3D Printing of Collagen/Oligomeric Proanthocyanidin/Oxidized Hyaluronic Acid Composite Scaffolds for Articular Cartilage Repair
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
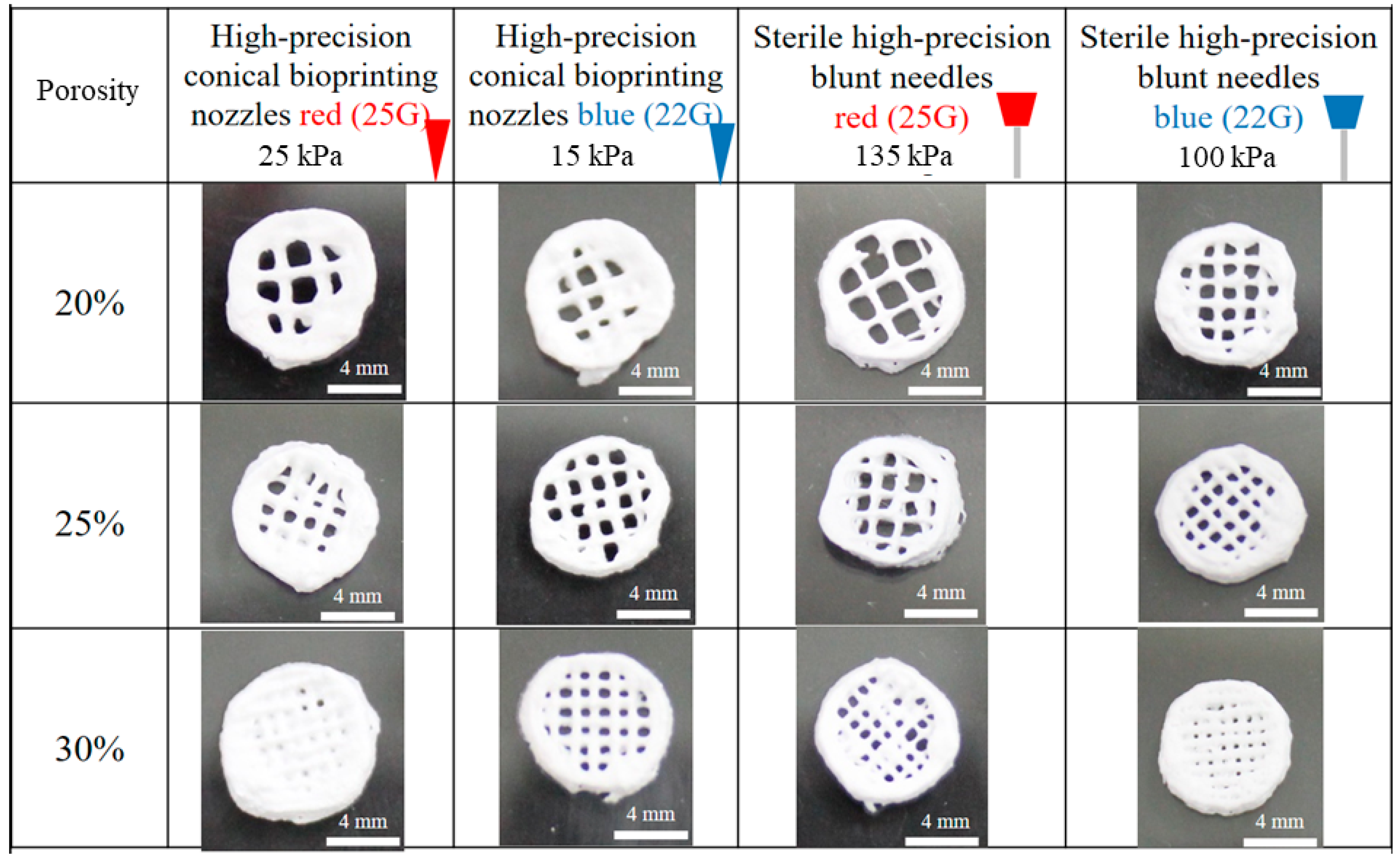
2.2. Fabrication of Scaffolds
2.3. Characterization of Materials
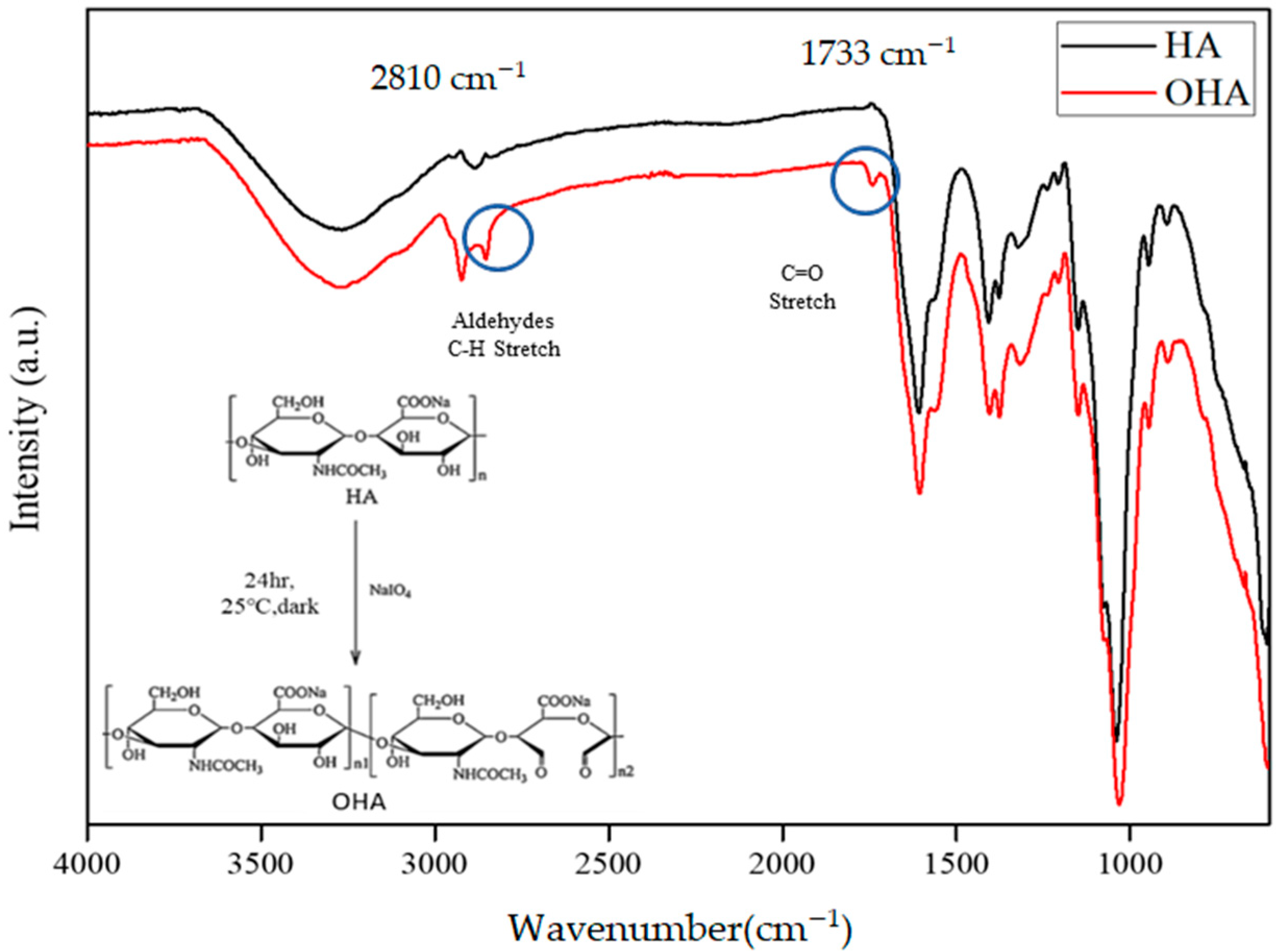
2.3.1. Fourier Transform Infra-Red (FTIR)
2.3.2. Scanning Electron Microscopy (SEM)
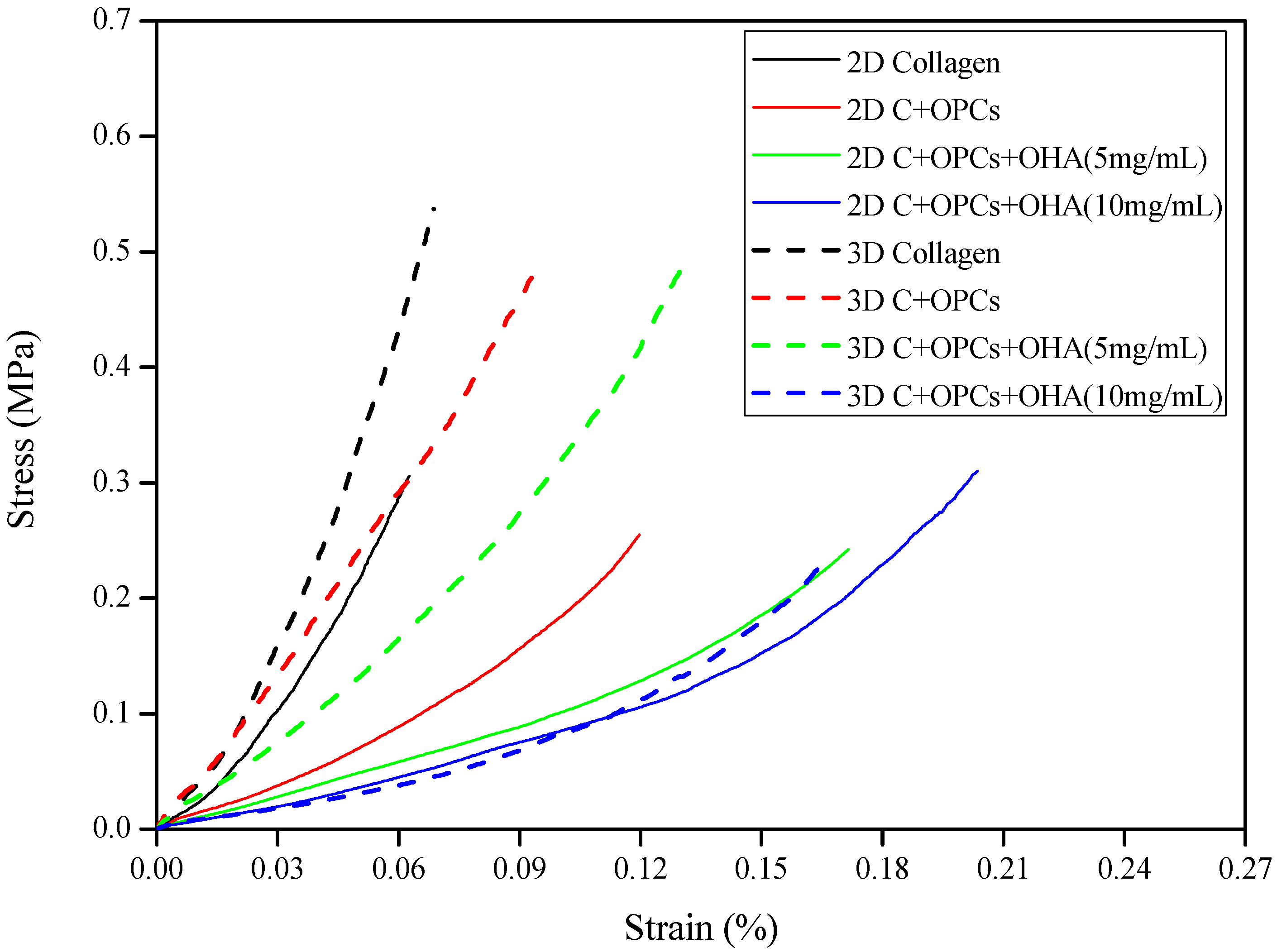
2.3.3. Mechanical Properties of the Scaffolds
2.3.4. Rheological Properties of the Hydrogel
2.4. In Vitro Experiments
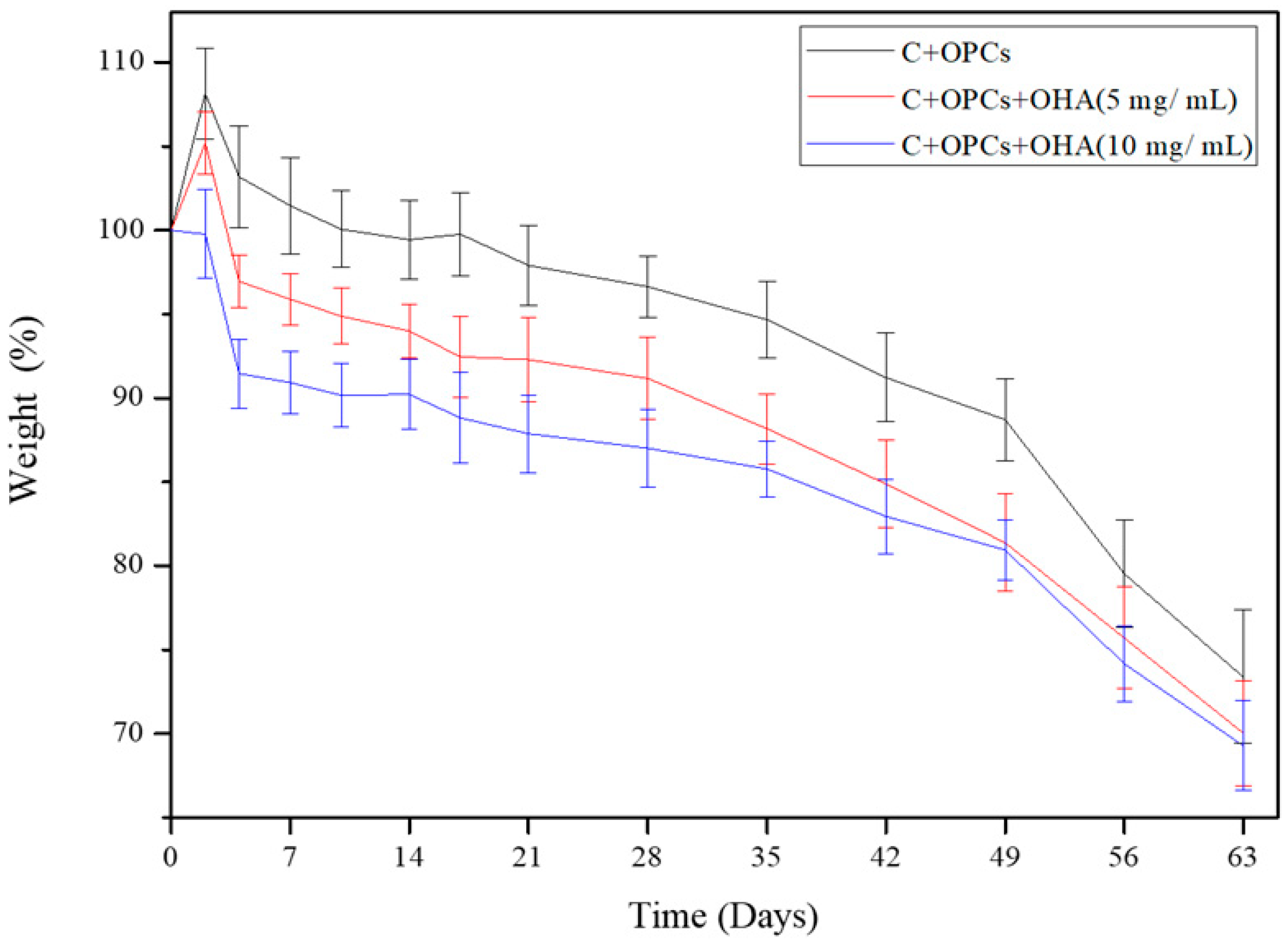
2.4.1. Degradation Rate
2.4.2. Bioactivity Evaluation
2.4.3. Cell Cytotoxicity and Cell Viability
2.5. In Vivo Tests
3. Results and Discussion
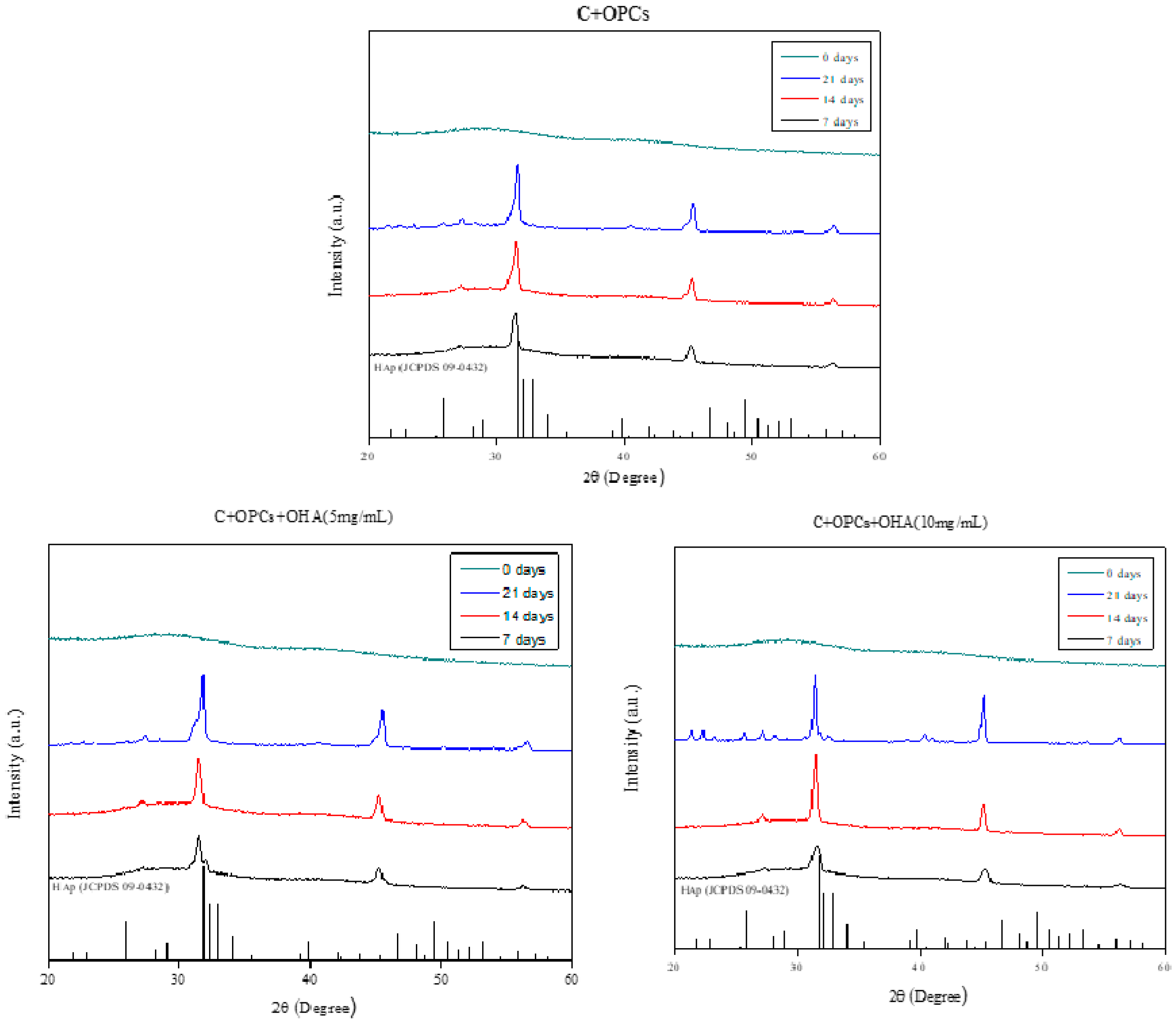
3.1. Characterizations of Collagen/OPC/OHA Composite Scaffolds
3.2. Degradation Behavior of Composite Scaffolds
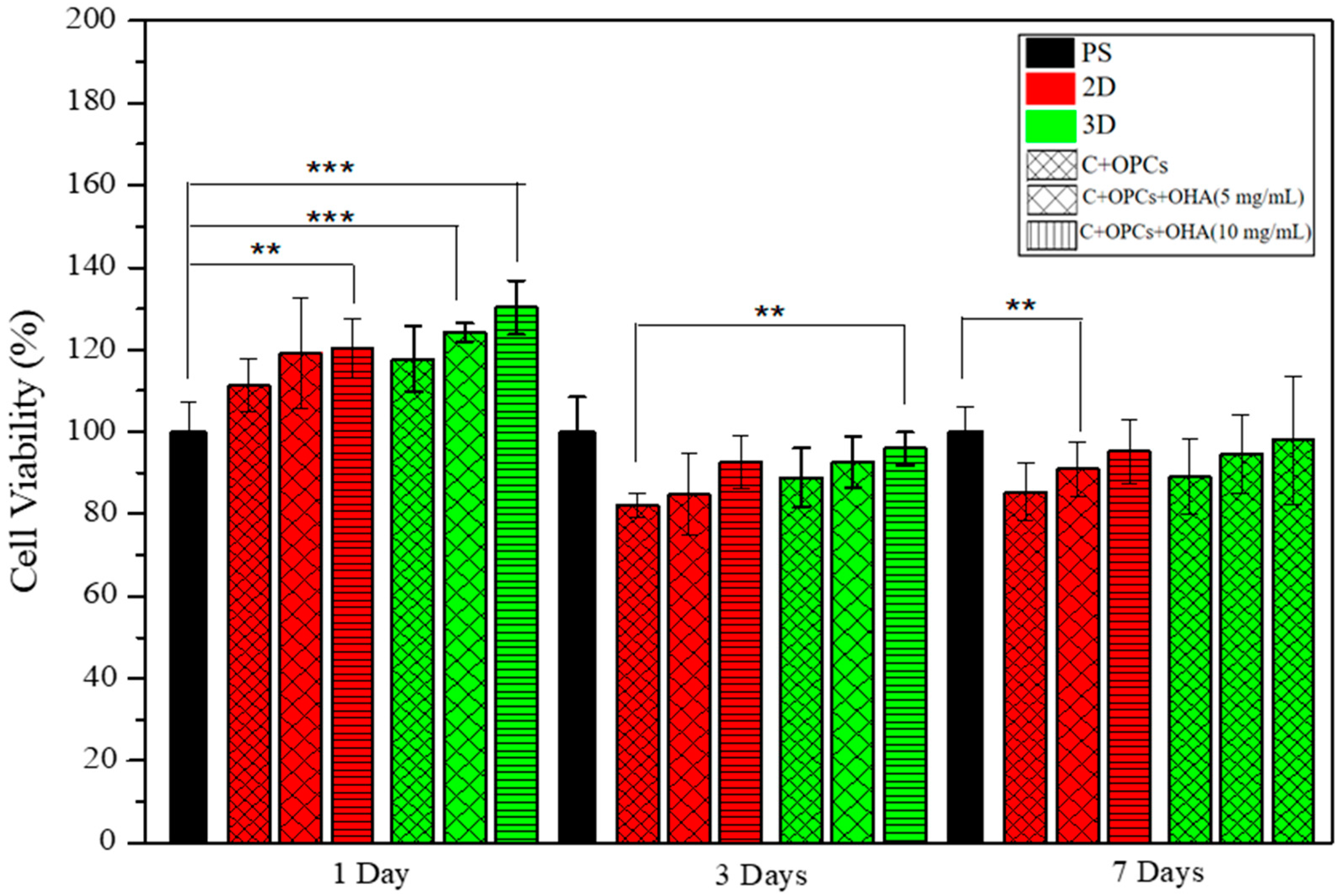
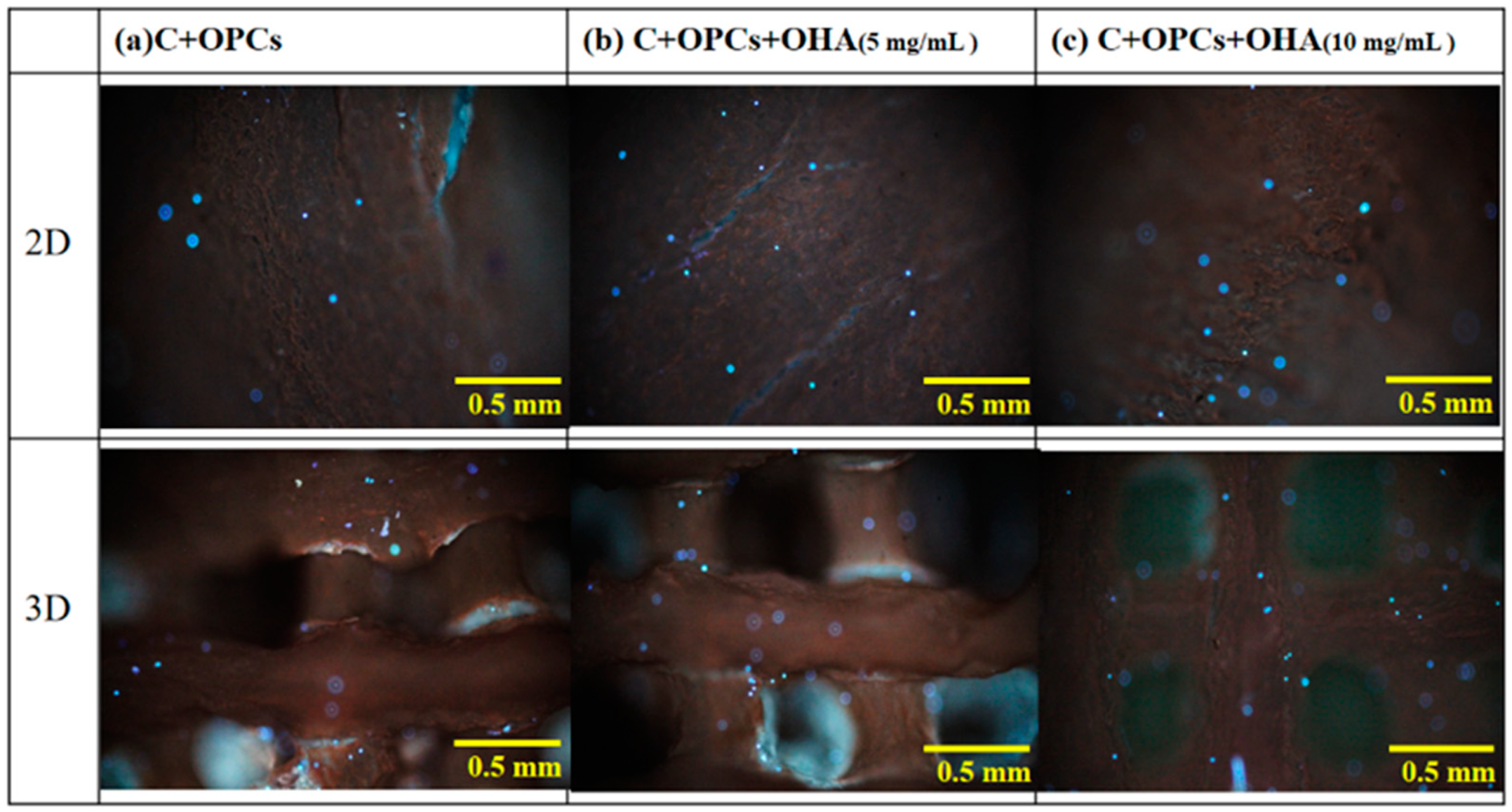
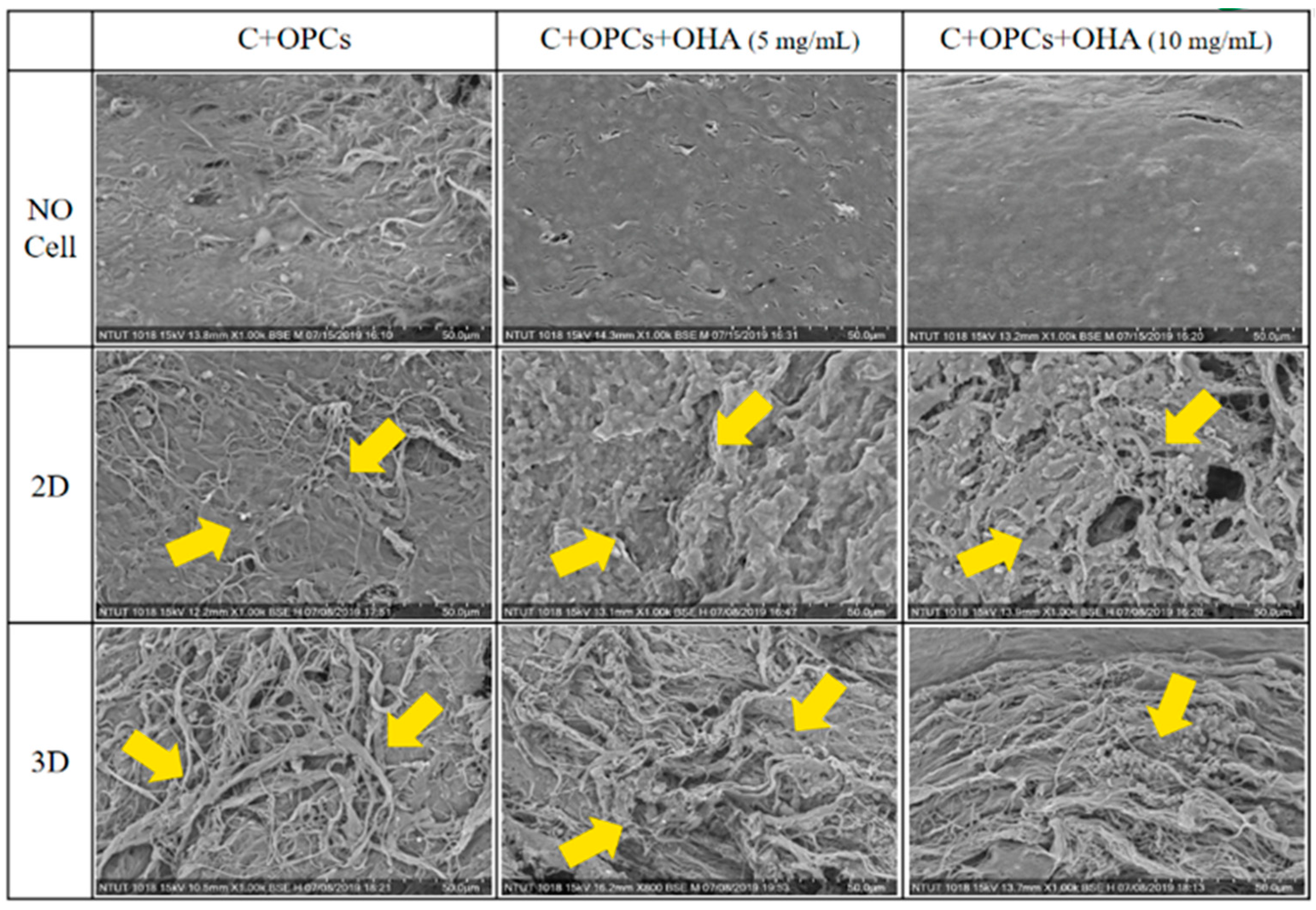
3.3. Bioactivity and Cell Viability
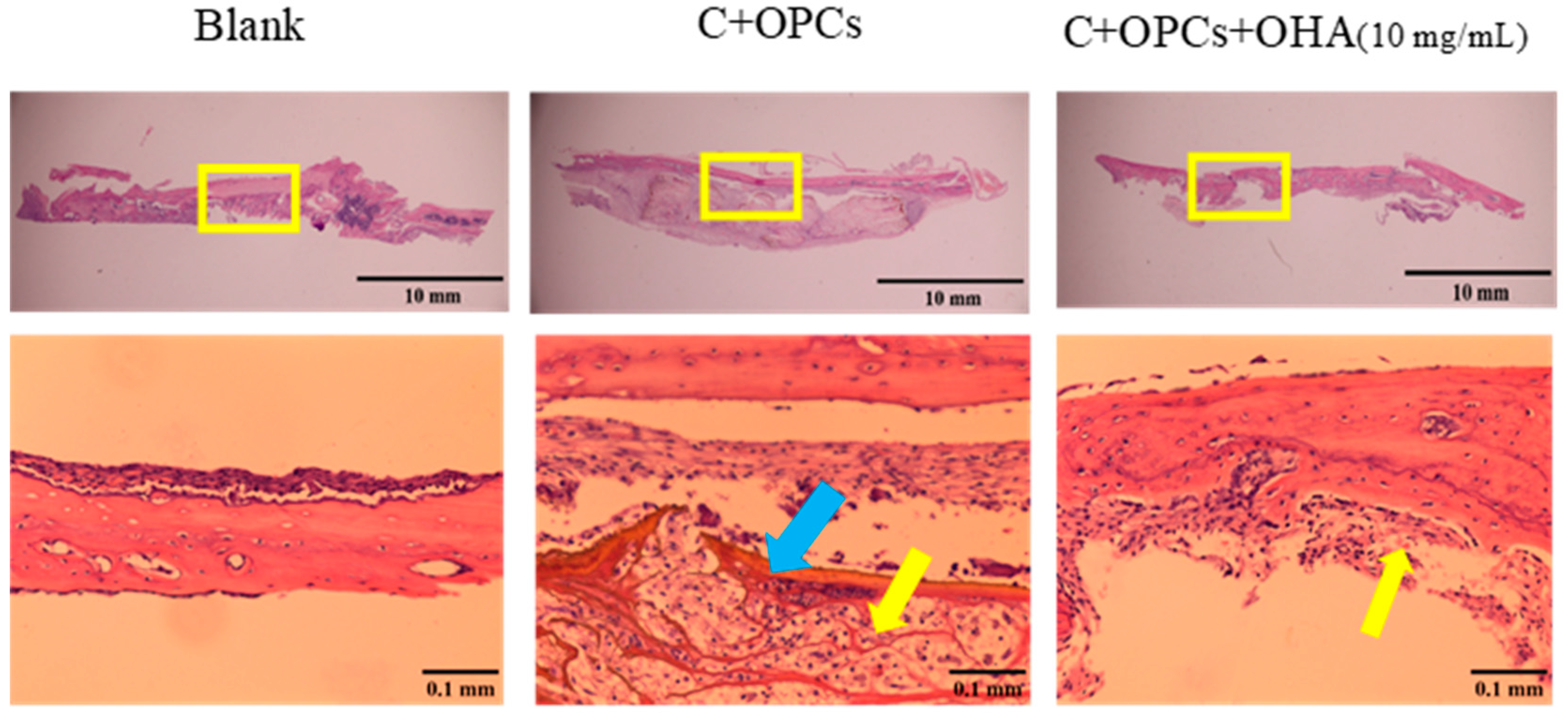
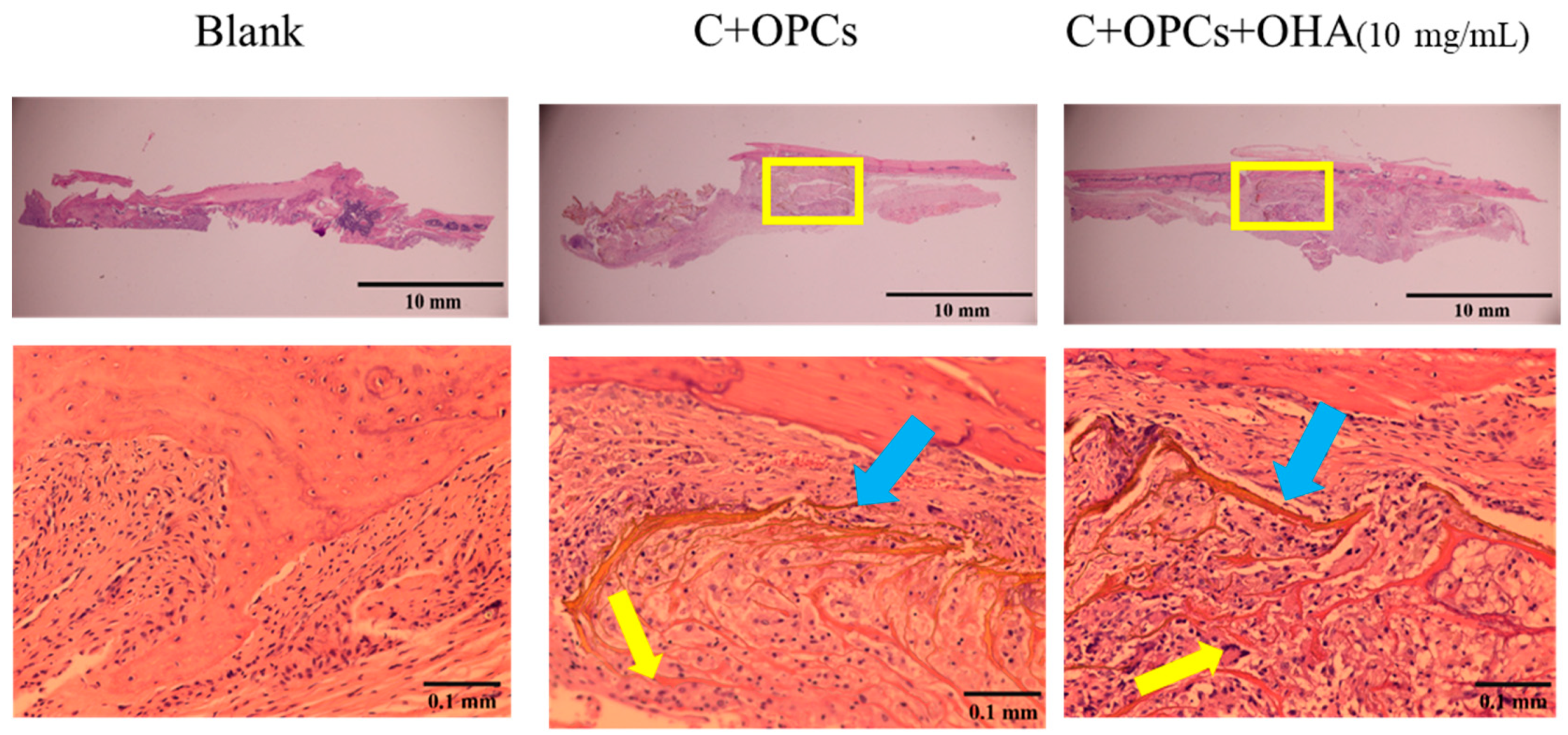
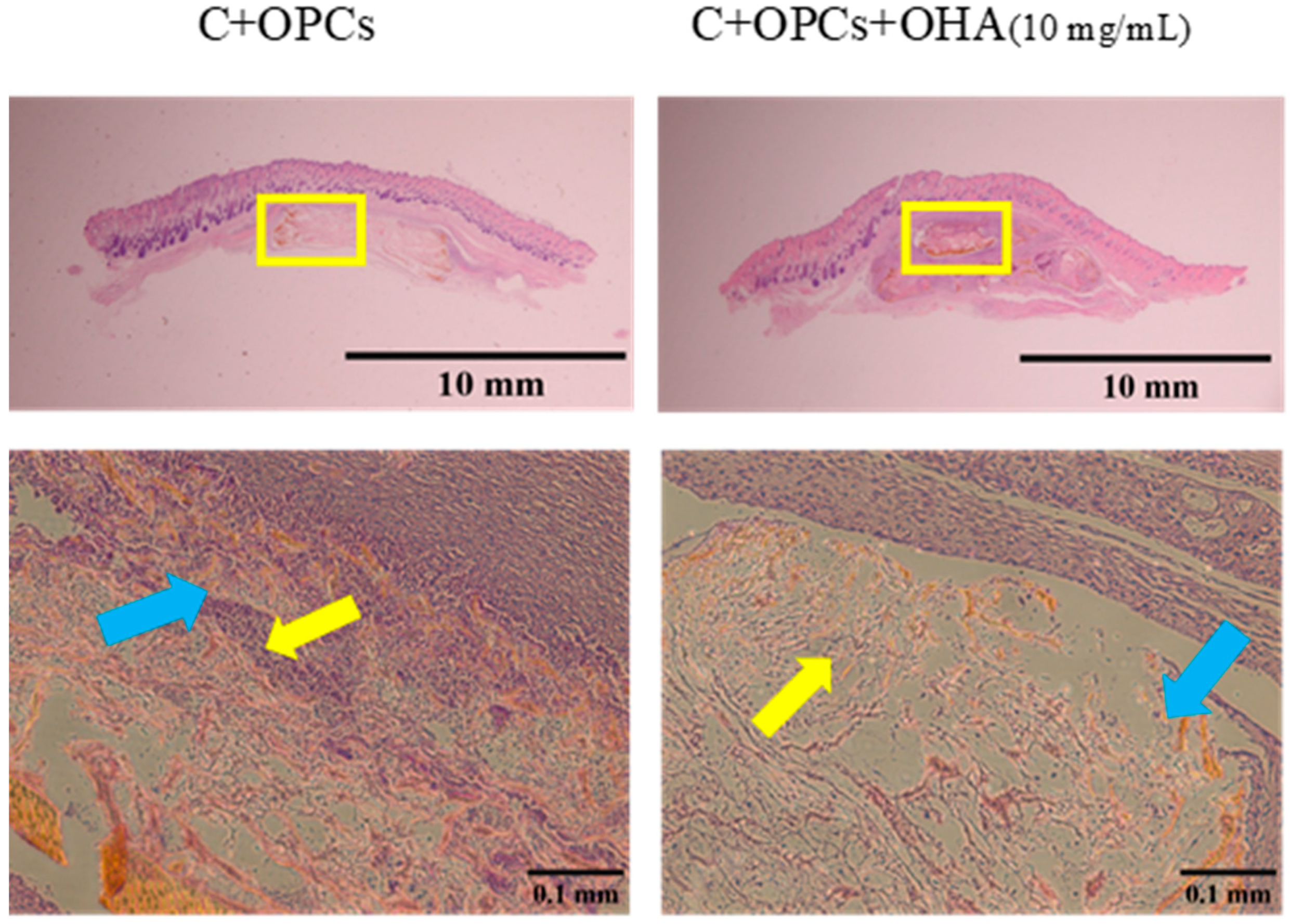
