispersible Magnetic/Plasmonic Heteronanostructures for Multimodality Biomedical Imaging. Nano Lett. 2019, 19, 3011–3018. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.-H.; Lin, T.-K.; Liang, Y.-C.; Chang, H.-W. Formation and Application of Core–Shell of FePt-Au Magnetic–Plasmonic Nanoparticles. Front. Chem. 2021, 9, 181. [Google Scholar] [CrossRef]
- Vidotti, M.; Carvalhal, R.F.; Mendes, R.K.; Ferreira, D.C.M.; Kubota, L.T. Biosensors Based on Gold Nanostructures. J. Braz. Chem. Soc. 2011, 22, 3–20. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, R.; Chai, Y.; Hong, C.; Guan, S. Preparation of a composite film electrochemically deposited with chitosan and gold nanoparticles for the determination of α-1-fetoprotein. Bioprocess Biosyst. Eng. 2010, 33, 613–618. [Google Scholar] [CrossRef]
- Jia, H.; Yang, T.; Zuo, Y.; Wang, W.; Xu, J.; Lu, L.; Li, P. Immunosensor for α-fetoprotein based on a glassy carbon electrode modified with electrochemically deposited N-doped graphene, gold nanoparticles and chitosan. Microchim. Acta 2017, 184, 3747–3753. [Google Scholar] [CrossRef]
- Xue, M.H.; Xu, Q.; Zhou, M.; Zhu, J.J. In situ immobilization of glucose oxidase in chitosan–gold nanoparticle hybrid film on Prussian Blue modified electrode for high-sensitivity glucose detection. Electrochem. Commun. 2006, 8, 1468–1474. [Google Scholar] [CrossRef]
- Shan, C.; Yang, H.; Han, D.; Zhang, Q.; Ivaska, A.; Niu, L. Graphene/AuNPs/chitosan nanocomposites film for glucose biosensing. Biosens. Bioelectron. 2010, 25, 1070–1074. [Google Scholar] [CrossRef]
- Rassas, I.; Braiek, M.; Bonhomme, A.; Bessueille, F.; Raffin, G.; Majdoub, H.; Jaffrezic-Renault, N. Highly Sensitive Voltammetric Glucose Biosensor Based on Glucose Oxidase Encapsulated in a Chitosan/Kappa-Carrageenan/Gold Nanoparticle Bionanocomposite. Sensors 2019, 19, 154. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, D.S.; Goulet, P.J.G.; Pieczonka, N.P.W.; Oliveira, O.N.; Aroca, R.F. Gold Nanoparticle Embedded, Self-Sustained Chitosan Films as Substrates for Surface-Enhanced Raman Scattering. Langmuir 2004, 20, 10273–10277. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Tenhu, H. Recent advances in polymer protected gold nanoparticles: Synthesis, properties and applications. Chem. Commun. 2007, 0, 4580–4598. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, Q.; Yang, X. Morphology study of gold–chitosan nanocomposites. J. Colloid Interface Sci. 2005, 282, 26–31. [Google Scholar] [CrossRef]
- Du, Y.; Luo, X.L.; Xu, J.J.; Chen, H.Y. A simple method to fabricate a chitosan-gold nanoparticles film and its application in glucose biosensor. Bioelectrochemistry 2007, 70, 342–347. [Google Scholar] [CrossRef]
- Huang, H.; Yang, X. Chitosan mediated assembly of gold nanoparticles multilayer. Colloids Surfaces A Physicochem. Eng. Asp. 2003, 226, 77–86. [Google Scholar] [CrossRef]
- Esumi, K.; Takei, N.; Yoshimura, T. Antioxidant-potentiality of gold–chitosan nanocomposites. Colloids Surfaces B Biointerfaces 2003, 32, 117–123. [Google Scholar] [CrossRef]
