Polyvinyl Alcohol/Chitosan and Polyvinyl Alcohol/Ag@MOF Bilayer Hydrogel for Tissue Engineering Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Ag@MOF
2.3. Preparation of Composites Hydrogels
2.3.1. PVA Hydrogels
2.3.2. PVA/CS Hydrogels
2.3.3. PVA/Ag@MOF Hydrogels
2.3.4. Bilayer Hydrogels
2.4. Characterization
2.4.1. FT-IR
2.4.2. XRD
2.4.3. SEM
2.4.4. TGA
2.4.5. Rheological Characterization
2.4.6. Water Retention Studies
2.4.7. In Vitro Degradation
2.4.8. Antibacterial Properties
2.4.9. Cytotoxicity Test
2.4.10. Cell Observations
2.4.11. Statistical Analysis
3. Results and Discussion
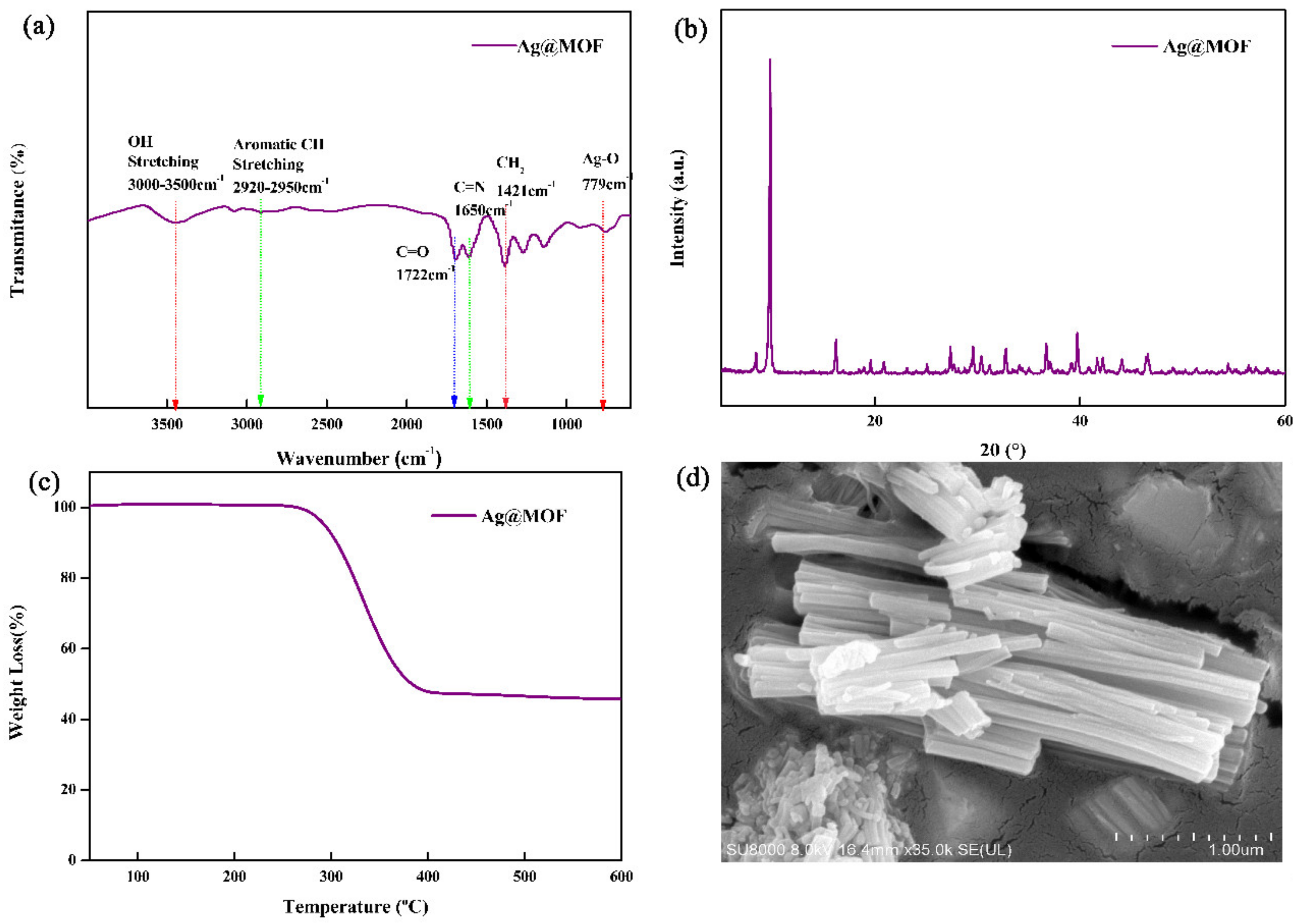
3.1. Structure and Morphology of Ag@MOF
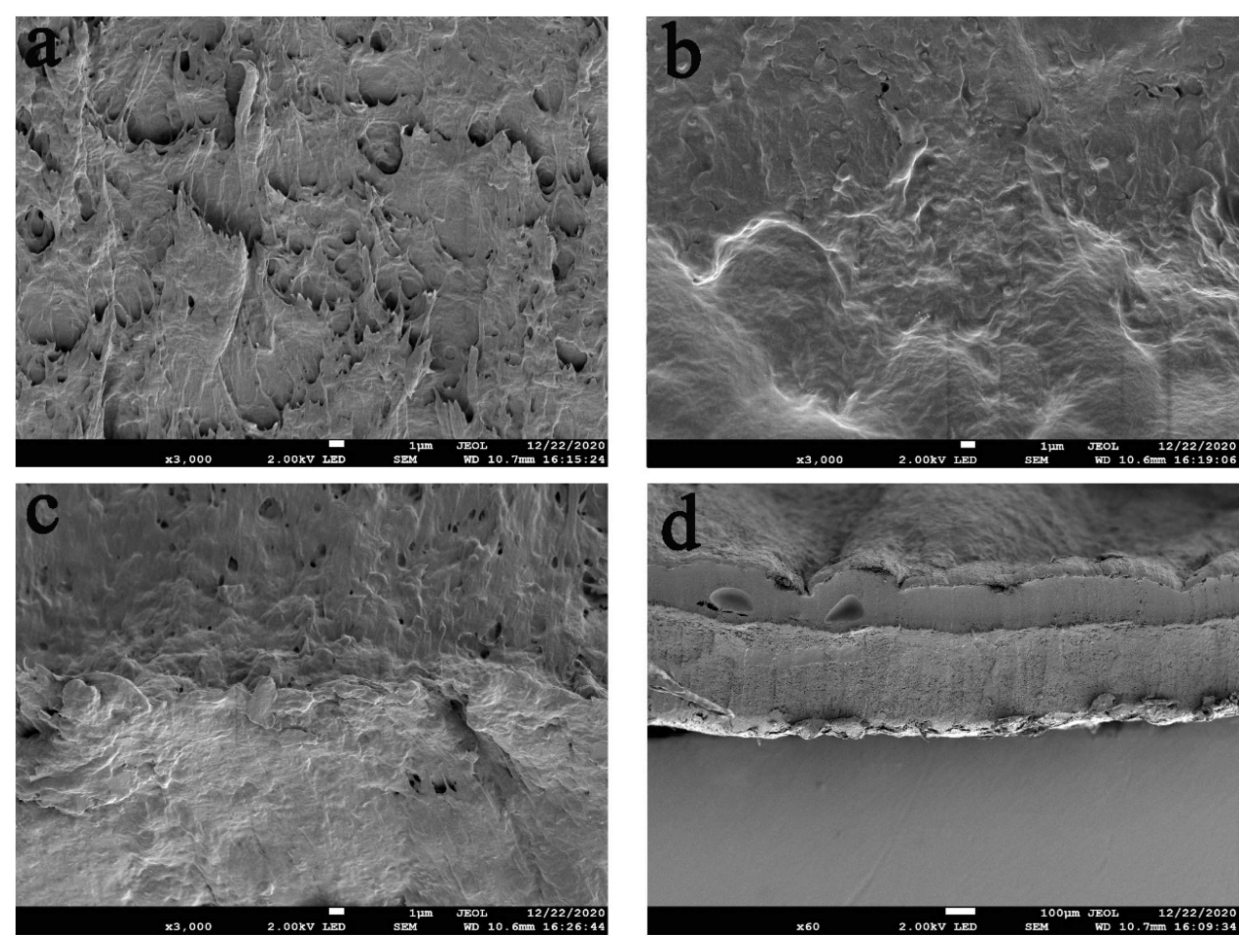
3.2. SEM of the Hydrogels
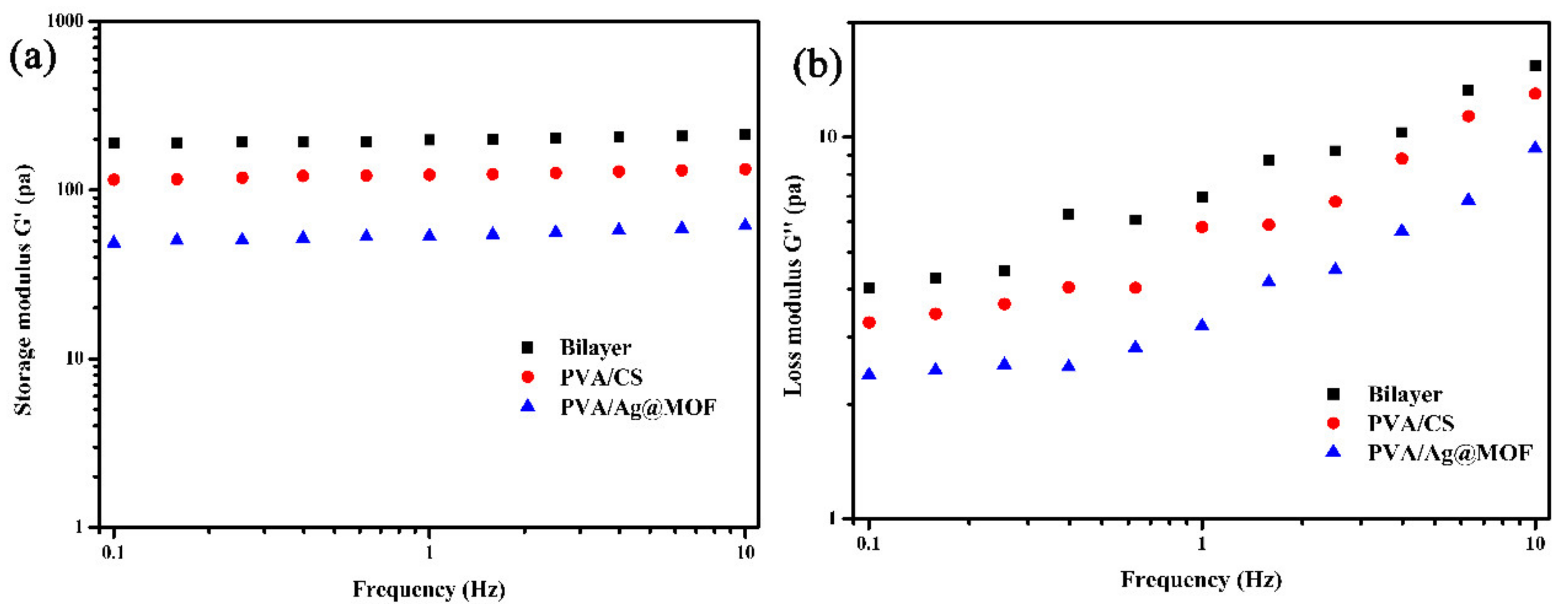
3.3. Rheological Analysis of Hydrogels
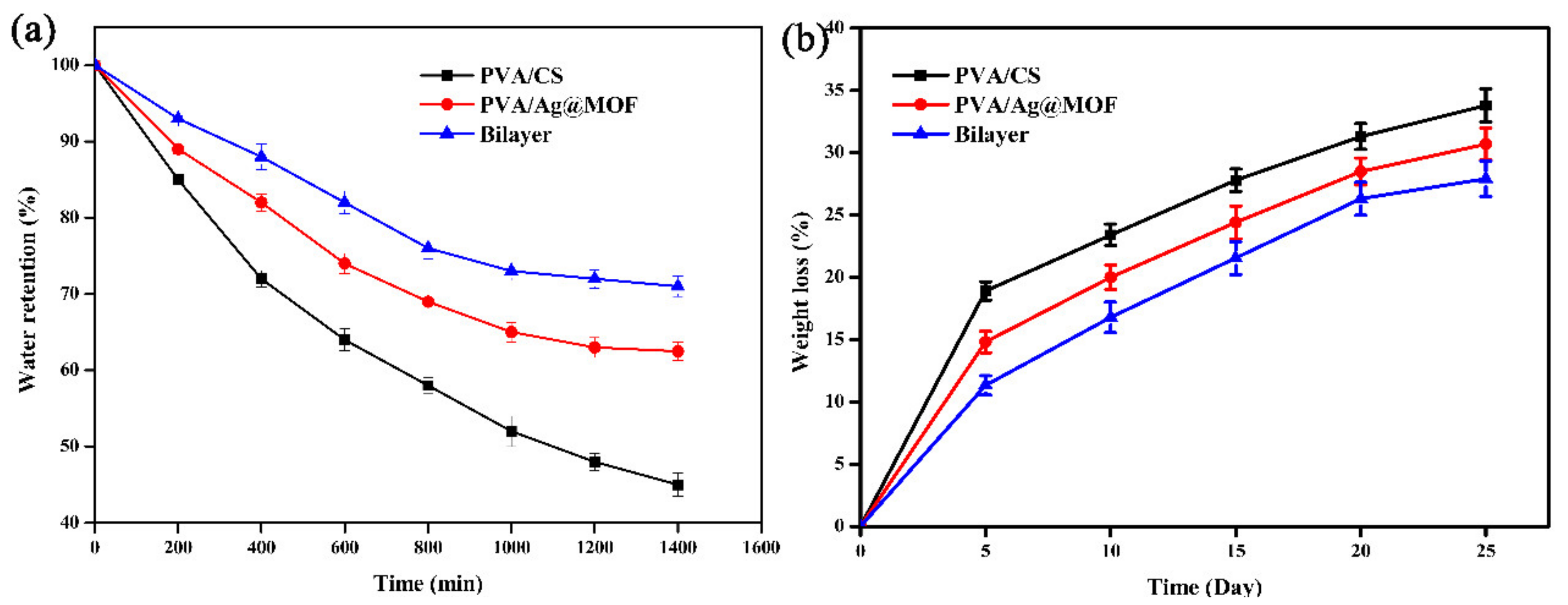
3.4. Water Retention of Hydrogels
3.5. Biodegradation of Hydrogels
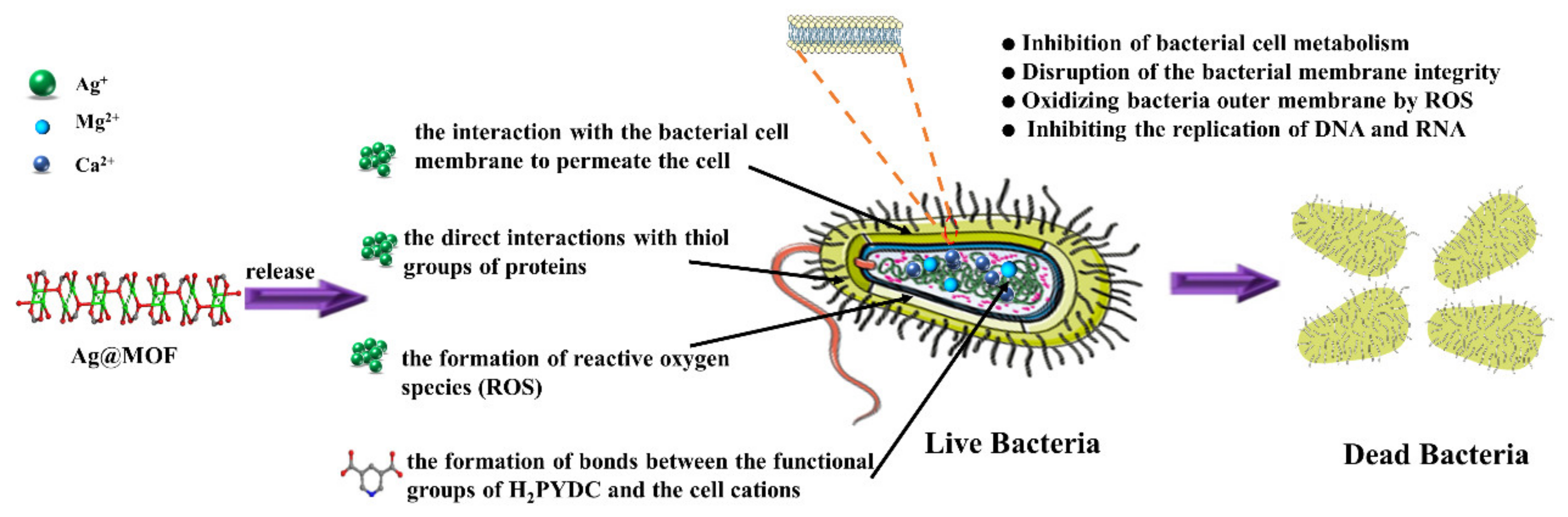
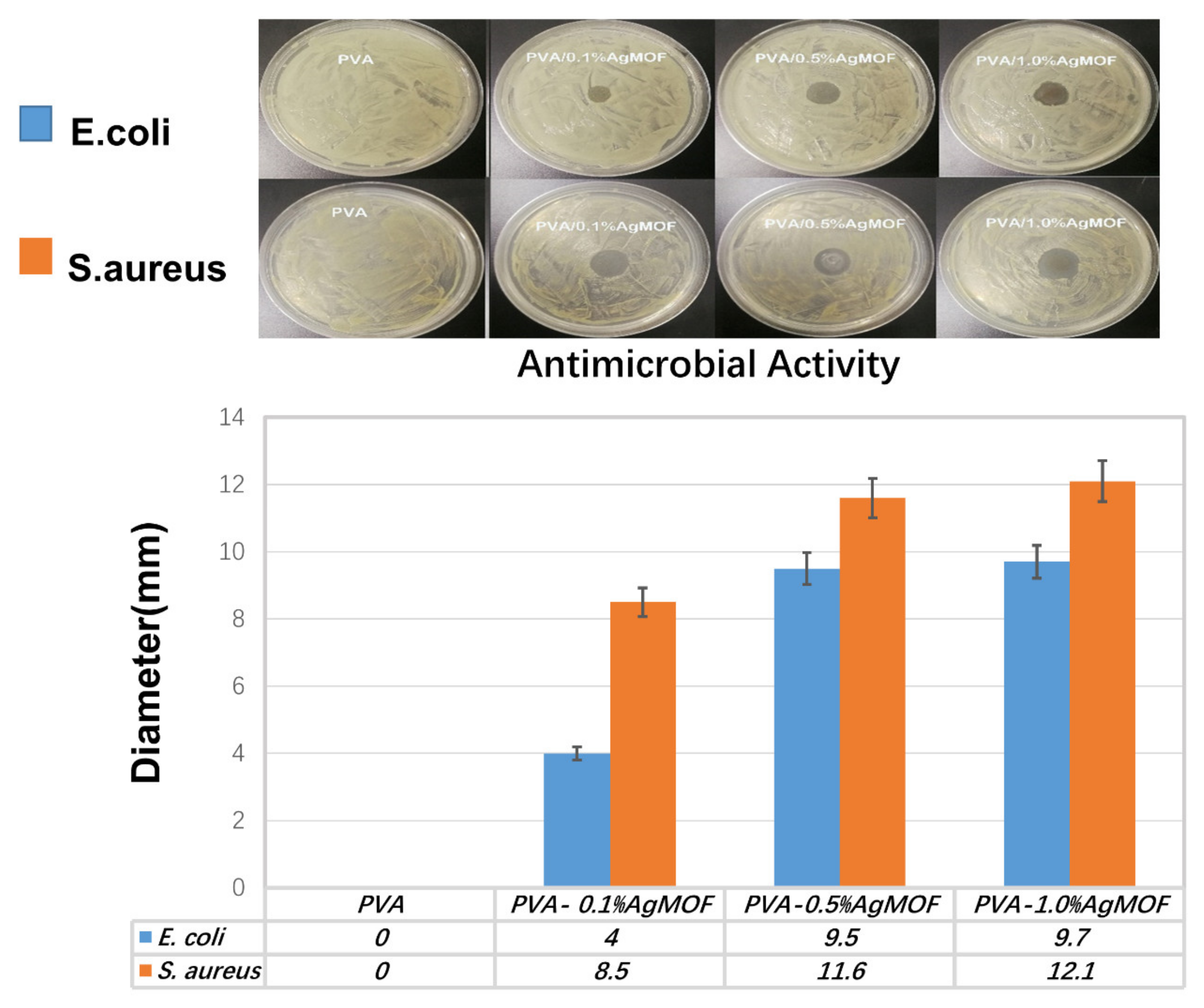
3.6. Antibacterial Properties of Hydrogels
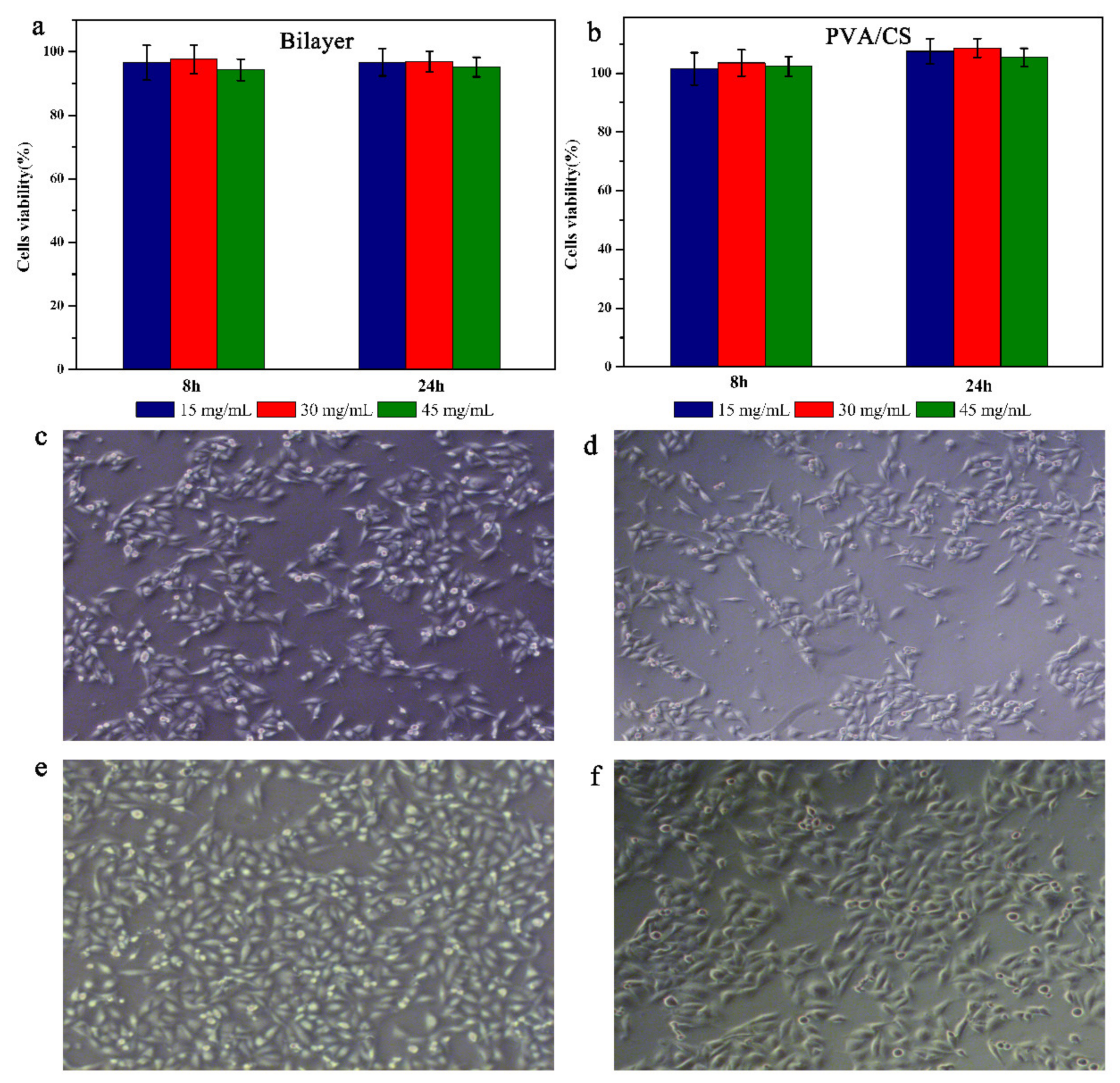
3.7. Biocompatibility and Cell Adhesion of Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guzelgulgen, M.; Ozkendir-Inanc, D.; Yildiz, U.H.; Arslan-Yildiz, A. Glucuronoxylan-based quince seed hydrogel: A promising scaffold for tissue engineering applications. Int. J. Biol. Macromol. 2021, 180, 729–738. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, K.; Du, L.; Cheng, Z.; Zhang, T.; Ding, J.; Li, W.; Xu, B.; Zhu, M. Construction of chitosan scaffolds with controllable microchannel for tissue engineering and regenerative medicine. Mater. Sci. Eng. C 2021, 126, 112178. [Google Scholar] [CrossRef]