3.4. In Vivo Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenberg, S.E.; VanHouten, J.; Lakomkin, N.; Ehrenfeld, J.; Jahangir, A.A.; Boyce, R.H.; Obremksey, W.T.; Sethi, M.K. Does Admission to Medicine or Orthopaedics Impact a Geriatric Hip Patient’s Hospital Length of Stay? J. Orthop. Trauma 2016, 30, 95–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curl, W.W.; Krome, J.; Gordon, E.; Rushing, J.; Smith, B.P.; Poehling, G.G. Cartilage injuries: A review of 31,516 knee arthroscopies. Arthrosc. J. Arthrosc. Relat. Surg. 1997, 13, 456–460. [Google Scholar] [CrossRef]
- Pathria, M.N.; Chung, C.B.; Resnick, D.L. Acute and Stress-related Injuries of Bone and Cartilage: Pertinent Anatomy, Basic Biomechanics, and Imaging Perspective. Radiology 2016, 280, 21–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunziker, E. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthr. Cartil. 2002, 10, 432–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.-C.; Chen, C.-H.; Chiu, L.-H.; Tsuang, Y.-H.; Bai, M.-Y.; Chung, R.-J.; Lin, Y.-H.; Hsieh, F.-J.; Chen, Y.-T.; Yan, T.-L. Facilitating in vivo articular cartilage repair by tissue-engineered cartilage grafts produced from auricular chondrocytes. Am. J. Sports Med. 2018, 46, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Raynauld, J.