- Ottonelli, M.; Zappia, S.; Demartini, A.; Alloisio, M. Chitosan-Stabilized Noble Metal Nanoparticles: Study of their Shape Evolution and Post-Functionalization Properties. Nanomaterials 2020, 10, 224. [Google Scholar] [CrossRef]
- Shen, J.-J.; He, J.; Ding, Y. Preparation, Stabilization, and Self-Assembly Of Gold Nanoparticles by Chitosan Derivatives. Adv. Mater. Lett. 2019, 10, 80–84. [Google Scholar] [CrossRef]
- Neagu, A.; Curecheriu, L.; Airimioaei, M.; Cazacu, A.; Cernescu, A.; Mitoseriu, L. Impedance spectroscopy characterization of relaxation mechanisms in gold–chitosan nanocomposites. Compos. Part B Eng. 2015, 71, 210–217. [Google Scholar] [CrossRef]
- Ding, L.; Hao, C.; Xue, Y.; Ju, H. A Bio-Inspired Support of Gold Nanoparticles−Chitosan Nanocomposites Gel for Immobilization and Electrochemical Study of K562 Leukemia Cells. Biomacromolecules 2007, 8, 1341–1346. [Google Scholar] [CrossRef]
- Huang, H.; Yang, X.; And, H.H.; Yang, X. Synthesis of Chitosan-Stabilized Gold Nanoparticles in the Absence/Presence of Tripolyphosphate. Biomacromolecules 2004, 5, 2340–2346. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Wu, J.; Sailor, M.J. Chitosan Hydrogel-Capped Porous SiO2 as a pH Responsive Nano-Valve for Triggered Release of Insulin. Adv. Funct. Mater. 2009, 19, 733–741. [Google Scholar] [CrossRef]
- Cui, D.; Szarpak, A.; Pignot-Paintrand, I.; Varrot, A.; Boudou, T.; Detrembleur, C.; Jérôme, C.; Picart, C.; Auzély-Velty, R. Contact-Killing Polyelectrolyte Microcapsules Based on Chitosan Derivatives. Adv. Funct. Mater. 2010, 20, 3303–3312. [Google Scholar] [CrossRef]
- Lavanya, K.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N. Temperature- and pH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater. Sci. Eng. C 2020, 111, 110862. [Google Scholar] [CrossRef]
- Bataglioli, R.A.; Taketa, T.B.; Neto, J.B.M.R.; Lopes, L.M.; Costa, C.A.R.; Beppu, M.M. Analysis of pH and salt concentration on structural and model-drug delivery properties of polysaccharide-based multilayered films. Thin Solid Films 2019, 685, 312–320. [Google Scholar] [CrossRef]
- Lin, Y.L.; Jen, J.C.; Hsu, S.H.; Chiu, M. Sciatic nerve repair by microgrooved nerve conduits made of chitosan-gold nanocomposites. Surg. Neurol. 2008, 70, S9–S18. [Google Scholar] [CrossRef]
- González-Campos, J.B.; Prokhorov, E.; Luna-Bárcenas, G.; Fonseca-García, A.; Sanchez, I.C. Dielectric relaxations of chitosan: The effect of water on the α-relaxation and the glass transition temperature. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 2259–2271. [Google Scholar] [CrossRef]
- Mott, N.F. Metal-Insulator Transition; Taylor & Francis: London, UK, 1990; ISBN 0-85066-783-6v. [Google Scholar]
- Psarras, G.C. Hopping conductivity in polymer matrix–metal particles composites. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1545–1553. [Google Scholar] [CrossRef]
- Hernández-Vargas, J.; González-Campos, J.B.; Lara-Romero, J.; Prokhorov, E.; Luna-Bárcenas, G.; Aviña-Verduzco, J.A.; González-Hernández, J.C. Chitosan/MWCNTs-decorated with silver nanoparticle composites: Dielectric and antibacterial characterization. J. Appl. Polym. Sci. 2014, 131, 40214. [Google Scholar] [CrossRef]