- Laird, N.Z.; Acri, T.M.; Chakka, J.L.; Quarterman, J.C.; Malkawi, W.I.; Elangovan, S.; Salem, A.K. Applications of nanotechnology in 3D printed tissue engineering scaffolds. Eur. J. Pharm. Biopharm. 2021, 161, 15–28. [Google Scholar] [CrossRef]
- Laird, N.Z.; Acri, T.M.; Tingle, K.; Salem, A.K. Gene- and RNAi-activated scaffolds for bone tissue engineering: Current progress and future directions. Adv. Drug Deliv. Rev. 2021, 174, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Wu, W.; Wei, Y.; Ren, L.; Lin, S.; Wu, J. Biomimetic gelatin/chitosan/polyvinyl alcohol/nano-hydroxyapatite scaffolds for bone tissue engineering. Mater. Des. 2021, 207, 109865. [Google Scholar] [CrossRef]
- Poormoghadam, D.; Ghollasi, M.; Babavalian, H.; Tabasi, A.; Shams, M.; Goodarzi, V.; Salimi, A. Modification and characterization of an innovative polyvinyl alcohol-45S5 bioactive glass nanocomposite scaffold containing Donepezil hydrochloride for bone tissue engineering applications. Mater. Lett. 2021, 300, 130160. [Google Scholar] [CrossRef]
- Sani, I.S.; Rezaei, M.; Khoshfetrat, A.B.; Razzaghi, D. Preparation and characterization of polycaprolactone/chitosan-g-polycaprolactone/hydroxyapatite electrospun nanocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2021, 182, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fang, H.; Zheng, S.; Li, L.; Jiao, Z.; Wang, H.; Nie, Y.; Liu, T.; Song, K. A biological functional hybrid scaffold based on decellularized extracellular matrix/gelatin/chitosan with high biocompatibility and antibacterial activity for skin tissue engineering. Int. J. Biol. Macromol. 2021, 187, 840–849. [Google Scholar] [CrossRef]
