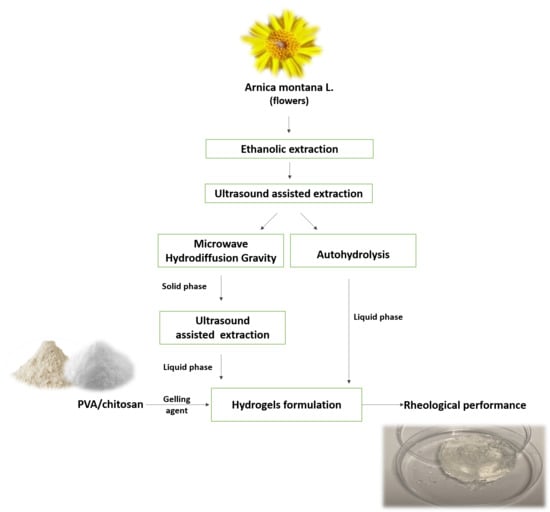
Valorization of Arnica montana Wastes after Extraction of the Ethanol Tincture: Application in Polymer-Based Matrices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
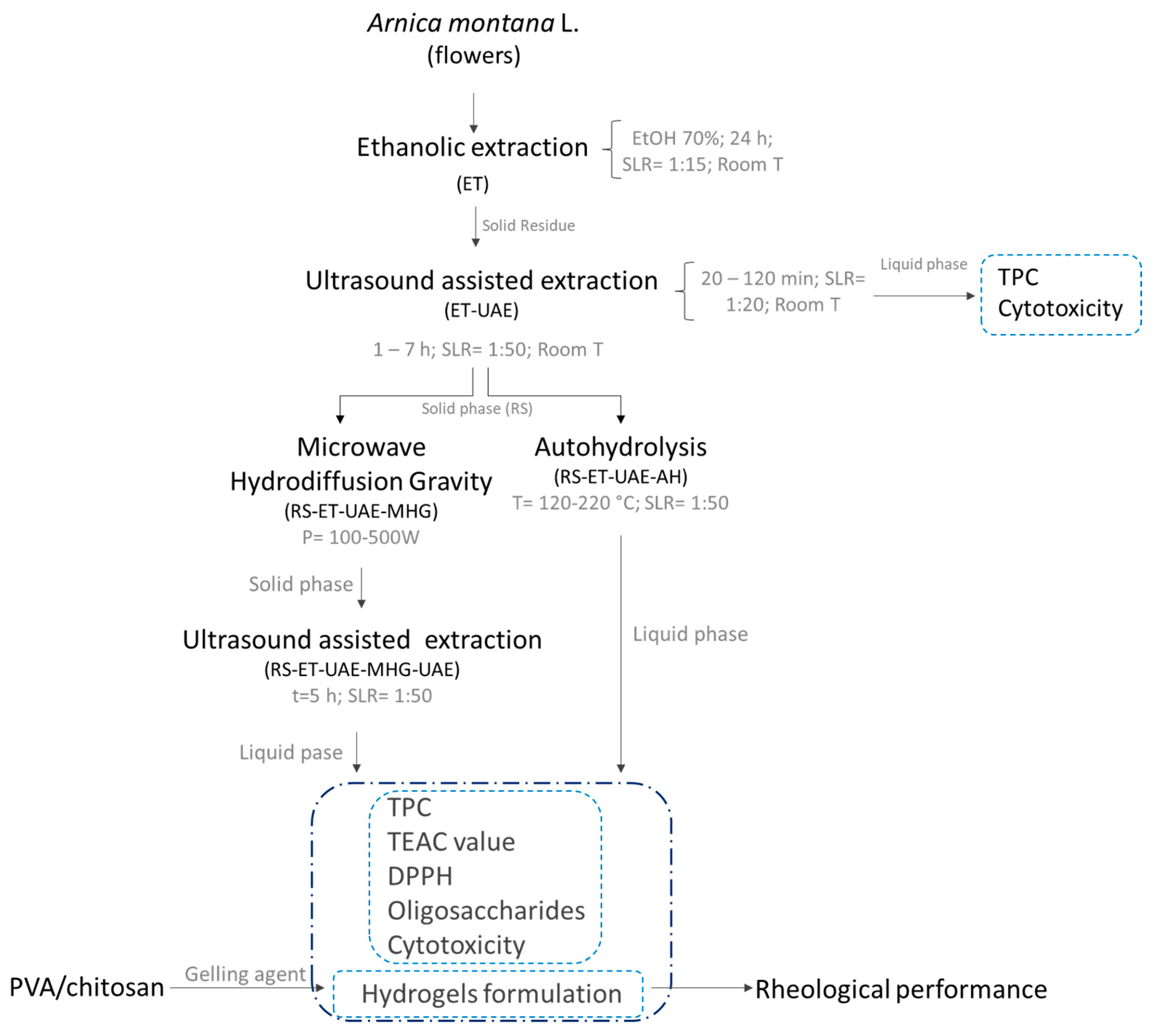
2.2. Extraction Technologies
2.2.1. Ethanolic Extraction
2.2.2. Ultrasound-Assisted Extraction
2.2.3. Pressurized Hot Water Extraction or Autohydrolysis
2.2.4. Microwave Hydrodiffusion Gravity-Ultrasound Assisted Extraction
2.3. Oligosaccharide Content
2.4. Antioxidant Activity
2.5. Total Phenolic Content
2.6. Molar Mass Distribution
2.7. Cytotoxicity
2.8. Formulation of Novel Topical Dressings
2.9. Rheology Testing
2.10. Statistical Analysis
3. Results and Discussion
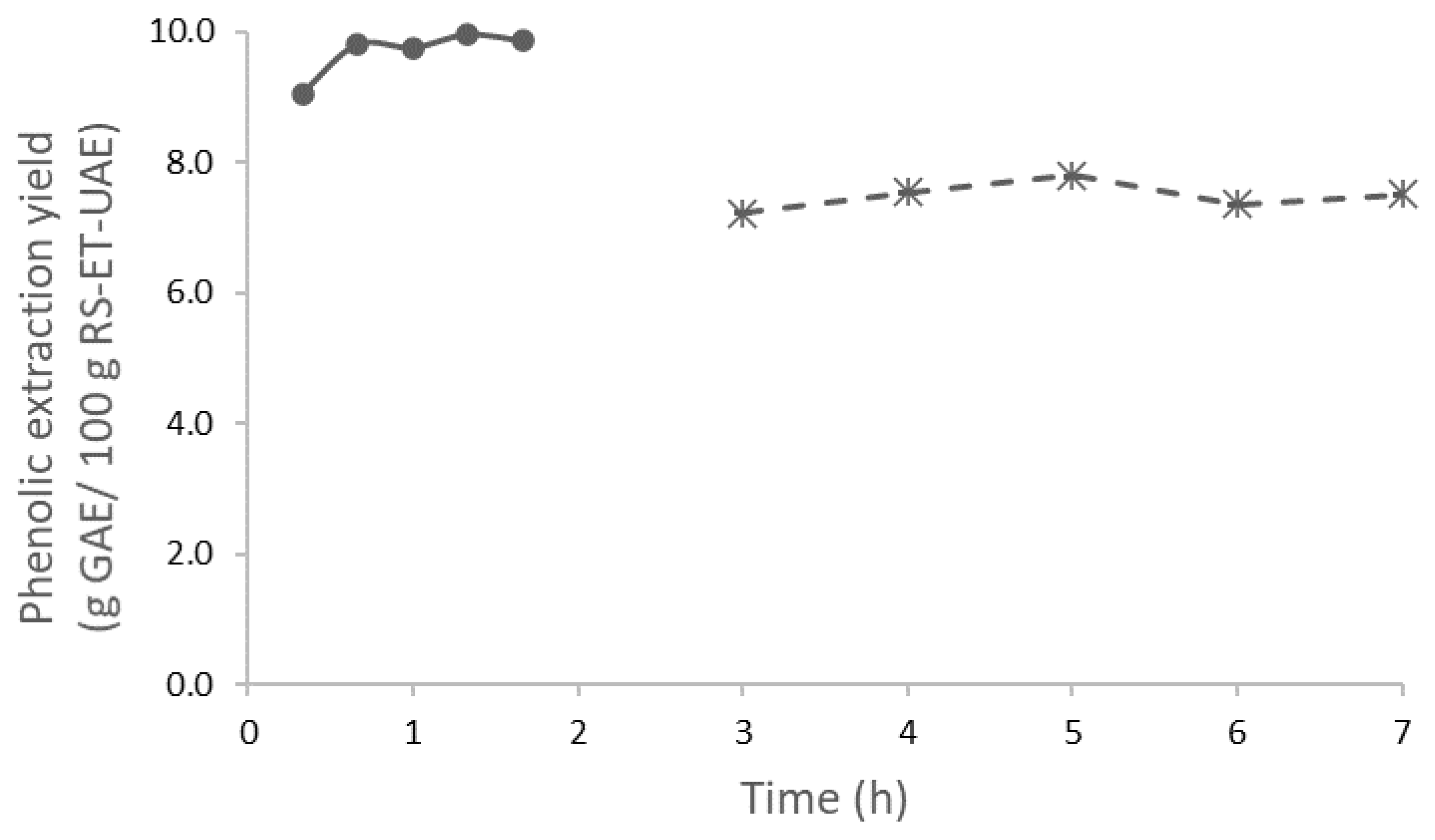
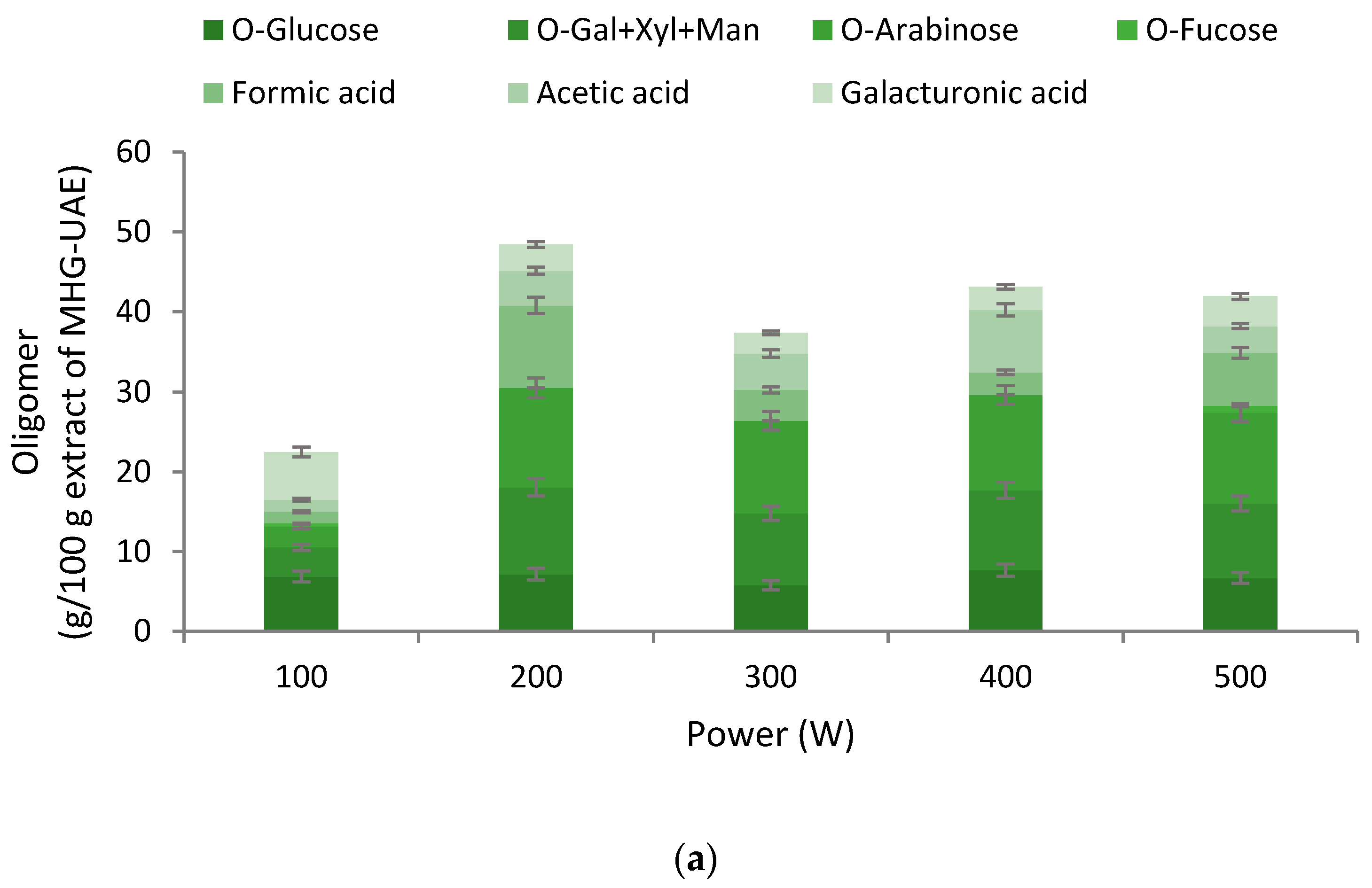
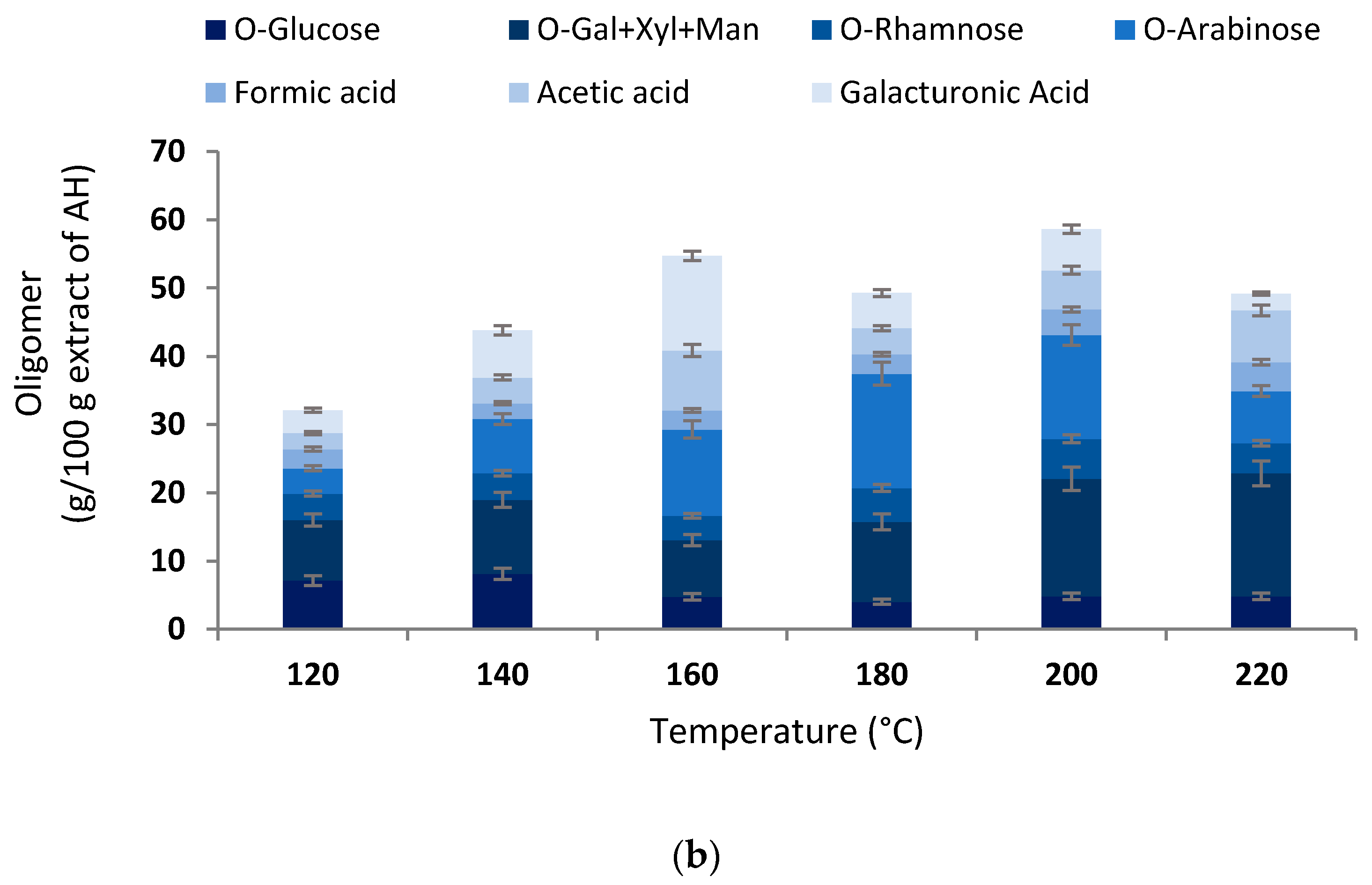
3.1. Extraction Yields, Chemical Features, and Antioxidant Properties
3.2. Cytotoxicity
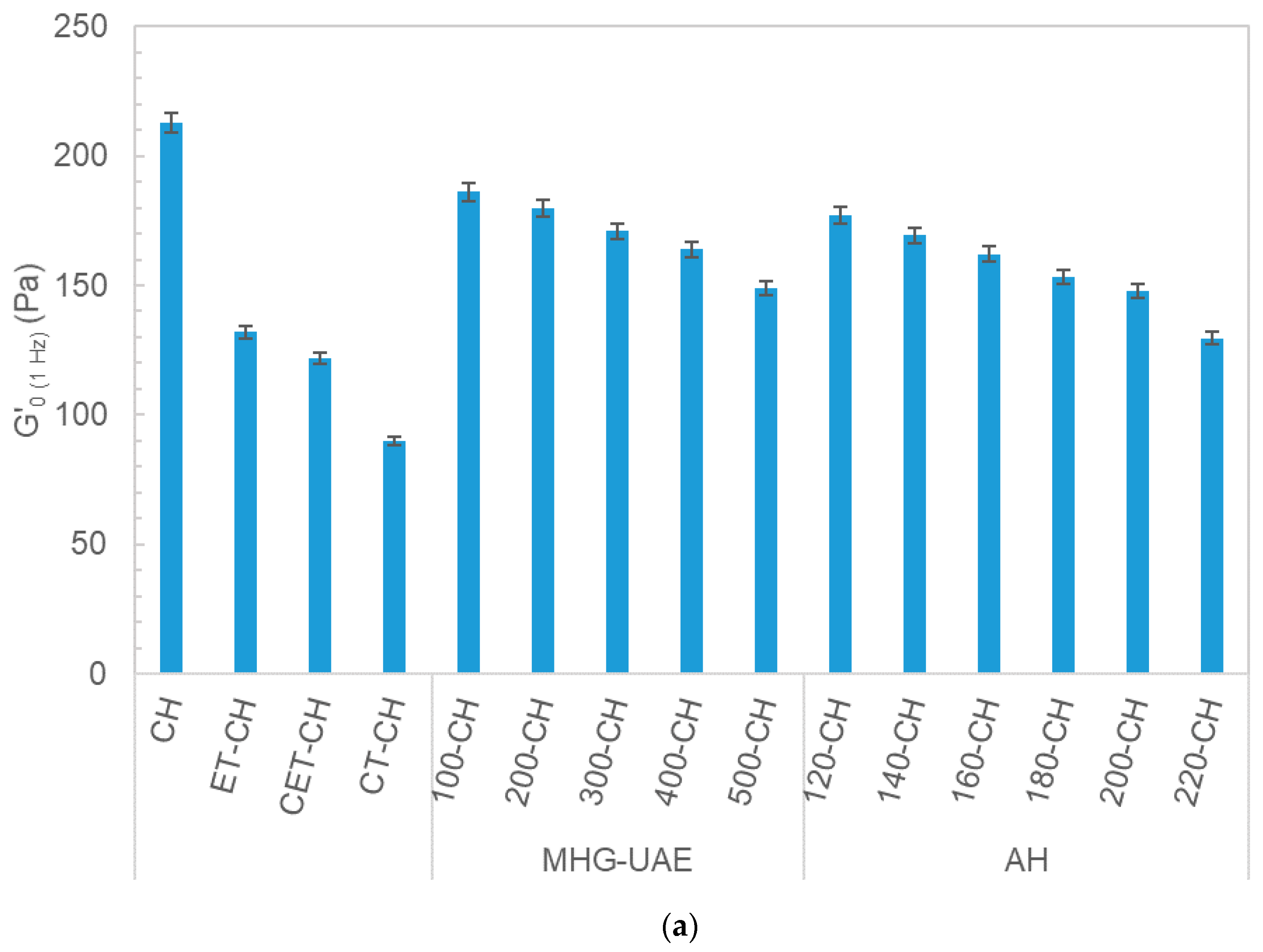
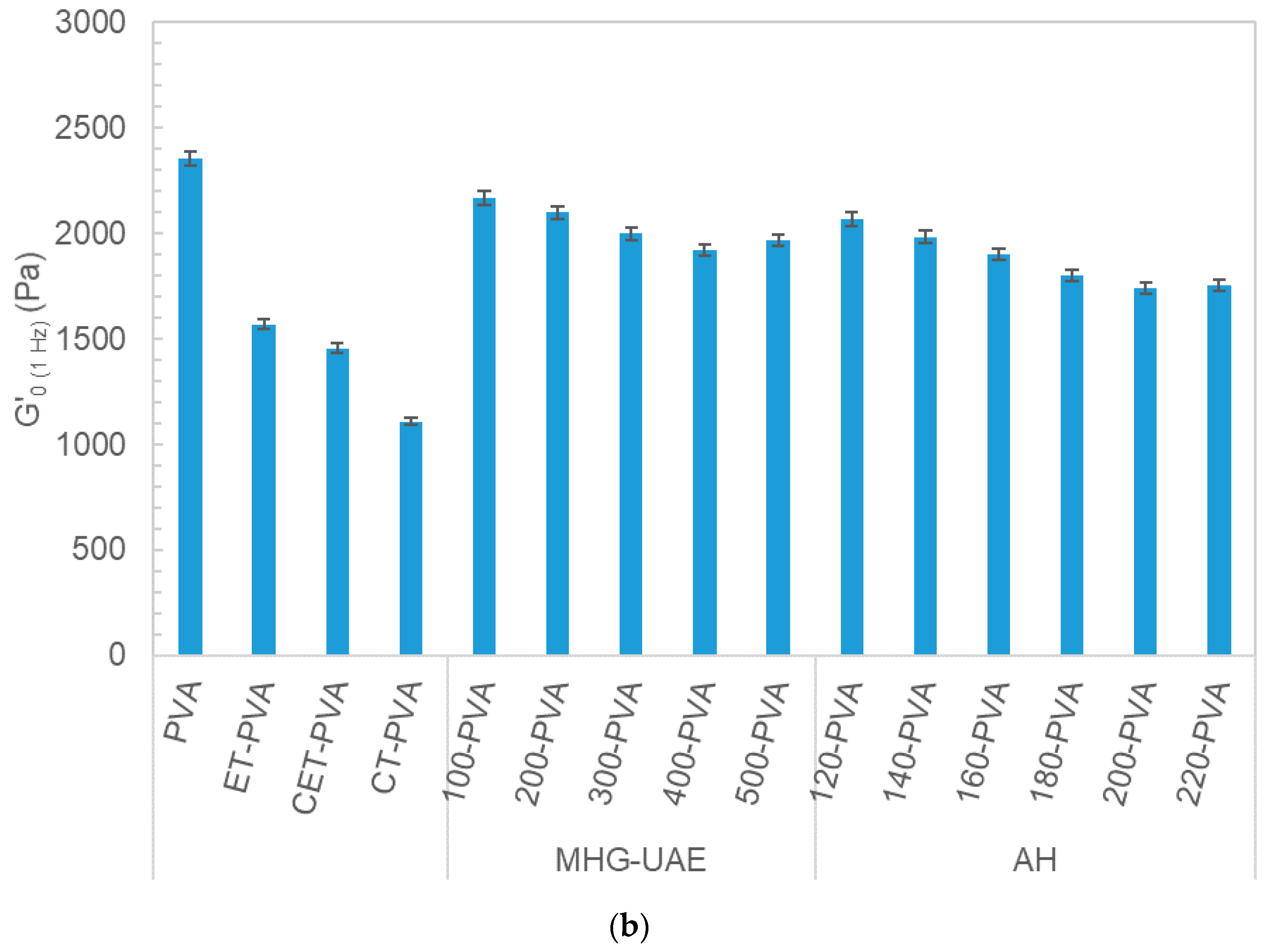
3.3. Rheology of Potential Topic Dressings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, T.; Debnath, J.; Nath, B.; Dash, S. Formulation and evaluation of an herbal cream for wound healing activity. Int. J. Pharm. Pharm. Sci. 2014, 6, 693–697. [Google Scholar]