-P.; Buckland-Wright, C.; Ward, R.; Choquette, D.; Haraoui, B.; Martel-Pelletier, J.; Uthman, I.; Khy, V.; Tremblay, J.-L.; Bertrand, C.; et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: A randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003, 48, 370–377. [Google Scholar] [CrossRef]
- Iannitti, T.; Lodi, D.; Palmieri, B. Intra-articular injections for the treatment of osteoarthritis. Drugs R D 2011, 11, 13–27. [Google Scholar] [CrossRef]
- Kennedy, M.I.; Whitney, K.; Evans, T.; Laprade, R.F. Platelet-Rich Plasma and Cartilage Repair. Curr. Rev. Musculoskelet. Med. 2018, 11, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-S.; Kim, C.-W.; Jung, D.-W. Management of Focal Chondral Lesion in the Knee Joint. Knee Surg. Relat. Res. 2011, 23, 185–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mankin, H.J. The response of articular cartilage to mechanical injury. J. Bone Jt. Surg. 1982, 64, 460–466. [Google Scholar] [CrossRef]
- Everhart, J.; Campbell, A.B.; Abouljoud, M.M.; Kirven, J.C.; Flanigan, D.C. Cost-efficacy of Knee Cartilage Defect Treatments in the United States. Am. J. Sports Med. 2019, 48, 242–251. [Google Scholar] [CrossRef]