- Zuluaga-Vélez, A.; Quintero-Martinez, A.; Orozco, L.M.; Sepúlveda-Arias, J.C. Silk fibroin nanocomposites as tissue engineering scaffolds—A systematic review. Biomed. Pharmacother. 2021, 141, 111924. [Google Scholar] [CrossRef]
- Ravishankar, K.; Venkatesan, M.; Desingh, R.P.; Mahalingam, A.; Sadhasivam, B.; Subramaniyam, R.; Dhamodharan, R. Biocompatible hydrogels of chitosan-alkali lignin for potential wound healing applications. Mater. Sci. Eng. C 2019, 102, 447–457. [Google Scholar] [CrossRef]
- Khan, M.I.; Paul, P.; Behera, S.K.; Jena, B.; Tripathy, S.K.; Stålsby Lundborg, C.; Mishra, A. To decipher the antibacterial mechanism and promotion of wound healing activity by hydrogels embedded with biogenic Ag@ZnO core-shell nanocomposites. Chem. Eng. J. 2020, 417, 128025. [Google Scholar] [CrossRef]
- Akhlaq, M.; Azad, A.; Ullah, I.; Nawaz, A.; Safdar, M.; Bhattacharya, T.; Uddin, A.; Abbas, S.; Mathews, A.; Kundu, S.; et al. Methotrexate-Loaded Gelatin and Polyvinyl Alcohol (Gel/PVA) Hydrogel as a pH-Sensitive Matrix. Polymers 2021, 13, 2300. [Google Scholar] [CrossRef]
- Figueroa-Pizano, M.; Vélaz, I.; Peñas, F.; Zavala-Rivera, P.; Rosas-Durazo, A.; Maldonado-Arce, A.; Martínez-Barbosa, M. Effect of freeze-thawing conditions for preparation of chitosan-poly (vinyl alcohol) hydrogels and drug release studies. Carbohydr. Polym. 