- Šutovská, M.; Capek, P.; Kočmalová, M.; Pawlaczyk, I.; Zaczyńska, E.; Czarny, A.; Uhliariková, I.; Gancarz, R.; Fraňová, S. Characterization and pharmacodynamic properties of Arnica montana complex. Int. J. Biol. Macromol. 2014, 69, 214–221. [Google Scholar] [CrossRef]
- Sharma, S.; Arif, M.; Nirala, R.K.; Gupta, R.; Thakur, S.C. Cumulative therapeutic effects of phytochemicals in Arnica montana flower extract alleviated collagen-induced arthritis: Inhibition of both pro-inflammatory mediators and oxidative stress. J. Sci. Food Agric. 2016, 96, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Kriplani, P.; Guarve, K.; Baghael, U.S. Arnica montana L.—A plant of healing: Review. J. Pharm. Pharmacol. 2017, 69, 925–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robledo, S.M.; Vélez, I.D.; Schmidt, T.J. Arnica tincture cures cutaneous leishmaniasis in golden hamsters. Molecules 2018, 23, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kriplani, P.; Guarve, K. Recent Patents on Anti-Cancer Potential of Helenalin. Recent Pat. Anticancer. Drug Discov. 2020, 15, 132–142. [Google Scholar]
- Pljevljakušić, D.; Janković, T.; Jelačić, S.; Novaković, M.; Menković, N.; Beatović, D.; Dajić-Stevanović, Z. Morphological and chemical characterization of Arnica montana L. under different cultivation models. Ind. Crops Prod. 2014, 52, 233–244. [Google Scholar] [CrossRef]
- Stefanache, C.P.; Peter, S.; Meier, B.; Danila, D.; Tanase, C.; Wolfram, E. Phytochemical composition of arnicae flos from wild populations in the northern area of the romanian eastern carpathians. Rev. Chim. 2015, 66, 784–787. [Google Scholar]
- Ganzera, M.; Egger, C.; Zidorn, C.; Stuppner, H. Quantitative analysis of flavonoids and phenolic acids in Arnica montana L. by micellar electrokinetic capillary chromatography. Anal. Chim. Acta 2008, 614, 196–200. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.M. Identification of hydroxycinnamoylquinic acids of arnica flowers and burdock roots using a standardized LC-DAD-ESI/MS profiling method. J. Agric. Food Chem. 2008, 56, 10105–10114. [Google Scholar] [CrossRef]