- Basad, E.; Wissing, F.R.; Fehrenbach, P.; Rickert, M.; Steinmeyer, J.; Ishaque, B. Matrix-induced autologous chondrocyte implantation (MACI) in the knee: Clinical outcomes and challenges. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 3729–3735. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Kohane, D.S.; Langer, R. Polymeric Biomaterials in Tissue Engineering. Pediatr. Res. 2008, 63, 487–491. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, Q.T.; Hwang, Y.; Chen, A.C.; Varghese, S.; Sah, R.L. Cartilage-like mechanical properties of poly (ethylene glycol)-diacrylate hydrogels. Biomaterials 2012, 33, 6682–6690. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, C.M.; Kadoya, Y.; Hozumi, K.; Okano-Kosugi, H.; Asada, S.; Kitagawa, K.; Nomizu, M.; Koide, T. A collagen-mimetic triple helical supramolecule that evokes integrin-dependent cell responses. Biomaterials 2010, 31, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Chevallay, B.; Herbage, D. Collagen-based biomaterials as 3D scaffold for cell cultures: Applications for tissue engineering and gene therapy. Med. Biol. Eng. Comput. 2000, 38, 211–218. [Google Scholar] [CrossRef]
- Wolf, K.; Alexander, S.; Schacht, V.; Coussens, L.M.; von Andrian, U.H.; van Rheenen, J.; Deryugina, E.; Friedl, P. Collagen-based cell migration models in vitro and in vivo. Semin. Cell Dev. Biol. 2009, 20, 931–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef]