2018, 195, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Nah, H.; Heo, D.N.; Kim, K.-H.; Seok, J.M.; Heo, M.; Moon, H.-J.; Lee, D.; Lee, J.S.; An, S.Y.; et al. Induction of osteogenic differentiation in a rat calvarial bone defect model using an In situ forming graphene oxide incorporated glycol chitosan/oxidized hyaluronic acid injectable hydrogel. Carbon 2020, 168, 264–277. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small 2019, 15, e1900046. [Google Scholar] [CrossRef]
- Aradmehr, A.; Javanbakht, V. A novel biofilm based on lignocellulosic compounds and chitosan modified with silver nanoparticles with multifunctional properties: Synthesis and characterization. Colloids Surfaces A: Physicochem. Eng. Asp. 2020, 600, 124952. [Google Scholar] [CrossRef]
- Biranje, S.S.; Madiwale, P.V.; Patankar, K.C.; Chhabra, R.; Bangde, P.; Dandekar, P.; Adivarekar, R.V. Cytotoxicity and hemostatic activity of chitosan/carrageenan composite wound healing dressing for traumatic hemorrhage. Carbohydr. Polym. 2020, 239, 116106. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M.; Velazquez, G. Composite films of regenerate cellulose with chitosan and polyvinyl alcohol: Evaluation of water adsorption, mechanical and optical properties. Int. J. Biol. Macromol. 2018, 117, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Omidi, M.; Chen, X. Development of the PVA/CS nanofibers containing silk protein sericin as a wound dressing: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2020, 149, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lei, Y.; Wang, D.; Li, C.; Cheng, J.; Wang, J.; Meng, W.; Liu, M. The