- Clauser, M.; Aiello, N.; Scartezzini, F.; Innocenti, G.; Dall’Acqua, S. Differences in the chemical composition of Arnica montana flowers from wild populations of north Italy. Nat. Prod. Commun. 2014, 9, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Kimel, K.; Godlewska, S.; Krauze-Baranowska, M.; PobŁocka-Olech, L. HPLC-DAD-ESI/MS analysis of Arnica TM constituents. Acta Pol. Pharm. Drug Res. 2019, 76, 1015–1027. [Google Scholar] [CrossRef]
- Balabanova, V.; Gevrenova, R.; Zheleva-Dimitrova, D. HPLC determination of phenolic acids in Arnicae flos. Comptes Rendus L’Academie Bulg. Des Sci. 2013, 66, 1547–1552. [Google Scholar]
- Nikolova, M.; Petrova, M.; Zayova, E.; Vitkova, A.; Evstatieva, L. Comparative study of in vitro, ex vitro and in vivo grown plants of Arnica montana-Polyphenols and free radical scavenging activity. Acta Bot. Croat. 2013, 72, 13–22. [Google Scholar] [CrossRef]
- Rywaniak, J.; Luzak, B.; Podsedek, A.; Dudzinska, D.; Rozalski, M.; Watala, C. Comparison of cytotoxic and anti-platelet activities of polyphenolic extracts from Arnica montana flowers and Juglans regia husks. Platelets 2015, 26, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, O.; Constantin, D.; Gaspar, A.; Toma, L.; Utoiu, E.; Moldovan, L. Evaluation of antioxidant and cytoprotective activities of Arnica montana L. and Artemisia absinthium