- Raemdonck, K.; Martens, T.F.; Braeckmans, K.; Demeester, J.; De Smedt, S. Polysaccharide-based nucleic acid nanoformulations. Adv. Drug Deliv. Rev. 2013, 65, 1123–1147. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef] [Green Version]
- Freeman, B.D.; Pinnau, I. Polymer Membranes for Gas and Vapor Separation; American Chemical Society: Washington, DC, USA, 1999. [Google Scholar]
- Lin, W.; Liu, Z.; Kampf, N.; Klein, J. The Role of Hyaluronic Acid in Cartilage Boundary Lubrication. Cells 2020, 9, 1606. [Google Scholar] [CrossRef]
- Singh, A.; Corvelli, M.; Unterman, S.A.; Wepasnick, K.A.; McDonnell, P.J.; Elisseeff, J.H. Enhanced lubrication on tissue and biomaterial surfaces through peptide-mediated binding of hyaluronic acid. Nat. Mater. 2014, 13, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chan, P.; Ho, K.; Fung, K.P.; Wang, J. Antioxidant activity of natural flavonoids is governed by number and location of their aromatic hydroxyl groups. Chem. Phys. Lipids 1996, 79, 157–163. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Fine, A.M. Oligomeric proanthocyanidin complexes: History, structure, and phytopharmaceutical applications. Altern. Med. Rev. 2000, 5, 144–151. [Google Scholar]