Study of Zeolitic Imidazolate Framework (ZIF-8) Doped Polyvinyl Alcohol/Starch/Methyl Cellulose Blend Film. Polymers 2019, 11, 1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niua, X.; Weiab, Y.; Liuc, Q.; Yangc, B.; Mac, N.; Lic, Z.; Zhaoab, L.; Chenab, W.; Huangab, D. Silver-loaded microspheres reinforced chitosan scaffolds for skin tissue engineering. Eur. Polym. J. 2020, 134, 109861. [Google Scholar] [CrossRef]
- Kovacova, M.; Markovic, Z.M.; Humpolíček, P.; Mičušík, M.; Švajdlenková, H.; Kleinová, A.; Danko, M.; Kubát, P.; Vajďák, J.; Capáková, Z.; et al. Carbon Quantum Dots Modified Polyurethane Nanocomposite as Effective Photocatalytic and Antibacterial Agents. ACS Biomater. Sci. Eng. 2018, 4, 3983–3993. [Google Scholar] [CrossRef] [PubMed]
- Slenters, T.V.; Hauser-Gerspach, I.; Daniels, A.U.; Fromm, K.M. Silver coordination compounds as light-stable, nano-structured and anti-bacterial coatings for dental implant and restorative materials. J. Mater. Chem. 2008, 18, 5359–5362. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Gao, Y.; Zhang, Y.; Cheng, T.; Ou, H.; Yang, L.; Liu, J.; Shi, L.; Liu, J. Silver-Decorated Polymeric Micelles Combined with Curcumin for Enhanced Antibacterial Activity. ACS Appl. Mater. Interfaces 2017, 9, 16880–16889. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.K.; Surekha, D.B.; Tripathi, M.; Anjum, M.; Muthu, M.S.; Tilak, R.; Agrawal, A.K.; Singh, S. Antibiofilm Potential of Silver Sulfadiazine-Loaded Nanoparticle Formulations: A Study on the Effect of DNase-I on Microbial Biofilm and Wound Healing Activity. Mol. Pharm. 2019, 16, 3916–3925. [Google Scholar] [CrossRef]