- Capaccioli, S.; Lucchesi, M.; Rolla, P.A.; Ruggeri, G. Dielectric response analysis of a conducting polymer dominated by the hopping charge transport. J. Phys. Condens. Matter 1998, 10, 5595. [Google Scholar] [CrossRef]
- Tjong, S.C.; Mai, Y.W. Physical Properties and Applications of Polymer Nanocomposites; Woodhead Pub: Cambridge, UK; Philadelphia, PA, USA, 2010; ISBN 978-0-85709-024-9. [Google Scholar]
- Bakeeva, I.V.; Kolesnikova, Y.A.; Kataeva, N.A.; Zaustinskaya, K.S.; Gubin, S.P.; Zubov, V.P. Gold nanoparticles as structurizing agents for the formation of hybrid nanocomposites. Russ. Chem. Bull. 2008, 57, 337–344. [Google Scholar] [CrossRef]
- Wu, Y.; Zuo, F.; Lin, Y.; Zhou, Y.; Zheng, Z.; Ding, X. Green and Facile Synthesis of Gold Nanoparticles Stabilized by Chitosan. J. Macromol. Sci. Part A 2014, 51, 441–446. [Google Scholar] [CrossRef]
- González-Campos, J.B.; Prokhorov, E.; Luna-Bárcenas, G.; Sanchez, I.C.; Lara-Romero, J.; Mendoza-Duarte, M.E.; Villaseñor, F.; Guevara-Olvera, L. Chitosan/silver nanoparticles composite: Molecular relaxations investigation by dynamic mechanical analysis and impedance spectroscopy. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 739–748. [Google Scholar] [CrossRef]
- Ogawa, K.; Hirano, S.; Miyanishi, T.; Yui, T.; Watanabe, T. A New Polymorph of chitosan. Macromolecules 1984, 17, 1001. [Google Scholar] [CrossRef]
- Demarger-Andre, S.; Domard, A. Chitosan carboxylic acid salts in solution and in the solid state. Carbohydr. Polym. 1994, 23, 211–219. [Google Scholar] [CrossRef]
- Zong, Z.; Kimura, Y.; Takahashi, M.; Yamane, H. Characterization of chemical and solid state structures of acylated chitosans. Polymer 2000, 41, 899–906. [Google Scholar] [CrossRef]
- Okuyama, K.; Noguchi, K.; Kanenari, M.; Egawa, T.; Osawa, K.; Ogawa, K. Structural diversity of chitosan and its complexes. Carbohydr. Polym. 2000, 41, 237–247. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; David, L.; Trombotto, S.; Lucas, J.-M.; Peniche-Covas, C.; Domard, A. Kinetics Study of the Solid-State Acid Hydrolysis of Chitosan: Evolution of the Crystallinity and Macromolecular Structure. Biomacromolecules 2010, 11, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Sabadini, R.C.; Gonçalves, T.S.; de Camargo, A.S.S.; Pawlicka, A.; Silva, M.M. Structural, morphological, thermal and electrochemical characteristics of chitosan: Praseodymium triflate based solid polymer electrolytes. Int. J. Green Energy 2019, 16, 1602–1610. [Google Scholar] [CrossRef]
- Mati-Baouche, N.; De Baynast, H.; Vial, C.; Audonnet, F.; Sun, S.; Petit, E.; Pennec, F.; Prevot, V.; Michaud, P. Physico-chemical, thermal, and mechanical approaches for the characterization of solubilized and solid state chitosans. J. Appl. Polym. Sci. 2015, 132, 41257. [Google Scholar] [CrossRef]
- Heilmann, A. Polymer Films with Embedded Metal. Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2003; ISBN 978-3-540-43151-0. [Google Scholar]