- Sugimoto, E.; Yamaguchi, M. Stimulatory effect of daidzein in osteoblastic MC3T3-E1 cells. Biochem. Pharmacol. 2000, 59, 471–475. [Google Scholar] [CrossRef]
- Kim, S.; Nimni, M.E.; Yang, Z.; Han, B. Chitosan/gelatin-based films crosslinked by proanthocyanidin. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2005, 75, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Jaurequi, J.; Tang, B.W.; Nimni, M.E. Proanthocyanidin: A natural crosslinking reagent for stabilizing collagen matrices. J. Biomed. Mater. Res. 2003, 65, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-Y.; Shyu, P.-C.; Dong, G.-C.; Chen, Y.-S.; Kuo, W.-W.; Yao, C.-H. Reconstruction of calvarial defect using a tricalcium phosphate-oligomeric proanthocyanidins cross-linked gelatin composite. Biomaterials 2009, 30, 1682–1688. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Chung, C.-M.; Chen, Y.-S.; Bau, D.-T.; Yao, C.-H. Rat bone marrow stromal cells-seeded porous gelatin/tricalcium phosphate/oligomeric proanthocyanidins composite scaffold for bone repair. J. Tissue Eng. Regen. Med. 2012, 7, 708–719. [Google Scholar] [CrossRef]
- Ebenezar, A.R.; Srinivasan, N.; Manimaran, V.S.; Srinivasulu, S.; Mahalaxmi, S. Application of a proanthocyanidin agent to improve the bond strength of root dentin treated with sodium hypochlorite. J. Conserv. Dent. 2011, 14, 306–308. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, I.W.; Lee, Y.M.; Lee, H.B.; Khang, G. Macroporous biodegradable natural/synthetic hybrid scaffolds as small intestine submucosa impregnated poly(d, l-lactide-co-glycolide) for tissue-engineered bone. J. Biomater. Sci. Polym. Ed. 2004, 15, 1003–1017. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, Y.; Jin, Q.; Takita, H. Geometry of Carriers Controlling Phenotypic Expression in BMP-Induced Osteogenesis and Chondrogenesis. J. Bone Jt. Surg. 2001, 83, S1–S105. [Google Scholar] [CrossRef]
- Roosa, S.M.M.; Kemppainen, J.M.; Moffitt, E.N.; Krebsbach, P.H.; Hollister, S.J. The pore size of polycaprolactone scaffolds has limited influence on bone regeneration in an in vivo model. J. Biomed. Mater. Res. Part A 2010, 92, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Shima, S.; Oyane, M. Plasticity theory for porous metals. Int. J. Mech. Sci. 1976, 18, 285–291. [Google Scholar] [CrossRef]
- Shin, Y.J.; Shafranek, R.T.; Tsui, J.H.; Walcott, J.; Nelson, A.; Kim, D.-H. 3D bioprinting of mechanically tuned bioinks derived from cardiac decellularized extracellular matrix. Acta Biomater. 2021, 119, 75–88. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-F.; Hsu, Y.-H.; Lin, Y.