- Bateman, F.L.; Kirejczyk, S.G.M.; Stewart, G.V.; Cutler, D.C.; Quilling, L.L.; Howerth, E.W.; Mayer, J. Effects of an enrofloxacin-silver sulfadiazine emulsion in the ears of rabbits with perforated tympanic membranes. Am. J. Veter- Res. 2019, 80, 325–334. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, T.; Hua, A.; Ma, S.; Zhang, Z.; Liu, L. Prolonged antimicrobial activity of silver core-carbon shell nanoparticles. Korean J. Chem. Eng. 2019, 36, 1882–1889. [Google Scholar] [CrossRef]
- Tian, X.; Jiang, X.; Welch, C.; Croley, T.R.; Wong, T.-Y.; Chen, C.; Fan, S.; Chong, Y.; Li, R.; Ge, C.; et al. Bactericidal Effects of Silver Nanoparticles on Lactobacilli and the Underlying Mechanism. ACS Appl. Mater. Interfaces 2018, 10, 8443–8450. [Google Scholar] [CrossRef]
- Travlou, N.A.; Algarra, M.; Alcoholado, C.; Cifuentes-Rueda, M.; Labella, A.M.; Lázaro-Martínez, J.M.; Rodriguez-Castellon, E.; Bandosz, T.J. Carbon Quantum Dot Surface-Chemistry-Dependent Ag Release Governs the High Antibacterial Activity of Ag-Metal–Organic Framework Composites. ACS Appl. Bio Mater. 2018, 1, 693–707. [Google Scholar] [CrossRef]
- He, Y.; Zhou, W.; Qian, G.; Chen, B. Methane storage in metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 5657–5678. [Google Scholar] [CrossRef]