- Astruc, D.; Daniel, M.-C.; Ruiz, J. Dendrimers and gold nanoparticles as exo-receptors sensing biologically important anions. Chem. Commun. 2004, 2637–2649. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-C.; Lee, S.-W.; Kang, I.-J. Preparation and Characterization of Chitosan-Gold Nanocomposites for Drug Delivery Application. Surf. Rev. Lett. 2010, 17, 165–172. [Google Scholar] [CrossRef]
- Shu, X.Z.; Zhu, K.J.; Song, W. Novel pH-sensitive citrate cross-linked chitosan film for drug controlled release. Int. J. Pharm. 2001, 212, 19–28. [Google Scholar] [CrossRef]
- Wan, Y.; Creber, K.A.M.; Peppley, B.; Bui, V.T. Ionic conductivity of chitosan membranes. Polymer 2003, 44, 1057–1065. [Google Scholar] [CrossRef]
- Prokhorov, E.; Luna-Bárcenas, G.; González-Campos, J.B.; Kovalenko, Y.; García-Carvajal, Z.Y.; Mota-Morales, J. Proton conductivity and relaxation properties of chitosan-acetate films. Electrochim. Acta 2016, 215, 600–608. [Google Scholar] [CrossRef]
- Viciosa, M.T.; Dionísio, M.; Silva, R.M.; Reis, R.L.; Mano, J.F. Molecular Motions in Chitosan Studied by Dielectric Relaxation Spectroscopy. Biomacromolecules 2004, 5, 2073–2078. [Google Scholar] [CrossRef]
- Kumar-Krishnan, S.; Prokhorov, E.; Luna-Barcenas, G. Molecular relaxation in Chitosan films in GHz frequency range. MRS Online Proc. Libr. 2014, 1613, 83–88. [Google Scholar] [CrossRef]
- Ostrowska-Czubenko, J.; Pieróg, M. State of water in citrate crosslinked chitosan membrane. Prog. Chem. Appl. Chitin Deriv. 2010, XV, 33–40. [Google Scholar]
- Einfeldt, J.; Meißner, D.; Kwasniewski, A. Contributions to the molecular origin of the dielectric relaxation processes in polysaccharides—The high temperature range. J. Non. Cryst. Solids 2003, 320, 40–55. [Google Scholar] [CrossRef]
- M Valenzuela-Acosta, E.; Prokhorov, E.; Arias de Fuentes, O.; Luna-Barcenas, G.; A. Mauricio-Sánchez, R.; A. Elizalde-Pena, E. Chitosan-Gold Nanocomposite for Copper Ions Detection. Curr. Nanosci. 2016, 12, 754–761. [Google Scholar] [CrossRef][Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strupiechonski, E.; Moreno-Ríos, M.; Ávila-Dávila, E.O.; Román-Doval, R.; Prokhorov, E.; Kovalenko, Y.; Zárate-Triviño, D.G.; Medina, D.I.; Luna-Barcenas, G. Relaxation Phenomena in Chitosan-Au Nanoparticle Thin Films. Polymers 2021, 13, 3214. https://doi.org/10.3390/polym13193214
Strupiechonski E, Moreno-Ríos M, Ávila-Dávila EO, Román-Doval R, Prokhorov E, Kovalenko Y, Zárate-Triviño DG, Medina DI, Luna-Barcenas G. Relaxation Phenomena in Chitosan-Au Nanoparticle Thin Films. Polymers. 2021; 13(19):3214. https://doi.org/10.3390/polym13193214
Chicago/Turabian StyleStrupiechonski, Elodie, Marisa Moreno-Ríos, Erika O. Ávila-Dávila, Ramón Román-Doval, Evgeny Prokhorov, Yuriy Kovalenko, Diana G. Zárate-Triviño, Dora I. Medina, and Gabriel Luna-Barcenas. 2021. "Relaxation Phenomena in Chitosan-Au Nanoparticle Thin Films" Polymers 13, no. 19: 3214. https://doi.org/10.3390/polym13193214
APA StyleStrupiechonski, E., Moreno-Ríos, M., Ávila-Dávila, E. O., Román-Doval, R., Prokhorov, E., Kovalenko, Y., Zárate-Triviño, D. G., Medina, D. I., & Luna-Barcenas, G. (2021). Relaxation Phenomena in Chitosan-Au Nanoparticle Thin Films. Polymers, 13(19), 3214. https://doi.org/10.3390/polym13193214