-C.; Nguyen, T.-T.; Chen, H.-W.; Nabilla, S.C.; Hou, S.-Y.; Chang, F.-C.; Chung, R.-J. 3D Printing of Collagen/Oligomeric Proanthocyanidin/Oxidized Hyaluronic Acid Composite Scaffolds for Articular Cartilage Repair. Polymers 2021, 13, 3123. https://doi.org/10.3390/polym13183123
Lee C-F, Hsu Y-H, Lin Y-C, Nguyen T-T, Chen H-W, Nabilla SC, Hou S-Y, Chang F-C, Chung R-J. 3D Printing of Collagen/Oligomeric Proanthocyanidin/Oxidized Hyaluronic Acid Composite Scaffolds for Articular Cartilage Repair. Polymers. 2021; 13(18):3123. https://doi.org/10.3390/polym13183123
Chicago/Turabian StyleLee, Chung-Fei, Yung-Heng Hsu, Yu-Chien Lin, Thu-Trang Nguyen, Hsiang-Wen Chen, Sasza Chyntara Nabilla, Shao-Yi Hou, Feng-Cheng Chang, and Ren-Jei Chung. 2021. "3D Printing of Collagen/Oligomeric Proanthocyanidin/Oxidized Hyaluronic Acid Composite Scaffolds for Articular Cartilage Repair" Polymers 13, no. 18: 3123. https://doi.org/10.3390/polym13183123
APA StyleLee, C.-F., Hsu, Y.-H., Lin, Y.-C., Nguyen, T.-T., Chen, H.-W., Nabilla, S. C., Hou, S.-Y., Chang, F.-C., & Chung, R.-J. (2021). 3D Printing of Collagen/Oligomeric Proanthocyanidin/Oxidized Hyaluronic Acid Composite Scaffolds for Articular Cartilage Repair. Polymers, 13(18), 3123. https://doi.org/10.3390/polym13183123