L. ethanolic extracts. Chem. Cent. J. 2012, 6, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merfort, I. Arnica: Current state of efficacy, pharmacokinetics and side effects. Z. Fur Phyther. 2010, 31, 188–192. [Google Scholar] [CrossRef]
- Gaspar, A.; Craciunescu, O.; Trif, M.; Moisei, M.; Moldovan, L. Antioxidant and anti-inflammatory properties of active compounds from Arnica montana L. Rom. Biotechnol. Lett. 2014, 19, 9353–9365. [Google Scholar]
- Oliveira, R.N.; Paranhos da Silva, C.M.; Moreira, A.P.D.; Mendonça, R.H.; Thiré, R.M. da S.M.; McGuinness, G.B. Comparative analysis of PVA hydrogels incorporating two natural antimicrobials: Punica granatum and Arnica montana tinctures. J. Appl. Polym. Sci. 2017, 134, 45392. [Google Scholar] [CrossRef]
- Sánchez-Aguinagalde, O.; Lejardi, A.; Meaurio, E.; Hernández, R.; Mijangos, C.; Sarasua, J.R. Novel hydrogels of chitosan and poly (Vinyl alcohol) reinforced with inorganic particles of bioactive glass. Polymers 2021, 13, 691. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar]
- Chhabra, P.; Mehra, L.; Mittal, G.; Kumar, A. A comparative study on the efficacy of chitosan gel formulation and conventional silver sulfadiazine treatment in healing burn wound injury at molecular level. Asian J. Pharm. 2017, 11, S489–S496. [Google Scholar]