- Barea, E.; Montoro, C.; Navarro, J. Toxic gas removal-metal–organic frameworks for the capture and degradation of toxic gases and vapours. Chem. Soc. Rev. 2014, 43, 5419–5430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Zhang, Z.; Li, W.; Lei, Z.; Cheng, N.; Tan, Y.; Mu, S.; Sun, X. Highly Exposed Active Sites of Defect-Enriched Derived MOFs for Enhanced Oxygen Reduction Reaction. ACS Sustain. Chem. Eng. 2019, 7, 17855–17862. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ganguly, S.; Chakraborty, A.; Mandal, A.; Das, D. Green Synthesis of Self Assembled Nanospherical Dysprosium MOFs: Selective and Efficient Detection of Picric Acid in Aqueous and Gas Phase. ACS Sustain. Chem. Eng. 2018, 7, 819–830. [Google Scholar] [CrossRef]
- Liu, M.; Wang, L.; Zheng, X.; Xie, Z. Zirconium-Based Nanoscale Metal–Organic Framework/Poly(ε-caprolactone) Mixed-Matrix Membranes as Effective Antimicrobials. ACS Appl. Mater. Interfaces 2017, 9, 41512–41520. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ye, J.; Zhang, D.; Xie, R.; Bogale, R.F.; Sun, Y.; Zhao, L.; Zhao, Q.; Ning, G. Silver carboxylate metal–organic frameworks with highly antibacterial activity and biocompatibility. J. Inorg. Biochem. 2014, 138, 114–121. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, G.; Wang, D.; Zheng, Y.; Li, Y.; Meng, W.; Zhang, X.; Du, F.; Lee, S. Ag@MOF-loaded chitosan nanoparticle and polyvinyl alcohol/sodium alginate/chitosan bilayer dressing for wound healing applications. Int. J. Biol. Macromol. 2021, 175, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Hu, X.; Wei, W.; Yu, H.; Li, J.; Zhang, J.; Dong, W. Investigation of Salecan/poly(vinyl alcohol) hydrogels prepared by freeze/thaw method. Carbohydr. Polym. 2015, 118, 60–69. [Google Scholar] [CrossRef]