- López-Hortas, L.; Le Juge, C.; Falqué, E.; Domínguez, H.; Torres, M.D. Bioactive extracts from edible nettle leaves using microwave hydrodiffusion and gravity and distillation extraction techniques. Process Biochem. 2020, 94, 66–78. [Google Scholar] [CrossRef]
- Moldovan, L.; Gaspar, A.; Toma, L.; Craciunescu, O.; Saviuc, C. Comparison of polyphenolic content and antioxidant capacity of five romanian traditional medicinal plants. Rev. Chim. 2011, 62, 299–303. [Google Scholar]
- Zheleva-Dimitrova, D.; Balabanova, V. Antioxidant and acetylcholinesterase inhibitory potential of Arnica montana cultivated in Bulgaria. Turk. J. Biol. 2012, 36, 732–737. [Google Scholar]
- Vidic, D.; Ćavar Zeljković, S.; Dizdar, M.; Maksimović, M. Essential oil composition and antioxidant activity of four Asteraceae species from Bosnia. J. Essent. Oil Res. 2016, 28, 445–457. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Hydrogel-Based Strategies to Advance Therapies for Chronic Skin Wounds. Annu. Rev. Biomed. Eng. 2019, 21, 145–169. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, A.R.; Eivazlou, R.; Razavi, S.M.A. Steady shear flow behavior of sage seed gum affected by various salts and sugars: Time-independent properties. Int. J. Biol. Macromol. 2016, 91, 1018–1024. [Google Scholar] [CrossRef]


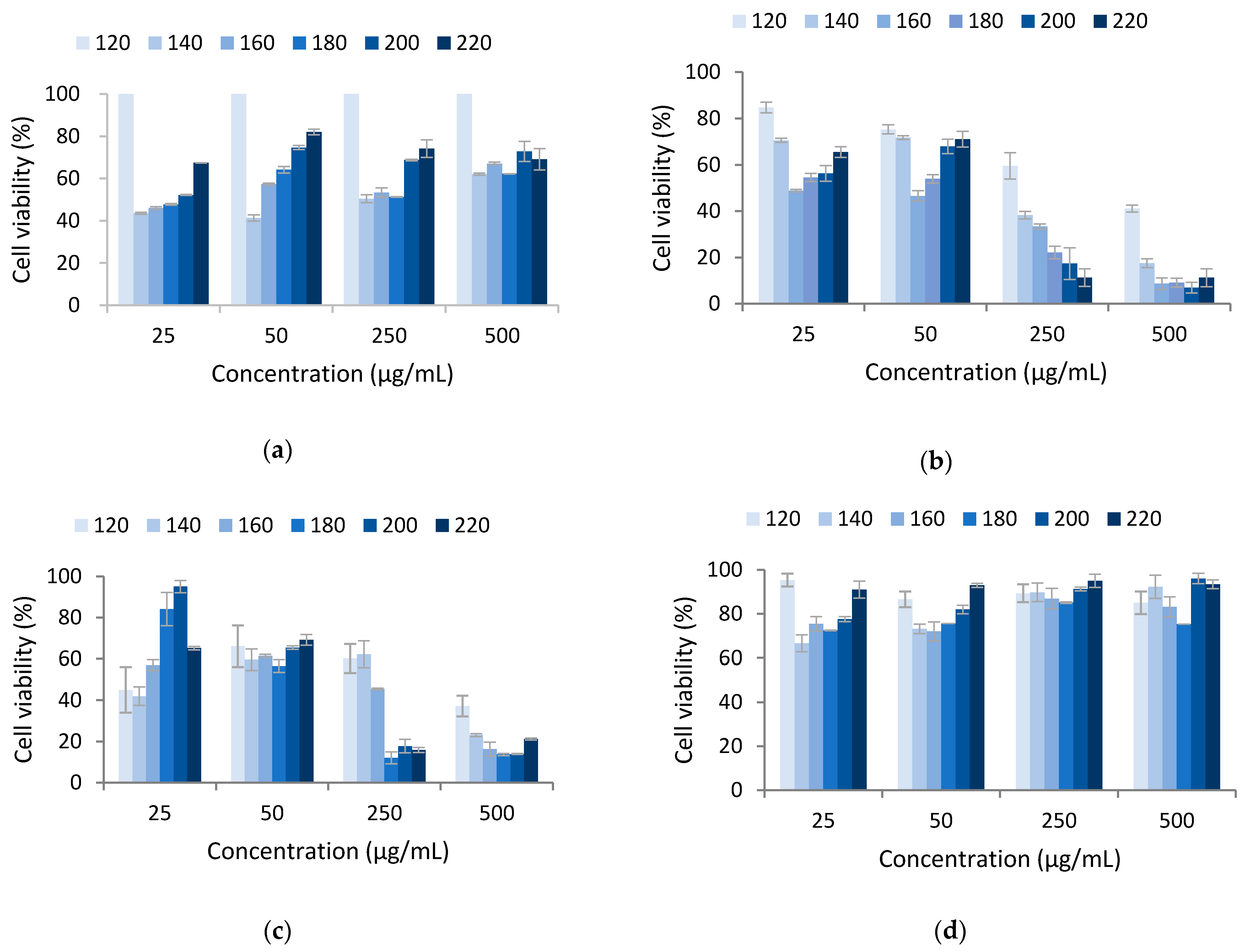

| Cell Viability (%) of Extracts Obtained at Different Microwave Powers (Extract Concentration; µg/mL) | |||||
|---|---|---|---|---|---|
| Cell Line | 100 W | 200 W | 300 W | 400 W | 500 W |
| A548 | 78.3 ± 1.5; (50) | 46.7 ± 0.6; (50) | 56.8 ± 1.6; (50) | 58.5 ± 0.3; (25) | 42.4 ± 1.3; (50) |
| HCT-116 | 63.8 ± 5.1; (25) | 49.2 ± 7.4; (250) | 52.9 ± 2.4; (500) | 65.3 ± 5.0; (500) | 57.0 ± 16.2; (50) |
| PSN1 | 69.4 ± 3.6; (25) | 69.9 ± 2.6; (25) | 53.0 ± 4.5; (25) | 50.4 ± 0.5; (25) | 65.5 ± 6.7; (25) |
| Cell Viability (%) Obtained at Different Extraction Times (Extract Concentration in µg/mL) | ||||||
|---|---|---|---|---|---|---|
| Cell Line | 20 min | 40 min | 60 min | 80 min | 100 min | 120 min |
| HCT-116 | 76.1 ± 5.3; (500) | 49.3 ± 3.1; (500) | 59.1 ± 5.9; (500) | 49.8 ± 3.8; (500) | 50.3 ± 2.2; (500) | 38.7 ± 2.4; (250) |
| PSN1 | 55.6 ± 1.4; (250) | 31.2 ± 0.8; (500) | 43.1 ± 1.1; (500) | 73.9 ± 1.9; (500) | 37.6 ± 1.0; (250) | 43.9 ± 1.1; (250) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flórez-Fernández, N.; Ferreira-Anta, T.; Torres, M.D.; Domínguez, H. Valorization of Arnica montana Wastes after Extraction of the Ethanol Tincture: Application in Polymer-Based Matrices. Polymers 2021, 13, 3121. https://doi.org/10.3390/polym13183121
Flórez-Fernández N, Ferreira-Anta T, Torres MD, Domínguez H. Valorization of Arnica montana Wastes after Extraction of the Ethanol Tincture: Application in Polymer-Based Matrices. Polymers. 2021; 13(18):3121. https://doi.org/10.3390/polym13183121
Chicago/Turabian StyleFlórez-Fernández, Noelia, Tania Ferreira-Anta, María Dolores Torres, and Herminia Domínguez. 2021. "Valorization of Arnica montana Wastes after Extraction of the Ethanol Tincture: Application in Polymer-Based Matrices" Polymers 13, no. 18: 3121. https://doi.org/10.3390/polym13183121
APA StyleFlórez-Fernández, N., Ferreira-Anta, T., Torres, M. D., & Domínguez, H. (2021). Valorization of Arnica montana Wastes after Extraction of the Ethanol Tincture: Application in Polymer-Based Matrices. Polymers, 13(18), 3121. https://doi.org/10.3390/polym13183121