- Bagri, L.P.; Saini, R.K.; Bajpai, A.K.; Choubey, R. Silver hydroxyapatite reinforced poly(vinyl alcohol)—starch cryogel nanocomposites and study of biodegradation, compressive strength and antibacterial activity. Polym. Eng. Sci. 2019, 59, 254–263. [Google Scholar] [CrossRef]
- Zandi, N.; Dolatyar, B.; Lotfi, R.; Shallageh, Y.; Shokrgozar, M.A.; Tamjid, E.; Annabi, N.; Simchi, A. Biomimetic nanoengineered scaffold for enhanced full-thickness cutaneous wound healing. Acta Biomater. 2021, 124, 191–204. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Wang, G.; Zhang, X.; Zheng, Y.; Lee, S.; Wang, D.; Yang, Y. Polyvinyl Alcohol/Chitosan and Polyvinyl Alcohol/Ag@MOF Bilayer Hydrogel for Tissue Engineering Applications. Polymers 2021, 13, 3151. https://doi.org/10.3390/polym13183151
Zhang M, Wang G, Zhang X, Zheng Y, Lee S, Wang D, Yang Y. Polyvinyl Alcohol/Chitosan and Polyvinyl Alcohol/Ag@MOF Bilayer Hydrogel for Tissue Engineering Applications. Polymers. 2021; 13(18):3151. https://doi.org/10.3390/polym13183151
Chicago/Turabian StyleZhang, Meng, Guohui Wang, Xin Zhang, Yuqi Zheng, Shaoxiang Lee, Dong Wang, and Yang Yang. 2021. "Polyvinyl Alcohol/Chitosan and Polyvinyl Alcohol/Ag@MOF Bilayer Hydrogel for Tissue Engineering Applications" Polymers 13, no. 18: 3151. https://doi.org/10.3390/polym13183151
APA StyleZhang, M., Wang, G., Zhang, X., Zheng, Y., Lee, S., Wang, D., & Yang, Y. (2021). Polyvinyl Alcohol/Chitosan and Polyvinyl Alcohol/Ag@MOF Bilayer Hydrogel for Tissue Engineering Applications. Polymers, 13(18), 3151. https://doi.org/10.3390/polym13183151




